Dear Editor
Respiratory failure is common and the most severe complication in Coronavirus SARS-CoV-2 disease of 2019 (COVID-19) [1]. We read with much interest the recent article in Your Journal by Ye Q. et al. describing the “cytokine storm” in COVID patients, illustrating that while a balanced immune response may eliminate invading microbes, an overwhelming response with elevated levels of multiple cytokines, as reflected in multiplex cytokine analysis, could induce collateral tissue damage and be harmful for the host [2]. Further, IL-6 and its downstream target CRP reflect this “cytokine storm” and seem to be good indicators of poor prognosis and respiratory failure in COVID-19 patients [3,4]. This acute immune response could contribute to the progression of COVID-19 disease but could also represent inflammatory noise, making it difficult to pinpoint specific inflammatory processes related to respiratory failure.
COVID-19 related pneumonia with severe respiratory failure is also characterized by enhanced fibrosis and extracellular matrix remodeling (ECM) [5]. We hypothesized that plasma markers reflecting inflammation and fibrosis in relevant tissues would be associated with respiratory failure in hospitalized COVID-19 patients. We focused on inflammatory markers that were independent of the “inflammatory noise” (reflected by CRP and ferritin) and renal effects, during the acute phase response.
Thirty-nine adult patients (≥18 years old) with confirmed COVID-19 were consecutively recruited between March 6 and April 14 to a clinical cohort study (Norwegian SARS-CoV-2 study; ClinicalTrials.gov, number NCT04381819). Clinical information and routine laboratory samples were collected at the earliest time-point after hospitalization. 1–3 plasma samples were collected at day 0–2 (within 48 h of admission), day 3–5 and day 7–10. Informed consents were obtained from all patients or next-of-kin if patients were incapacitated of giving consent. The study was approved by the South-Eastern Norway Regional Health Authority (reference number: 106624).
Respiratory failure was defined as arterial partial pressure of oxygen to fraction of inspired oxygen ratio (P/F ratio) < 40 kPa (300 mm Hg) during hospitalization regardless of need for mechanical ventilator support, corresponding to the threshold in the Acute Respiratory Distress Syndrome (ARDS) Berlin definition [6].
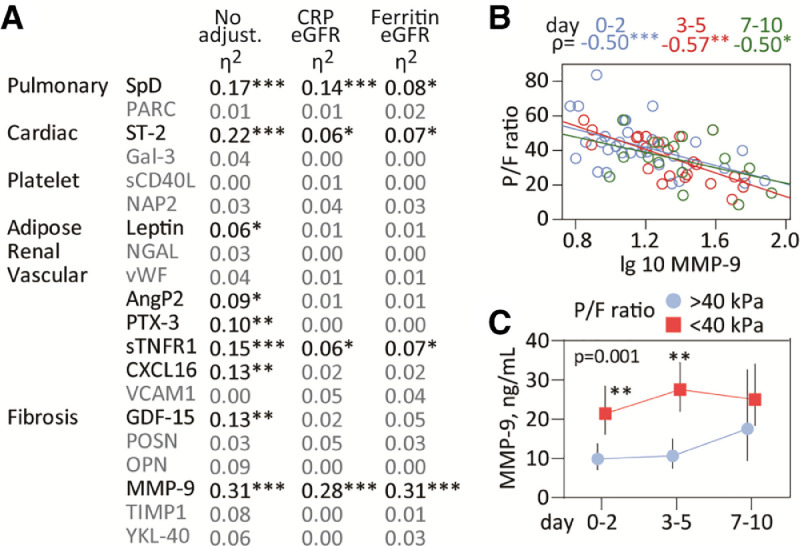
A list of the various markers in relation to tissues and functions is given in Fig. 1 A. Plasma markers were measured in duplicate by enzyme immunoassays using commercially available antibodies (R&D Systems, Minneapolis, MN) in a 384 format with intra-assay coefficient of variation <5%.
Fig. 1.
