Abstract
The clinical management of COVID-19 is challenging. Medical imaging plays a critical role in the early detection, clinical monitoring and outcomes assessment of this disease. Chest x-ray radiography and computed tomography) are the standard imaging modalities used for the structural assessment of the disease status, while functional imaging (namely, positron emission tomography) has had limited application. Artificial intelligence can enhance the predictive power and utilization of these imaging approaches and new approaches focusing on detection, stratification and prognostication are showing encouraging results. We review the current landscape of these imaging modalities and artificial intelligence approaches as applied in COVID-19 management.
The Clinical Presentation of COVID-19
COVID-19 is primarily a respiratory tract infection caused by the SARS-CoV2 virus. Infection spreads from human to human via small droplets and potentially aerosol.1 The incubation period for COVID-19 ranges from 2 to 14 days, averaging 5.2 days.2 During this time, an asymptomatic patient can transmit the virus to other people, making it a highly contagious infection.2 The typical symptoms include cough, sneezing, fever, malaise, body aches, and shortness of breath.2 The virus initially infects and replicates in upper airways with subsequent spread involving the lung parenchyma (the alveolar sacs). However, some patients present solely with digestive symptoms and the virus is only isolated in stool samples, without symptomatic involvement of the airways. Thus, fecal-oral transmission has been purported.2 The diagnosis is based on reverse polymerase chain reaction testing of nasopharyngeal swab, although newer and faster tests are becoming available, including the one performed on saliva.3
SARS-CoV2 may infect any individual, but elderly, immunocompromised, and those with pre-existing cardiovascular and pulmonary conditions are at a higher risk of severe disease and death.4 Most of the morbidity due to COVID-19 results from involvement of the respiratory tract. However, although infrequent, there is an increasing concern for neurologic manifestations of COVID-19, such as encephalitis and myopathy occurring in younger patients.5 , 6
Some patients with COVID-19 may rapidly progress to develop acute respiratory distress syndrome (ARDS), which is characterized by rapid onset of widespread inflammation in the lungs, limiting the adequate air exchange. This is due to the diffuse injury to the alveolar cells caused both by the viral infection and the immune response to it. Clinically, these patients are typically dyspneic, tachypneic, and cyanotic.6 With limited efficacy of direct anti-viral therapy, the management is primarily based on supportive care including mechanical ventilation, with severe cases requiring extracorporeal membrane oxygenation,7 fluid and electrolyte management, and anti-coagulation in selected cases. The mortality rate of patients with ARDS ranges from 35 to 50% but can be as high as 80% in patients with COVID-19.7 A possible reason for the very high mortality rate in these patients could be an association with an increased risk of thromboembolic disease including pulmonary emboli. Recent data suggest an incidence up to 31% in the critically ill patients.8
The Immunologic basis of COVID-19
SARS-CoV2 is a positive-sense, single-strand RNA, beta-coronavirus.9 RNA viruses have a greater mutation rate than DNA viruses, which is one reason why SARS-CoV2 is highly adaptive and sustainable.10 This virus comprises 4 main proteins: spike, membrane, envelope and nucleocapsid proteins.11 SARS-Cov2 enters the host cells, which are primarily pneumocyte type II cells, using the angiotensin-cell converting enzyme II receptor.12 Once the virus attaches and enters the host cell (through endocytosis), its single-stranded RNA replicates more copies of itself using the RNA-dependent RNA polymerase within the host cell cytoplasm. This viral progeny then acquires a new envelope from the cell membrane and exits the host cell.12 The immune response that is elicited by the host against the virus results in damage to the lungs and is composed of both cellular and humoral components. The CD-4 T helper cells mediate an immune response to the viral infection. The CD8 T cytotoxic cells secrete perforin, granzyme, interferon-gamma, and a wide range of chemokines, including IL-1, IL-6, IL-8, IL-21, TNF-beta and MCP-1. These are implicated in orchestrating a “cytokine storm” that causes severe alveolar destruction and leads to ARDS.13 The humoral arm of the immune system mediated by B cells releases antibodies specific to viral antigens (spike protein on the SARS-Cov2 envelope).
