Abstract
Background
Cell proliferation and angiogenesis are important in progression of cancerous processes. Differentiating cutaneous T-cell lymphoma (CTCL) from its mimicking dermatoses and prognosticating it are challenging.
Aim
This study assesses cell proliferation and angiogenesis in different CTCL subtypes using immunohistochemistry (IHC) for Ki67 and CD31 to testify their usability in differentiating CTCL from mimicking dermatoses and discriminating CTCL subtypes from each other with correlation to clinicopathological parameters and disease advancement.
Patients and Methods
IHC for Ki67 and CD31 were applied to skin biopsies from 81 patients divided into CTCL (n=59) and dermatoses (n=22) groups. Hot-spot analysis was used to score Ki67 and CD31 microvascular density (MVD) semiquantitatively. Statistical analysis was performed to compare Ki67 index and MVD between CTCL and dermatoses. CTCL subgroups were compared to each other. Ki67 index and CD31 were compared to age, gender, skin and nodal involvement, blood tumor burden and TNMB stage.
Results and Conclusion
There were significant differences in proliferation index and MVD between dermatoses and CTCL, and between dermatoses and all CTCL subtypes with exception of Ki67 in early mycosis fungoides (MF) and CD31 in patch lesions. Increased cell proliferation and MVD were significantly associated with older age, T3 and 4 skin involvement, significant nodes (N1-3), positive blood tumor burden (B1,2) in CTCL and TNMB stage of MF. Both markers differentiated significantly late from early MF, classic MF from its variants and non-MF CTCL from total MF, but not from late MF. In conclusion, Ki67 and CD31 expression in skin biopsies using IHC reproduces the role of proliferation and angiogenesis in the differential diagnosis and prognostication of CTCL being expressed at higher levels in aggressive than indolent CTCL. Therapeutic targeting of cell proliferation and angiogenesis may improve patient’s outcome in CTCL. Usability of these markers into patient’s stratification should be considered in further studies.
Keywords: Ki67, CD31, cutaneous T-cell lymphoma, mycosis fungoides, TNMB stage
Introduction
Cutaneous T-cell lymphomas (CTCLs) constitute a heterogeneous group of extranodal non-Hodgkin lymphomas (NHL) that accounts for about 4% of all NHL. The annual age-adjusted incidence of CTCLs is approximately 6 cases/million and men are affected twice as women. The incidence increases with age, with an average onset between 50 and 60 years, while children and young adults are rarely affected. CTCL classification is complex, nonetheless mycosis fungoides (MF) is the most frequent subtype accounting for approximately 50% to 70%.1 CTCLs might mimic several non-neoplastic dermatologic conditions, posing a diagnostic challenge to both dermatologists and pathologists, especially at early disease stages. Moreover, it is very challenging to prognosticate this disease,2,3 and numerous prognostic markers have been sought including advanced age, clinical stage, Sézary cell count, lactate dehydrogenase (LDH), beta-2 microglobulin, neutrophil/lymphocyte ratio, staphylococcus aureus infection, cell size, detection of differentiation marker abnormalities, molecular features and proliferation indices e.g. Ki67.3,4
Tumor microenvironment is critical for initiation and progression of cancerous growth which is dependent on establishment of a functional vascular network to support cell proliferation by the process of angiogenesis.5 Recent studies verified the crucial role of skin microenvironment in CTCL progression.2,6 Angiogenesis is highly pertinent to the biology and therapy of NHL,2,6 being more prominent in aggressive rather than indolent lymphoproliferative disorders.7 During the progression of CTCLs, malignant T cells produce several angiogenic factors, such as podoplanin, lymphatic vessel hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor-C (VEGF-C), VEGF-R3 and lymphotoxin alpha (LTα), which promote angiogenesis and lymphangiogenesis.8 Angiogenesis can be quantified by microvessel density (MVD) using immunohistochemistry (IHC) for CD34, CD31 or von Willebrand factor. Previous studies reported significantly higher MVD in primary CTCL compared to normal skin.9,10 Furthermore, CD31 expression in MF has been proposed as a marker of disease advancement.11 Thus, anti-angiogenic therapies can be used to target CTCL tumor vasculature or malignant tumor cells directly or through combinations with other drugs. Preliminary clinical data indicated therapeutic advantages associated with strategies targeting dual compartments, as well as multiple angiogenic pathways within the tumor microenvironment.5,6
This study assesses cell proliferation and angiogenesis in different subtypes of CTCLs using IHC for Ki67 and CD31, respectively, comparing the expression status with a group of CTCL lymphoma mimickers to testify their usability in differentiating CTCL from the mimicking dermatoses and discriminating CTCL subtypes from each other. Cell proliferation and angiogenesis are correlated with the clinicopathological parameters and disease advancement in patients with CTCL.
Patients and Methods
Patients, Clinical and Histopathological Classifications
The study was performed on archived paraffin blocks for skin lesions obtained from eighty-one patients including: fifty-nine patients diagnosed with CTCL and twenty-two patients diagnosed with non-neoplastic dermatological conditions that mimics CTCL (inflammatory dermatoses). As angiogenesis is an established feature of psoriasis, it was excluded to evade misleading comparison. Biopsies were referred from the Department of Dermatology and Oncology Center at Faculty of Medicine, Mansoura University, Egypt, to the Pathology Department Laboratory at the same institute in the period from January 2014 to August 2019. Cases were included based on the availability of clinicopathological data and archived tissue material for further study. Hematoxylin and eosin (H&E)-stained histopathological sections prepared from the non-neoplastic dermatoses were reviewed in conjunction with the clinical features to confirm the diagnosis. The diagnosis and subtyping of CTCLs were based on clinical evaluation, histopathological (H&E) examination and immunohistochemical phenotyping (antibodies for CD3, CD4, CD8, CD7 for all biopsies and CD20 and CD30 for late MF and non-MF CTCL and whenever indicated to ascertain the diagnosis), according to the 2018 update of the WHO-EORTC (World Health Organization/European Organization of Research and Treatment of Cancer revision) classification and diagnostic criteria.1,12 Staging of CTCLs was assessed according to the TNMB system (Tumor Node Metastasis Blood) released by the International Society for Cutaneous Lymphomas/European Organization of Research and Treatment of Cancer revision on 2018.1 Relevant demographic and clinicopathological data were collected from medical records of the above-mentioned departments.
