Abstract
Background
Viral infections are known to exacerbate asthma in adults. Previous studies have found few patients with asthma among severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia cases. However, the relationship between SARS-CoV-2 infection and severe asthma exacerbation is not known.
Objective
To assess the frequency of asthma exacerbation in patients with asthma hospitalized for SARS-CoV-2 pneumonia and compare symptoms and laboratory and radiological findings in patients with and without asthma with SARS-CoV-2 pneumonia.
Methods
We included 106 patients between March 4 and April 6, 2020, who were hospitalized in the Chest Diseases Department of Strasbourg University Hospital; 23 had asthma. To assess the patients' asthma status, 3 periods were defined: the last month before the onset of COVID-19 symptoms (p1), prehospitalization (p2), and during hospitalization (p3). Severe asthma exacerbations were defined according to Global INitiative for Asthma guidelines during p1 and p2. During p3, we defined severe asthma deterioration as the onset of breathlessness and wheezing requiring systemic corticosteroids and inhaled β2 agonist.
Results
We found no significant difference between patients with and without asthma in terms of severity (length of stay, maximal oxygen flow needed, noninvasive ventilation requirement, and intensive care unit transfer); 52.2% of the patients with asthma had Global INitiative for Asthma step 1 asthma. One patient had a severe exacerbation during p1, 2 patients during p2, and 5 patients were treated with systemic corticosteroids and inhaled β2 agonist during p3.
Conclusions
Our results demonstrate that patients with asthma appeared not to be at risk for severe SARS-CoV-2 pneumonia. Moreover, SARS-CoV-2 pneumonia did not induce severe asthma exacerbation.
Key words: Coronavirus, COVID-19, SARS-CoV-2, SARS-CoV-2 pneumonia, Asthma, Exacerbation, France, Europe
Abbreviations used: ACE2, Angiotensin-converting enzyme 2; aOR, Adjusted odd ratio; COVID-19, Coronavirus disease 2019; CT, Computed tomography; GINA, Global INitiative for Asthma; p1, The last month before the onset of COVID-19 symptoms; p2, During hospitalization; p3, After hospitalization; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
What is already known about this topic? Patients with asthma are rare in epidemiological studies of severe acute respiratory syndrome coronavirus 2 pneumonia.
What does this article add to our knowledge? Being asthmatic is not a risk factor for severe acute respiratory syndrome coronavirus 2.
How does this study impact current management guidelines? Severe acute respiratory syndrome coronavirus 2 pneumonia may not induce severe asthma exacerbation.
Introduction
At the end of December 2019, Chinese public health authorities reported several cases of acute respiratory syndrome in Wuhan, Hubei, China.1 Since then, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has spread across the globe, leading to a public health emergency of international concern on January 30, 2020, and resulting in a pandemic.2 The most common symptoms of COVID-19 are fever and respiratory symptoms, including cough, sore throat, and shortness of breath.3 Other noted symptoms include fatigue, myalgia, diarrhea, abdominal pain, and recently anosmia, dysgeusia,4 and confusion.5 Many patients also develop lymphopenia and pneumonia with characteristic pulmonary ground glass opacity changes on chest computed tomography (CT).6 , 7
The pathophysiology mechanisms are not yet well characterized. After inhalation of SARS-CoV-2 through aerosolized uptake, the virus likely binds to epithelial cells from the nasal cavity, starts replicating, and reaches the lower respiratory tract. The main receptor for the SARS-CoV-2 spike is angiotensin-converting enzyme 2 (ACE2), which is expressed in several organs such as the lung, heart, kidney, intestine, and endothelial cells. For about 20% of the infected patients, the disease will progress to a pneumoniae through propagation of SARS-CoV within type II cells through ACE2 and will compromise the alveolo-capilar space. It may result in a diffuse alveolar damage and fibrosis. A hyperinflammatory syndrome called “cytokine storm” consisting in fever, cytopenias, hyperferritinemia, diffuse alveolar damage, and hypercytokinemia may occur during this phase. It may lead to multiorgan failure and high rate of mortality. From an immunologic point of view, it is characterized by increased IL-2, IL-7, granulocyte-CSF, IFN-γ–inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and TNF-α.8, 9, 10, 11
Viral infections exacerbated asthma in up to 41% of adult patients. Rhinoviruses were the most frequently detected type of virus. Other respiratory tract viruses, including respiratory syncytial virus, influenza viruses, coronaviruses, human metapneumoviruses, parainfluenza viruses, adenoviruses, and bocaviruses, have also been detected in patients with asthma exacerbation.12
Until now, coronaviruses seemed to make only a minor contribution to asthma exacerbation in adults.12 Although the Centers for Disease Control and Prevention stated that patients with moderate to severe asthma could be at a greater risk of more severe COVID-19,13 no published data support this statement at this time. However, Zhang et al14 reported that allergic disease and asthma were not risk factors for SARS-CoV-2 infection in 140 Chinese patients.