The Imaging landscape for COVID-19
Structural imaging
The role of medical imaging in the clinical management of COVID19 is evolving. At the frontline, chest x-ray (CXR), computed tomography (CT), and even ultrasonography have been performed to complement the clinical diagnosis and monitor the clinical course of the disease (Table 1 ). More recently, more articulated guidelines for CT imaging in COVID-19 patients have been released, including the Fleischner society guidelines, which posits that imaging is not indicated in asymptomatic individuals or even those with mild symptoms suspected to have COVID-19.14 It is primarily reserved for patients with moderate to severe features of COVID19 regardless of the test results as a means of medical triage and complimenting the diagnosis and may be limited to patients with functional impairment and/or hypoxemia after recovery from COVID-19.14
Table 1.
Imaging modalities in COVID-19 management
| Imaging modality | Indications | Key findings | Strengths | Limitations |
|---|---|---|---|---|
| CXR | Routine assessment | Bilateral diffuse patchy and peripheral predominant opacities | Serial assessment of prominent features, easy to perform, portable, low radiation | May be negative in the initial stage/ |
| CT | Baseline, assessment of clinical progression, sequelae. | bilateral ground-glass, consolidative, opacities; crazy paving pattern. | Optimal structural information. | Limited/nil functional information, high radiation. |
| PET | No definite clinical use | Diffuse FDG uptake | Assessment of severity of the disease. | Expensive, lengthier study, limited added benefit. |
CXR imaging can be negative for the first few days of symptomatic infection. When positive, it shows bilateral diffuse patchy opacities with some bibasilar sparing (Fig 1 ).13 CT findings of COVID-19 typically include bilateral ground-glass, consolidative, or rounded opacities, showing a more peripheral pattern of distribution (Fig 2 ). In about 20% of the cases, there may be a crazy paving pattern (interlobular septal thickening superimposed on ground-glass opacities) (Fig 3 ).16 Serial CT imaging may be performed to assess the density and the pattern of these opacities to determine progression or regression of the disease.14 Typically, there is no evidence of lung cavitation, discrete nodules, pleural effusion or lymphadenopathy. If these CT imaging features are present in a COVID-19 positive patient, it should raise the suspicion of super infection. There is, however, a growing number of cases that have associated pulmonary emboli.15, 16, 17 (Fig 4 )
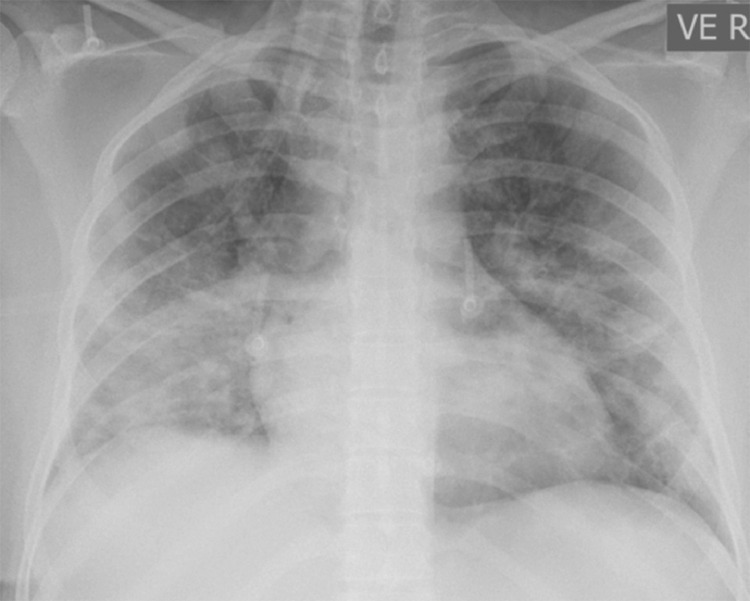
FIG 1.
Chest Plain film radiograph of a patient with pulmonary COVID-19: Peripheral predominant opacification bilateral lungs and blurring of the right hemidiaphragm. Basilar distributed opacities on chest radiography remain characteristic for COVID-19 infection.
