Abstract
The novel coronavirus, later identified as SARS-CoV-2, originating from Wuhan in China in November 2019, quickly spread around the world becoming a pandemic. Despite the knowledge of previous coronaviruses, such as those responsible for the SARS and MERS-CoV epidemic, there is no drug or prophylaxis treatment to this day. The rapid succession of scientific findings on SARS-CoV-2 provides a significant number of potential drug targets. Nevertheless, at the same time, the high quantity of clinical data, generated by a large number of rapidly infected people, require accurate tests regarding effective medical treatments. Several in vitro and in vivo studies were rapidly initiated after the outbreak of the pandemic COVID-19. Initial clinical studies revealed the promising potential of remdesivir that demonstrated a powerful and specific in vitro antiviral activity for COVID-19. Promising effects appear to be attributable to hydroxychloroquine. Remdesivir and hydroxychloroquine are being tested in ongoing randomized trials. In contrast, oseltamivir was not effective and corticosteroids are not currently recommended. However, few data from ongoing clinical trials are identifying low molecular weight heparins, innate immune system stimulating agents, and inflammatory modulating agents as potential effective agents.
The authors assume that the current pandemic will determine the need for a systematic approach based on big data analysis for identifying effective drugs to defeat SARS-Cov-2. This work is aimed to be a general reference point and to provide an overview as comprehensive as possible regarding the main clinical trials in progress at the moment.
Keywords: Clinical trials, Coronavirus, SARS-CoV-2, COVID-19, Pharmacological treatments, Therapies
1. Introduction
On December 31, 2019, the Wuhan Municipal Health Commission (China) reported to the World Health Organization a cluster of pneumonia cases of unknown etiology in the city of Wuhan, in the Chinese province of Hubei. On January 9, 2020, the Chinese Center for Disease Control and Prevention reported that a new coronavirus (SARS-CoV-2) has been identified as the causative agent of respiratory disease, later called COVID-19 (CoronaVirusDisease-19) (Zhu et al., 2020).
As of April 18, 2020, there have been more than 2.2 million recorded cases and over 80,000 deaths in over 22 countries (“Situation Report n.89,” 2020).
COVID-19 syndrome is similar to Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS-CoV). This similarity was confirmed by the rapid viral genetic makeup sequencing that evidenced the proximity among the pathogens (Sanders et al., 2020).
The availability of the entire genomic sequence of the virus on one hand and isolated virus specimens (made available by the researchers who obtained them) may be useful to quickly refine the knowledge on the peculiar features of this new coronavirus. Above all, this information could help us significantly to develop reliable diagnostic tools and effective therapeutic regimens.
SARS-CoV-2, a single-stranded RNA-wrapped virus penetrates cells by binding to a receptor called Angiotensin Converting Enzyme 2 (ACE2) (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team China CDC, 2020).
The viral particle uses host cell receptors and endosomes to penetrate cells. A serine protease, TMPRSS2, facilitates entry into cells (Hoffmann et al., 2020).
Once inside the cell, the viral polyproteins that encode the replicase-transcriptase complex are synthesized. The virus then synthesizes RNA through its RNA-dependent RNA polymerase (Y. Chen et al., 2020c; Fehr and Perlman, 2015).
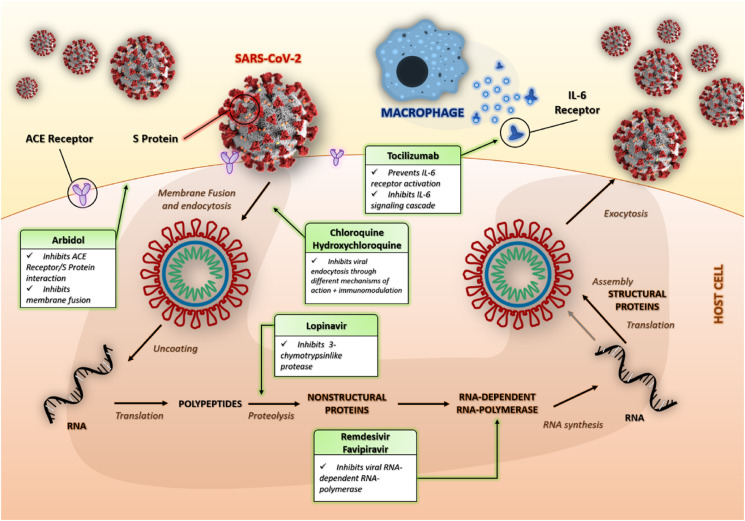
These stages of the viral life cycle provide potential targets for drug therapy (Fig. 1 ). World Health Organization (WHO) states that there are currently no specific drugs against SARS-CoV-2 (“WHO to accelerate research and innovation for new coronavirus,” 2020).
Fig. 1.
Schematic representation of the life cycle of SARS-CoV-2. The main targets of some of the drug therapies described are also indicated.
To date, there is no evidence supported by randomized clinical trials (RCTs) that demonstrate the efficacy of drugs for the treatment of suspected or confirmed patients with COVID-19.
Besides, there are no clinical trials supporting prophylaxis therapy. Some drugs that are already in use or are being tested for other diseases are being used for COVID-19. For other drugs, preclinical tests have started giving a possible use. There are over 600 ongoing clinical trials (“ClinicalTrials.gov,” n.d.).
This narrative review summarizes the current evidence related to the main treatments proposed for COVID-19 and provides a summary of current clinical experiences for the treatment of this disease.
2. Materials and methods
A literature review was carried out through three databases: PubMed, EMBASE, and Cochrane Library, to identify relevant articles written in English and published until April 20, 2020. Search terms included: COVID-19, severe acute respiratory syndrome from SARS-CoV-2, SARS-CoV-2, SARS-CoV, in combination with treatment, trial, and pharmacology.
The WHO publications database on COVID-19 has also been evaluated (“Global research on coronavirus disease (COVID-19),” 2020).
The research identified 1400 articles. Due to the lack of concluded Randomized Controlled Trial (RCT), clinical cases, case reports, and review articles have been considered. The authors independently reviewed the titles and abstracts for inclusion. Ongoing and completed clinical trials were identified using the search term COVID-19 infection on ClinicalTrials.gov.
2.1. General clinical characteristics of SARS-CoV-2
The disease caused by SARS-CoV-2 called COVID-19 has a rather broad symptomatology, usually similar to SARS, MERS and a standard flu: fever (83–98%), cough (59–82%), shortness of breath (19–55%), and muscle ache (11–44%) (Huang et al., 2020).
These symptoms are often associated with sore throat, tiredness and general weakness, headache, confusion, etc. (Huang et al., 2020)
Some studies have highlighted different symptoms, such as hemoptysis, lower levels of white blood cells, thrombocytopenia, increased levels of C-reactive protein, etc. (“The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China,” 2020; D. Wang et al., 2020a).
The clinical course of the disease may also differ in severity and progression. Already ill, weak and/or elderly patients can experience a rapid course of the disease, up to death (D. Wang et al., 2020a).
Patients with hypoxemia can quickly degenerate into acute respiratory distress syndrome (ARDS), severe sepsis and even multiple organ collapse within 7 days (Paraskevis et al., 2020).