The aims of the present study were to assess the frequency of asthma exacerbation in patients with asthma hospitalized for SARS-CoV-2 pneumonia, and to compare the symptoms and biological and radiological findings in patients with and without asthma with SARS-CoV-2 pneumonia.
Methods
Study design and population
This was a monocentric, retrospective, cohort study using clinical, radiological, and laboratory data obtained from patients hospitalized at the Chest Diseases Department of Strasbourg University Hospital.
The study population consisted of 106 patients with SARS-CoV-2 pneumonia hospitalized between March 4 and April 6, 2020. Since the outbreak of COVID-19, 60 of 86 beds in our department have been devoted to the treatment of SARS-CoV-2 pneumonia, including noninvasive ventilatory support.
SARS-CoV-2 pneumonia diagnosis was established on the basis of the presence of all of the following:
-
•
Clinical symptoms: fever, asthenia, dyspnea, tachypnea, cough, chest pain, and/or crackles.
-
•
Chest CT manifestations indicating SARS-Cov-2 pneumonia according to the literature: ground glass opacity, bilateral involvement, peripheral distribution, multilobar involvement, consolidations.15, 16, 17 All CT images were acquired using an 80-row CT scanner (Aquilion PRIME SP, Canon Medical Systems, Otawara, Japan) without injection. Quality of imaging was chosen according to the patient age and body mass index. When possible, patients were instructed to hold their breath and raise their arms above their head to minimize artifacts. Images were reconstructed with a slice thickness of 1 mm in the mediastinal and parenchymal windows and transmitted to postprocessing workstations for multiplanar and maximum intensity projection reconstructions.
-
•
Positive laboratory confirmation of SARS-CoV-2 infection (centralized in Strasbourg Hospital Viral Laboratory). Quantitative real-time PCR tests for SARS-CoV-2 nucleic acid were performed using nasopharyngeal swabs.18 The primer and probe sequences target 2 regions on the RdRp gene and are specific to SARS-CoV-2. Assay sensitivity is approximately 10 copies/reaction.
Among the 106 patients with SARS-CoV-2 pneumonia, 23 had a clinical diagnosis of asthma based on the clinical history recorded by medical staff. All the clinical data were verified with the patient's general practitioner and specialists (allergists, pulmonologists).
Because we are a reference center for asthma, almost all SARS-CoV-2 pneumonia cases in patients with asthma hospitalized at the emergency room of Strasbourg University Hospital were referred to our department.
To assess the patient's asthma status, we defined 3 periods. The first period was the last month before the onset of COVID-19 symptoms (p1). The second period was the prehospitalization period between the beginning of the SARS-CoV-2 infection symptoms and the first day of hospitalization (p2). The third period was during hospitalization (p3).
Severe asthma exacerbations were assessed during p1 and p2, and defined as a deterioration in asthma resulting in hospitalization or emergency room treatment or the need for oral steroids for more than 3 consecutive days.19
During p3, we defined symptoms corresponding to asthma as breathlessness and wheezing requiring systemic corticosteroids and inhaled β2 agonist.
This study was performed in accordance with the Declaration of Helsinki and with Good Clinical Practice guidelines. We received approval from the Clinical Research Ethic Committee of Strasbourg University Hospital (CE-2020-56). Oral informed consent was obtained from all patients. All personal data remained confidential and were anonymized.
Data collection
Demographic, clinical, laboratory, and radiological data were recorded during routine health care. All study variables were reported on a standardized data-collection sheet.
For all patients, the clinical data included the delay before onset of COVID-19 symptoms and hospitalization; symptoms at admission (eg, fever, asthenia, anorexia, myalgia, confusion, odynophagia, nasal obstruction, rhinorrhea, acute anosmia, sneezes, gastrointestinal symptoms, cough, dyspnea, chest tightness, respiratory frequency, wheezing, and crackles); maximum oxygen flow, noninvasive ventilation need, intensive care unit transfer for mechanical ventilation or death; smoking habits (past smoker, current smoker, and the smoking index); and health issues such as hypertension, obesity (defined by body mass index ≥ 30 kg/m2), cardiopathy, diabetes mellitus, chronic renal insufficiency, lung cancer, and obstructive sleep apnea.
Lung CT images were analyzed and the global percentage of abnormal lung parenchyma visually estimated and classified by 2 experienced radiologists into 4 categories following the French Society of Thoracic Imaging guidelines20: absent/minimal (<10%), moderate (10%-25%), extensive (26%-50%), and severe/critical (>50%).
Laboratory results comprised neutrophil, lymphocyte, and eosinophil counts, and C-reactive protein level.