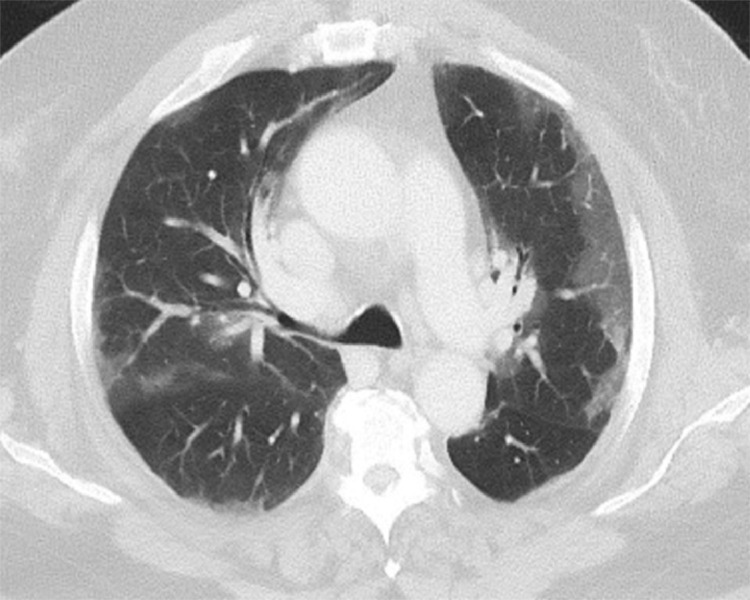
FIG 2.
CT scan of a patient with pulmonary COVID-19: Axial image with characteristic peripheral ground-glass opacities with visible vessels coursing within the opacities. It could represent inflammation of the bronco vascular sheath or a response to hypoxemia.
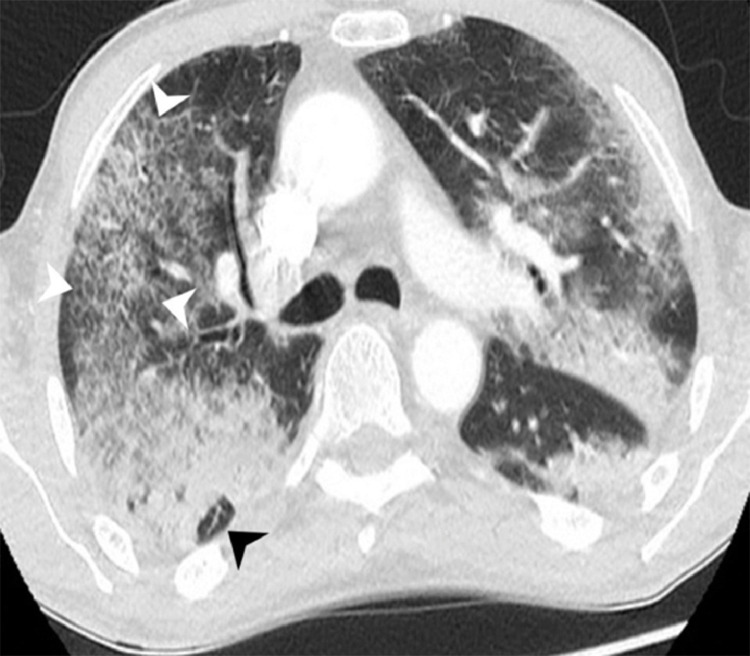
FIG 3.
CT image of a patient with severe pulmonary COVID-19: Axial image with late stage changes to COVID-19. Several consolidations are seen in both lungs. Within the right posterior upper lobe, an area of arcade-like appearance is seen (black arrowhead). Within the anterior right upper lobe an area with a “crazy paving” pattern is seen with ground glass opacification and overlying interlobular septal thickening (white arrowheads).
