Abstract
BACKGROUND
There are controversial ideas about the application of metabolic indices for the prediction of nonalcoholic steatohepatitis (NASH). In this study, we evaluated some novel metabolic indices for the screening of NASH.
METHODS
This prospective case-control study was performed in a gastroenterology outpatient clinic. Consecutively selected patients with persistently elevated aminotransferase levels and evidence of fatty liver in ultrasonography were enrolled. Those with other etiologies of aminotransferase elevation were excluded. The remaining was presumed to have NASH. The control group consisted of age and sex-matched subjects with normal liver function tests and liver ultrasound examinations.
RESULTS
Finally, 94 patients with steatohepatitis and 106 controls were included in the project. The mean liver fat content (LFC), aspartate aminotransferase, and alanine aminotransferase levels were significantly lower in the control group than in the NASH group. LFC was independently associated with the presence of NASH in logistic regression analysis. LFC had a good area under the curve for the prediction of NASH in ROC (receiver operating characteristic curve) analysis.
CONCLUSION
LFC seems to be a reliable metabolic index for the detection of patients with NASH.
Keywords: Fatty liver, Non-alcoholic steatohepatitis, Metabolic syndrome, Metabolic index, Body mass index, HOMA-IR
INTRODUCTION
Large body of evidence has demonstrated the relationship between non-alcoholic steatohepatitis (NASH) and metabolic syndrome (MS).1-2 Some experts believe that NASH is the hepatic manifestation of MS.2 Body mass index (BMI) and waist circumference were the primary metabolic indices that were used to evaluate insulin resistance (IR). The existence of some NASH subjects with normal BMI suggested that the mentioned indices had limitations in defining the IR status.3 Further research showed the importance of visceral adipose tissue instead of subcutaneous fat tissue in the pathogenesis of MS and NASH.4 Therefore, newer metabolic indices were developed to accurately estimate the degree of IR. The aim of this study was to define the accuracy of some new metabolic indices in predicting the presence of NASH.
MATERIALS AND METHODS
TEthical considerations
The protocol of this study was approved by the Ethics Committee of Tehran University of Medical Sciences (Registration number: IR.TUMS.MEDICINE.REC.1397.781). The participants were enrolled in the study after completing the written informed consent (file:///C:/Users/internet/Downloads/mmn3fm90rrdhsw.pdf).
Study design and patient selection criteria
This prospective case-control study was conducted in a gastroenterology clinic of a general hospital. The study started in January 2018 and terminated in January 2019. All consecutively selected referred patients with persistent elevation of serum aminotransferases and evidence of fatty liver at ultrasonography were included. The upper normal alanine aminotransferase level was set at 37.5 (U/L) in men and 36 (U/L) in women based on the previous research in a sample of Iranian general population.5 Those with hepatitis B (n = 1), hepatitis C (n = 1), hepatotoxic medications during the previous 3 months (n = 3), alcoholic hepatitis (n = 3), autoimmune hepatitis (n = 1), Wilson disease (n = 1), congestive heart failure (n = 3), chronic kidney disease (n = 7), and cancer of any origin (n = 1) were excluded from the study. The remaining subjects were presumed as having NASH. The control group consisted of healthy individuals who accompanied the patients in the same clinic during the study period. They were included if they had normal liver ultrasonography and routine laboratory (including liver function) tests. The controls were age and sex-matched (block matching method) with the cases. Matching was performed by the statistician from a cohort of 200 healthy candidates. All liver ultrasonography examination was performed by a single expert radiologist.