Immunohistochemistry (IHC)
For the 81 included skin biopsies, IHC was conducted on about 4µm-thick paraffin sections on heat fixed positively charged slides. Deparaffinization, rehydration and epitope exposure were performed with use of 0.01 M citrate buffer (pH 6.0) for 10 minutes in microwave. Activity of endogenous peroxidase was blocked by 3% hydrogen peroxide incubation for 10 min. All sections were rinsed with phosphate buffer saline (PBS) and incubated for one hour at room temperature with the primary antibodies directed against: monoclonal mouse anti-human Ki67; a cell proliferation marker; (Dako corporation product clone: MIB-1, isotype: IgG1, kappa, ready to use for immunohistochemical staining of formalin-fixed, paraffin-embedded tissues, code: IR626, corresponding to a1002bp Ki67 molecule), and monoclonal mouse anti-human CD31 antibody; a neoangiogenesis and stem cell marker; (Dako corporation product clone: JC70A species, IgG1, kappa, cell culture supernatant dialyzed against 0.05 mol/L Tris-HCl, pH 7.2, and containing 15 mmol/L NaN3, concentrate, dilution 1:100). Standard avidin-biotin-peroxidase technique was applied using diaminobenzidine (DAB, 5 minutes incubation) for visualization and hematoxylin for counterstaining (30 seconds). Appropriate negative controls, consisting of histologic sections processed without the addition of primary antibody, were prepared, along with a positive control sections prepared from tonsillar tissue for CD31, while the basal epidermal cells were considered as an internal positive control for Ki67.
Evaluation of IHC Reaction
IHC reactions were evaluated by two pathologists (who were blinded to the patients’ clinical data) with a multi-headed light microscope (Olympus, Tokyo, Japan). Evaluation was done initially independently, then consensus review was performed until agreement was achieved regarding each slide.11,13,14
Proliferation (Ki67) Scoring
Using “hot-spot” analysis on light microscope, a semi-quantitative assessment of the average percentage of Ki67 positively stained cells among the total number of cutaneous cell infiltrate was performed. The hot-spots were selected upon initial overview of the entire section at a low magnification (×40), followed by analysis of at least three selected fields with the highest index (hot-spots) within the infiltrate (or adding more fields with the aim to count 300 cells, due to low cellularity in some biopsies) using the light microscope on x200 magnification (0.09 mm.). Only nuclear staining was considered positive. Staining intensity is not relevant provided that counterstaining is appropriate.13,14 For statistical analysis, Ki67 index was classified into low (<20%) and high (≥20%) categories.15
Microvessel Density (CD31) Scoring
CD31 expression was observed in the cytoplasm and cell membranes of endothelial cells of dermal microvessels. The sections were first scanned at low power (x40) to identify the hot-spots (areas of highest microvessel density [MVD] count). Afterward, five non-overlapping hot-spot areas were examined under x200 magnification (0.09 mm.), and the sum of microvessel count was recorded.10,11
Ethical Standards
The study was performed on archived material obtained from paraffin tissue blocks and kept at pathology laboratory at the study setting. The work proposal was approved by the Institutional Research Board (IRB, code R.19.12.691) at Faculty of Medicine, Mansoura University. Anonymity of all subjects was maintained through the study by using patient’s code numbers instead of names. All the study procedures were done in accordance with the current revision of Helsinki declaration for dealing with human subjects in research studies.
Statistical Analysis
Computer-fed data were analyzed using IBM Corp. SPSS (International Business Machines Corporation Statistical Product and Service Solutions), released 2013 for Windows, Version 22.0. Armonk, NY: IBM Corp. Qualitative data were described using numbers and percentages. Quantitative data were described using median (minimum and maximum) or mean±SD (standard deviation) after testing normality using Kolmogorov–Smirnov test. Significance of the obtained results was judged at the 0.05 level. For quantitative data between groups, Mann–Whitney U-test was used to compare 2 independent groups and Kruskal–Wallis (KW) test was used to compare more than 2 independent groups with Mann–Whitney U-test to detect pair-wise comparison. χ2Chi-Square test and Fischer exact test (FET) were used whenever appropriate. The Spearman’s rank-order correlation was used to determine the strength and direction of a linear relationship between two non-normally distributed continuous variables and/or ordinal variables.
Results
Study Group Criteria
The study included eighty-one patients distributed into two groups: a CTCL group (lymphoma group) including fifty-nine patients, and a CTCL mimickers group (non-neoplastic inflammatory dermatoses group) comprised of twenty-two patients including: 14 cases (63.6%) of eczema, 5 cases (22.7%) of lichen planus and 3 cases (13.7%) of pseudolymphoma (two cases of Jessner’s lymphocytic infiltrate and a case of cutaneous B-cell pseudolymphoma). The patients ranged in age from 2 to 96 years with mean ages of 44.75±17.7 and 37.41±8.05 years for the CTCL group and the dermatoses groups, respectively, imparting no statistical age difference between groups. Most patients in the CTCL group were males (57.6%), while most patients in the dermatoses group were females (68.2%), resulting in a statistically significant (p=0.04) gender difference between groups (Table 1).
Table 1.
Age and Gender Distribution and Comparison Between the Study Groups
| Lymphoma Group | Non-Neoplastic Group | Total | p value | |
|---|---|---|---|---|
| Age | ||||
| Range/y | 2–96 | 16–55 | 2–96 | 0.07 |
| Mean±SD/y | 44.75±17.7 | 37.41±8.05 | 42.88±16.03 | |
| Age group (n, %) | ||||
| ≤40y | 24 (40.7%) | 15 (68.2%) | 39(48.1%) | |
| >40y | 35 (59.3%) | 7 (31.8%) | 42(51.9%) | |
| Gender (n, %) | ||||
| Male | 34(57.6%) | 7(31.8%) | 41(50.6%) | 0.04* |
| Female | 25(42.4%) | 15(68.2%) | 40 (49.4%) | |
| M/F ratio | 1.4: 1 | 1:2.1 | ||
| Total (n, %) | 59 (100%) | 22 (100%) | 81 (100%) |
Note: *p value is statistically significant if ≤0.05.
Abbreviations: y, years; SD, standard deviation.