2.2. Analysis of ongoing clinical trials
Currently, there is no sufficient evidence that any existing antiviral drugs can effectively treat SARS-CoV-2 pneumonia. However, there are several ongoing clinical trials of potential anti-COVID-19 therapies.
The search terms COVID-19 and SARS-CoV-2 inserted on Clinicaltrials.gov web site produced 692 active studies with 516 specific studies for COVID-19 as of April 18, 2020. Of these 516 studies, approximately 220 (including ongoing and completed ones) provided possible alternative drug therapies for COVID-19 in adult patients.
Among these 220 investigations, 120 are interim studies, with 36 placebo-controlled studies. Other researches concern Phase-4 with 18, Phase-3 with 46, Phase-2 with 54, and Phase-1 with 18 studies.
For 84 studies, it was not possible to define the exact phase of the trials. Therapies were divided into two categories according to their goal.
The first category includes drugs that act directly against the coronavirus, both by inhibiting viral proliferation and by blocking viral entry into human cells. The second category of drugs involves the modulation of the human immune system, both by increasing the innate response and by inhibiting the inflammatory processes that cause lung injury.
Fig. 1 shows the main mechanisms of action of the treatments subject to this narrative review.
Table 1 summarizes the current pharmacological treatments for SARS-COV-2 identified in the present work through the Clinicaltrials.gov website (“ClinicalTrials.gov,” n.d.).
Table 1.
Summary of current pharmacological treatments for SARS-COV-2 identified through Clinicaltrials.gov website (“ClinicalTrials.gov,” n.d.).
| AGENT | PHARMACOLOGICAL TARGET/ACTION | ClinicalTrials.gov IDENTIFIER | ADULT ADMINISTRATION | SAMPLE | TYPE OF TRIAL/ESTIMATED STUDY COMPLETION | |
|---|---|---|---|---|---|---|
| DRUGS ACTING DIRECTLY AGAINST THE CORONAVIRUS | REMDESIVIR | RNA-dependent RNA-polymerase inhibitor | NCT04292899 | A loading dose of 200 mg, followed by daily maintenance doses of 100 mg, for 5–10 days. | Adults with moderate/severe COVID-19 (also mechanically ventilated) | Phase 3 Randomized Study/May 2020 |
| NCT04292730 | A loading dose of 200 mg, followed by daily maintenance doses of 100 mg, for 5–10 days. | Adults with moderate/severe COVID-19 (also mechanically ventilated) | Phase 3 Randomized Study/May 2020 | |||
| NCT04257656 | A loading dose of 200 mg, followed by daily maintenance doses of 100 mg, for 9 days. | Adults with moderate/severe COVID-19 (also mechanically ventilated) | Phase 3 Randomized, Double-blind, Placebo-controlled, Multicenter Study/Completed on March 30, 2020 | |||
| NCT04280705 | A loading dose of 200 mg, followed by daily maintenance doses of 100 mg, for 9 up to 10 days. | Adults with moderate/severe COVID-19 (also mechanically ventilated) | Multicenter, Adaptive, Randomized Blinded Controlled Trial/April 2023 | |||
| NCT04292899 | A loading dose of 200 mg, followed by daily maintenance doses of 100 mg, for 5–10 days. | Adults with moderate/severe COVID-19 (also mechanically ventilated) | Phase 3 Randomized Study/May 2020 | |||
| NCT04292730 | A loading dose of 200 mg, followed by daily maintenance doses of 100 mg, for 5–10 days. | Adults with moderate/severe COVID-19 (also mechanically ventilated) | Phase 3 Randomized Study/May 2020 | |||
| FAVIPINAVIR | RNA-dependent RNA-polymerase inhibitor | NCT04359615 | Under Investigation | Adult confirmed COVID-19 patients. | Randomized, double-blind, controlled, clinical trial/May 3, 2020 | |
| NCT04346628 | Favipiravir administered orally, 1800 mg on the first dose (day 1) followed by 800 mg twice daily for the next 9 days (days 2–10). | Patients with laboratory confirmation of infection with SARS-CoV-2 and who are PCR positive (within five days or fewer prior to enrollment) and asymptomatic with breath difficulties | A Phase-2 Randomized, Open Label Study/April 2021 | |||
| NCT04349241 | A loading dose of 2400–3000 mg every 12 h twice, followed by maintenance doses of 1200–1800 mg every 12 h. | Adults with moderate/severe COVID-19 | Interventional Phase-3 Study/October 1, 2020 | |||
| LOPINAVIR/RITONAVIR | Coronavirus protease 3CL1 inhibitors | NCT04252885 | Group A) ordinary treatment plus a lopinavir (200 mg) and ritonavir (50 mg) (oral, q12h, every time 2 tablets of each, taking for 7–14 days). Group B) ordinary treatment plus a regimen of arbidol (100 mg) (oral, 200 mg each time, taking for 7–14 days). | Adults in sputum, throat swab, lower respiratory tract secretion, blood and other samples, the nucleic acid of the novel coronavirus was positive, or the sequencing of the virus gene was highly homologous with the known novel coronavirus | A Randomized, Open-label, Phase-4 Controlled Study/May 30, 2020 | |
| IVERMECTIN | Dissociation of the preformed IMPα/β1 heterodimer | NCT04343092 | Ivermectin 12 mg/weekly) + Hydroxychloroquine 400mg/daily + azithromycin 500 mg daily | Patients with confirmed COVID-19 | Interventional Phase-1 study/August 1, 2020 | |
| CHLOROQUINE and HYDROXY-CHLOROQUINE | Increase of endosomal pH | NCT04303507 | A loading dose of 10 mg base/kg (four 155 mg tablets for a 60 kg subject), followed by 155 mg daily (250 mg chloroquine phosphate salt/200 mg hydroxychloroquine sulfate) for 90 days. | Adult workers in a healthcare facility delivering direct care to patients with either proven or suspected COVID-19. | A double-blind, randomized, placebo-controlled trial/April 2021 | |
| NCT04308668 | Hydroxychloroquine: 200 mg tablet; 800 mg orally once, followed in 6 to 8 h by 600 mg, then 600 mg once a day for 4 consecutive days | Adults with confirmed COVID-19 or contact with COVID-19 positive subjects within 4 days | Interventional Phase-3 Randomized trial/April 21, 2020 | |||
| RECOMBINANT HUMAN ANGIOTENSIN-CONVERTING ENZYME 2 (APN01) | Blocker of protein S | NCT04287686 | 0.4 mg/kg rhACE2 IV BID | Adults with moderate/severe COVID-19 | Randomized, Open Label, Controlled Clinical Study/April 2020 | |
| UMIFENOVIR | Inhibitor of membrane fusion between the viral capsid and the cell membrane of the target cell | NCT04260594 | Arbidol tablets: take 2 tablets/time, 3 times/day for 14–20 days | Adults with moderate/severe COVID-19 without need of ventilation | Randomized, Open, Multicenter Phase-4 Study/July 1, 2020 | |
| NCT04255017 | Group 1) supportive treatment. Group 2) Arbidol hydrochloride 0.2g once,3 times a day,2 weeks. Group 3) Oseltamivir 75 mg once, twice a day,2 weeks. Group 4) Lopinavir/ritonavir 500 mg once, twice a day,2 weeks | Adults with confirmed COVID-19 | Interventional Phase-4/June 1, 2020 | |||