Metabolic indices
The body mass index (BMI) was calculated by dividing weight to squared height. The unit was kg/m2. The waist circumference (WC) was measured as the largest abdominal diameter in mid-distance between the lower rib and iliac crest. The unit for measurement was centimeter. The lipid accumulation product (LAP) is a new tool for the estimation of body fat. It is calculated by a formula that applies triglyceride (TG) and WC.6 Visceral adiposity index (VAI) is an indicator of central obesity and estimates the amount of visceral fat tissue.7 It seems to be a reliable predictor of IR syndrome. Body round index (BRI) is another marker used for the estimation of abdominal fat.8 It is suggested that BRI might be related to MS. The values near to one indicated the linear body shape and values more that one reveals a round body configuration. The body shape index (BSI) is a novel metabolic marker that is calculated based on WC, which is standardized for weight and height.9 Homeostasis model assessment (HOMA) was a pure laboratory indicator of IR that was used for the prediction of NASH in this experiment. Liver fat content (LFC) was calculated by a formula applying aspartate aminotransferase (AST), AST/ALT ratio, diabetes mellitus, MS status, and fasting serum insulin.10 The accuracy of the mentioned model for THE estimation of LFC was significant. LFC was the only metabolic index that used the combination of laboratory investigation and metabolic data for the evaluation of NASH status in this research.
Laboratory assessments
All laboratory examinations were done after an overnight fasting state (about 8 hours) according to the previous surveys.11-12 The measurements were performed in a standard condition based on the manufacturers’ kits manual.
Sample size calculation method
The estimation of sample size was based on the formula [N= (t/d)2 *(1-p)/p; (t = 1.96, p = 0.32 and d = 0.02)] by considering the mean incidence of NASH in the previous studies.1,13 The alpha was set as 0.05 and the value of beta was considered as 0.02 in the sample size formula. Finally, the sample size of 200 was defined in this study.
Statistical analysis
Continuous variables were provided as mean (SD) and the categorical ones were shown as frequency (%). The logistic regression analysis was applied and the regression coefficient (Pearson rank) was calculated, to define which metabolic index would be related to NASH. The ROC analysis was used and the area under the curve (AUC) was calculated to determine which metabolic index could accurately predict NASH.
RESULTS
Finally, 94 patients with NASH and 106 controls were included in the analysis. The comparisons regarding laboratory assessments, metabolic indices, MS components, and smoking status between the patients with NASH and control groups are presented in table 1.
Table 1. The participants’ characteristics regarding the laboratory assessments, metabolic indices, and metabolic syndrome components .
