Abstract
Introduction: This study aimed to perform a meta-analysis on the prevalence of metabolic syndrome (MetS) among patients with asthma and to measure the association asthma has with MetS.
Methods: The Web of Science, Medline, Scopus, Embase and Google Scholar were searched using the "Asthma", "Metabolic Syndrome", "Dysmetabolic Syndrome", "Cardiovascular Syndrome", "Insulin Resistance Syndrome", "Prevalence", "Odds Ratio", "Cross-Sectional Studies", and "Case-Control Studies" keywords. All observational studies reporting the prevalence of MetS among people with and without asthma were included in the study. In the presence of heterogeneity, random-effects models were used to pool the prevalence and odds ratios (OR), as measures of association in cross-sectional and case-control/ cohort studies, respectively.
Results: The prevalence of MetS among patients with asthma (8 studies) and the OR comparing the prevalence of MetS among patients with and without asthma (5 studies) were pooled separately. The pooled prevalence of MetS among patients with asthma was found to be 25% (95% confidence interval (CI): 13%–38%). In contrast, the overall pooled OR for MetS in patients with asthma, compared to healthy controls, was 1.34 (95% CI: 0.91–1.76), which was not statistically significant.
Conclusion: The prevalence of MetS was relatively high in patients with asthma. Furthermore, the odds of MetS was higher in patients with asthma, compared to healthy controls, although this difference was not statistically significant. More original studies among different populations are needed in order to more accurately examine the association between asthma and MetS, as well as the relationship asthma has with the individual components of MetS.
Keywords: Metabolic Syndrome, Prevalence, Asthma, Epidemiology, Meta-analysis
Introduction
Asthma is one of the most common respiratory ailments and although there are a number of treatments available, these are not always effective for patients with severe clinical symptoms.1-3 A recent literature search reported that obesity was an important factor that disrupts the control of asthma symptoms and results in a reduced response to treatment.4 Indeed, a robust epidemiological association between asthma and obesity has recently become well established.5-8 Asthma has also been associated with other components of metabolic syndrome (MetS), such as hypertension and insulin resistance, irrespective of elevated body mass.8-11 According to reports from the World Health Organization (WHO), obesity has shown a drastic increase in recent decades. For instance, more than one-third of the American population were estimated to be obese from 2009 to 2010.12 Obesity is a major risk factor for several other diseases, such as: diabetes, arthritis, cardiovascular problems, obstructive sleep apnea and cancer.13 Also, as shown in several cross-sectional studies, obesity has a strong relationship with asthma,14 as a predisposing factor.15 Moreover, obesity is an obstacle in asthma control and treatment, and weight loss has been associated with improved clinical symptoms of asthma and lung function.7,16,17 The cellular mechanism by which obesity affects asthma is still under debate.18 Although inflammatory cell infiltration occurs in obesity, it has not been reliably observed in those suffering from asthma and so other mechanisms, such as hyperinsulinemia, dyslipidemia and hyperglycemia should be taken into account, rather than solely focusing on the inflammatory condition.19
MetS is characterized as a syndrome in which three of the following symptoms exist: disturbed fasting glucose metabolism, dyslipidemia, obesity and hypertension.20-22 Also, increased risk of coronary heart disease, atherosclerosis and diabetes have a direct link with MetS.21-23 Hepatic failure, sleep apnea and chronic inflammatory states, including asthma, are also common clinical signs of MetS.21-23 Although the association between asthma and MetS has been examined in a number of studies, to the best of our knowledge no meta-analysis has investigated the association between asthma and MetS. Thus, we aimed to conduct a meta-analysis to examine the association between asthma and MetS using published observational studies.
Materials and Methods
Search strategy and study selection
This meta-analysis was conducted using the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA) guidelines.24 All cross-sectional, case-control and cohort studies reporting the prevalence of MetS among people with and without asthma were included in the study.
The following keywords were identified using the medical subject headings (MESH) in Medline: “Asthma”, “Metabolic Syndrome”, “Dysmetabolic Syndrome”, “Cardiovascular Syndrome”, “Insulin Resistance Syndrome”, “Prevalence”, “Odds Ratio”, “Cross-Sectional Studies”, and “Case-Control Studies”. These keywords were used to search the following databases: Web of Science, Medline, Scopus, Embase and Google Scholar.