| NATURAL KILLERS CELLS | Enhance innate immune system responses, in particular Natural Killers Cells | NCT04280224 | Conventional treatment plus NK cells. Participants will receive conventional treatment plus twice a week of NK cells (0.1–2*10E7 NK cells/kg body weight). | Adults with moderate/severe COVID-19 | Interventional Phase-1/September 30, 2020 | |
| RECOMBINANT TYPE 1 INFERFERON | Broad spectrum antiviral activity | NCT04293887 | Standard treatment + recombinant human interferon α1β 10ug Bid by nebulization for 10 days. | Adults with moderate/severe COVID-19 | Multi-center, randomized, open, blank-controlled, Phase-1, multi-stage clinical study/May 30, 2020 | |
| DRUGS MODULATING THE HUMAN IMMUNE SYSTEM | ANTICITOKINE AND IMMUNO-MODULATORY AGENTS | TOCILIZUMAB + FAVIPINAVIR: Anti-IL-6 anti-inflammatories + RNA-dependent RNA-polymerase inhibitor | NCT04310228 | Group 1) Favipiravir Combined with Tocilizumab. Group 2) Favipinavir. Group 3) Tocilizumab: first dose is 4-8 mg/kg and the recommended dose is 400 mg. FAVIPINAVIR: On the 1st day, 1600 mg each time, twice a day; from the 2nd to the 7th day, 600 mg each time, twice a day. No more than 7 days | COVID-19 positive patients with increased levels of IL-6. | Interventional Randomized study/May 2020 |
| SARILUMAB: Anti-IL-6 anti-inflammatories | NCT04315298 | Single intravenous (IV) dose of sarilumab. Two doses (high an low) under investigation. | Hospitalized severe COVID-19 patients | Adaptive Phase-2/3 Randomized, Double-Blind, Placebo-Controlled study/March 9, 2021 | ||
| BEVACIZUMAB: inhibitor of vascular endothelial growth factor A (VEGF-A) | NCT04275414 | Under ECG monitoring, give bevacizumab 500 mg + 0.9% sodium chloride solution 100 ml via intravenous drip, time is no less than 90min. | Severe/Critical COVID-19 patients. | Pilot Phase-2 and Phase-3 Study/April 2020 | ||
| FINGOLIMOD: internalizer of S1P receptors, which sequesters lymphocytes in lymph nodes | NCT04280588 | Each patient in the fingolimod treatment group was given 0.5 mg of fingolimod orally once daily, for three consecutive days | Severe COVID-19 patients | Interventional Phase-2/July 1, 2020 | ||
| ECULIZUMAB: Distal complement inhibitor | NCT04288713 | Eculizumab 900 mg IV every 7 days. Eculizumab is given IV over 30 min without the need of a pump (although one can be used if available). | COVID-19 positive patients | Expanded access study | ||
| THALIDOMIDE | Inhibitor of TNF-alpha synthesis | NCT04273529 | 100 mg,po,qn, for 14 days. | Adults with mild/severe COVID-19 | Interventional Phase-2 Randomized/May 30, 2020 | |
| NCT04273581 | E100 mg/d,qn, for 14 days. | Adults with mild/severe COVID-19 | Interventional Phase-2 Randomized/April 30, 2020 | |||
| CORTICOSTEROIDS | Inhibitory action against the production of T cells (T lymphocytes), and the release of pro-inflammatory interleukins, such as IL-2, IL-1, IL-6 and IL-12, but also TNF-alpha, the INF-gamma. | NCT04263402 | 2 Groups. 1) Methylprednisolone ( < 40 mg/d) 2) Methylprednisolone (40~80 mg/d) | Adults with moderate/severe COVID-19 | Open, Prospective/Retrospective, Randomized Controlled Cohort Phase-4 Study/June 1, 2020 | |
| LOW MOLECULAR WEIGHT HEPARINS | Antithrombotic activity, with an increase in the ratio between anti-Xa and anti-IIa activity | NCT04345848 | Therapeutic doses of subcutaneous low-molecular-weight heparin (enoxaparin) or intravenous unfractionated heparin, from admission until the end of hospital stay or clinical recovery. | Adults with moderate/severe COVID-19 | Interventional Phase-3 Randomized/November 30, 2020 | |
| NCT04362085 | Therapeutic anticoagulation with LMWH or UFH (high dose nomogram). | Patients with COVID-19 and hospital admitted | 2-arm, parallel, pragmatic, multi-centre, open-label randomized controlled Phase-3 trial/November 2020 |
3. Results
3.1. RNA polymerase- RNA dependent inhibitors
3.1.1. Remdesivir
Remdesivir (GS-5734) was discovered and synthesized in 2017 by Siegel et al. to counter Ebola and other emerging viruses (Siegel et al., 2017).
Later on, there were different pre-clinical and randomized clinical studies that have respectively analyzed its mechanisms of action and its efficacy against these microorganisms (Agostini et al., 2018). It has broad-spectrum antiviral activity against RNA viruses. It is a prodrug, the structure of which resembles adenosine (Siegel et al., 2017).
Therefore, it can incorporate into the nascent viral RNA and inhibit the RNA-dependent RNA polymerase, stopping the replication of the viral genome (Mulangu et al., 2019).
Remdesivir has previously been shown to exhibit antiviral activity against several coronaviruses, including SARS-CoV and MERS-CoV, in vitro and in vivo (Agostini et al., 2018; Sheahan et al., 2017). In a recent in vitro study, remdesivir has also been shown to inhibit SARS-CoV-2 (M. Wang et al., 2020b).
The current dose under consideration is a single 200 mg, followed by a 100 mg daily infusion, for a period of time of 5–10 days. Different trials are underway to evaluate the use of remdesivir against coronavirus (Table 1).
The most common severe adverse effect of remdesivir is a reversible increase in transaminases, with possible kidney damage (Sheahan et al., 2020).
The AIFA (Italian Medicines Agency) is sponsoring two randomized, open-label, multicenter phase 3 studies, which will shed light on the effectiveness of remdesivir as an antiviral agent compared to supportive care. (“ClinicalTrials.gov,” n.d.).
Preliminary results from some ongoing studies randomized trials suggest to include this agent for the treatment of COVID-19. Because of this, the FDA recently approved the emergency use of remdesivir for the treatment of COVID-19 (“FDA Allows For ‘Emergency Use’ of Remdesivir, Experimental Coronavirus Drug,” 2020).
3.1.2. Favipiravir
Favipiravir or 6-fluoro-3-hydroxy-2-pyrazinecarboxamide (T-705) was developed in 2002 and was approved for medical use in Japan in 2014 (Furuta et al., 2002).
It has been found to have strong and selective inhibitory activity against Influenza viruses. Besides, Favipiravir is also considered as a novel viral RNA polymerase inhibitor (Furuta et al., 2013).
In 2018, Favipiravir was also studied as a potential countermeasure against neglected and emerging RNA viruses (Delang et al., 2018).
Therefore, similar to remdesivir, favipiravir works as an RNA-dependent RNA polymerase inhibitor structurally resembling endogenous guanine (Furuta et al., 2017).
Competitive inhibition may reduce viral replication. Although most of the information on this antiviral comes from its activity against Ebola and the H1N2 virus, favipiravir also demonstrated extensive activity against other RNA viruses (Furuta et al., 2017).