CTCL Subtypes, Patterns of Involvement and Staging
Table 2 shows the subtypes, patterns of involvement and staging of CTCLs. MF comprised most of CTCLs (83%) and were classified as classic and variant forms (67.4% and 32.6% respectively). Classic MF was mostly presented as patches in 15 cases (30.6%), followed by plaques in 10 cases (20.4%), while erythrodermic and tumor subtypes were confirmed in 8 cases (16.4%). The most frequent MF variant was the hypopigmented variant (28.6%). Folliculotropic and poikilodermic variants were diagnosed in one case each. Non-MF CTCLs comprised 17% of lymphomas, of which peripheral T-cell lymphoma (PTCL) was the most frequent (60%) and T-cell lymphoblastic lymphoma was the least frequent (one case; 10%). Regarding skin involvement, most cases revealed patches, papules, and/or plaques covering >10% of the skin surface and were categorized as T2a (33.9%) and T2b (18.6%) respectively. Limited patches (T1a), papules, and/or plaques (T1b) covering <10% of the skin surface were detected in 22% and 8.5% of patients. About 13.6% of patients had tumors ≥1cm in diameter, meanwhile only 2 cases were presented with confluence of erythema covering >80% of the body surface area and were categorized as T4. Clinically abnormal peripheral lymph nodes were identified in 22% of patients, but most patients (78%) revealed no nodal involvement. Upon laboratory investigations, two patients revealed low blood tumor burden with atypical (Sézary) cells forming >5% of peripheral blood lymphocytes, and one patient had a high blood tumor burden with a Sézary cell count >1000/µL. Only one patient (1.7%) – diagnosed with T-cell lymphoblastic lymphoma – was presented with widespread metastatic deposits notably involving the spleen and mediastinum. MF was staged according to the 2017 World Health organization (WHO) staging scheme. Most MF patients (79.6%) were presented at an early stage (IA and B) and the remainder (20.4%) were presented at late stages (IIB, IIIA and B, IVA).
Table 2.
Subtyping, Patterns of Involvement and Staging of Cutaneous T-Cell Lymphomas (Lymphoma Group)
| Variables | Frequencies (n, %) |
|---|---|
| Subtypes (n=59) | |
| Mycosis fungoides (MF) (n, % of total) | 49 (83%) |
| Classic MF (n, % of MF) | 33 (67.4%) |
| Patch MF | 15 (30.6%) |
| Plaque MF | 10 (20.4%) |
| Tumor MF | 6 (12.3%) |
| Erythrodermic MF | 2 (4.1%) |
| MF variants (n, % of MF) | 16 (32.6%) |
| Hypopigmented MF | 14 (28.6%) |
| Folliculotropic MF | 1 (2%) |
| Poikilodermic MF | 1 (2%) |
| Non-MF cutaneous T-cell lymphomas (n, % of total) | 10 (17%) |
| PTCL (n, % of non-MF CTCL) | 6 (60%) |
| CD30+ pcALCL (n, % of non-MF CTCL) | 3 (30%) |
| T-cell lymphoblastic lymphoma (n, % of non-MF CTCL) | 1 (10%) |
| Skin (n=59) | |
| T1a | 13 (22%) |
| T1b | 5 (8.5%) |
| T2a | 20 (33.9%) |
| T2b | 11 (18.6%) |
| T3 | 8 (13.6%) |
| T4 | 2 (3.4%) |
| Lymph node (n=59) | |
| No clinically abnormal peripheral lymph nodes (N0) | 46 (78%) |
| Clinically abnormal peripheral lymph nodes (N1,2,3) | 13 (22%) |
| Blood (n=59) | |
| B0 | 56 (94.9%) |
| B1 | 2 (3.4%) |
| B2 | 1 (1.7%) |
| Metastasis (n=59) | |
| M0 | 58 (98.3) |
| M1 | 1 (1.7%) |
| Stage* (n=49) | |
| Early stage (n, % of MF) | 39 (79.6%) |
| IA | 19 (38.8%) |
| IB | 20 (40.8%) |
| Late stage (n, % of MF) | 10 (20.4%) |
| IIB | 7 (14.3%) |
| IIIA | 1 (2%) |
| IIIB | 1 (2%) |
| IVA | 1 (2%) |
Notes: *Staging parameters are applicable only for the 49 MF cases.
Abbreviations: n, number; PTCL, peripheral T-cell lymphoma; pcALCL, primary cutaneous anaplastic large cell lymphoma.
Immunohistochemistry (IHC)
Tumor Cell Proliferation
The median Ki67 expression score for lymphoma mimickers was 2, as 95.5% of non-neoplastic dermatoses revealed a low index for this proliferation marker with only one case of Jessner’s lymphocytic infiltrate that had a high index, while the median Ki67 score for the CTCL group was 8, and 37.3% of biopsies revealed a high proliferation index (Figure 1) rendering a significant statistical difference (p=0.04). In an ascending order, median Ki67 expression scores of: 4 for MF variants, 5 for early stage, 7 for total MF, 8 for classic MF, 35 for late stage MF and 55 for non-MF CTCLs were recorded when separated into subgroups. Most of early stage MF and MF variants displayed a low Ki67 index (92.3% and 87.5%, respectively). Total MF revealed high index in 24.5% of cases, classic MF revealed a high Ki67 index in 33.3% of biopsies, whereas all non-MF CTCL and late stage MF had high Ki67 indices. Comparing the Ki67 index for each of the aforementioned CTCL subgroups with the dermatoses group revealed a statistically significant increase in cell proliferation for total MF (p=0.04), classic MF (p=0.01), and MF variants (p=0.03), a highly significant increase in late stage MF (p<0.001) and non-MF CTCLs (p<0.001), but no significant difference was recorded when early stage MF was compared to dermatoses group. Separating patch only lesions from plaque lesions also revealed no difference from dermatoses (p=1 and 0.07, respectively). Furthermore, comparing Ki67 index within the CTCL subgroups revealed highly significant increase when late MF stages were compared to early stage, and when non-MF CTCL was compared to MF (p<0.001 each), but no difference was recorded between non-MF CTCL and late MF. Ki 67 index was significantly higher in classic MF as compared to MF variants (p=0.03) (Table 3).