| Variables | Control (N = 94) | NASH (N = 126) | p value |
| Age (year) | 52.10 ± 2.98 | 51.23 ± 2.34 | 0.07 |
| Fasting blood sugar | 105.30 ± 14.31 | 110.08 ± 15.34 | 0.05 |
| Triglyceride | 161.51 ± 84.91 | 166.69 ± 99.82 | 0.69 |
| Cholesterol | 182.90 ± 36.44 | 191.68 ± 35.59 | 0.81 |
| Low density lipoprotein | 104.35 ± 31.39 | 107.24 ± 32.35 | 0.52 |
| Height | 166.70 ± 7.78 | 166.67 ± 7.69 | 0.97 |
| Weight | 88.05 ± 11.08 | 88.35 ± 9.56 | 0.84 |
| Aspartate aminotransferase | 21.33 ± 6.74 | 42.69 ± 16.26 | < 0.001 |
| Alanine aminotransferase | 23.08 ± 15.40 | 43.40 ± 16.55 | < 0.001 |
| Systolic blood pressure | 12.18 ± 0.56 | 14.19 ± 0.52 | 0.05 |
| Waist circumference | 117.38 ± 12.93 | 118.87 ± 13.06 | 0.42 |
| Body mass index | 31.92 ± 5.32 | 31.99 ± 4.48 | 0.92 |
| Body shape index | 0.09 ± 0.009 | 0.09 ± 0.008 | 0.55 |
| Body round index | 8.21 ± 2.38 | 8.46 ± 2.52 | 0.47 |
| Visceral adiposity index | 3.02 ± 1.55 | 3.21 ± 1.89 | 0.46 |
| Lipid accumulative product | 103.65 ± 65.81 | 110.67 ± 80.05 | 0.50 |
| AST to ALT ratio | 0.47 ± 0.18 | 0.85 ± 0.395 | < 0.001 |
| Liver fat content | 10.26 ± 3.89 | 15.86 ± 7.59 | < 0.001 |
| Homeostasis model assessment | 4.45 ± 2.24 | 5.14 ± 2.30 | 0.03 |
| High density lipoprotein | 34.88 ± 3.70 | 34.25 ± 3.56 | 0.05 |
| Insulin | 18.24 ± 5.64 | 20.92 ± 5.72 | 0.03 |
| Alkaline phosphatase | 273.50 ± 157.51 | 303.40 ± 179.89 | 0.06 |
| Male sex (n, %) | 43(45.7%) | 57(53.8%) | 0.25 |
| Diabetes (n, %) | 73(77.7%) | 87(82.1%) | 0.43 |
| Metabolic syndrome (n, %) | 73(77.7%) | 87(82.1%) | 0.43 |
| Smoking (n, %) | 73(77.7%) | 77(72.6%) | 0.41 |
| Hypertension (n, %) | 73(77.7%) | 77(72.6%) | 0.41 |
AST, Aspartate transaminase; ALT, Alanine aminotransferase
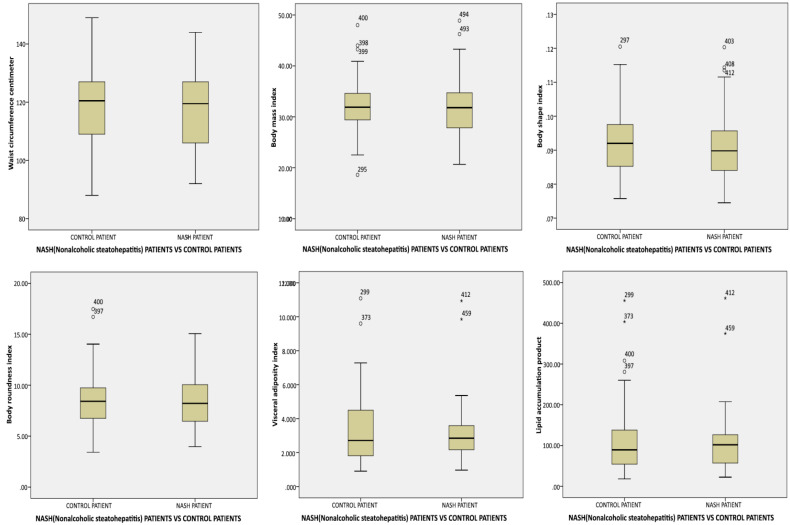
The comparison of mean metabolic indices values between the patients with NASH and controls is demonstrated in figure 1.
Fig. 1.
The comparison of mean metabolic indices between patients with non-alcoholic steatohepatitis and control group (Box plot)
The logistic regression analysis to define which metabolic index is independently associated with NASH is presented in table 2.
Table 2. The logistic regression analysis to define which metabolic index is independently associated with non-alcoholic steatohepatitis .
| Metabolic index | Odds ratio | p value | 95% Confidence interval | |
| Upper limit | Lower limit | |||
| Waist circumference | 1.01 | 0.06 | 1.001 | 2.005 |
| Body mass index | 0.40 | 0.08 | 0.39 | 1.05 |
| Body shape index | 0.01 | 0.67 | 0.001 | 1.32 |
| Body round index | 1.25 | 0.63 | 0.50 | 3.15 |
| Visceral adiposity index | 0.15 | 0.06 | 0.04 | 0.67 |
| Lipid accumulation product | 1.05 | 0.07 | 1.01 | 1.09 |
| Homeostasis model assessment | 0.22 | 0.08 | 0.04 | 1.22 |
| Liver fat content | 1.89 | < 0.001 | 1.50 | 2.40 |
The ROC (receiver operating characteristic curve) analysis and calculated area under the curve (AUC) of metabolic indices for prediction of NASH are shown in table 3.