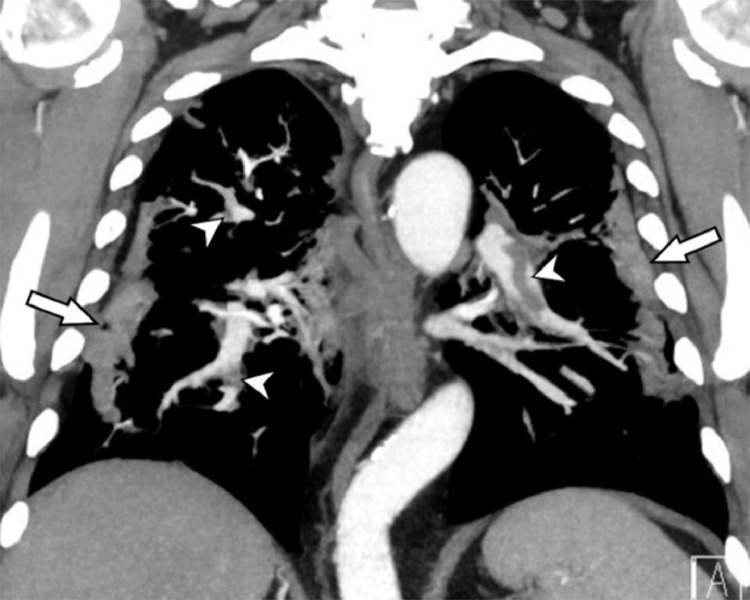
FIG 4.
CT with intravenous contrast in a patient with pulmonary COVID-19: Coronal computed tomography image shows several pulmonary emboli (white arrowheads) in the left lower lobe artery, right upper lobe and right middle lobe segmental arteries. Characteristic COVID-19 consolidations in the periphery are also appreciated on this soft tissue window (white arrows).
Though not broadly reflected on the population statistics with regards to the cardinal symptoms and outcomes of the present pandemic,18 there was early concern of the neurological complications related to this specific virus.19 It has been postulated that the neurologic symptoms are mostly observed in patients with severe ARDS20 but soon the clinical manifestations (incl. headache, nausea, and vomiting, acute cerebrovascular events, impaired consciousness) were recognized also in patients without severe respiratory infection.21 The SARS-CoV-2 infection linkage to a prothrombotic state causing venous and arterial thromboembolism and elevated D-dimer levels has been primarily implicated as a cause.22 Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia.22 The secondary effects of the immunologic and inflammatory responses directed towards the nervous system are likely causative but the neuroinvasive propensity, demonstrated as a common feature of coronaviruses is also thought to hold true for the SARS-CoV2, which probably enters and affects the medullary neurons during the latency period due to its propensity for endothelial cells disruption. This possibly contributes to the acute respiratory failure in certain patients.23 Finally, a subset of patients experience peripheral nervous system manifestations such anosmia and ageusia.24
Currently, the imaging features related to the neurologic complications of the virus are consistent with stroke related to large vessel occlusion and encephalopathy (Fig 5 ) with reported leptomeningeal enhancement and cranial nerve palsies,25 , 26 which in the vast majority are seen in subjects with severe alternate manifestations of Covid-19 infection.27 , 28 It is occasionally quite difficult to distinguish the ischemic events related to the viral replication from pre-existing competing vascular risk factors and mechanisms and larger cohorts with well documented co-morbidities and clinical details are required. Nonetheless, the anecdotal experience suggests that in a significant amount of cases associated with diverse neurological signs, even when the imaging has been generally regarded as unrevealing or often normal.
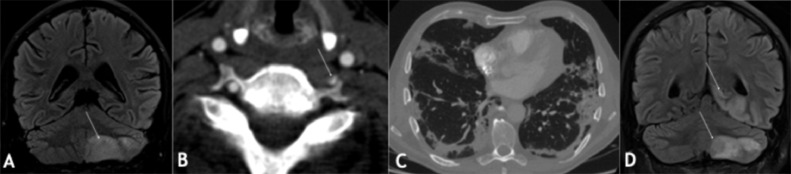
FIG 5.