Inclusion and exclusion criteria
All English language articles that were published from inception to June 2018 were imported into Endnote X6. Firstly, the title and abstracts of all identified papers were examined and then the full-texts of all relevant articles were examined. The full-text articles were then meticulously examined by two researchers trained (NI and SS) using the specified criteria and any disagreements were resolved by consulting a third researcher (NK).
The location of the study, study design, time, sampling procedures and statistical analyses were also examined in the selection process. Non-original articles, such as reviews, were excluded but research letters published in highly prestigious journal were included. Moreover, all studies on people suffering from other diseases were excluded. Finally, an e-mail was sent to the corresponding author of any articles which could not be included in the meta-analysis, due to missing details.
Data extraction and quality assessment
The data extraction was conducted by the two aforementioned researchers and familiar with data extraction based on formulated a research question i.e. using the PICO model (NI and SS) and any disagreements were resolved by consulting the previously mentioned third researcher (NK). The following variables were extracted for the analyses: study specifications (first author’s name, study design, location and date of publication), participant details (age, sample size, and each study groups’ health status, in relation to asthma and MetS). The quality of reporting in each study was examined using the STROBE checklist (22 items) and only those which reached the minimum acceptable level of quality (>15 items) were included in the analysis stage 25. The Newcastle-Ottawa tool26 was also used to examine the risk of bias in the included studies, where the minimum and maximum scores possible were 0 and 9 for case-control and cohort studies and 0 and 10 for cross-sectional studies. Studies with scores ≥6, 3<score<6, and score <3 were considered to be low risk , moderate risk and high risk, respectively. This tool examined the risk of bias in three aspects (selection , comparability and outcome/exposure) for the cross-sectional, case-control and cohort studies included in the current meta-analysis.
All researchers involved in searching different databases, data extraction and quality assessment had previous experience in conducting or collaboration in a meta-analysis study.
Statistical analysis
A random-effects model was used to pool the OR of MetS among patients with and without asthma, to identify heterogeneity among the studies. Heterogeneity between the studies was determined using the I2 index. Meta-regression for different variables including age, study design, MetS diagnostic criteria and publication date were used to determine the source of heterogeneity, when the I2 index was found to be higher than 0.6. In addition, sensitivity analysis was perform to explore the impact of excluding or including studies in analysis based on sample size, MetS diagnostic criteria and study design. Finally, publication bias was examined using a Funnel plot and Egger’s test.27 All statistical analyses were performed using Stata software version 13 (Stata Corp, College Station, TX, USA).
Results
The process of article selection is illustrated in Figure 1. This shows that 370 studies were identified at the first stage of the search process and an additional 26 articles were found through searching the reference lists of these articles. Thirty-seven articles were excluded due to duplication of the data. Following a review of all remaining abstracts, 342 papers were excluded due to duplication (n=4) and irrelevant data (n=338) and the full text versions of 17 articles were downloaded for detailed analysis. Following the assessment of full-texts, six papers were excluded due to inappropriate study design (n=2), duplication (n=1) and irrelevant data (n=3). Finally, seven cross-sectional studies, one case-control study and three cohort studies were considered for the final stage of the study. However, only five studies had information regarding the association between MetS and asthma using an OR. All studies included in this systematic review and meta-analysis were published in English during the period January 2010 to June 2018, and the ages of the participants ranged from 12-78 years old. More detailed information regarding the studies included in this systematic review and meta-analysis are provided in Table 1. The results of the risk of bias assessment for the studies included in the meta-analysis are presented in Appendix Table 1.
Figure 1.