Table 3. The ROC analysis and calculated area under the curve of metabolic indices for the prediction of non-alcoholic steatohepatitis .
| Metabolic index | Area under curve | p value | 95% Confidence interval | |
| Upper limit | Lower limit | |||
| Waist circumference | 0.47 | 0.45 | 0.39 | 0.55 |
| Body mass index | 0.48 | 0.71 | 0.40 | 0.57 |
| Body shape index | 0.46 | 0.35 | 0.38 | 0.54 |
| Body round index | 0.48 | 0.56 | 0.39 | 0.56 |
| Visceral adiposity index | 0.50 | 0.97 | 0.42 | 0.58 |
| Lipid accumulation product | 0.51 | 0.89 | 0.42 | 0.59 |
| Homeostasis model assessment | 0.46 | 0.32 | 0.38 | 0.54 |
| Liver fat content | 0.77 | < 0.001 | 0.70 | 0.84 |
The ROC analysis for evaluating the AUC of metabolic indices for the prediction of NASH is shown in figure 2.
Fig. 2.
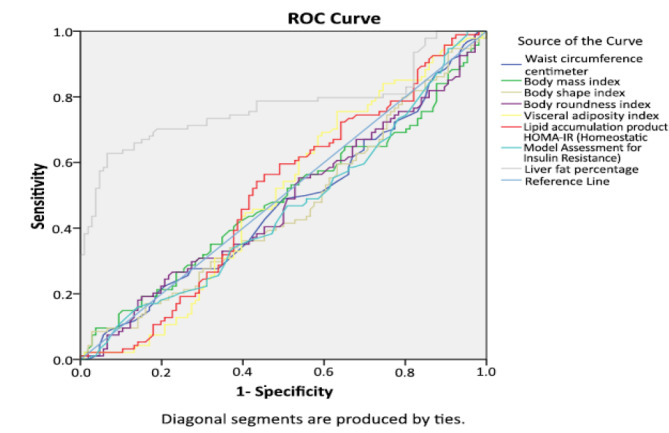
The ROC analysis for evaluating the area under the curve of metabolic indices for prediction of NASH
DISCUSSION
This study showed the independent correlation between LFC and the presence of NASH. Moreover, the ROC analysis showed a good AUC for LFC in the prediction of NASH. This finding is in parallel to the large body of evidence that showed the association between LFC and NASH. We should emphasize that these investigations estimated LFC based on magnetic resonance imaging (MRI) technique.14-16 However, we used a formula for liver fat estimation that had good sensitivity and specificity considering MRI as the gold standard method.9
VAI was the metabolic index that was not associated with NASH in our experiment. This is in parallel with previous results that showed no association between the mentioned index and liver histology in patients with NASH.17-19 Meanwhile, reports about the relationship between VAI and HOMA exists.17
BRI was another metabolic index that showed no association with NASH in this research. In the meantime, one experiment showed no superiority of BRI to the traditional metabolic indices for the detection of NASH.20 This result is in contrast to the previous reports that showed a strong association between the mentioned index and NASH.21,22
BSI was the next metabolic index that was not related to NASH in our study. This result is opposite to the previous reports that showed an association between the mentioned index and NASH.22
LAP was the last metabolic index that showed no relationship with NASH in this project. This finding is in accordance with the previous results that showed LAP could not predict LFC.23 However, there are reports about the association between the mentioned index and liver steatosis .24,25
HOMA was the only pure laboratory marker that did not show an independent association with NASH in the logistic regression analysis. This is in contrast with the previous reports that showed a strong relationship between the mentioned index and NASH.26,26-28
The possible explanation for the above-mentioned controversies would be the concomitant use of several metabolic indices at the same time in our project. Although some associations were observed in univariate analysis between some applied metabolic indices and the presence of NSAH (data are not shown), LFC was the only independent predictor of NASH in multivariate logistic regression analysis. Another reason for obtaining these results might be due to the characteristics of the control group in our survey. The mean BMI was more than 30, the mean WC was 117 cm, and the frequency of MS, DM, and hypertension was 77.7% in the controls. In other words, the control group consisted of a considerable number of obese subjects with a high propensity to IR.
Strengths
To define the best metabolic index for NASH screening, some important novel indices were evaluated at the same time in this investigation. Besides, we assessed a considerable amount of NASH risk factors (including metabolic syndrome components and smoking status) in this experiment.
Limitations
First, considering the cross-sectional design of this investigation only the association between metabolic indices and NASH was evaluated. The cause and effect model could not be evaluated due to the study design.