Neurological manifestations of COVID-19 on MRI (A-D): COVID-19 positive patient with word-finding difficulties, bilateral incoordination, right homonymous hemianopia 15 days after COVID-19 symptoms (cough, shortness of breath, fever, myalgia, loss of appetite) onset. The admission MRI showed an acute infract on the left cerebellum (arrow in A) with FLAIR hyperintense signal as well as thrombus in the left vertebral artery (arrow in B), The lung CT showed typical severe findings of Covid-19 infection with peripheral predominant foci of consolidation bilaterally (C). In the course of the disease, the patient experienced pulmonary embolism, occlusive deep vein thrombosis in the left lower limb, and progressive ischaemic infarcts in the posterior circulation (arrows in D) as demonstrated by FLAIR hyperintense signal.
Another functional imaging modality of interest in the context is the dual energy CT. By acquisition of 2 separate CT series at respectively high and low kilovolt peak, it is possible to calculate iodine density, average effective atomic number, or virtual mono energetic images.29 DCET showing low iodine density suggests a decreased blood flow within the opacifications, which is in contrast with an increase in iodine density in the rim of these opacifications. This observed phenomenon may be explained by the hypoxemia possibly related to microemboli.30
Positron emission tomography imaging
Positron emission tomography (PET) is the hallmark of functional imaging. 18F-fluorodeoxyglucose (FDG) is the staple radiotracer that is used for the assessment of metabolic activity of disease processes, especially malignant diseases. Infectious/inflammatory processes can be intensely FDG-avid given the role of cytokines in the upregulation of glucose receptors leading to increased FDG uptake.31 FDG-PET may be used for the assessment of the degree of infection or inflammatory response to it in infectious lung diseases. FDG-PET has been used in various infectious diseases, such as HIV/AIDS, toxoplasmosis, and osteomyelitis, among others.32 PET scan is generally not used in the management of pneumonia but may offer the following benefits - (1) early identification of inflammatory processes which cannot be seen by CT scan alone, (2) quantifiable assessment of disease progression/regression based on the delta difference in the signal reflecting changes in the inflammatory/infectious process, and (3) early detection of sequelae such as pleuritis, abscess or sepsis.32 Given that it has been shown to be useful for imaging lung infections, such as tuberculosis and atypical pneumonia,33 its potential role in COVID19 management, albeit small may be extrapolated (Fig 6 ).
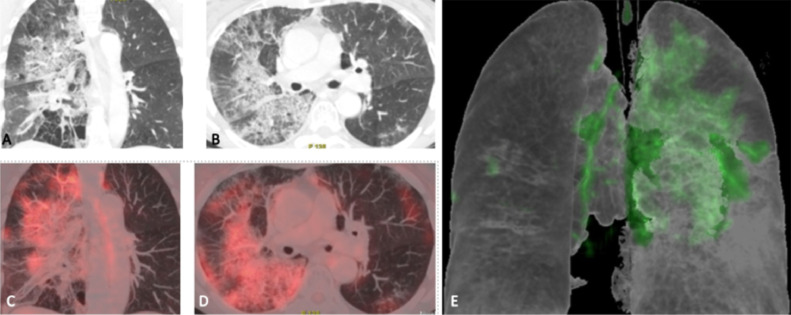
FIG 6.
COVID-19 findings on FDG PET/CT (A-E): COVID-19 positive patients with respiratory manifestations. The FDG-PET/CT showed multilobar patchy opacities on coronal and axial CT images (A, B) with diffusely increased FDG uptake of moderate intensity on coronal and axial PET/CT images (C, D) and on 3 dimensional reconstructed image (E) (Courtesy of Dr. Maldonado, Hospital Quiron, Madrid, Spain).