Disease-relevant variables for asthma were age at onset (stratified on “<18,” “18-50,” and “>50 years”); eosinophil count before the beginning of COVID-19; allergic status based on clinical history and/or skin test results; spirometry (FEV1 and FEV1/forced vital capacity); severity of asthma (the number of severe exacerbations during the last 12 months,19 number of hospitalizations in the intensive care unit, step treatment according to Global Initiative for Asthma [GINA] 2019 guidelines); comorbidities (ie, gastroesophageal reflux, obstructive sleep apnea, hyperventilation syndrome, chronic rhinosinusitis with polyposis, anti-inflammatory drug hypersensitivity, paradoxical vocal cord motion disorder); and asthma control, during the last 4 weeks before the beginning of SARS-CoV-2 infection based on the GINA guidelines (well controlled, partly controlled, or uncontrolled).21
Statistical analysis
We summarized continuous measures as medians and interquartile ranges and categorical variables as frequencies and proportions. We tested comparisons between patients with and without asthma using Fisher exact or χ2 test for categorical variables and the Wilcoxon rank-sum test for numerical variables. Because of the absence of randomization and to adjust for possible confusing factors, we performed multivariable logistic regression analysis using a binomial generalized linear model to determine the adjusted odds ratio (aOR). Variables with P less than .1 in the univariate analysis were selected as independent variables in the multivariate model. To evaluate whether asthma is associated with and a risk factor for severe COVID-19 outcomes, we used propensity scores to adjust for confounding variables. The propensity score allows analyzing an observational nonrandomized study so that it mimics some of the particular characteristics of a randomized controlled trial as it accounts for systematic differences in baseline characteristics between subjects with and without asthma when estimating the effect of asthma on severe COVID-19 outcomes. Propensity scores were generated by multiple logistic regression using known risk factors for COVID-19 (age, sex, hypertension, diabetes, body mass index ≥ 30 kg/m2, and heart failure) as independent variables. Finally, we used a generalized linear mixed-efiect model with subjects as a random efiect to evaluate change in asthma exacerbation (logistic regression) and change in eosinophil counts (Gaussian regression) during the 3 difierent time periods. Statistical analyses were performed using R Software, version 3.6.3 (https://cran.rproject.org). P less than .05 was considered significant.
Results
Study population
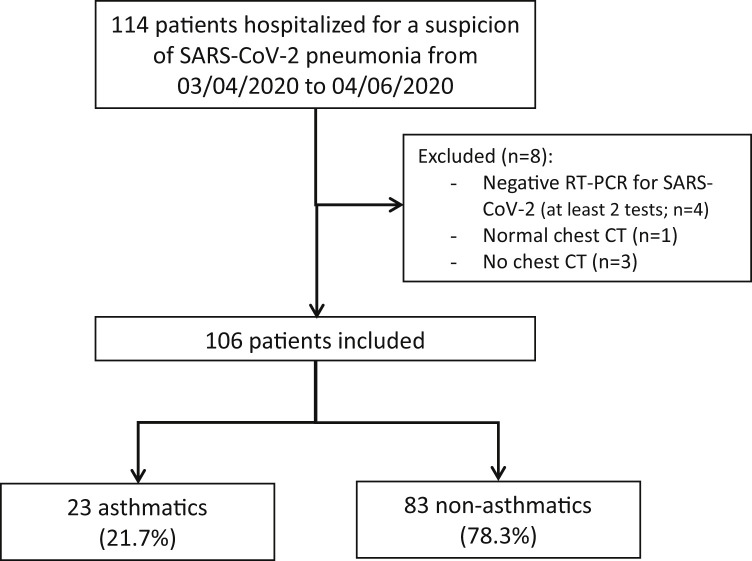
A total of 114 patients were hospitalized for suspicion of SARS-CoV-2 pneumonia between March 4 and April 6, 2020, in a dedicated COVID-19 Unit in the Chest Diseases Department of Strasbourg University Hospital. Of these patients, 106 were included in the present study (Figure 1 ). Their clinical characteristics are described in Table I .
Figure 1.
Flowchart of patient inclusion.
Table I.
Clinical characteristics of included patients
| Characteristic | Total (n = 106) | Patients without asthma (n = 83) | Patients with asthma (n = 23) | P value |
|---|---|---|---|---|
| Sex | .63 | |||
| Female | 40 (37.7) | 30 (36.1) | 10 (43.5) | |
| Male | 66 (62.3) | 53 (63.9) | 13 (56.5) | |
| Age (y) | 63.5 (54.2-72.0) | 65.0 (56.0-72.0) | 59.0 (49.5-9.5) | .37 |
| Delay between onset of symptoms and hospitalization (d) | 7.0 (4.2-10.0) | 7.0 (4.0-10.0) | 7.0 (5.0-9.0) | .90 |
| Comorbidity | ||||
| Obesity (body mass index > 30 kg/m2) | 42 (39.6) | 33 (39.8) | 9 (39.1) | >.99 |
| Hypertension | 45 (42.5) | 37 (44.6) | 8 (34.8) | .48 |
| Diabetes | 23 (21.7) | 19 (22.9) | 4 (17.4) | .78 |
| Chronic heart disease | 6 (5.7) | 5 (6.0) | 1 (4.3) | >.99 |
| Chronic renal insufficiency | 5 (4.8) | 4 (4.9) | 1 (4.3) | >.99 |
| Lung cancer | 7 (6.6) | 6 (7.2) | 1 (4.3) | >.99 |
| Obstructive sleep apnea | 14 (13.2) | 12 (14.5) | 2 (8.7) | .73 |
| Smokers | ||||
| Past smokers | 33 (31.1) | 23 (27.7) | 10 (43.5) | .20 |
| Current smokers | 6 (5.7) | 5 (6.0) | 1 (4.3) | >.99 |
| Smoking index∗ | 0.0 (0.0-20.0) | 0.0 (0.0-20.0) | 0.0 (0.0-15.0) | .60 |
Data are given as n (%) or median (interquartile range).