Inflammatory markers and respiratory failure during COVID-19 disease. A) Circulating markers measured in the study reflecting inflammation in relevant tissues or cells (pulmonary, adipose, cardiac, renal, platelets) or related to function (fibrogenesis, vascular inflammation). The table shows correlations between the P/F ratio and plasma markers during the course of the study. Partial eta2 is shown in unadjusted analysis (but with time as fixed factor) and following adjustment with eGFR and CRP or eGFR and ferritin. B) Spearman correlation between MMP-9 and P/F ratio at different time-points (day 0–2 blue, day 3–5 red, day 7–10 green) during the course of the study. C) Temporal course of MMP-9 during COVID-19 infection according to respiratory failure. Data are presented as back-transformed estimated marginal means with 95% confidence intervals from the mixed model analysis (see statistical methods) and the P-value for the group effect (i.e. respiratory failure) is given on the graph. *p<0.01, **p<0.01, ***p<0.001. SpD, surfactant protein D; PARC/CCL18, pulmonary and activation-regulated chemokine; ST-2, suppression of tumorigenesis-2; Gal-3, Galectin-3 ; sCD40L, soluble CD40 ligand; NAP2/CXCL7, neutrophil activating peptide; NGAL, neutrophil gelatinase-associated lipocalin; vWF, von Willebrand factor; AngP2, angiopoietin 2; PTX-3, pentraxin 3; sTNFR1, soluble tumor necrosis factor receptor type 1; CXCL16, C-X-C Motif Chemokine Ligand 16; VCAM1, vascular cell adhesion molecule 1; GDF-15, growth differentiation factor; POSN, periostin; OPN, osteopontin; MMP-9, matrix metallopeptidase 9; TIMP1, tissue inhibitor of matrix metalloproteinase; YKL-40 also known as chitinase-3-like protein 1. . (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Patient characteristics were compared using student's t-test or chi-square for continuous and categorical variables, respectively (Table 1 ). We screened for associations between the temporal profile of the inflammatory markers and respiratory failure using multivariate analysis of covariance (MANCOVA) with inflammatory markers as dependent, time-group as fixed and P/F ratio (continuous), eGFR and CRP or ferritin as covariates. Markers of interest were visualized in scatterplots and spearman correlation with the P/F ratio assessed at each time-point. Finally, the association between marker and respiratory failure was evaluated in a generalized linear mixed model with patient number as random factor, respiratory failure and time as fixed and including eGFR and CRP or ferritin as covariates. Post-hoc testing at individual time-points was performed with the same adjustments. P-values are two-sided and considered significant when <0.05.
Table 1.
Characterization of the study group at baseline according to respiratory failure.
| Respiratory failure |
||
|---|---|---|
| No (n = 18) | Yes (n = 21) | |
| Women, n (%) | 7 (39) | 3 (14)* |
| Age, years | 60 (18) | 62 (13) |
| Time from symptoms, days | 8.8 (4.0) | 10.2 (3.4) |
| P/F ratio | 52 (12) | 29 (8)*** |
| Current smoker, n (%) | 5 (28) | 3 (14) |
| Comorbidities, n (%) | ||
| Chronic cardiac disease | 5 (28) | 4 (19) |
| Chronic lung disease | 4 (22) | 5 (24) |
| Chronic kidney disesae | 2 (11) | 2 (10) |
| Obesity/Diabetes | 3 (17) | 4 (19) |
| Leukocytes, x109/L | 5.1 (2.1) | 8.0 (3.5)** |
| Lymphocytes, x109/L | 1.24 (0.50) | 0.91 (0.33)* |
| Monocyte, x109/L | 0.48 (0.20) | 0.41 (0.19) |
| Neutrophils, x109/L | 3.3 (1.8) | 6.8 (3.4)*** |
| Platelets | 192 (57) | 211 (63) |
| eGFR, mL/min/1.73m2 | 82 (31) | 81 (32) |
| Ferritin, ng/mLa | 430 [248, 757] | 1151 [827, 1545]⁎⁎ |
| CRP, mg/La | 44 [18,67] | 117 [47,177]** |
Continuous data are given as mean±standard deviation.
median [25th, 75th percentile].
p<0.05,.
p<0.01 vs. patients with no respiratory failure. P/F ratio: arterial partial pressure of oxygen (PaO2) to fraction of inspired oxygen (FIO2). Respiratory failure defined as PO2/FiO2 ratio < 40 kPa.