For the treatment of COVID-19, doses at the upper limit of the average dosage range should be considered. Appropriate doses of favipiravir against coronavirus are still under investigation. Some trials are testing protocols with attack doses of 1800–2400 mg, followed by maintenance doses ranging from 300 mg to 1800 mg (Table 1). (Sissoko et al., 2016).
Favipiravir is generally well tolerated. However, knowledge about safety in higher dose regimens is limited (Chinello et al., 2017; Dong et al., 2020; Kumagai et al., 2015). Favipiravir can cause hyperuricaemia, increase in transaminases, decrease in the number of neutrophils, diarrhea (Furuta et al., 2013).
Currently, there is no sufficient information available to support the use of favipiravir for COVID-19.
One study compared favipiravir with Umifenovir. After seven days of therapy, a significant difference was found between patients treated with umifenovir or favipiravir (C. Chen et al., 2020a), with better results returned by the latter.
These data suggest that further clinical trials on the efficacy of favipiravir for the treatment of COVID-19 are required. Despite little scientific evidence and the limited number of clinical trials available, in March 2020, favipiravir was approved by the National Medical Products Administration of China as the first anti-COVID-19 drug in China.
3.2. Viral protease inhibitors
3.2.1. Lopinavir/ritonavir
Lopinavir and ritonavir are used in combination as therapeutic drugs for HIV (Croxtall and Perry, 2010).
Although coronaviruses encode a different enzyme class of protease (cysteine protease), there is theoretical evidence that lopinavir and ritonavir can also inhibit the coronaviral protease 3CL1 (Chan et al., 2015; Chu et al., 2004; De Wilde et al., 2014).
The Lopinavir/ritonavir combination is being investigated in a clinical study against COVID-19 in patients with moderate and severe COVID-19 (Table 1). However, it has shown only little benefit (Agostini et al., 2018).
In another study in patients with severe COVID-19 (ChiCTR2000029308), no benefits of lopinavir/ritonavir were observed compared to standard care (Cao et al., 2020).
Further information on the use of lopinavir/ritonavir for the treatment of COVID-19 mainly comes from clinical cases and retrospective, non-randomized, and small studies, which make it difficult to ascertain the effect of the treatment (Chan et al., 2003; Yao et al., 2020).
The current lopinavir/ritonavir dose protocol under investigation consists of doses of lopinavir (200 mg) and ritonavir (50 mg) every 12 h for 7–14 days. (“Diagnosis and treatment protocol for novel coronavirus pneumonia,” 2020).
Although further clinical trials are underway on lopinavir/ritonavir, current data do not support lopinavir/ritonavir in COVID-19 treatment. This because of significant drug on drug interactions and their potential adverse reactions. According to a recent RCT, about 50% of patients treated with lopinavir/ritonavir experienced at least one adverse effect and 14% of patients had to interrupt the therapy (Cao et al., 2020).
The main adverse effects of lopinavir/ritonavir include gastrointestinal disorders (up to 30%) and hepatotoxicity (between 2% and 10%) (“Lexicomp Database Online,” 2016). More serious adverse effects are represented by hepatotoxicity, pancreatitis, abnormalities in cardiac conduction (Cao et al., 2020; Chu et al., 2004).
3.2.2. Ivermectin
Ivermectin has been studied since 1946 against avid diphtheria. It was considered as an enigmatic multifaceted ‘wonder’ drug in 2017 (Crump, 2017).
More recently it has also been used (as already approved by the FDA) as an anti-parasitic against scabies and equally against HIV, Zika, Dengue, West Nile, and Influenza viruses (Wagstaff et al., 2012).
Its mechanism of action involves the dissociation of the preformed IMPα/β1 heterodimer, responsible for the nuclear transport of loads of viral proteins (Caly et al., 2012).
Since nuclear transport of viral proteins is essential for the replication cycle and inhibition of the host's antiviral response, focusing on the nuclear transport process can be a viable therapeutic approach against RNA viruses (Yang et al., 2020).
Recently, an in vivo study has shown Ivermectin's ability to reduce viral RNA up to 5000 times after 48 h of SARS-CoV-2 infection (Caly et al., 2020).
It is currently under study in COVID-19 positive patients, with a dosage of 12 mg per week, together with hydroxychloroquine (400 mg/day + azithromycin 500 mg/day) (Table 1).
With a known safety profile for pesticide use, further studies will be needed to define and to establish the appropriate dosage of Ivermectin in the treatment of COVID-19.
3.3. Drugs inhibiting the fusion between cell membrane and virus
3.3.1. Chloroquine and hydroxychloroquine
As known as antimalarial and anti-autoimmune agents, hydroxychloroquine and chloroquine can also block virus infection by increasing the endosomal pH, necessary to the fusion of the membrane between the virus and the host cell (Savarino et al., 2003).
Recently, in vitro tests revealed the ability to reduce the number of viral copies of SARS-CoV-2 (Lan et al., 2020).
A news briefing from China reported that chloroquine has been successfully used to treat over 100 COVID-19 cases. The results demonstrated increased viral clearance and reduced disease progression (Gao et al., 2020). However, the final data have not yet been published, preventing the validation of these statements.
According to some authors, adding azithromycin to hydroxychloroquine in 6 patients resulted in numerically higher viral clearance (6/6, 100%) compared to hydroxychloroquine monotherapy (8/14, 57%) (Gautret et al., 2020).
Another prospective randomized trial of 30 patients performed in China with 400 mg daily for 5 days in combination with standard therapies (supportive therapy, interferon, and other antivirals) did not reveal any difference in virological outcomes (J. Chen et al., 2020b).
The dosage of chloroquine for the treatment of oral SARS-CoV-2 is 500 mg twice a day (Colson et al., 2020; “Diagnosis and treatment protocol for novel coronavirus pneumonia,” 2020).
Different dosages are also being studied, as a loading dose of 10 mg base/kg (four 155 mg tablets for a 60 kg subject), followed by 155 mg daily (250 mg chloroquine phosphate salt/200 mg hydroxychloroquine sulfate) for 90 days.
The lack of scientific evidence and RCT results do not allow the identification of an optimal dose to guarantee the safety and efficacy of chloroquine. Further results from ongoing studies will be needed to outline the optimal dose.
However, data related to these molecules are quite controversial – Lancet recently published a peer-reviewed paper on hydroxychloroquine – an observational study stated that the risk of cardiac arrhythmias outweighed its beneficial use. (“RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis,” 2020).
The same paper was retracted a few days later after a number of criticisms by more than 120 researchers. Therefore, the last conclusive results are still to be demonstrated. (“RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis,” 2020).
Chloroquine phosphate is contraindicated in case of hypersensitivity to the active principle. From a toxicological point of view, it may cause cardiovascular effects (such as prolongation of the QTc tract), hemolysis and other hematological effects, hypoglycaemia and central nervous system disorders (Colson et al., 2020; “Lexicomp Database Online,” 2016).
The characteristic cardiovascular toxicity of chloroquine phosphate makes it not suitable for cardiopathic patients.