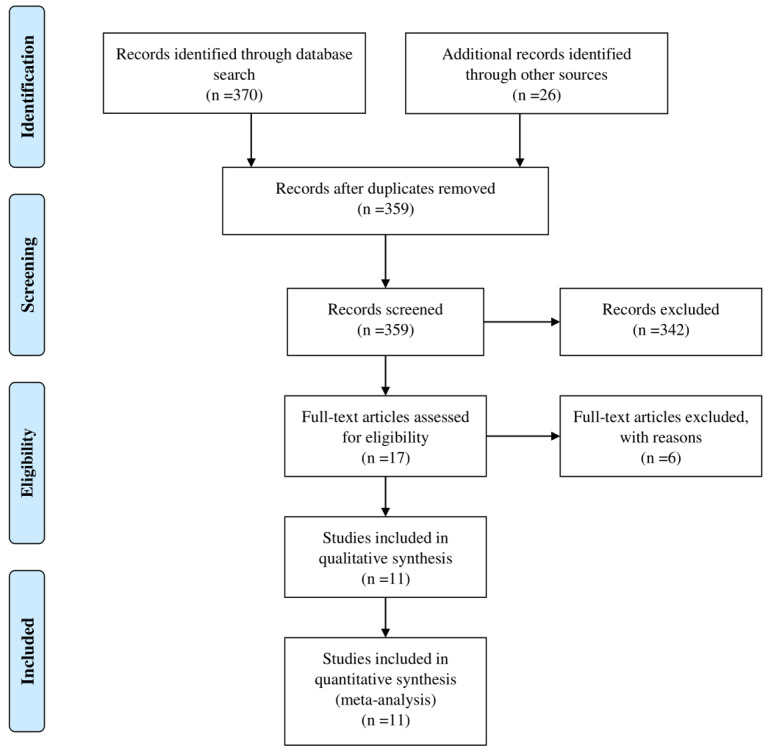
Follow diagram of the systematic review & meta-analysis of asthma and MetS.
Table 1. Eligible studies reporting the prevalence of metabolic syndrome in patients with asthma .
| First Author | Country | Study Design | MetS Criteria | DOP | Age Range | Mean Age | Group | Gender | Sample Size | N. | MetS Prev. (%) |
| Forno29 | U.S. | Cross-Sectional | Three of the five criteria: fasting glucose ≥ 110 mg/dL; WC ≥ 75th percentile; fasting TG ≥ 100 mg/dl; HDL ≤ 50 mg/dL; and SBP ≥ 90th percentile | 2015 | 12-17 | - | - | Both | 1429 | 224 | 15.7 |
| Male | 745 | ||||||||||
| Female | 684 | ||||||||||
| No asthma | Both | 1334 | 58 | 9.02 | |||||||
| Asthma | Both | 95 | 7 | 15.77 | |||||||
| Aydin30 | Turkey | Case-Control | Modified WHO diagnostic criteria | 2013 | Postmenopausal | Asthma=57.5±13.9/Control=59.6±12.8 | - | Female | 75 | 12 | 26.7 |
| Assad28 | U.S. | Longitudinal Analysis | ATP-III criteria | 2013 | - |
24.9±3.6 (Male=24.6±3.6/ Female= 25.1±3.7) |
No asthma | Both | 4017 | 88 | 2.2 |
| Male | 1900 | 53 | 2.8 | ||||||||
| Female | 2117 | 36 | 1.7 | ||||||||
| Asthma | Both | 602 | 19 | 3.2 | |||||||
| Male | 185 | 3 | 1.6 | ||||||||
| Female | 417 | 16 | 3.8 | ||||||||
| Ahmed31 | Pakistan | Cross-Sectional | - | 2016 | - | - | - | Both | 154 | 46 | 29.87 |
| Male | 80 | ||||||||||
| Female | 74 | ||||||||||
| Adeyeye9 | Nigeria | Cross-Sectional | The international diabetes Federation (IDF) criteria | 2012 | 14-78 |
46.5±17.2 (Male= 44.9±16.2/ Female=47.6±17.7) |
- | Both | 158 | 28 | 17.7 |
| Male | 63 | 13 | 20.6 | ||||||||
| Female | 95 | 15 | 15.8 | ||||||||