Smoking index = number of cigarettes smoked per day × years of tobacco use.
Clinical symptoms and biological and radiological status of all patients
The clinical symptoms and biological and radiological status of all patients are described in Table II . In univariate analysis, dyspnea, chest tightness, and wheezing were significantly associated with the asthmatic status of the patients. Once adjusted in multivariate analysis, only chest tightness was more frequent in patients with asthma than in patients without asthma (aOR, 4.798; 95% CI, 1.111-21.783).
Table II.
Clinical symptoms and biological and radiological status of all patients
| Characteristic | Total (n = 106) | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| Patients without asthma (n = 83) | Patients with asthma (n = 23) | P value | aOR | P value | ||
| Symptoms | ||||||
| Fever | 99 (93.4) | 78 (94) | 21 (91.3) | .64 | ||
| Asthenia | 90 (84.9) | 71 (85.5) | 19 (82.6) | .75 | ||
| Anorexia | 72 (67.9) | 54 (65.1) | 18 (78.3) | .31 | ||
| Confusion | 7 (6.6) | 6 (7.2) | 1 (4.3) | >.99 | ||
| Myalgia | 49 (46.2) | 37 (44.6) | 12 (52.2) | .64 | ||
| Pharyngalgia | 5 (4.7) | 4 (4.8) | 1 (4.3) | >.99 | ||
| Nasal obstruction | 10 (9.4) | 8 (9.6) | 2 (8.7) | >.99 | ||
| Acute anosmia | 23 (21.7) | 16 (19.3) | 7 (30.4) | .26 | ||
| Sneezes | 8 (7.5) | 4 (4.8) | 4 (17.4) | .07 | ||
| Rhinorrhea | 9 (8.5) | 6 (7.2) | 3 (13.0) | .40 | ||
| Gastrointestinal symptoms | 49 (46.2) | 38 (45.8) | 11 (47.8) | >.99 | ||
| Cough | 93 (87.7) | 71 (85.5) | 22 (95.7) | .29 | ||
| Dyspnea | 84 (79.2) | 62 (74.7) | 22 (95.7) | .04 | 5.8 (1.0-109.9) | .10 |
| Chest tightness | 12 (11.3) | 5 (6.0) | 7 (30.4) | .004 | 4.8 (1.1-21.8) | .04 |
| Pulmonary auscultation | ||||||
| Wheezing | 11 (10.4) | 5 (6.0) | 6 (26.1) | .01 | 2.5 (0.4-12.8) | .29 |
| Crackles | 89 (84.0) | 71 (85.5) | 18 (78.3) | .52 | ||
| Biology | ||||||
| Lymphocytes | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) | 1.0 (0.8-1.2) | .26 | ||
| Eosinophils (admission) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .17 | ||
| Eosinophils (discharge) | 0.1 (0.0-0.2) | 0.1 (0.0-0.2) | 0.1 (0.0-0.2) | .52 | ||
| Chest CT | ||||||
| <10% | 14 (13.2) | 7 (8.4) | 7 (30.4) | .01 | ||
| 10%-25% | 45 (42.5) | 38 (45.8) | 7 (30.4) | .24 | ||
| 25%-50% | 29 (27.4) | 23 (27.7) | 6 (26.1) | >.99 | ||
| >50% | 18 (17.0) | 15 (18.1) | 3 (13.0) | .76 | ||
| Ventilation support | ||||||
| Oxygen therapy | 96 (90.6) | 73 (88.0) | 23 (100) | .11 | ||
| Noninvasive ventilation | 15 (14.2) | 11 (13.2) | 4 (17.4) | .74 | ||
AIC, Akaike information criterion.
Data are given as n (%) or median (interquartile range).
Model fit: AIC: 104.68.
Bold indicates statistical significance (P < .05).
Eosinopenia (eosinophil count, ≤0.05 g/L) was found in 88.7% of all patients and lymphopenia (lymphocyte count, ≤1.0 g/L) in 60.4% at admission, with no difference between the 2 groups.
The global severity of lung parenchyma involvement was similar in the 2 groups. In a propensity score–adjusted analysis, being asthmatic was not a risk factor for having more involvement of the lung parenchyma (OR, 0.904; 95% CI, 0.409-1.790; P = .786).