Hydroxychloroquine is contraindicated in case of hypersensitivity to the active principle. The drug can cause idiosyncratic reactions and, secondarily, cardiotoxicity (to a lesser extent than chloroquine). (Lim et al., 2009; D. Zhou et al., 2020a)
3.3.2. Recombinant human angiotensin-converting enzyme 2 (APN01)
It is thought that the recombinant human angiotensin-converting enzyme 2 (rhACE2) may prevent the entry of SARS-CoV-2 into the host cell, by blocking the protein S responsible for the interaction between the virus and cellular ACE2.
In a recent study, rhACE2 was reported to inhibit SARS-CoV-2 replication in cell and embryonic stem cell-derived organoids by a factor of 1000–5000 times (Penninger et al., 2020).
The administration of rhACE2 may reduce the serum level of Angiotensin II, depriving the ACE enzyme of its substrate. This mechanism could prevent further activation of the ACE2 angiotensin receptor and thus preserve pulmonary vascular integrity and prevent ARDS (Khan et al., 2017).
rhACE2 has already undergone a phase II test for ARDS. A small pilot study in China is now evaluating the biological and physiological role of rhACE2 in COVID-19 pneumonia, primarily as a treatment for ARDS (Table 1) (“ClinicalTrials.gov,” n.d.).
The administration of recombinant human angiotensin-converting enzyme 2 is currently under investigation in a randomized, open Label, controlled clinical study.
3.3.3. Arbidol Hydrochloride (umifenovir)
Arbidol Hydrochloride is an antiviral agent that inhibits membrane fusion between the viral capsid and the cell membrane of the target cell (Chiou and Riegelman, 1971; Ford et al., 1979; Suzuki and Sunada, 1997; Tamblyn, 1994).
It is approved by Russia and China against the Influenza virus and arbovirus (Kadam and Wilson, 2017).
It is currently being tested as a single agent (“ClinicalTrials.gov,” n.d.) and in comparison with Oseltamivir and the lopinavir/ritonavir combination (Table 1).
Umifenovir is contraindicated in case of hypersensitivity to the active principle. The most common adverse reactions include gastrointestinal disturbances, allergic reactions, increased transaminase levels (Kadam and Wilson, 2017).
Current scientific evidence does not support the use of Arbidol for COVID-19. In a randomized clinical trial, favipiravir demonstrated supremacy in terms of therapeutic results (C. Chen et al., 2020a).
3.4. Innate immune system enhancing agents
3.4.1. Natural Killer cells
The higher mortality rate from COVID-19 has been observed among elderly patients, which can be explained by the weakening of the immune system with age. There are numerous approaches to enhance innate immune system responses. Natural Killer (NK) cells are an important component of the innate immune system and ensure a rapid response to viral infections. Previous studies have shown that lung migration of NK cells and macrophages plays a significant role in the clearance of SARS-CoV-2 (Chen et al., 2010).
A phase 1 study is underway in China. (“ClinicalTrials.gov,” n.d.). This study is analyzing the response of patients to the combination of ordinary therapy plus NK cells twice a week (Table 1).
The results of this study will help to understand if adding NK cells can help achieve viral clearance in COVID-19 pneumonia, especially in elder and fragile patients.
3.4.2. Recombinant type 1 interferon
Interferons are secreted by virus-infected cells. When used alone or in combination with other drugs, they have a broad-spectrum antiviral effect against HCV, respiratory syncytial virus, SARS-CoV, and MERS-CoV (Cinatl et al., 2003; Sheahan et al., 2020).
Recombinant type 1 interferon is currently under study in a multi-center, randomized, open, blank-controlled, Phase-1, multi-stage clinical study, that should be completed on May 30, 2020. (NCT04293887) (“ClinicalTrials.gov,” n.d.).
3.5. Inflammatory response attenuating agents
3.5.1. Anticitokine and immunomodulatory agents (Tocilizumab, sarilumab, bevacizumab, Eculizumab, and Fingolimod)
Another potential therapeutic class for COVID-19 is that of monoclonal antibodies, directed against the main inflammatory cytokines, or other components of the innate immune response.
The reason for their use lies in the pathophysiology of damage to the lungs and other organs caused by COVID-19, which induces an amplified immune response, named “cytokine storm” (Mehta et al., 2020).
IL-6 seems to be a key factor in this inflammation as evidenced by clinical results from Chinese research (F. Zhou et al., 2020b).
So perhaps, monoclonal antibodies directed towards IL-6 may reverse this process and lead to clinical improvements. Tocilizumab, approved by the FDA for the treatment of rheumatoid arthritis, has been used on a small number of serious cases of COVID-19 and provided promising results. Following the preliminary data available, the administration of a single 400 mg dose is associated with clinical improvement in 91% of patients, assessed as better respiratory function and rapid reduction of fever (Xu et al., 2020). The lack of a control group requires confirmation with more rigorous data that will have to be provided by additional studies (“ClinicalTrials.gov,” n.d.).
Tocilizumab is also studied in administration combined with Favipinavir, to analyze a possible synergistic action of the two drugs (Table 1). The results of this study are expected to be available by the end of May/June 2020. Tocilizumab can lead to an increased risk of infections, especially of the upper airways, increased AST transaminases, hypertension, hematological effects, hepatotoxicity, gastrointestinal perforation, hypersensitivity reactions to the active principle (Sheppard et al., 2017; Xu et al., 2020).
Sarilumab, another IL-6 receptor antagonist, approved for rheumatoid arthritis, is being tested in an adaptive phase-2/3 randomized, double-blind, placebo-controlled study in hospitalized severe COVID-19 patients (Table 1) (“Sanofi and Regeneron begin global Kevzara® (sarilumab) clinical trial program in patients with severe COVID-19,” 2020).
Among monoclonal antibodies or immunomodulatory agents which were studied in China and approved for large-scale use in the USA we have Fingolimod (immunomodulating agent, used to treat refractory multiple sclerosis; NCT04280588) bevacizumab (anti-vascular endothelial growth factor medication; NCT04275414), and Eculizumab (antibody inhibitor terminal complement; NCT04288713) (“ClinicalTrials.gov,” n.d.).
3.5.2. Thalidomide
In this spasmodic search for drugs that can guarantee a therapy for SARS-CoV-2, we can mention Thalidomide - an antiangiogenic, anti-inflammatory, and anti-fibrotic agent. Thalidomide is an inhibitor of TNF-alpha synthesis, was used as a treatment for multiple inflammatory diseases, such as Behçets' disease and Crohn's disease (Vargesson, 2015).
Preclinical studies showed that thalidomide was effective in the treatment of H1N1-infected mice by reducing the infiltration of inflammatory cells and the production of pro-inflammatory cytokines (Zhu et al., 2014).
In the wake of this evidence, current studies are focusing on immunomodulatory effects that could reduce lung damage caused by the strong immune response to SARS-CoV-2 (Table 1) (“ClinicalTrials.gov,” n.d.).
However, it is eaully important to underline that thalidomide can cause some adverse effects such as severe birth defects or embryo-fetal death, even with a single dose, if taken during pregnancy (Boylen et al., 1963).
Besides, this molecule may also induce severe hepatotoxicity (Hamadani et al., 2007; Hanje et al., 2006).
3.5.3. Corticosteroids
Systemic glucocorticoids are currently contraindicated in SARS-CoV-2 infection, as they can slow viral clearance (Russell et al., 2020).
Observational studies showed a delay in viral clearance from the respiratory tract that is associated with some complications including psychosis ed hyperglycemia by using corticosteroid combinations in patients with SARS and MERS (Arabi et al., 2018; Stockman et al., 2006).