| Uzunlulu32 | Turkey | Case-Control | The international diabetes Federation (IDF) criteria | 2011 | - |
Asthma=43.83±10.98 Control= 42.01±9.21 |
No asthma | Both | 98 | 33 | 33.7 |
| Male | 17 | 6 | 35.3 | ||||||||
| Female | 81 | 27 | 33.3 | ||||||||
| Asthma | Both | 90 | 33 | 36.7 | |||||||
| Male | 20 | 6 | 30 | ||||||||
| Female | 70 | 27 | 38.6 | ||||||||
| Pantoja-Alcantar33 | Mexico | Cross-Sectional | ATP III criteria | 2012 | - | - | - | Both | 39 | 11 | 28.2 |
| Singh34 | India | Cross-Sectional | The NCEP ATP III definition | 2016 | 17-59 | - | - | Both | 60 | 20 | 33.3 |
| Male | 28 | ||||||||||
| Female | 32 | ||||||||||
| Del-Rio-Navarro35 | Mexico | Cross-Sectional | de Ferranti criteria | 2010 | Adolescent | - | - | Both | 174 | 72 | 41.34 |
The association between MetS and asthma
The prevalence of MetS was pooled across the 8 studies and was estimated to be 25% (95% confidence interval (CI): 13–38%) among patients with asthma (Figure 2).Furthermore, the association between MetS and asthma was examined across the 5 studies with ORs, which found that the odds of MetS was 34% higher in patients with asthma, compared to a healthy control group, but this was not statistically significant [odds ratio (OR): 1.34; 95% confidence interval (CI): 0.91-1.76] (Figure 3). More detailed information about the studies comparing the prevalence of MetS between patients with and without asthma are presented in Table 2.
Figure 2.
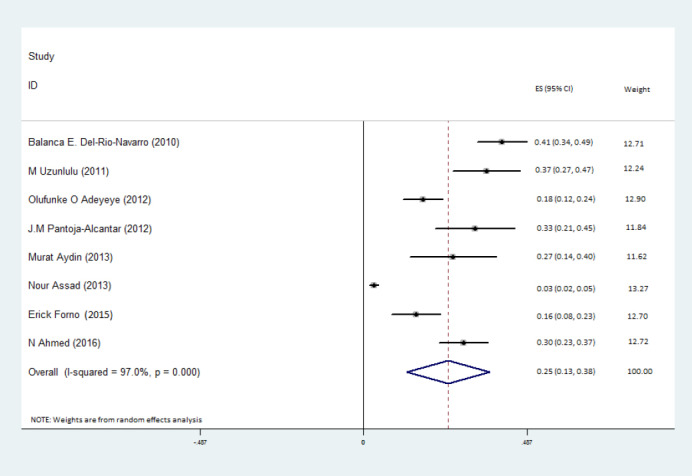
Forest plot of metabolic syndrome prevalence among patients with asthma.
Figure 3.
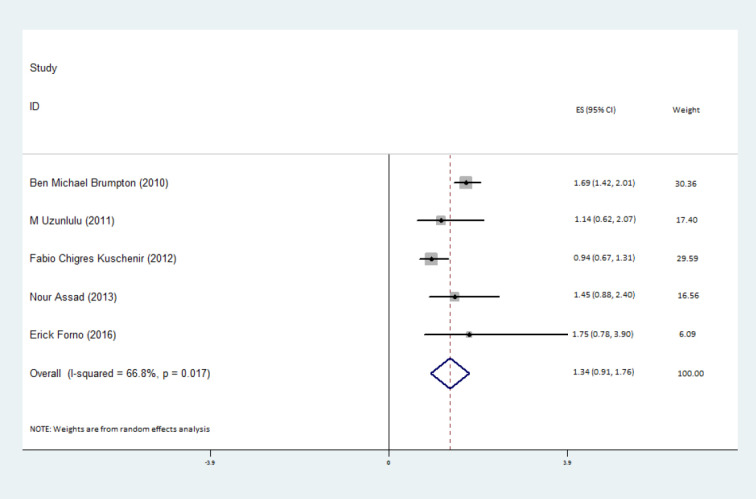
Forest plot of metabolic syndrome odds among patients with asthma, compared with healthy controls.