Clinical characteristics of patients with asthma
The clinical characteristics of patients with asthma are described in Table III . We found that 52.2% of the patients were GINA 1 and were not receiving any inhaled corticosteroid, and 39.1% were GINA 4 and 5. Only 1 patient was treated with biotherapy and oral corticosteroids. Among patients with asthma, 63.6% were well controlled and 23.4% were partially controlled. Eleven of the 23 patients with asthma had at least 1 severe exacerbation in the previous year, and 68.2% were considered suffering from allergic asthma according to their clinical history. The median eosinophil count before SARS-CoV-2 infection was 0.19 (0.11-0.30) g/L.
Table III.
Clinical characteristics of patients with asthma
| Characteristic | Total patients with asthma (n = 23) | No exacerbation (n = 17) | Exacerbation (n = 6) |
|---|---|---|---|
| Age at onset (y) | |||
| <18 | 6 (26.1) | 4 (23.5) | 2 (33.3) |
| 18-50 | 13 (56.5) | 10 (58.8) | 3 (50.0) |
| >50 | 4 (17.4) | 3 (17.6) | 1 (16.7) |
| Spirometry∗ | |||
| FEV1 (% predicted) | 85.5 (73.3-88.2) | 85.5 (82.5-86.8) | 78.0 (60.8-88.9) |
| FEV1/FVC (%) | 70 (64.3-88.2) | 77.1 (68.0-86.1) | 67.5 (60.3-73.6) |
| Allergy (based on clinical history)† | 15 (68.2) | 11 (68.8) | 4 (66.7) |
| Family atopy† | 12 (54.5) | 8 (50.0) | 4 (66.7) |
| Positive skin prick test result‡ | 5 of 8 | 2 of 3 | 3 of 5 |
| Symptoms to† | |||
| Birch pollen | 6 (27.3) | 5 (31.3) | 1 (16.7) |
| Grass pollen | 6 (27.3) | 4 (25.0) | 1 (16.7) |
| Pets (cat or dog) | 4 (18.2) | 2 (12.5) | 2 (33.3) |
| House dust | 11 (50.0) | 8 (50.0) | 3 (50.0) |
| Desensitization† | 3 (13.6) | 3 (18.8) | 0 (0.0) |
| Rhinoconjunctivitis exacerbated (February 2020)† | 2 (9.1) | 2 (12.5) | 0 (0.0) |
| Severity of asthma | |||
| Exacerbations during the last 12 mo | 0.5 (0.0-1.0) | 0 (0.0-1.0) | 1 (0.3-1.0) |
| Almost 1 hospitalization in intensive care unit in life | 1 (4.3) | 0 (0.0) | 1 (16.7) |
| Daily dose of inhaled corticosteroids during the last month | 0 (0.0-820.0) | 0 (0.0-600.0) | 600 (50.0-1210.0) |
| GINA step 1 | 12 (52.2) | 10 (58.8) | 2 (33.3) |
| GINA step 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| GINA step 3 | 2 (8.7) | 1 (5.9) | 1 (16.7) |
| GINA step 4 | 5 (21.7) | 4 (23.5) | 1 (16.7) |
| GINA step 5 | 4 (17.4) | 2 (11.8) | 2 (33.3) |
| Comorbidity | |||
| Gastroesophageal reflux | 12 (52.2) | 8 (47.1) | 4 (66.7) |
| Hyperventilation syndrome | 1 (4.4) | 0 (0.0) | 1 (0.2) |
| Chronic rhinosinusitis with polyposis | 1 (4.4) | 0 (0.0) | 1 (16.7) |
| Anti-inflammatory drug hypersensitivity | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Paradoxical vocal cord motion disorder | 3 (13.0) | 2 (11.8) | 1 (16.7) |
| Obesity | 9 (39.1) | 6 (35.3) | 3 (50.0) |
| Asthma control† | |||
| Well controlled | 14 (63.6) | 9 (56.3) | 5 (83.3) |
| Partly controlled | 6 (27.3) | 6 (37.5) | 0 (0.0) |
| Uncontrolled | 2 (9.1) | 1 (6.25) | 1 (16.7) |
FVC, Forced vital capacity.
Data are given as n (%) or median (interquartile range). Birch pollen symptoms: rhinitis, conjunctivitis, or asthma on March or April. Grass pollen symptoms: symptoms in May and June. House dust: symptoms in contact with domestic dust.
Spirometry data were obtained from the patient's doctors.
n = 22; for 1 patient we did not obtain any information because he was immediately transferred to the intensive care unit. For others, medical information was obtained from patients and medical report from their general practitioner and specialists.
Positive threshold for skin test: mean diameter of the wheal ≥3 mm compared with negative control (n = 8).