Furthermore, a meta-analysis of 10 observational studies with 6548 patients with pneumonia, published in 2019, found that corticosteroids were associated with an increased risk of mortality and about two times greater risk of secondary infections (Ni et al., 2019).
Whether methylprednisolone administration may or may not help suppress unwanted immune reactions is still a controversial topic. Therefore, studies have been launched to investigate and assess their efficacy and safety, methylprednisolone in particular.
The rationale for the use of corticosteroids lies in their inhibitory action against the production of T cells (T lymphocytes), and the release of pro-inflammatory interleukins, such as IL-2, IL-1, IL-6, and IL-12, but also TNF-alpha, the INF-gamma (Table 1).
Today, the limited data available and the lack of proven benefits with corticosteroids warn against their clinical use in COVID-19 positive patients.
3.5.4. Low molecular weight heparins
Low molecular weight heparins (LMWH) are glycosaminoglycans obtained by the fractionation of heparin. They are used in the prophylaxis of post-surgical venous thromboembolism and non-surgical patients with acute pathology and reduced mobility.
A Chinese retrospective analysis of 415 cases of severe hospitalized COVID-19 patients suggested that in subjects with coagulation activation, administration of unfractionated or LMWH heparin for at least one week could lead to a greater chance of survival. The positive therapeutic effect would be evident only in those patients who show a high level of D-dimer (6 times of the maximum values) or a high score on a scale of sepsis-induced coagulopathy (SIC score> 4).
More hemorrhagic adverse events were observed in heparin-treated patients with normal D-dimer values (Siddiqi and Mehra, 2020).
This study presents an important series of limits, but it represents the only cognitive element available we have at present regarding LMWH for COVID-19.
The use of LMWH in COVID-19 patients can be applied only in subjects with 4–6 times higher levels than normal or a SIC score> 4. Since this indication is based on preliminary evidence, this use may be considered after a careful case-by-case evaluation only.
It is also important to remember that this retrospective study indicates that not only would patients who do not show equal levels of clotting activation not benefit from the administration of heparin, but their clinical condition could even get worse (McGonagle et al., 2020).
Low molecular weight heparins are currently under investigation in some interventional randomized phase-3 trials (Table 1), in adults with moderate/severe COVID-19, or COVID-19 hospitalized patients.
The evidence on the therapeutic use of LMWH in COVID-19 patients is incomplete and with important concerns regarding safety. Therefore, randomized studies to evaluate its clinical efficacy and safety will be necessary.
3.5.5. Hyperimmune plasma
The administration of passive polyclonal antibodies (Ab) found in the plasma, has been used in the past to improve the survival rate of patients with acute respiratory syndromes of viral etiology, to provide immediate immunity to the patient (Mair-Jenkins et al., 2015).
Zhang et al. have shown that SARS-CoV-2 convalescent plasma contains Ab neutralizing against the virus involved (Zhang et al., 2005).
Some preliminary studies carried out in China and South Korea, on a sample of 27 patients aged between 28 and 75 years, reported good results after performing the Convalescent Plasma Transfusion (CPT) (Zhang and Liu, 2020).
The CPT protocols studied in these trials were different, and vary from a minimum of a single dose of 200 ml of convalescent plasma with neutralizing antibody titers> 1: 640, to a maximum of 2400 ml (Duan et al., 2020; Zhang et al., 2020).
All patients in these studies received CPT between day 6 and day 50 after symptom onset or hospitalization (Ye et al., 2020).
Despite these encouraging results, these studies showed high bias, due to the limited size of the sample, poor methodological methods for the selection of participants, the dosage of CPT and duration of therapy, etc.
Some clinical trials are in progress on samples of 10–100 patients, treated with plasma infusions from 300 ml to 500 ml, in a time interval of up to 4 h (“ClinicalTrials.gov,” n.d.).
Currently, there is not enough scientific evidence available on the effectiveness of the use of autoimmune plasma. Evidence that can be provided by the results of the ongoing trials.
3.6. Vaccines
Thanks to the sequencing of the viral genome of SARS-CoV-2, multiple nucleic acid vaccines are being tested, focusing on the specific sequence that codes for the viral S protein.
The mRNA 1273 vaccine conceived by Moderna consists of a synthetic strand of mRNA that codes for the viral spike protein. The evaluation of its effectiveness is ongoing (NCT04283461). (“ClinicalTrials.gov,” n.d.).
As for Moderna's 1273 mRNA, INO-4800 produced by Inovio Pharmaceuticals, it is also a genetic vaccine which, inoculated in humans, is translated into proteins that trigger a targeted immune response (NCT04336410). (“ClinicalTrials.gov,” n.d.).
The simple structure of nucleic acids avoids the risk of incorrect folding, which instead could occur in recombinant protein-based vaccines (Sheahan et al., 2020).
However, the way of administration, the interval between administrations and the quantity supplied in genetic vaccines are key factors, and therefore these will be investigated carefully as they may significantly influence the immunogenicity of this kind of vaccine (Sheahan et al., 2020).
The vaccine developed by Oxford University is composed of a non-replicating adenovirus vector. The fact of not being replicant makes the vaccine safer in children and in people with pre-existing diseases. It is currently undergoing clinical trials (NCT04324606). (“ClinicalTrials.gov,” n.d.).
Nonavax is developing a vaccine based on nanoparticles that use antigens deriving from S protein. Thanks to the encapsulation, these nanoparticles can be combined with antigenic epitopes causing a lymphocyte proliferation and cytokine production (Al-Halifa et al., 2019).
The University of Queensland is working on a stabilized subunit vaccine based on molecular clamp technology, which would allow recombinant viral proteins to remain durably in their pre-fusion form. Similar vaccines have been used against Ebola and Influenza. (“UQ COVID-19 vaccine shown to induce potent protective response in pre-clinical trials,” 2020).
3.7. Limitations
Some limitations may be attributed to this work. First of all, as per the literature analysis, this was carried out only on 3 search engines: PubMed, EMBASE, and Cochrane Library using just certain specific keywords. This method may exclude some papers.
Besides, the importance and the necessity to discover a new system to fight the novel coronavirus means that there are daily news, updates, and publications that cannot be considered for practical reasons in this publication. Some studies in progress at this moment may be completed at the time of publication of this manuscript.
Due to the lack of large-sample randomized clinical trials small sample studies have also been included.
However, this work is aimed to be a general reference point and to provide an overview as comprehensive as possible regarding the main clinical trials in progress at the moment.
4. Conclusions
The COVID-19 pandemic represents the largest global public health crisis of this century. We present an overview of the possible therapeutic options currently under investigation and the future perspectives of this disease. The current clinical studies that were quickly launched at the beginning of the pandemic and were underway in April 2020 are summarized. Most of them are based on the administration of therapeutic agents previously used for other pathological conditions.
These agents can be divided into two broad categories, 1) those that can directly interfere with the virus replication cycle, and 2) those based on immunotherapeutic approaches aimed at modulating immune responses.
Although vaccines and antibodies targeting SARS-CoV-2 are under study, these still require careful evaluation of efficacy and safety. Therefore, to date, drug therapy is the only available approach for a rapid response to the pandemic.