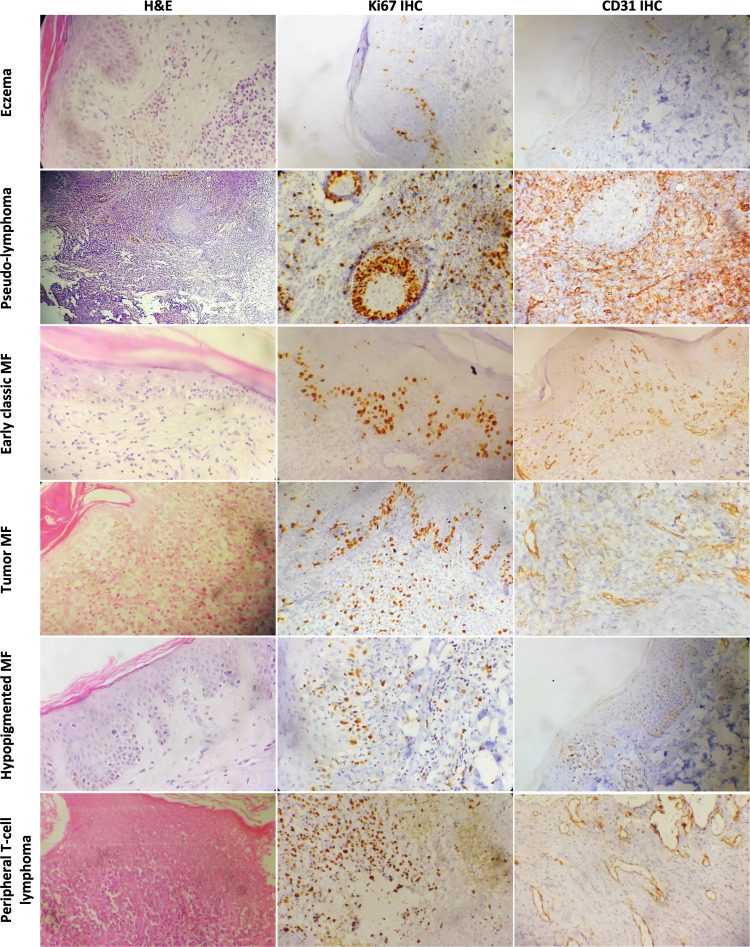
Figure 1.
Ki67 and CD31 IHC expression compared in different non-neoplastic dermatoses and CTCL subtypes (x200).
Table 3.
Comparison of Proliferation (Ki67) and Angiogenesis (CD31) Between CTCLs and Lymphoma Mimickers (Non-Neoplastic Dermatoses)
| Expression | Non-Neoplastic n=22 | Lymphoma n=59 | MF n=49 | Non-MF CTCL n=10 | Early Stage MF n=39 | Late Stage MF n=10 | Classic MF n=33 | MF Variants n=16 |
|---|---|---|---|---|---|---|---|---|
| Ki67 | ||||||||
| Median | 2 | 8 | 7 | 55 | 5 | 35 | 8 | 4 |
| Range | 1–25 | 1–75 | 1–70 | 40–75 | 1–30 | 20–70 | 2–70 | 1–55 |
| Low index (n, %) | 21(95.5%) | 37(62.7%) | 37(75.5%) | 0 (0.0) | 36(92.3%) | 0 (0.0) | 22(66.7%) | 14(87.5%) |
| High index (n, %) | 1(4.5%) | 22(37.3%) | 12(24.5%) | 10(100.0%) | 3(7.7%) | 10(100.0) | 11(33.3%) | 2(12.5%) |
| Test of significance with non-neoplastic |
χ2=8.45 p=0.004* |
χ2=4.04 p=0.04* |
FET p<0.001* |
FET p=1.0 |
FET p<0.001* |
χ2=6.41 p=0.01* |
χ2=4.27 p=0.03* |
|
| Test of significance between subgroups | Vs. total MF χ2=20.25 p<0.001* Vs. late MF FET P=1.0 |
FET p<0.001* |
χ2=4.27 p=0.03* |
|||||
| CD31 | ||||||||
| Median | 20 | 37 | 27 | 71 | 25 | 68 | 38 | 21.5 |
| Range | 9–59 | 12–94 | 12–79 | 61–94 | 12–60 | 58–79 | 20–79 | 12–62 |
| Test of significance with non-neoplastic | Z=4.31 p<0.001* |
Z=2.96 p=0.003* |
Z=4.47 p<0.001* |
Z=2.08 p=0.03* |
Z=4.22 p<0.001* |
Z=3.93 p<0.001* |
Z=3.71 p<0.001* |
|
| Test of significance between subgroups | Vs. total MF z=4.28 p<0.001* Vs. late MF z=0.94 p=0.35 |
Z=4.61 p<0.001* |
Z=3.71 p<0.001* |
|||||
| Test of significance between CD31 and Ki67 | rs=0.922 <0.001* |
|||||||
Notes: χ2; chi-Square test, Z; Mann–Whitney U-test; rs; Spearman correlation coefficient, *p value is statistically significant if ≤0.05.
Abbreviation: FET, Fischer exact test.
Angiogenesis
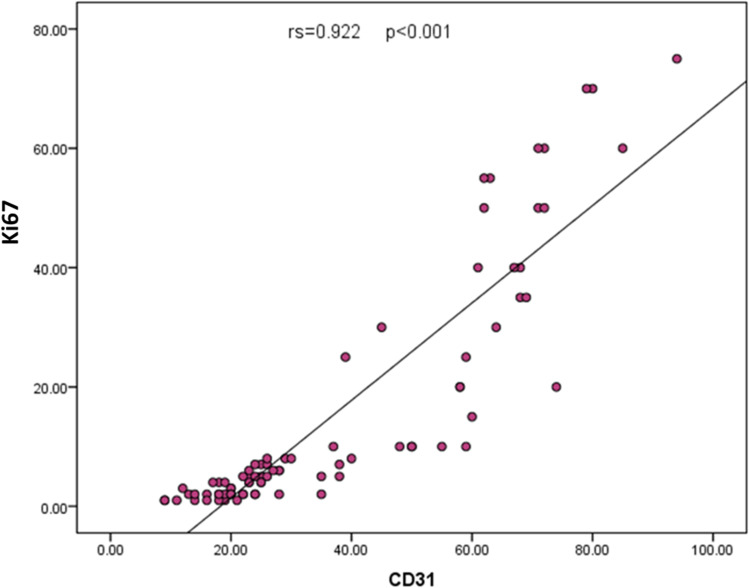
The total MVD was determined in the dermis using CD31 immunohistochemistry (Figure 1). Overall, non-neoplastic dermatoses exhibited a lower median MVD compared to the CTCL group with a highly significant difference in-between (20 versus 37, p<0.001). The highest MVD in the dermatoses group was noticed in pseudolymphomas (50–59). Among subgroups, late stage MF and non-MF CTCL subgroups attained the highest median MVD (68 and 71, respectively), meanwhile, MF variants and early MF revealed the lowest median MVD (21.5 and 25, respectively). Comparing the non-neoplastic group to the lymphoma subgroups revealed a significant MVD increase in total MF, early stage MF (p=0.03 each), non-MF CTCL, late stage MF, classic MF and MF variants (p<0.001 each). Among subgroups, there was a significantly higher MVD (p<0.001) when non-MF CTCL compared to MF, late stage MF was compared to early stage, and when classic MF was compared to MF variants (Table 3). There was no difference in MVD between late stage MF and non-MF CTCL. Separating patch only lesions from plaque lesions revealed no difference between patch MF and the non-neoplastic dermatoses (KW; p=0.07) but there was a significant difference with plaque lesions (KW; p=0.001). Concordant Ki67 and CD31 expression was observed among studied cases (p<0.001, Figure 2).