Patient outcomes
Twenty-one patients (19.8%) required intensive care unit management and mechanical ventilation. No difference was found between the groups with and without asthma. Eighty of 106 patients had a favorable outcome and were discharged from the hospital. No death was observed in the asthmatic group. Length of stay (P = .64), maximal oxygen flow needed (P = .83), and the frequency of noninvasive ventilation were similar in the 2 groups. In a propensity score–adjusted analysis, being asthmatic was not a risk factor for transfer to the intensive care unit (aOR, 1.065; 95% CI, 0.272-3.522; P = .92) or for longer length of stay (aOR, 1.614; 95% CI, 0.586-4.585; P = .36). The eosinophil counts returned back to normal for 74.5% of all patients at discharge. In a generalized linear mixed-efiect model, eosinophil counts changed significantly over the 3 time periods (P < .001), but not asthma (P = .29).
Comparison of asthma exacerbation over time
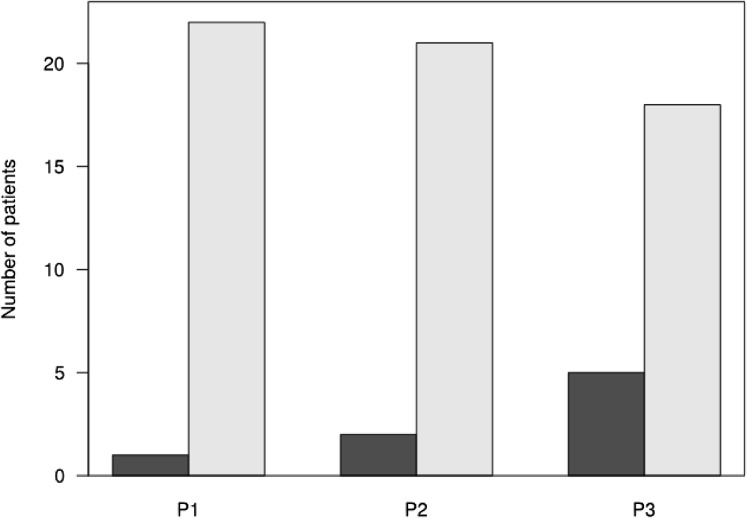
In the first period (p1), only 1 patient had a severe exacerbation. In the second period (p2), 2 patients developed a severe exacerbation. In the third period (p3), 5 patients were being treated with systemic corticosteroids because of asthma symptoms. For 3 of them, systemic corticosteroids were started only in p3. Using exacerbation as a dependent variable in a generalized linear mixed-efiect model, no time-period effect was found (P = .09; Figure 2 ).
Figure 2.
Number of patients treated or not with oral corticosteroids during the 3 periods (dark gray) p1 and p2: Number of patients with a severe asthma exacerbation; p3: Number of patients treated with oral corticosteroids and β2 agonist; (light gray) no treatment with oral corticosteroids, and β2 agonist during p1, p2, and p3. No time period effect (P = .09).
Discussion
In the eastern part of France, we have been facing a severe COVID-19 epidemic. At the height of the epidemic, Strasbourg University Hospital had 429 beds in conventional medicine units and 207 in an intensive care unit devoted to COVID-19. As our department is a reference center for asthma, any case of SARS-CoV-2 pneumonia in patients with asthma was primarily referred to us. In the same period, we estimated that 5 patients with a clinical history of asthma had been hospitalized in other medical departments. This could explain why we had a higher prevalence of patients with asthma in our population (21.7%) than mentioned in previous publications.14 , 22 , 23 The clinical history of asthma was carefully verified with the patient, the general practitioner, and when possible with the specialist (allergist or pulmonologist). The clinical history was recorded by 2 different doctors on 2 different occasions.
We did not observe any increase in severe exacerbation with the development of SARS-CoV-2 pneumonia. This result is not in line with other respiratory viruses. Viral respiratory infections are detected in up to 85% of asthma exacerbations in children and approximately half the exacerbations in adults.12 , 24 , 25 During asthma exacerbation in adults, the most frequently detected viruses are rhinoviruses (42.1%), respiratory syncytial virus (13.6%), herpes simplex virus (12.3%), influenza virus (10%), parainfluenzavirus (5.3%), coronaviruses (8.4%), metapneumovirus (5.3%), Bocavirus (6.9%), and adenovirus (3.8%).26 Papadopoulos et al12 noted that reported respiratory infection rates for coronaviruses among children usually range from less than 1% to 9%, with varied distribution between subtypes, and that coronavirus infections are often associated with other viruses. For adults coming into the emergency department for acute asthma exacerbation, coronaviruses have been found in only 4% of induced sputum samples.27 No data are available on the asthma exacerbation rate and SARS-CoV-1 or Middle East respiratory syndrome coronavirus infection. Overall, coronaviruses seem to make a minor contribution to acute asthma exacerbation.
In contrast to other respiratory viruses, SARS-CoV-2 may not be a risk factor for severe asthma exacerbation. Several hypotheses can be raised.