Of 39 COVID-19 patients, 21 developed respiratory failure and was dominated by male sex, high leukocyte and neutrophil counts, low lymphocyte counts and high CRP and ferritin levels (Table 1).
MANCOVA in relation to the P/F ratio revealed that multiple markers were associated the P/F ratio in the unadjusted model (Fig. 1A). However, after adjustment for eGFR, CRP and ferritin only surfactant protein D (SpD), MMP-9, sTNFR1 and ST-2 remained associated with respiratory failure. Of these, the association between P/F ratio and MMP-9 was not affected by these adjustments, and the focus of further analysis.
Correlation analysis at individual time-points (Fig. 1B) revealed consistent correlations for MMP-9 (ρ between −0.50 and −0.57). Evaluation of MMP-9 temporal course according to respiratory failure (i.e. P/F <40 kPa, Fig. 1C) revealed a strong group effect (p = 0.001 and p = 0.007 when adjusting for eGFR and either ferritin or CRP, respectively), with significantly higher MMP-9 levels at admission and 3–5 days. MMP-9 correlated strongly with neutrophil counts (partial ƞ2=0.24, p<0.001). Further adjustment with neutrophil or platelet counts, a history of chronic cardiac or pulmonary disease had no effect on the association between MMP9 and respiratory failure.
In the present study, markers reflecting inflammation and ongoing fibrogenesis and vascular inflammation were associated with respiratory function as reflected by the P/F ratio in hospitalized COVID-19 patients. However, upon adjustment of the systemic inflammatory storm as reflected by the acute phase respondent CRP or ferritin as a marker of hyperinflammation and macrophage activation as well as eGFR, only MMP-9 was convincingly associated with the P/F ratio and distinguished patients with and without respiratory failure.
MMP-9 belongs to a family of proteases that degrade ECM proteins and has been widely studied in acute lung injury and chronic lung disease [7]. While present in low quantities in heathy lungs, MMP-9 is abundant in lung diseases characterized by tissue remodeling such as asthma, pulmonary fibrosis and COPD [8]. In acute lung injury, MMP-9 released from neutrophils promotes inflammation and degradation of the alveolar capillary barrier, further stimulating migration of inflammatory cells and destruction of lung tissue [7]. We found, however to this end, no reports of MMP-9 during COVID-19 disease, although coronavirus infection of human monocytes enhanced MMP-9 secretion [9]. Hsu et al. reported a marked increase in plasma MMP-9 activity in critically ill patients who developed ARDS, strongly negatively correlated with P/F ratio, similar to our findings [10]. While the cellular origin and molecular pathways involved in MMP-9 release during COVID-19 infection are unknown, the distinct and early up-regulation, independent of the “inflammatory noise” due to the acute phase response and kidney function, suggest further studies are warranted.
Our study suggests that MMP-9 may be an early indicator of respiratory failure in COVID-19 patients and underscore the role ECM remodeling and fibrosis in this disorder. Treatment modalities targeting MMP-9 activity or neutrophil activation could be of importance in COVID-19 lung disease.
Declaration of Competing Interest
None.
Acknowledgments
Author contributions
Conception and design: TU, PA and LH. Data analysis and acquisition: TU, JCH, ARH, KEM, AL, GKB, SD, AMDR, LH. Interpretation of the data: TU, PA and LH. Drafting or revision of the manuscript: TU, PA, LH. Final approval of the manuscript: all authors.
Sources of support
This study received funding from the Research Council of Norway grant no 312780 and has received private donation from Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner.
References
- 1.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 doi: 10.1136/bmj.m1966. 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herold T., Jurinovic V., Arnreich C. Elevated levels of interleukin-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grifoni E., Valoriani A., Cei F. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med [DOI] [PMC free article] [PubMed]
- 6.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Davey A., McAuley D.F., O'Kane C.M. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J. 2011;38:959–970. doi: 10.1183/09031936.00032111. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson J.J., Senior R.M. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 9.Desforges M., Miletti T.C., Gagnon M., Talbot P.J. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130:228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu A.T., Barrett C.D., DeBusk G.M. Kinetics and role of plasma matrix metalloproteinase-9 expression in acute lung injury and the acute respiratory distress syndrome. Shock. 2015;44:128–136. doi: 10.1097/SHK.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]