At present, some of them have already shown promising results in preliminary studies and were approved for wider use. However, no effective therapy has been identified yet. Therefore, no standard therapeutic protocol is available.
Since the beginning of the pandemic, some news and social media have disseminated incorrect information about COVID-19 and possible therapies (Orso et al., 2020). The Internet is an excellent source of data, but anyone can share and disseminate incorrect information that can endanger people's health. This should be seriously considered by the competent authorities (Kadam and Atre, 2020; Rovetta and Bhagavathula, 2020; Waszak et al., 2018).
It is imperative that all actors involved in the fight against coronavirus follow the findings of scientific research, and not be misled by information without scientific basis.
Some medicines presented some activity against COVID-19. However, there is no reliable evidence on prognosis and safety. In order to find a safe and effective therapeutic protocol or vaccine as soon as possible, international collaboration between authorities and research centers is needed.
Future review of the literature will be effective to shed light on the efficacy of the therapies under study, guiding research towards the development of a safe and effective treatment protocol.
Authors’ contribution
Dr. Giulio Nittari: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing - review & editing.
Dr. Graziano Pallotta: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing - review & editing.
Prof. Seyed Khosrow Tayebati: Supervision; Validation; Writing - review & editing.
Prof. Francesco Amenta: Supervision.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Authors declare they have no conflict of interests.
Acknowledgements
Fig. 1 contains a graphical illustration of SARS-CoV-2 which was used under a Public Domain License. We would like to thanks the authors of that illustration: Alissa Eckert, MS; Dan Higgins, MAM. The media comes from the Centers for Disease Control and Prevention's Public Health Image Library (PHIL), with identification number #23312.
References
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Halifa S., Gauthier L., Arpin D., Bourgault S., Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A., Al Khatib K., Alraddadi B., Shalhoub S., Abdulmomen A., Qushmaq I., Mady A., Mady O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Balkhy H.H., Balkhy A., Deeb A.M., Al Mutairi H., Al-Dawood A., Merson L., Hayden F.G., Fowler R.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018 doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Boylen J.B., Horne H.H., Johnson W.J. Teratogenic effects OF thalidomide and related substances. Lancet. 1963 doi: 10.1016/S0140-6736(63)91346-1. [DOI] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Wagstaff K.M., Jans D.A. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antivir. Res. 2012 doi: 10.1016/j.antiviral.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang Jingli, Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li Huadong, Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li Hui, Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang Juan, Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P., Cai J.P., Chu H., Zhou J., Chen H., Qin C., Yuen K.Y. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERSCoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015 doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M.L., Tse M.W., Que T.L., Peiris J.S.M., Sung J., Wong V.C.W., Yuen K.Y. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003 [PubMed] [Google Scholar]
- Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J., Yin P., Wang X. medRxiv; 2020. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010 doi: 10.1128/jvi.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., Ling Y., Huang D., Song S., Zhang D., Quian Z., Li T., Shen Y., Lu H. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J. Zhejiang Univ. 2020;49:1–10. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020 doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinello P., Petrosillo N., Pittalis S., Biava G., Ippolito G., Nicastri E. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Neglected Trop. Dis. 2017 doi: 10.1371/journal.pntd.0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou W.L., Riegelman S. Pharmaceutical applications of solid dispersion systems. J. Pharmaceut. Sci. 1971 doi: 10.1002/jps.2600600902. [DOI] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004 doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003 doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrialsgov U.S. Natl. Libr. Med. https://clinicaltrials.gov [WWW Document], n.d. accessed 4.10.20.
- Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall J.D., Perry C.M. LopinavirRitonavir: a review of its use in the management of HIV-1 infection. Drugs. 2010 doi: 10.2165/11204950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Crump A. Ivermectin: enigmatic multifaceted “wonder” drug continues to surprise and exceed expectations. J. Antibiot. (Tokyo) 2017 doi: 10.1038/ja.2017.11. [DOI] [PubMed] [Google Scholar]
- De Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., Van Nieuwkoop S., Bestebroer T.M., Van Den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014 doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 2018 doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Diagnosis and treatment protocol for novel coronavirus pneumonia Natl. Heal. Comm. State adm. Tradit. Chinese med. 2020. http://www.kankyokansen.org/uploads/uploads/files/jsipc/protocol_V6.pdf [WWW Document] accessed 4.14.20.
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020 doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Yong, Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Yeqin, Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. Unit. States Am. 2020 doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fda Allows For Emergency Use of Remdesivir Experimental Coronavirus Drug Time. 2020. https://time.com/5831062/fda-allows-emergency-use-remdesivir/ [WWW Document] accessed 5.2.20.
- Fehr A.R., Perlman S. Coronaviruses: Methods and Protocols. 2015. Coronaviruses: an overview of their replication and pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.L., Stewart A.F., Rubinstein M.H. The assay and stability of chlorpropamide in solid dispersion with urea. J. Pharm. Pharmacol. 1979;31:726–729. doi: 10.1111/j.2042-7158.1979.tb13645.x. [DOI] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013 doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 2017 doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Fukuda Y., Kuno M., Kamiyama T., Kozaki K., Nomura N., Egawa H., Minami S., Watanabe Y., Narita H., Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002 doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020 doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Global research on coronavirus disease (Covid-19) 2020. World Heal. Orgainization.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov [WWW Document] accessed 4.18.20. [Google Scholar]
- Hamadani M., Benson D.M., Copelan E.A. Thalidomide-induced fulminant hepatic failure [1] Mayo Clin. Proc. 2007 doi: 10.4065/82.5.638. [DOI] [PubMed] [Google Scholar]
- Hanje A.J., Shamp J.L., Thomas F.B., Meis G.M. Thalidomide-induced severe hepatotoxicity. Pharmacotherapy. 2006 doi: 10.1592/phco.26.7.1018. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam A.B., Atre S.R. Negative impact of social media panic during the COVID-19 outbreak in India. J. Trav. Med. 2020 doi: 10.1093/jtm/taaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. U.S.A. 2017 doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017 doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y., Murakawa Y., Hasunuma T., Aso M., Yuji W., Sakurai T., Noto M., Oe T., Kaneko A. Lack of effect of favipiravir, a novel antiviral agent, on the QT interval in healthy Japanese adults. Int. J. Clin. Pharmacol. Therapeut. 2015 doi: 10.5414/CP202388. [DOI] [PubMed] [Google Scholar]
- Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR test results in patients recovered from COVID-19. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexicomp Database Online . 2016. Lexicomp Inc.http://online.lexi.com [WWW Document] accessed 3.17.20. [Google Scholar]