First, SARS-CoV-2 may be a disease mainly of the upper and lower respiratory tract, causing ENT infection and pulmonary lesions. ACE2 has been shown to be the functional receptor of SARS-CoV-1.28 This receptor is abundantly expressed in type I and type II pneumocytes, whereas bronchial epithelial cells exhibit only weak staining.29 , 30 ACE2 has also been identified as the receptor of novel SARS-CoV-2,31 indicating that alveolar pneumocytes in the lung could be a possible entry site for SARS-CoV-2. However, ACE expression is not limited to the lungs, and extrapulmonary spread of SARS-CoV in ACE2-positive tissues has been observed. The same can be expected for SARS-CoV-2.32 This is in accordance with the suggestion that COVID-19 seems to induce a specific pathology of the alveolo-capillary space leading to severe altered gas exchanges and pulmonary-specific vasculopathy.33 , 34 Very recently it has been suggested that ACE2 expression in nasal epithelium was inversely related to allergic sensitization.35
The second hypothesis involves the role of eosinophil cells. In our population, 88.7% of patients had a low eosinophil count at admission. The eosinophil count had returned to normal at discharge for 74.5% of patients. Liu et al36 described similar results with a low eosinophil count at admission and demonstrated that the increase in eosinophils may be an indicator of COVID-19 improvement. In their study, during the period of lower eosinophil count, the SARS-CoV-2 RNA test result remained positive, and after the eosinophil count returned to normal, the SARS-CoV-2 RNA test result was negative within 5 days. The normalization of eosinophil count is also associated with improved symptoms and chest X-rays.36 However, that previous study included patients without asthma. In our patients, we did not find any significant difference in eosinophil counts between patients with and without asthma.
The role of eosinophils in asthma exacerbation is widely published. Since the first article on the relationship between eosinophil count and asthma severity,37 several publications have confirmed a relationship between eosinophil count and frequency of asthma exacerbation.38 Decreasing the number of eosinophils in eosinophilic asthma is associated with a reduction in severe exacerbation, use of systemic corticosteroids, or biotherapy (eg, anti–IL-5 or anti–IL-5 receptor).39 , 40 In SARS-CoV-2 pneumonia, postmortem examination did not find any eosinophils in the alveol or in the bronchi and bronchioles.41
The third hypothesis is on the role of inhaled corticosteroids. In in vitro models, inhaled corticoids alone or in association with bronchodilators inhibit human coronavirus-229E replication, partly by inhibiting receptor expression and/or endosomal function and reducing cytokine production (IL-6, IL-8). This suggests that these drugs modulate infection-induced inflammation in the airways.42
Roughly half of our patients with asthma (52.2%) had GINA step 1 asthma and did not use inhaled corticosteroids on a regular basis; 63.6% of them were well controlled. We did not find any difference between the age of patients with and without asthma. Moreover, the classical factors associated with SARS-CoV-2 pneumonia (hypertension, obesity, diabetes) were found in both groups. In contrast, the classical factors responsible for uncontrolled asthma, such as tobacco smoke, obesity, and obstructive sleep apnea, were not more frequent in the group with asthma. This suggests that the risk factors for hospitalization in our patients were related more to the risk factors of SARS-CoV-2 pneumonia than to asthma.
Furthermore, most of our patients with asthma were considered to have allergic asthma (68.2%). In adults, the frequency of allergic asthma is approximately 50%.43 , 44 However, the diagnosis of clinical allergy was based on a suggestive clinical history. For only a few patients, skin tests were performed. Further studies showed that pollen may affect the development and severity of viral infection, decreasing the IFN and proinflammatory chemokine response of airway epithelia to the virus.45 However, only 2 (9.1%) patients with asthma had rhinoconjunctivitis before the SARS-CoV-2 pneumonia symptoms, and none of them required oral corticosteroids for asthma during p1, p2, and p3. Thus, pollen allergy appeared not to be the reason for asthma exacerbation in our patients.
The severity and outcome of SARS-CoV-2 pneumonia were similar in the 2 groups in terms of lung parenchyma involvement, oxygen flow, frequency of transfer to the intensive care unit, and duration of hospitalization. This suggests that asthma would not be a risk factor for the development of severe SARS-CoV-2 pneumonia.
Possible limitations of our study could be linked to a monocentric recruitment of patients and low number of patients with asthma included in the study. However, this reflects the findings of other larger studies,14 demonstrating a low frequency of patients with asthma with severe SARS-CoV-2 pneumonia.
Conclusions
Our results demonstrate that patients with asthma do not experience differences in clinical symptoms or biological and radiological status in SARS-CoV-2 pneumonia. The published risk factors associated with SARS-CoV-2 pneumonia (ie, hypertension, obesity, diabetes) were found in both patients with and without asthma. In contrast, we did not find the usual risk factors responsible for uncontrolled asthma. This suggests that the COVID-19 infection was the main factor leading to hospitalization. Moreover, in contrast to other viral respiratory infections, SARS-CoV-2 pneumonia did not appear to induce severe asthma exacerbation. Larger studies would be desirable to confirm our results and understand the mechanisms explaining such findings.