Second, the diagnosis of patients with NASH was not based on liver biopsy. Besides, rare etiologies that cause elevation of serum aminotransferase levels (like PSC and alpha one antitrypsin deficiency) were not checked in the study. Therefore, the possibility of selection bias exists in this experiment.
Future direction
Defining a reliable metabolic index will help the clinicians to screen the patients with NASH with the propensity to the complications of metabolic syndrome. Improving metabolic conditions reduces cardiovascular morbidities and concomitant health care expenses. LFC was the metabolic indicator that used the combination of laboratory assessments and metabolic conditions for the prediction of NASH. It seems to be a reasonable approach to search for a novel metabolic indicator that applies a mixture of laboratory and anthropometric data for a more reliable screening of NASH in the general population.
CONCLUSION
LFC might be an appropriate tool for screening of patients with NASH in the general population.
Acknowledgments
The authors thank Mr. Hejrani and Mrs. Taleh from Sina Hospital Research Development Center for their kind assistance in typing and preparing the draft. We also would like to express our gratitude to Professor Neda Moslemi and Arsia Jamali from TUMS for the critical review of the manuscript.
Please cite this paper as:
Jamali R, Ebrahimi M, Faryabi A, Ashraf H. Which Metabolic Index is Appropriate for Predicting Non-alcoholic Steatohepatitis? Middle East J Dig Dis 2020;12:99-105. doi: 10.34172/mejdd.2020.168.
Footnotes
ETHICAL APPROVAL There is nothing to be declared.
CONFLICT OF INTEREST The authors declare no conflict of interest related to this work.
References
- 1.Jamali R, Khonsari M, Merat S, Khoshnia M, Jafari E, Bahram Kalhori A. et al. Persistent alanine aminotransferase elevation among the general Iranian population: prevalence and causes. World J Gastroenterol. 2008;14:2867–71. doi: 10.3748/wjg.14.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa Y, Itoh M. Metabolic syndrome and NAFLD/NASH. Nihon Shokakibyo Gakkai Zasshi. 2017;114:834–8. doi: 10.11405/nisshoshi.114.834. [DOI] [PubMed] [Google Scholar]
- 3.Fusillo S, Rudolph B. Nonalcoholic fatty liver disease. Pediatr Rev. 2015;36:198–205. doi: 10.1542/pir.36-5-198. [DOI] [PubMed] [Google Scholar]
- 4.Yu AH, Duan-Mu YY, Zhang Y, Wang L, Guo Z, Yu YQ. et al. Correlation between Non-Alcoholic Fatty Liver Disease and Visceral Adipose Tissue in Non-Obese Chinese Adults: A CT Evaluation. Korean J Radiol. 2018;19:923–9. doi: 10.3348/kjr.2018.19.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamali R, Pourshams A, Amini S, Deyhim MR, Rezvan H, Malekzadeh R. The upper normal limit of serum alanine aminotransferase in Golestan Province, northeast Iran. Arch Iran Med. 2008;11:602–7. [PubMed] [Google Scholar]
- 6.Dai H, Wang W, Chen R, Chen Z, Lu Y, Yuan H. Lipid accumulation product is a powerful tool to predict non-alcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond) 2017;14:49. doi: 10.1186/s12986-017-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol. 2014;2014:730827. doi: 10.1155/2014/730827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Q, Zhang K, Li Y, Zhen Q, Shi J, Yu Y. et al. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han Chinese people in Northeast China: a cross-sectional study. Diabet Med. 2018;35:1580–7. doi: 10.1111/dme.13787. [DOI] [PubMed] [Google Scholar]
- 9.Giudici KV, Martini LA. Comparison between body mass index and a body shape index with adiponectin/leptin ratio and markers of glucose metabolism among adolescents. Ann Hum Biol. 2017;44:489–94. doi: 10.1080/03014460.2017.1327617. [DOI] [PubMed] [Google Scholar]