- Lim H.S., Im J.S., Cho J.Y., Bae K.S., Klein T.A., Yeom J.S., Kim T.S., Choi J.S., Jang I.J., Park J.W. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by plasmodium vivax. Antimicrob. Agents Chemother. 2009 doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., Makki S., Rooney K.D., Nguyen-Van-Tam J.S., Beck C.R. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J. Infect. Dis. 2015 doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Mbaya O.T., Proschan M., Mukadi D., Manzo M.L., Nzolo D., Oloma A.T., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Clifford Lane H., Muyembe-Tamfum J.J., Sivahera B., Camara M., Kojan R., Walker R., Dighero-Kemp B., Cao H., Mukumbayi P., Mbala-Kingebeni P., Ahuka S., Albert S., Bonnett T., Crozier I., Duvenhage M., Proffitt C., Teitelbaum M., Moench T., Aboulhab J., Barrett K., Cahill K., Cone K., Eckes R., Hensley L., Herpin B., Higgs E., Ledgerwood J., Pierson J., Smolskis M., Sow Y., Tierney J., Sivapalasingam S., Holman W., Gettinger N., Vallée D., Nordwall J. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit. Care. 2019 doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso D., Federici N., Copetti R., Vetrugno L., Bove T. Infodemic and the spread of fake news in the COVID-19-era. Eur. J. Emerg. Med. 2020 doi: 10.1097/mej.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D., Kostaki E.G., Magiorkinis G., Panayiotakopoulos G., Sourvinos G., Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020 doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninger J.M., Mirazimi A., Montserrat N. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;29 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retracted: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of Covid-19: a multinational registry analysis . 2020. Lancet.https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext [WWW Document] accessed 5.30.20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rovetta A., Bhagavathula A.S. COVID-19-related web search behaviors and infodemic attitudes in Italy: infodemiological study. J. Med. Internet Res. 2020 doi: 10.2196/19374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020 doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sanofi and Regeneron begin global Kevzara® (sarilumab) clinical trial program in patients with severe Covid-19 Sanofi. 2020. http://www.news.sanofi.us/2020-03-16-Sanofi-and-Regeneron-begin-global-Kevzara-R-sarilumab-clinical-trial-program-in-patients-with-severe-COVID-19 [WWW Document]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 2003 doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., MacKman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020 doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard M., Laskou F., Stapleton P.P., Hadavi S., Dasgupta B. Tocilizumab (actemra) Hum. Vaccines Immunother. 2017 doi: 10.1080/21645515.2017.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L., Neville S., Carra E., Lew W., Ross B., Wang Q., Wolfe L., Jordan R., Soloveva V., Knox J., Perry J., Perron M., Stray K.M., Barauskas O., Feng J.Y., Xu Y., Lee G., Rheingold A.L., Ray A.S., Bannister R., Strickley R., Swaminathan S., Lee W.A., Bavari S., Cihlar T., Lo M.K., Warren T.K., Mackman R.L. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 2017 doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- Sissoko D., Laouenan C., Folkesson E., M'Lebing A.B., Beavogui A.H., Baize S., Camara A.M., Maes P., Shepherd S., Danel C., Carazo S., Conde M.N., Gala J.L., Colin G., Savini H., Bore J.A., Le Marcis F., Koundouno Fara Raymond, Petitjean F., Lamah M.C., Diederich S., Tounkara A., Poelart G., Berbain E., Dindart J.M., Duraffour S., Lefevre A., Leno T., Peyrouset O., Irenge L., Bangoura N., Palich R., Hinzmann J., Kraus A., Barry T.S., Berette S., Bongono A., Camara M.S., Chanfreau Munoz V., Doumbouya L., Harouna Souley, Kighoma P.M., Koundouno Fara Roger, Réné Lolamou, Loua C.M., Massala V., Moumouni K., Provost C., Samake N., Sekou C., Soumah A., Arnould I., Komano M.S., Gustin L., Berutto C., Camara D., Camara F.S., Colpaert J., Delamou L., Jansson L., Kourouma E., Loua M., Malme K., Manfrin E., Maomou A., Milinouno A., Ombelet S., Sidiboun A.Y., Verreckt I., Yombouno P., Bocquin A., Carbonnelle C., Carmoi T., Frange P., Mely S., Nguyen V.K., Pannetier D., Taburet A.M., Treluyer J.M., Kolie J., Moh R., Gonzalez M.C., Kuisma E., Liedigk B., Ngabo D., Rudolf M., Thom R., Kerber R., Gabriel M., Di Caro A., Wölfel R., Badir J., Bentahir M., Deccache Y., Dumont C., Durant J.F., El Bakkouri K., Gasasira Uwamahoro M., Smits B., Toufik N., Van Cauwenberghe S., Ezzedine K., Dortenzio E., Pizarro L., Etienne A., Guedj J., Fizet A., Barte de Sainte Fare E., Murgue B., Tran-Minh T., Rapp C., Piguet P., Poncin M., Draguez B., Allaford Duverger T., Barbe S., Baret G., Defourny I., Carroll M., Raoul H., Augier A., Eholie S.P., Yazdanpanah Y., Levy-Marchal C., Antierrens A., Van Herp M., Günther S., de Lamballerie X., Keïta S., Mentre F., Anglaret X., Malvy D. Experimental treatment with favipiravir for Ebola virus disease (the jiki trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016 doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Situation Report n 89 . 2020. World Heal. Organ.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [WWW Document] accessed 4.18.20. [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006 doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Sunada H. Comparison of nicotinamide, ethylurea and polyethylene glycol as carriers for nifedipine solid dispersion systems. Chem. Pharm. Bull. 1997 doi: 10.1248/cpb.45.1688. [DOI] [PubMed] [Google Scholar]
- Tamblyn S.E. Pandemic planning in Canada. Eur. J. Epidemiol. 1994 doi: 10.1007/BF01719688. [DOI] [PubMed] [Google Scholar]
- The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (Covid-19) in China Zhonghua Liuxingbingxue Zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team China CDC The epidemiological characteristics of an outbreak of 2019 novel coronaviurs diseases. Vital Surveillances. 2020 [PMC free article] [PubMed] [Google Scholar]
- Uq Covid19 vaccine shown to induce potent protective response in pre-clinical trials . 2020. Univ. Queensl.https://www.uq.edu.au/news/article/2020/04/uq-covid-19-vaccine-shown-induce-potent-protective-response-pre-clinical-trials [WWW Document] accessed 5.5.20. [Google Scholar]
- Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res. Part C Embryo Today - Rev. 2015 doi: 10.1002/bdrc.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012 doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszak P.M., Kasprzycka-Waszak W., Kubanek A. The spread of medical fake news in social media – the pilot quantitative study. Heal. Policy Technol. 2018 doi: 10.1016/j.hlpt.2018.03.002. [DOI] [Google Scholar]
- Who to accelerate research and innovation for new coronavirus . 2020. World Heal. Orgainization.https://www.who.int/news-room/detail/06-02-2020-who-to-accelerate-research-and-innovation-for-new-coronavirus [WWW Document] accessed 4.18.20. [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. chinaXiv; 2020. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.N.Y., Atkinson S.C., Wang C., Lee A., Bogoyevitch M.A., Borg N.A., Jans D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020 doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020 doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., Xia X., Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., Chen Q., Zhang L., Zhong Q., Zhang X., Zou Y., Zhang S. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.S., Chen J.T., Liu Y.X., Zhang Z.S., Gao H., Liu Y., Wang X., Ning Y., Liu Y.F., Gao Q., Xu J.G., Qin C., Dong X.P., Yin W.D. A serological survey on neutralizing antibody titer of SARS convalescent sera. J. Med. Virol. 2005 doi: 10.1002/jmv.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020 doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Shi X., Ju D., Huang H., Wei W., Dong X. Anti-inflammatory effect of thalidomide on H1N1 influenza virus-induced pulmonary injury in mice. Inflammation. 2014 doi: 10.1007/s10753-014-9943-9. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]