The role of Artificial intelligence in COVID-19 imaging
Few would argue that AI is causing a paradigm shift in health care and there might be value in the application of AI to the current COVID-19 outbreak. Applications of AI in COVID-19 range from predicting risk of infection to curation of health records and diagnostic imaging.34 , 35 There is a strong interest for AI applications in COVID-19 lung CT and X-ray imaging. A comprehensive review of this landscape is beyond the scope of this article, but we will highlight a few key areas of development. Almost all AI models being designed for COVID-19 have a clinical focus. There are several major competitions focusing on this domain, including the MIT - COVID-19 challenge and the Imaging COVID19 AI.36 , 37 Within structural imaging, the main areas where AI has been applied include detection of COVID-19 findings on CXR/CT.38 There are several studies that have showcased AI models that differentiate imaging features of COVID-19 from those of community acquired pneumonia and have shown a variable rate of accuracy and predictive power.39, 40, 41, 42, 43, 44, 45 (Table 2 ) Most recently, quantitative imaging approaches driven by machine learning, such as Radiomics have also been leveraged as a tool for automated diagnosis of COVID-19.46 While most of these studies show fairly sizeable training datasets and impressive accuracy rates, there is a tremendous opportunity to homogenize/standardize these models to create a unified platform for AI-based detection of COVID-19 on CXR/CT. Beyond that, AI-based approaches have been used for the quantification of disease progression for the purpose of severity assessment.47 These models focus on the calculating the delta difference in the extent/spread of the patchy opacities in the lungs, and study other features such as hyperinflation/hyperlucency and vascular changes.
Table 2.
Key studies in AI for COVID-19 imaging
| Publication | Focus | Methodology | Results |
|---|---|---|---|
| Li, Lin, et al40 | Distinguishing COVID-19 from other pneumonia. | 4536 3D volumetric chest CT exams from 3506 patients acquired at 6 medical centers. Deep learning neural network methodology used. | COVID-19 identified on CT (AUROC 0.96). Community acquired pneumonia identified on CT (AUROC 0.95). Overlap found in CT imaging findings of all viral pneumonias with other chest diseases. |
| Wang, Linda, et al41 | Detection of COVID-19 on CXR. | 13,975 CXR images across 13,870 patient cases. Deep convolutional neural network. |
N/A. (Open-source tool for public use). |
| Wang, Shuai, et al42 | Detection of COVID-19 on CT. | CT images from 99 patients (of which 55 cases were of typical viral pneumonia and 44 of COVID-19). Convolutional neural net. | AUC of 0.90 (internal validation) and 0.78 (external validation). Sensitivity of 80.5% and 67.1%, specificity of 84.2% 76.4%, accuracy of 82.9% and 73.1%, the negative prediction value of 0.88 and 0.81. |
| Apostolopoulos, Ioannis D., et al43 | Detection of COVID-19 on CXR. |
1427 X-ray images (of which 224 images were of COVID-19 disease, 700 images of common bacterial pneumonia, and 504 images of normal conditions). Transfer Learning. | Accuracy, sensitivity, and specifcity obtained is 96.78%, 98.66%, and 96.46%, respectively. |
| Narin, Ali, et al44 | Comparing the performance of various deep learning methods for detection of COVID-19 on CXR. | Convolutional neural network-based models (ResNet50, InceptionV3 and Inception-ResNetV2 | Pre-trained ResNet50 model provides the highest classification performance with 98% accuracy among other 2 proposed models (97% accuracy for InceptionV3 and 87% accuracy for Inception-ResNetV2. |
| Afshar, Parnian, et al45 | Detection of COVID-19 on CXR. | Convolutional neural network. | Accuracy of 95.7%, Sensitivity of 90%, Specificity of 95.8%, and Area Under the Curve (AUC) of 0.97. Pretraining improved accuracy to 98.3% and specificity to 98.6%. |
| Fang M, et al46 | Detection of COVID-19 on CT. | 75 pneumonia patients (46 with COVID-19, 29 other types of pneumonias). Radiomics + Support vector machine | AUCs of 0.862 and 0.826 in the training set and the test set, respectively. Predictive ability is not affected by gender, age, chronic disease and degree of severity |
| Tang Z, et al47 | Severity assessment of COVID-19 | 176 COVID-19 patients. Random forest. | Accuracy 87.5% True positive rate 93.3%, True negative rate 74.5%. |
These AI methodologies can be considered as a “radiologist assistant” as a form of computer-aided diagnosis useful in settings where the expert radiologist is overburdened or unavailable. A more “automated” model may be useful to non-imaging physicians in more urgent settings. On the other hand, within a clinical trial setting, such models can assess qualitative changes and provide quantitative scores of pathophysiologic processes, such as fibrosis, hyperinflation, and changes in vascular flow; which can be used as secondary or surrogate diagnostic and prognostic biomarkers.