Acknowledgments
We acknowledge the staff of the Chest Diseases Department of Strasbourg University Hospital for their unwavering dedication and their efforts in the care of patients, especially during this pandemic period. We thank the COVID-19 pneumonia and Asthma group members Matthieu Canuet, Eva Chatron, Tristan Degot, Sandrine Hirschi, Moustapha Hussein, Justine Leroux, Natacha Moutard, Flore Muller, Michele Porzio, Mohammed Rahli, Armelle Schuller, Mekki Tamir, Hasibe Yucel, and Adel Zouzou for their involvement with patients.
Footnotes
Conflicts of interest: F. de Blay received financial support from clinical grants from Aimmune, Stallergènes-Greer, ALK, Novartis, AstraZeneca, DBV, Sanofi, and GlaxoSmithKline and has board membership with Aimmune, Stallergènes-Greer, ALK, Novartis, AstraZeneca, DBV, and Sanofi. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus situation report. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200407-sitrep-78-covid-19.pdf?sfvrsn=bc43e1b_2 Available from: Accessed July 23, 2020.
- 3.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19)—symptoms. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html Available from: Accessed July 23, 2020.
- 4.American Academy of Otolaryngology-Head and Neck Surgery COVID-19 Anosmia Reporting Tool. 2020. https://www.entnet.org/content/reporting-tool-patients-anosmia-related-covid-19 Available from: Accessed July 23, 2020. [DOI] [PubMed]
- 5.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 9.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55:2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Erratum in: Intensive Care Med. 2020;46:1294–1297. doi: 10.1007/s00134-020-05991-x. Intensive Care Med 2020;46:846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadopoulos N.G., Christodoulou I., Rohde G., Agache I., Almqvist C., Bruno A. Viruses and bacteria in acute asthma exacerbations--a GA2 LEN-DARE systematic review. Allergy. 2011;66:458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19). People with moderate to severe asthma. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html Available from: Accessed July 23, 2020.
- 14.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 15.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 16.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J., Wu X., Zeng W., Guo D., Fang Z., Chen L. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Real-time RT-PCR assays for the detection of SARS-CoV-2. https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2 Available from: Accessed July 23, 2020.
- 19.Kuna P., Peters M.J., Manjra A.I., Jorup C., Naya I.P., Martínez-Jimenez N.E. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61:725–736. doi: 10.1111/j.1742-1241.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Société Française de Radiologie (SFR) La société d’Imagerie Thoracique propose un compte-rendu structuré de scanner thoracique pour les patients suspects de COVID-19. SFR e-Bulletin. 2020. https://ebulletin.radiologie.fr/actualites-covid-19/societe-dimagerie-thoracique-propose-compte-rendu-structure-scanner-thoracique Available from: Accessed July 23, 2020.
- 21.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2019. https://ginasthma.org/reports/2019-gina-report-global-strategy-for-asthma-management-and-prevention/ Available from: Accessed July 23, 2020.
- 22.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8:436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng X.-Y., Xu Y.-J., Guan W.-J., Lin L.-F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol. 2018;163:845–853. doi: 10.1007/s00705-017-3700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wark P.A.B., Johnston S.L., Moric I., Simpson J.L., Hensley M.J., Gibson P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn J.H., Li W., Choe H., Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci CMLS. 2004;61:2738–2743. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 30.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P. COVID-19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189:1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A. Association of respiratory allergy, asthma and expression of the SARS-CoV-2 receptor, ACE2. J Allergy Clin Immunol. 2020;146:203–206. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bousquet J., Chanez P., Lacoste J.Y., Barnéon G., Ghavanian N., Enander I. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 38.Price D.B., Rigazio A., Campbell J.D., Bleecker E.R., Corrigan C.J., Thomas M. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3:849–858. doi: 10.1016/S2213-2600(15)00367-7. [DOI] [PubMed] [Google Scholar]
- 39.Busse W., Chupp G., Nagase H., Albers F.C., Doyle S., Shen Q. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143:190–200. doi: 10.1016/j.jaci.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Busse W.W. Biological treatments for severe asthma: a major advance in asthma care. Allergol Int. 2019;68:158–166. doi: 10.1016/j.alit.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. J Clin Pathol. 2020;73:239–242. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaya M., Nishimura H., Deng X., Sugawara M., Watanabe O., Nomura K. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58:155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Froidure A., Mouthuy J., Durham S., Chanez P., Sibille Y., Pilette C. Asthma phenotypes and IgE responses. Eur Respir J. 2016;47:304–319. doi: 10.1183/13993003.01824-2014. [DOI] [PubMed] [Google Scholar]
- 44.Doyen V., Casset A., Divaret-Chauveau A., Khayath N., Peiffer G., Bonniaud P. Diagnosis of allergy in asthma. Rev Mal Respir. 2020;37:243–256. doi: 10.1016/j.rmr.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Gilles S., Blume C., Wimmer M., Damialis A., Meulenbroek L., Gökkaya M. Pollen exposure weakens innate defense against respiratory viruses. Allergy. 2020;75:576–587. doi: 10.1111/all.14047. [DOI] [PubMed] [Google Scholar]