Table 2. Prevalence of metabolic syndrome in patients with asthma compared to healthy controls .
| First Author | DOP | Study Design | Assessment of Asthma | Country | Age Range | Mean Age | Type of Effect | Gender | Group |
Effect
(95% CI) |
| Kuschnir36 | 2018 | Cross-sectional | Self-administered questionnaire | Brazil | 12-17 | 14.6±1.6 | Prevalence Ratio | Both | Current Asthma | 0.94 (0.67-1.31) |
| Severe Asthma | 2.43 (1.39-2.27) | |||||||||
| Forno29 | 2015 | Cross-sectional | Diagnosed by a doctor or other health care professional/Spirometry | U.S. | 12-17 | - | Crude OR | Both | Current Asthma | 1.75 (0.78-3.90) |
| Brumpton37 | 2013 | Prospective cohort | Self-administered questionnaire | Norway | 19-55 | - | Adjusted OR | Both | Current Asthma | 1.57 (1.31-1.87) |
| Assad28 | 2013 | Longitudinal analysis | Self-reported presence of asthma symptoms | U.S. | - |
24.9±3.6 (Male=24.6± 3.6/ Female= 25.1±3.7) |
Crude OR | Both | Current Asthma | 1.45 (0.88-2.40) |
| Male | 0.57 (0.18-1.83) | |||||||||
| Female | 2.25 (1.25-4.03) | |||||||||
| Uzunlulu32 | 2011 | Case-control | Diagnosed by a pulmonologist | Turkey | - |
Asthma= 43.83±10.98 Control= 42.01±9.21 |
Crude OR | Both | Current Asthma | 1.14 (0.62-2.07) |
| Male | 0.78 (0.19-3.17) | |||||||||
| Female | 1.30 (0.66-2.53) |
Heterogeneity and meta-regression
The I2 statistics was used to assess heterogeneity across the studies. A statistically significant level of heterogeneity was found across the studies estimating the prevalence of MetS among patients with asthma ((I2=97.0%, P = 0.001) (Figure 2). Meta-regression was then used to find the source of the heterogeneity among the studies, which found that age (P = 0.61), study design (P = 0.19), MetS diagnostic criteria (P = 0.12) and publication date (P = 0.29) were not responsible for the reported heterogeneity. Moreover, statistically significant heterogeneity was found for the estimated association between MetS and asthma status (I2=66.8%, P = 0.017) (Figure 2). Meta-regression of this data showed that age (P = 0.43), study design (P = 0.98), and publication date (P = 0.99) were not responsible for the observed heterogeneity. As the aforementioned variables were not significant, no subgroup-analysis was conducted. Sensitivity analysis showed that there was no influential study across the studies estimating the associations between MetS and asthma status.
Publication bias
No publication bias was found for the association between MetS and asthma status (Egger’s test: P = 0.46). The corresponding Funnel plot is shown in Figure 4.
Figure 4.
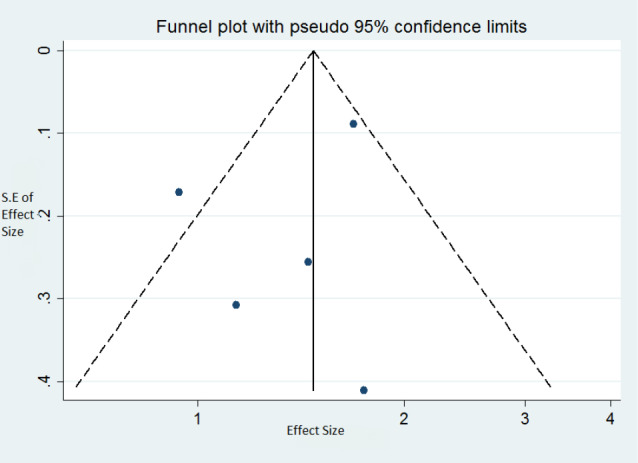
Funnel plot assessing publication bias on the association between metabolic syndrome and asthma status.