A much more challenging task is to detect early signs (or “predict”) neurological manifestations or complications of COVID-19. The scenario of new neurological impairment on a previously healthy subject with typical COVID-19 lab findings and respiratory symptoms is an ideal case for definite diagnosis; however, healthy otherwise subjects presenting with non-specific headache and dizziness in absence of other COVID-19 related evidence may pose a diagnostic challenge. For instance, patients with slurred speech, unilateral weakness, nerve palsies, disorientation, hallucinations, and neuropsychiatric disorders in the background of existing neurological diseases.48 Furthermore, patients on immunosuppressive medications for autoimmune diseases, such as multiple sclerosis, who may be at a higher risk for developing complications, pose extreme but real scenarios that will benefit from an AI-assisted diagnosis. So far, there has been no initiative undertaken for these patient groups and certainly non-specific brain MRI features pose difficulties for an early diagnosis. However, a worthy goal is to create an algorithm that will combine “hidden” patterns in “rich” images as distinct from other diseases, taking into account clinical signs and laboratory findings to conduct risk assessment, possibly early diagnosis, and prediction of long-term effects. Additionally, predictive analytics can triage mildly or moderately ill patients in need of beds, and who can safely go home, with neurology hospital resources stretched thin, analogously to a recently published study for respiratory symptoms.49
The volume of evidence and experience with imaging for COVID-19 is growing exponentially. And AI is becoming increasingly employed to automate and/or improve the analysis and interpretation of these studies. As we enter what might be the later stages of the pandemic, hopefully, we are becoming more adept and nuanced in how we use imaging and AI to manage patients with COVID-19 better. These experiences, along with the trained AI models shall serve us to optimize imaging-based management of other infectious/inflammatory lung diseases in the future as well.
References
- 1.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: Early experience from California. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazir T, Kayani RR, Nazir SR, Manzoor S, Taha N. Clinical and biochemical features of nCOVID -19. Adv J Biomed Sci. 2019;4:3–7. [Google Scholar]
- 4.Benny R, Khadilkar SV, Raghu P, et al. COVID 19: neuromuscular manifestations. Ann Indian Acad Neurol. 2020;23:40. doi: 10.4103/aian.AIAN_309_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respi Med. 2020;8:433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020;7:112–123. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok FA, Kruip MJ, Van der Meer NJ, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ysrafil AI. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response [published online ahead of print, 2020 Apr 18] Diabetes Metab Syndr. 2020;14:407‐412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denison MR. Severe acute respiratory syndrome coronavirus pathogenesis, disease and vaccines: an update. Pediatr Infect Dis J. 2004;23:S207–S214. doi: 10.1097/01.inf.0000144666.95284.05. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Investig. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng M-Y, Lee E, Yang J, et al. Imaging profile of the COVID-19 infection: Radiologic findings and literature review. Radiol Soc N Am. 2020 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong HYF, Lam HYS, Fong AH, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019 doi: 10.1148/radiol.2020201160. [published online ahead of print, 2019 Mar 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology. 2020;295 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotzinger DC, Beigelman-Aubry C, von Garnier C, Qanadli SD. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–59. doi: 10.1016/j.thromres.2020.04.011. Epub 2020 Apr 11. PMID: 32302782; PMCID: PMC7151364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Wang X, Yang P, et al. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. [published online ahead of print, 2020 Apr 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Docherty K, Butt J, de Boer R, et al. Deaths from Covid-19: Who are the forgotten victims? medRxiv. 2020 [Google Scholar]