Figure 2.
Scatter diagram showing concordance between Ki67 and CD31expression among the studied cases.
Correlations of Proliferation and Angiogenesis with Advancement of CTCLs
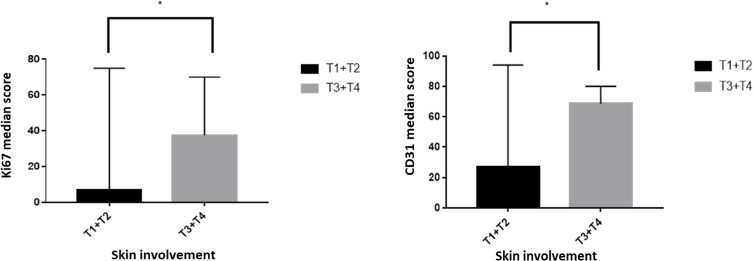
In lymphoma patients, advanced patient’s age was significantly associated with higher Ki67 scores and MVD (p<0.001). Likewise, male gender was significantly associated with Ki67 scores (p=0.02), but not with the MVD. Concerning skin involvement, biopsies obtained from patients presented with tumors ≥1cm or confluence of erythema covering >80% of the body surface area (T3 and T4) revealed significantly high Ki67 scores and MVD (p≤0.001) in contrast to the biopsies obtained from patients with cutaneous patches, papules and or plaques (T1 and T2, Figure 3). Moreover, clinically abnormal peripheral lymph nodes correlated significantly with higher proliferation and angiogenesis (p≤0.001) as compared to negative nodes. Significant differences were also observed when Ki67 expression and MVD were compared (p=0.007 and p=0.019, respectively) among patients with blood tumor burden (low or high) and patients with negative/insignificant blood involvement. In respect to the MF stage, biopsies from patients at stage II or III disease revealed a significantly higher Ki67 expression and MVD in contrast to biopsies from stage I patients (p≤0.001) with an overall increase in proliferation and angiogenesis in late stage disease (Table 4).
Figure 3.
Comparison of Ki67 (left panel, *p=0.001) and CD31 (right panel, *p<0.001) expression in relation to the pattern of skin involvement by cutaneous T-cell lymphoma.
Table 4.
Correlations of Proliferation (Ki67) and Angiogenesis (CD31) with Age, Gender and Advancement of the Disease
| Variables | Ki67 | CD31 | ||
|---|---|---|---|---|
| Ki67 Score (Median, Range) | Test of Significance | CD31 MVD (Median, Range) | Test of Significance | |
| Age/years | rs=0.536 p<0.001* |
rs=0.485 p<0.001* |
||
| ≤40y | 3(1.0–70) | z=4.57 p<0.001* |
22.5(9–80) | z=4.04 p<0.001* |
| >40y | 10(1.0–75) | 42.5(16–94) | ||
| Gender (n=59) | ||||
| Male | 7 (1–75) | z=2.25 p=0.02* |
28(9–94) | z=1.81 p=07 |
| Female | 4 (1–70) | 23.5(11–85) | ||
| Skin (n=59) | ||||
| T1 | 5(1–40)a | KW p=0.001* |
24.5(12–68)a | KW p<0.001* |
| T2 | 7(1–75)a | 30(14–94)ab | ||
| T3 | 35(20–70)b | 67.5(58–80)c | ||
| T4 | 65(60–70)b | 75(71–79)bc | ||
| Lymph node (n=59) | ||||
| N0 | 5 (1–60) | z=5.56 p<0.001* |
25(9.0–74) | z=5.34 p<0.001* |
| N1,2,3 | 52.5(20–75) | 68.5(58–94) | ||
| Blood (n=59) | ||||
| B0 | 7.5(1–75) | z=2.68 p=0.007* |
32.5(12–94) | z=2.34 p=0.019* |
| B1,2 | 70 (55–70) | 79(63–80) | ||
| Metastasis (n=59) | ||||
| M0 | 8 (1–75) | Z=1.62 p=0.11 |
36 (12–94) | Z=1.59 p=0.11 |
| M1 | 70 (70–70) | 80(80–80) | ||
| Stage* (n=49) | ||||
| Early stage (stage I) | 5(1–30)A | KW p<0.001* |
25(12–60)A | KW p=0.001* |
| Late stages | ||||
| II | 30(5–50)B | 64(22–74)BC | ||
| III | 65(60–70)C | 75(71–79)BD | ||
| IV | 40(40–40)ABC | 67(67–67)ACD | ||
Notes: abc, ABCD Similar superscripted letters denote non-significant difference within same column, Z; Mann–Whitney U-test, *p value is statistically significant if ≤0.05.
Abbreviation: KW; Kruskal–Wallis test.
Discussion
The present study intended to analyze tumor cell proliferation and angiogenesis in relation to the clinicopathological features and disease advancement in different subtypes of CTCL among a cohort of Egyptian patients. The potential utility of these markers to differentiate CTCL from its mimickers is also addressed with the secondary aim to highlight the demographic profiles, relative frequencies, common clinicopathological features and staging distribution of this relatively rare entity in our locality.