- 10.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM. et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–72. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Razavizade M, Jamali R, Arj A, Matini SM, Moraveji A, Taherkhani E. The effect of pioglitazone and metformin on liver function tests, insulin resistance, and liver fat content in nonalcoholic Fatty liver disease: a randomized double blinded clinical trial. Hepat Mon. 2013;13:e9270. doi: 10.5812/hepatmon.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamali R, Razavizade M, Arj A, Aarabi MH. Serum adipokines might predict liver histology findings in non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:5096–103. doi: 10.3748/wjg.v22.i21.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamali R, Arj A, Razavizade M, Aarabi MH. Prediction of Nonalcoholic Fatty Liver Disease Via a Novel Panel of Serum Adipokines. Medicine (Baltimore) 2016;95:e2630. doi: 10.1097/MD.0000000000002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel J, Bettencourt R, Cui J, Salotti J, Hooker J, Bhatt A. et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9:692–701. doi: 10.1177/1756283X16656735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Graffy PM, Reeder SB, Hernando D, Li K. Quantification of Liver Fat Content With Unenhanced MDCT: Phantom and Clinical Correlation With MRI Proton Density Fat Fraction. AJR Am J Roentgenol. 2018;211:W151–W157. doi: 10.2214/AJR.17.19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajmera V, Park CC, Caussy C, Singh S, Hernandez C, Bettencourt R. et al. Magnetic Resonance Imaging Proton Density Fat Fraction Associates With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018;155:307–10 e2. doi: 10.1053/j.gastro.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díez-Rodríguez R, Ballesteros-Pomar MD, Calleja-Fernández A, González-De-Francisco T, González-Herráez L, Calleja-Antolín S. et al. Insulin resistance and metabolic syndrome are related to non-alcoholic fatty liver disease, but not visceral adiposity index, in severely obese patients. Rev Esp Enferm Dig. 2014;106:522–8. [PubMed] [Google Scholar]
- 18.Vongsuvanh R, George J, McLeod D, van der Poorten D. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. J Hepatol. 2012;57:392–8. doi: 10.1016/j.jhep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Ercin CN, Dogru T, Genc H, Celebi G, Aslan F, Gurel H. et al. Insulin resistance but not visceral adiposity index is associated with liver fibrosis in nondiabetic subjects with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2015;13:319–25. doi: 10.1089/met.2015.0018. [DOI] [PubMed] [Google Scholar]
- 20.Liu PJ, Ma F, Lou HP, Zhu YN. Body roundness index and body adiposity index: two new anthropometric indices to identify metabolic syndrome among Chinese postmenopausal women. Climacteric. 2016;19:433–9. doi: 10.1080/13697137.2016.1202229. [DOI] [PubMed] [Google Scholar]
- 21.Procino F, Misciagna G, Veronese N, Caruso MG, Chiloiro M, Cisternino AM. et al. Reducing NAFLD-screening time: A comparative study of eight diagnostic methods offering an alternative to ultrasound scans. Liver Int. 2019;39:187–96. doi: 10.1111/liv.13970. [DOI] [PubMed] [Google Scholar]
- 22.Motamed N, Rabiee B, Hemasi GR, Ajdarkosh H, Khonsari MR, Maadi M. et al. Body Roundness Index and Waist-to-Height Ratio are Strongly Associated With Non-Alcoholic Fatty Liver Disease: A Population-Based Study. Hepat Mon. 2016;16:e39575. doi: 10.5812/hepatmon.39575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F. et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171:561–9. doi: 10.1530/EJE-14-0112. [DOI] [PubMed] [Google Scholar]
- 24.Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010;10:98. doi: 10.1186/1471-230X-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macut D, Tziomalos K, Božić-Antić I, Bjekić-Macut J, Katsikis I, Papadakis E. et al. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulationproduct in women with polycystic ovary syndrome. Hum Reprod. 2016;31:1347–53. doi: 10.1093/humrep/dew076.Epub2016Apr12. [DOI] [PubMed] [Google Scholar]
- 26.Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A. et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145–51. doi: 10.1016/j.jhep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Boza C, Riquelme A, Ibañez L, Duarte I, Norero E, Viviani P. et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15:1148–53. doi: 10.1381/0960892055002347. [DOI] [PubMed] [Google Scholar]
- 28.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]