- 19.Bernard-Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningo-encephalitis concomitant to SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online ahead of print February 27, 2020] J Med Virol. 2020;10 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinkin M, Gao V, Kahan J, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 25.Vaira LA, Salzano G, Deiana G, et al. Anosmia and ageusia: Common findings in COVID-19 patients. Laryngoscope. 2020 doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hounsfield GN. Computerized transverse axial scanning (tomography): Part 1. Description of system. Br J Radiol. 1973;46:1016–1022. doi: 10.1259/0007-1285-46-552-1016. [DOI] [PubMed] [Google Scholar]
- 30.Lang M, Som A, Dexter PM, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;S1473-3099:30367–30374. doi: 10.1016/S1473-3099(20)30367-4. [published online ahead of print, 2020 Apr 30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Love C, Tomas MB, Tronco GG, et al. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–1368. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 32.Vaidyanathan S, Patel C.N, Scarsbrook A.F., et al. FDG PET/CT in infection and inflammation—Current and emerging clinical applications. Clin Radiol. 2015;70:787–800. doi: 10.1016/j.crad.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Win Z, Todd J, Al-Nahhas A. FDG-PET imaging in Pneumocystis carinii pneumonia. Clin Nucl Med. 2005;30:690–691. doi: 10.1097/01.rlu.0000178784.58278.36. [DOI] [PubMed] [Google Scholar]
- 34.Shweta FN, Murugadoss K, Awasthi S, et al. Augmented curation of unstructured clinical notes from a massive EHR system reveals specific phenotypic signature of impending COVID-19 diagnosis. arXiv preprint arXiv:2004.09338. 2020 doi: 10.7554/eLife.58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi F, Wang J, Shi J, et al. Review of artificial intelligence techniques in imaging data acquisition, segmentation and diagnosis for covid-19. IEEE Rev Biomed Enineering. 2020 doi: 10.1109/RBME.2020.2987975. [DOI] [PubMed] [Google Scholar]
- 36.Available at:https://covid19challenge.mit.edu/ (last visited 4/29/20).
- 37.Available at:https://imagingcovid19ai.eu/ (last visited 5/10/20).
- 38.Mei X, Lee HC, Diao K, et al. Artificial intelligence for rapid identification of the coronavirus disease 2019 (COVID-19) medRxiv. 2020 [Google Scholar]
- 39.Shi F, Wang J, Shi J, et al. Review of artificial intelligence techniques in imaging data acquisition, segmentation and diagnosis for covid-19. IEEE Rev Biomed Eng. 2020 doi: 10.1109/RBME.2020.2987975. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Qin L, Xu Z, et al. Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Wong A.COVID-Net: A tailored deep convolutional neural network design for detection of COVID-19 cases from chest radiography images. arXiv preprint arXiv:2003.09871. 2020. [DOI] [PMC free article] [PubMed]
- 42.Wang S, Kang B, Ma J, et al. A deep learning algorithm using CT images to screen for Corona Virus Disease (COVID-19) MedRxiv. 2020 doi: 10.1007/s00330-021-07715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apostolopoulos ID, Mpesiana TA. Covid-19: automatic detection from x-ray images utilizing transfer learning with convolutional neural networks. Phys Eng Sci Med. 2020;1 doi: 10.1007/s13246-020-00865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narin A, Kaya C, Pamuk Z. Automatic detection of coronavirus disease (covid-19) using x-ray images and deep convolutional neural networks. arXiv preprint arXiv:2003.10849. 2020. [DOI] [PMC free article] [PubMed]
- 45.Afshar P, Heidarian S, Naderkhani F, et al. Covid-caps: A capsule network-based framework for identification of covid-19 cases from x-ray images. arXiv preprint arXiv:2004.02696. 2020. [DOI] [PMC free article] [PubMed]
- 46.Fang M, He B, Li L, et al. CT radiomics can help screen the coronavirus disease 2019 (COVID-19): A preliminary study. Sci China Inf Sci. 2020;63 [Google Scholar]
- 47.Tang Z, Zhao W, Xie X, et al. Severity assessment of coronavirus disease 2019 (COVID-19) using quantitative features from chest CT images. arXiv preprint arXiv:2003.11988. 2020.
- 48.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X, Coffee M, Bari A, et al. Towards an artificial intelligence framework for data-driven prediction of coronavirus clinical severity. CMC Comput Mater Contin. 2020;63 [Google Scholar]