Demographically, CTLC patients had a lower mean age as compared to worldwide studies,1,16-18 but matched one national study.10 However, dermatoses patients exhibited the average mean age defined for these conditions.19–21 Lymphomas predominated in males, while the dermatoses dominated in females as reported in previous studies.1,17,22 According to the WHO-EORTC classification, MF comprised 83% of CTCLs of which 32.6% were MF variants. Patch MF was the most frequent classic MF clinical form, while erythrodermic MF was the least frequently encountered form. Hypopigmented MF was the most frequent variant among our patients followed by the folliculotropic MF (FMF) and this may be attributed to the high frequency of former in our locality or the possibility of under diagnosis of the later.10,23-25 PTCL was more frequent in this study than CD30+ pcALCL. Nonetheless, ethnic and environmental factors may have influenced this disease distribution.17,18 In harmony with previous reports,17,18,26 about 5% of our patients had blood involvement, 17% presented with T3 and T4 skin lesions, 22% revealed significant peripheral lymph nodes and most MF patients (79.6%) were presented at an early stage (IA and B).
Noteworthy, poor prognosis in MF/SS is related to late TNMB stage, age above 60y, large cell transformation, elevated blood LDH or MF variants precisely FMF that runs an aggressive course being less responsive to skin-directed therapies due to the deep tumor infiltration.1,12,22 In contrast, hypopigmented MF commonly presents as asymptomatic macules or patches at stage I and slowly progresses over many years.23 For non-MF CTCLs, skin involvement of multiple anatomical sites is a limiting poor prognostic factor,17 while PTCL patients commonly have an unfavorable International Prognostic Index (IPI).27
In the past decade, the prognostic role of proliferation and angiogenesis markers has been investigated in CTCL, but has not yet been confirmed. Ki67 expression is an absolute requirement for cell proliferation and its rate is imperative for grading neoplasms and predicting their outcome. IHC for Ki67 is the most popular consistent approach to assess proliferation in tissues. Earlier studies reported that proliferation markers can significantly distinguish between high- and low-grade NHL.28 Applying Ki67 index on NHL of different types, a cut-off value of 45% differentiated indolent from aggressive follicular lymphoma and a cut-off value of 70% distinguished patients with diffuse large B-cell lymphoma (DLBCL) with good and poor outcomes when combined with other prognostic factors.28 In mantle cell lymphoma (MCL), a high Ki67 proliferation index was associated with poor survival and with blastoid variants, a 20% cut-off value was clinically meaningful, and a value of 30% in nodal biopsy was correlated with the leukemic MCL in bone marrow.15 A recent study used IHC to analyze Ki67 on 17 MF/SS skin biopsies, where the proliferation index varied from 5% to 50% (mean 16.23) and the skin proliferation index was observed to increase with expansion of tumor burden measured as blood T cell receptor Vβ (TCR-Vβ) clonality, indicating that skin and blood compartments are interconnected.29 Among previous studies of CTCL, all proliferation markers had predictive values regarding the disease severity and prognosis. Comparing the Ki67 proliferation rate, it was significantly higher in advanced MF as compared to early MF and parapsoriasis or other inflammatory dermatoses and it was also connected with shorter survival and metastasis.30,31 Furthermore, a statistically significant difference in Ki67 expression was observed when non-MF CTCL was compared to inflammatory dermatoses.30 In both, MF and non-MF CTCL, Ki67 expression highly correlated with the expression of other proliferation markers (MCM-3 and MCM-7). Considering skin involvement in MF, T1b had a significantly higher expression of Ki67 than T1a with similar observations between T2b and T2a.30 Our findings were in line with previous reports showing a statistically significant difference in Ki67 index between CTCL and the mimicking inflammatory dermatoses. High proliferation indices (above 20) were recorded in all late stage MF, all non-MF CTCLs and 33.3% of classic MF rendering highly significant statistical differences when compared to dermatoses group (p≤0.001). Although most MF variants had a low proliferation index, the existing difference was still enough to differentiate MF variants from the dermatoses group (p=0.03). Nonetheless, the difference between early stage MF and the dermatoses group did not achieve a statistical significance mostly due to scarcity of lymphoma cell infiltrate at this phase.
Comparing our subgroups to each other, higher Ki67 proliferation index was able to differentiate late stage from early MF. Unexpectedly, classic MF revealed higher Ki67 index than MF variants. To explain this finding, we have to indicate that hypopigmented MF was the most predominant MF variant in this study, and 11 out of 14 patients were presented at stage I disease with only patch skin lesions that revealed scattered haloed epidermal cells and mild superficial dermal infiltrate on microscopy. Although a higher Ki67 index for non-MF CTCL rendered a significant difference when compared to total MF (p<0.001), there was no difference when non-MF CTCL was compared to late stage MF, therefore it seems that the actual difference exists between non-MF CTCL and early MF. In accordance with the previous reports,15,28-31 our correlations between Ki-67 index and the prognostic parameters of CTCL were significant in respect to: advanced patient’s age, male gender, tumor or extensive erythrodermic skin involvement, abnormal peripheral lymph nodes, blood tumor burden and late stage disease.
Angiogenesis plays an important pathogenic role in CTLC initiation and progression by stimulating homing, proliferation, survival and metastasis of lymphoma cells via autocrine mechanisms involving VEGF-VEGF receptor axis, chemokine CXCR4/CXCL12 axis or JAK3/STAT5 signaling pathway. In general, MVD scores trend to be highest in aggressive NHL including Burkitt’s lymphoma and PTCL, intermediate in DLBCL and low in indolent follicular lymphoma.6 The present study revealed that high MVD in skin biopsies can segregate CTCLs and all its studied subgroups from the mimicking dermatoses and moreover, differentiate clinically aggressive CTCLs from indolent ones. The MVD was significantly increased in all CTCLs, classic MF, late stage MF, MF variants and non-MF CTCL when compared to lymphoma mimicking dermatoses (p<0.001). Similarly, a significant increase was observed when comparing total MF or early stage MF (p=0.03) to the non-neoplastic group. In accordance with our results, Jankowska-Konsur et al,11,32 documented a significantly higher CD31 expression and podoplanin (a lymphangiogenesis marker) in MF as compared to benign dermatoses. Exceptionally, we observed high MVD in pseudolymphomas. This finding has not been examined before in CTCL, however, overlapping MVD ranges were documented in cutaneous B-cell lymphoma compared with B-pseudolymphoma.33 Also, we did not find a significant MVD difference when comparing patch only MF lesions from non-neoplastic dermatoses but there was a significant difference with plaque lesions. Therefore, infiltration of the skin seems to have more important statistical significance than the extent of the lesions in early MF stages.10
In this study, MVD was found to be useful in differentiating non-MF CTCL from MF, late stage from early stage MF, and classic MF from MF variants, being much more increased in the former subtype of each comparison (p<0.001). In congruence to the proliferation index, differentiation between late stage MF and the non-MF CTCL was not possible based on MVD. It is well documented that the microenvironment of lymphomas accelerates angiogenesis through release of angiogenic factors (VEGF family, bFGF and placenta growth factor; PIGF) by tumoral cells or tumor stromal cells.6 In early MF with patch lesions, lymphoma cell infiltrates and the associated stromal cells may be very scant,22 and likewise in the hypopigmented MF the tumor cells present in single units in or in small groups.23 Thus, angiogenesis may remain unpronounced under these circumstances due to insufficiency of angiogenic factors, yet, a more pronounced microvasculature develops later in the course of malignancy with the more infiltrative lesions as seen in late MF and non-MF CTCL.