Discussion
The present study revealed that the prevalence of MetS was a lot higher (25%) in patients with asthma, meaning that this syndrome needs to be addressed and controlled in patients with asthma. On the other hand, although the present study found that the odds of being diagnosed with MetS were higher in patients with asthma, compared to healthy controls, this association was not statistically significant. However, these results should be interpreted with caution as the number of published studies included in the meta-analysis was not high and there may not have been sufficient statistical power to adequately examine the association between MetS and asthma. Furthermore, the associations asthma had with the components of MetS were not reported in these studies. This is problematic, as asthma may have completely different relationships with the different components of MetS. In addition, according to the research evidence, obesity is just one of the building blocks of MetS which increases the risk of asthma.38
Several mechanisms may be responsible for the relationship between asthma and MetS, such as localised and systemic inflammation, oxidative hazard and insulin resistance. System and airway inflammation have previously been identified as having a key role in the relationship between obesity and asthma. Enhanced pro-inflammatory cytokines, such as leptin, tumor necrosis factor α and interleukins are secreted from the adipose tissues of obese patients with asthma, which triggers further systemic inflammatory response.39,40 Macrophage proliferation and the differentiation of lung tissue are also plausible consequences of airway inflammation.39 However, clinical investigations have not supported these hypotheses.41 Another mechanism which has previously been mentioned is that insulin resistance may lead to airway dysfunction, since the association between asthma and insulin resistance has also been reported in a number of different studies.8,42 The reduction of glucose consumption and the induction of abnormal fat metabolism in muscles, which is concomitant with impaired mitochondrial energy production, may also contribute to skeletal muscle respiratory resistance. Therefore, respiratory muscle malfunction may lead to the airflow impediment observed in asthma.43 Regarding oxidative stress, the enhanced production of systemic reactive oxygen species (ROS) has been observed in obese patients, while asthmatics show low levels of endogenous antioxidants.44,45 However, it is not yet clear whether airway oxidative stress occurs as a consequence of systemic ROS formation, due to obesity.39
A number of regional and age-group studies have found asthma to have a relationship with MetS and its clinical manifestations, such as hypertension and hypertriglyceridemia.47-49 However, in a study conducted by Assad et al no significant link between asthma and MetS was found, after adjustments for BMI were made.28 Thus, it remains unclear whether those with MetS who have a normal weight also have an elevated risk of asthma. Even in vivo studies using animals have not produced conclusive results.
Lipoprotein dysfunction reported in asthma pathogenesis, suggests the use of statins could be beneficial in these patients, although there is still some debate on this issue.49-51 In addition to cholesterol metabolism regulating the effects of statins in asthmatics, the anti-inflammatory properties of these drugs have been thought to have a role in asthma control.44,52-54 In particular, Lovastatin has been shown to diminish the differentiation and proliferation of asthmatic bronchial fibroblasts in vitro.55 Furthermore, Atorvastatin and Simvastatin attenuate the number of inflammatory cells found in saliva specimens.28 In addition, bisphosphonate, such as alendronate, have been shown to provide protection against asthma via reducing eosinophilic airway inflammation by reducing the regulation of several cytokines.56 Indeed, the role of diet and exercise should not be overlooked in the treatment of asthmatic patients who are overweight or obese.21,57 Supplements such as retinoids, retinoic acid and fenretinide exert their beneficial effect by reducing the inflammatory response.58,59 While it is clear that these agents can reduce bronchial obstruction, more research is needed to ascertain the potential protective impacts of novel therapies to control MetS in asthmatic patients, since these may be useful in treating non-responding or uncontrolled asthma.60,61 Chemokines and their receptors have been shown to be involved in adipose tissue enlargement and the initiation of insulin resistance.62,63 Thus, the inhibition of chemokine receptors could potentially be helpful in asthmatic patients with MetS.64
Limitations of the study
Although the present study is the first meta-analysis to investigate the association between MetS and asthma, there are some limitations that should be considered when interpreting the results. Firstly, the number of studies included was less than 10 and the power was insufficient to examine publication bias. In addition, the source of heterogeneity could not be identified in the meta-regression due to a lack of statistical power and neither could subgroup-analysis be undertaken. Finally, more original research is need in different populations and among different age and sex groups, in order to increase the generalizability of any future meta-analyses.
Conclusion
The current meta-analysis found the prevalence of MetS was relatively high in patients with asthma. Furthermore, the odds of MetS was higher in patients with asthma than that found among healthy controls, although this difference was not statistically significant. More original studies in different populations are needed to examine the association asthma has with MetS, as well as the relationship that asthma has with the individual components of MetS.
Acknowledgments
We would like to thank Social Determinants of Health Research Center of Shahid Beheshti University of Medical Sciences for financial support.
Competing interests
None to declared.