In the current study, we observed a positive association between MVD and the studied prognostic factors namely: advanced age, tumorous and extensive erythrodermic skin involvements versus patches and plaques (T3 and 4), clinically abnormal peripheral lymph nodes (N1-3) versus negative nodes, positive versus negative blood tumor burden, and late MF stages (II, III) versus early stage (I). Our results were consistent with preceding reports that verified the association between increased MVD and LVD and progressive skin involvement (T3 and 4), nodal involvement and higher MF stages using IHC for CD31 and podoplanin, respectively.11,32 A supplementary study has linked shorter survival and metastasis to the significantly increased MVD in advanced stages of MF using CD34 IHC.31 In the same context, circulating endothelial progenitor cells and VEGF levels were correlated with tumor volumes in CTCL.6 Our findings support the notion that angiogenesis not only contributes to the growth of tumor mass but also facilitates the spread of neoplastic cells and dissemination of malignancy. On the contrary, a study using CD31 to count both MVD and vascular sprouts proved higher counts in early MF tumor specimens in comparison to normal skin but no associations were found with respect to prognostic factors including lesion type whether patch or plaque, MF variant, skin lesion severity or disease duration. This was attributed to sampling of the most affected skin lesion instead of the most chronic lesion or the small number of patients in each group.10 As ascertained in a prior study,32 it is important to note that Ki67 expression and CD31 were concordant in all subtypes of CTCL and in all investigated prognostic parameters in this work.
In conclusion, expression of Ki67 and CD31 in skin biopsies using IHC reproduces the role of cell proliferation and angiogenesis in the differential diagnosis and prognostication of CTCL. Ki67 and CD31 were expressed at higher levels in aggressive than indolent CTCL. They could be used to differentiate most CTCLs from tested mimicking non-neoplastic dermatoses (with exclusion of psoriasis). The only exception was the early MF that cannot be differentiated using Ki67 as tumor cell proliferation is not an evident finding at this stage. Likewise, patch only MF that showed no MVD difference with dermatoses. Because of the overlap in the ranges of Ki67 and CD31 MVD in CTCL and pseudolymphomas, both markers may not be useful as a diagnostic tool in such individual cases. Both markers can efficiently differentiate late MF from early MF, non-MF CTCL from total MF and the classic MF from its variants notably the hypopigmented, but are of no utility in differentiating late MF from non-MF CTCL. Increased cell proliferation and angiogenesis were associated with older age and diseases advancement parameters in CTCL and with TNMB stage of MF, thus therapeutic targeting of cell proliferation and angiogenesis may improve patient’s outcome in CTCL. Usability of these markers into patient’s stratification should be considered in further studies.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Leukemia and lymphoma society. Cutaneous T-cell lymphoma facts. 2018: 3–36. 800.955.4572 I. www.LLS.org. Available from: https://www.lls.org/sites/default/files/file_assets/PS96_CTCL_Booklet_Final.pdf. Accessed March17, 2020.
- 2.Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and sézary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70(2):205.e1–2. doi: 10.1016/j.jaad.2013.07.049 [DOI] [PubMed] [Google Scholar]
- 3.Litvinov IV, Tetzlaff MT, Thibault P, et al. Gene expression analysis in Cutaneous T-Cell Lymphomas (CTCL) highlights disease heterogeneity and potential diagnostic and prognostic indicators. Oncoimmunol. 2017;6(5):e1306618. doi: 10.1080/2162402X.2017.1306618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulitzer M. Cutaneous T-cell lymphoma. Clin Lab Med. 2017;37(3):527–546. doi: 10.1016/j.cll.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan J. Antiangiogenic therapies in non-hodgkin’s lymphoma. Curr Cancer Drug Targets. 2011;11(9):1030–1043. doi: 10.2174/156800911798073014 [DOI] [PubMed] [Google Scholar]
- 6.Tanase C, Popescu ID, Enciu AM, et al. Angiogenesis in cutaneous T-cell lymphoma-proteomic approaches. Oncol Lett. 2019;17(5):4060–4067. doi: 10.3892/ol.2018.9734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribatti D, Nico B, Ranieri G, Specchia G, Vacca A. The role of angiogenesis in human non-Hodgkin lymphomas. Neoplasia. 2013;15(3):231–238. doi: 10.1593/neo.121962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagherani N, Smoller BR. An overview of cutaneous T cell lymphomas. F1000Res. 2016;5:1882. doi: 10.12688/f1000research.8829.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazur G, Woźniak Z, Wróbel T, Maj J, Kuliczkowski K. Increased angiogenesis in cutaneous T-cell lymphomas. Pathol Oncol Res. 2004;10(1):34–36. doi: 10.1007/bf02893406 [DOI] [PubMed] [Google Scholar]
- 10.Bosseila M, Sayed Sayed K, El-Din Sayed SS, Abd El Monaem NA. Evaluation of angiogenesis in early mycosis fungoides patients: dermoscopic and immunohistochemical study. Dermatology. 2015;231(1):82–86. doi: 10.1159/000382124 [DOI] [PubMed] [Google Scholar]
- 11.Jankowska-Konsur A, Kobierzycki C, Grzegrzolka J, et al. Expression of CD31 in mycosis fungoides. Anticancer Res. 2016;36(9):4575–4582. doi: 10.21873/anticanres.11006 [DOI] [PubMed] [Google Scholar]
- 12.Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703–1714. doi: 10.1182/blood.2019002852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga Z, Cassoly E, Li Q, et al. Standardization for Ki-67 assessment in moderately differentiated breast cancer. A retrospective analysis of the SAKK 28/12 study. PLoS One. 2015;10(4):e0123435. doi: 10.1371/journal.pone.0123435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathol. 2017;49(2):166–1671. doi: 10.1016/j.pathol.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Jeong TD, Chi HS, Kim MS, Jang S, Park CJ, Huh JR. Prognostic relevance of the Ki-67 proliferation index in patients with mantle cell lymphoma. Blood Res. 2016;51(2):127–132. doi: 10.5045/br.2016.51.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CN, Hsu CK, Chang KC, Wu CL, Chen TY, Lee JY. Cutaneous lymphomas in Taiwan: a review of 118 cases from a medical center in southern Taiwan. Dermatol Sin. 2018;36:16–24. doi: 10.1016/j.dsi.2017.08.004 [DOI] [Google Scholar]
- 17.Liu K-L, Tsai W-C, Lee C-H, Di Napoli A. Non-mycosis fungoides cutaneous lymphomas in a referral center in Taiwan: a retrospective case series and literature review. PLoS One. 2020;15(1):e0228046. doi: 10.1371/journal.pone.0228046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez O, Sowash M, Mosojane KI, et al. A retrospective review of cutaneous lymphoma in Botswana. Int J Dermatol. 2020;59(3):352–358. doi: 10.1111/ijd.14690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya M, Kaur I, Kumar B. Lichen planus: a clinical and epidemiological study. J Dermatol. 2000;27(9):576–582. doi: 10.1111/j.1346-8138.2000.tb02232.x [DOI] [PubMed] [Google Scholar]
- 20.Lipsker D, Mitschler A, Grosshans E, Cribier B. Could Jessner’s lymphocytic infiltrate of the skin be a dermal variant of lupus erythematosus? An analysis of 210 cases. Dermatology. 2006;213(1):15–22. doi: 10.1159/000092832 [DOI] [PubMed] [Google Scholar]
- 21.Richard MA, Corgibet F, Beylot-Barry M, et al. Sex- and age-adjusted prevalence estimates of five chronic inflammatory skin diseases in France: results of the « OBJECTIFS PEAU » study. J Eur Acad Dermatol Venereol. 2018;32(11):1967–1971. doi: 10.1111/jdv.14959 [DOI] [PubMed] [Google Scholar]
- 22.Vaidya T, Badri T. Mycosis fungoides In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020-2019. Available from https://www.ncbi.nlm.nih.gov/books/NBK519572/. Accessed 5March 2020. [PubMed] [Google Scholar]
- 23.Hassab-El-Naby HM, El-Khalawany MA. Hypopigmented mycosis fungoides in Egyptian patients. J Cutan Pathol. 2013;40(4):397–404. doi: 10.1111/cup.12093 [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Halim M, El-Nabarawy E, El Nemr R, Hassan AM. Frequency of hypopigmented mycosis fungoides in Egyptian patients presenting with hypopigmented lesions of the trunk. Am J Dermatopathol. 2015;37(11):834–840. doi: 10.1097/DAD.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 25.Nashan D, Faulhaber D, Ständer S, Luger TA, Stadler R. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;156(1):1–10. doi: 10.1111/j.1365-2133.2006.07526.x [DOI] [PubMed] [Google Scholar]
- 26.Eder J, Kern A, Moser J, Kitzwögerer M, Sedivy R, Trautinger F. Frequency of primary cutaneous lymphoma variants in Austria: retrospective data from a dermatology referral center between 2006 and 2013. J Eur Acad Dermatol Venereol. 2015;29(8):1517–1523. doi: 10.1111/jdv.12907 [DOI] [PubMed] [Google Scholar]
- 27.Rizvi MA, Evens AM, Tallman MS, Nelson BP, Rosen ST. T-cell non-Hodgkin lymphoma. Blood. 2006;107(4):1255–1264. doi: 10.1182/blood-2005-03-1306 [DOI] [PubMed] [Google Scholar]
- 28.Broyde A, Boycov O, Strenov Y, Okon E, Shpilberg O, Bairey O. Role and prognostic significance of the Ki-67 index in non-Hodgkin’s lymphoma. Am J Hematol. 2009;84(6):338–343. doi: 10.1002/ajh.21406 [DOI] [PubMed] [Google Scholar]
- 29.Cristofoletti C, Bresin A, Picozza M, et al. Blood and skin-derived sézary cells: differences in proliferation-index, activation of PI3K/AKT/mTORC1 pathway and its prognostic relevance. Leukemia. 2019;33(5):1231–1242. doi: 10.1038/s41375-018-0305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jankowska-Konsur A, Kobierzycki C, Reich A, Grzegrzolka J, Maj J, Dziegiel P. Expression of MCM-3 and MCM-7 in primary cutaneous T-cell lymphomas. Anticancer Res. 2015;35(11):6017–6026. [PubMed] [Google Scholar]
- 31.Maj J, Jankowska-Konsur AM, Hałoń A, Woźniak Z, Plomer-Niezgoda E, Reich A. Expression of CXCR4 and CXCL12 and their correlations to the cell proliferation and angiogenesis in mycosis fungoides. Postepy Dermatol Alergol. 2015;32(6):437–442. doi: 10.5114/pdia.2015.48034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jankowska-Konsur A, Kobierzycki C, Grzegrzółka J, et al. Podoplanin expression correlates with disease progression in mycosis fungoides. Acta Derm Venereol. 2017;97(2):235–241. doi: 10.2340/00015555-2517 [DOI] [PubMed] [Google Scholar]
- 33.Schaerer L, Schmid MH, Mueller B, Dummer RG, Burg G, Kempf W. Angiogenesis in cutaneous lymphoproliferative disorders: microvessel density discriminates between cutaneous B-cell lymphomas and B-cell pseudolymphomas. Am J Dermatopathol. 2000;22(2):140–143. doi: 10.1097/00000372-200004000-00009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Leukemia and lymphoma society. Cutaneous T-cell lymphoma facts. 2018: 3–36. 800.955.4572 I. www.LLS.org. Available from: https://www.lls.org/sites/default/files/file_assets/PS96_CTCL_Booklet_Final.pdf. Accessed March17, 2020.