Ethical approval
Not applicable.
Funding
The present study was supported by Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant No. 20974).
References
- 1.Ulrik CS, Claudius BK, Tamm M, Harving H, Siersted HC, Backer V. et al. Effect of asthma compliance enhancement training on asthma control in patients on combination therapy with salmeterol/fluticasone propionate: a randomised controlled trial. Clin Respir J. 2009;3(3):161–8. doi: 10.1111/J.1752-699X.2009.00129.x. [DOI] [PubMed] [Google Scholar]
- 2.Becker AB, Abrams EM. Asthma guidelines: the global initiative for asthma in relation to national guidelines. Curr Opin Allergy Clin Immunol. 2017;17(2):99–103. doi: 10.1097/aci.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 3.National AE, Prevention P. Expert Panel Report 3 (Epr-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol Pract. 2007;120(5 Suppl):S94. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Reddel HK, Hurd SS, Fitzgerald JM. World Asthma Day Gina 2014: a global asthma strategy for a global problem. Int J Tuberc Lung Dis. 2014;18(5):505–6. doi: 10.5588/ijtld.14.0246. [DOI] [PubMed] [Google Scholar]
- 5.Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five european countries: results of a 2008 survey. Eur Respir Rev. 2010;19(116):150–7. doi: 10.1183/09059180.00002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershon AS, Wang C, Guan J, To T. Burden of comorbidity in individuals with asthma. Thorax. 2010;65(7):612–8. doi: 10.1136/thx.2009.131078. [DOI] [PubMed] [Google Scholar]
- 7.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–6. doi: 10.1164/rccm.200611-1717oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thuesen B, Husemoen L, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma‐like Symptoms In Adults. Clin Exp Allergy. 2009;39(5):700–7. doi: 10.1111/j.1365-2222.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 9.Adeyeye OO, Ogbera AO, Ogunleye OO, Brodie-Mens AT, Abolarinwa FF, Bamisile RT. et al. Understanding asthma and the metabolic syndrome - a Nigerian report. Int Arch Med. 2012;5(1):20. doi: 10.1186/1755-7682-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brumpton BM, Camargo CA Jr, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the hunt study. Eur Respir J. 2013;42(6):1495–502. doi: 10.1183/09031936.00046013. [DOI] [PubMed] [Google Scholar]
- 11.Park J, Kim TB, Joo H, Lee JS, Lee SD, Oh YM. Diseases concomitant with asthma in middle-aged and elderly subjects in Korea: a population-based study. Allergy Asthma Immunol Res. 2013;5(1):16–25. doi: 10.4168/aair.2013.5.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence Of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazzola M, Segreti A, Calzetta L, Rogliani P. Comorbidities of asthma: current knowledge and future research needs. Curr Opin Pulm Med. 2013;19(1):36–41. doi: 10.1097/mcp.0b013e32835b113a. [DOI] [PubMed] [Google Scholar]
- 14.Weiss St, Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med. 2004;169(8):963–8. doi: 10.1164/rccm.200303-403Ws. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115(5):897–909. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 16.Boulet LP. Asthma and obesity. Clin Exp Allergy. 2013;43(1):8–21. doi: 10.1111/j.1365-2222.2012.04040.X. [DOI] [PubMed] [Google Scholar]
- 17.McGinley B, Punjabi NM. Obesity, metabolic abnormalities, and asthma: establishing causal links. Am J Respir Crit Care Med. 2011;183(4):424–5. doi: 10.1164/rccm.201009-1525ED. [DOI] [PubMed] [Google Scholar]
- 18.Lugogo NL, Kraft M, Dixon Ae. Does obesity produce a distinct asthma phenotype? J Appl Physiol. 2009;108(3):729–34. doi: 10.1152/japplphysiol.00845.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111(11):1448–54. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 22.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9(1):48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sovio U, Skow A, Falconer C, Park MH, Viner RM, Kinra S. Improving prediction algorithms for cardiometabolic risk in children and adolescents. J Obes. 2013;2013:684782. doi: 10.1155/2013/684782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Plos Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S. et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. Plos One. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assad N, Qualls C, Smith LJ, Arynchyn A, Thyagarajan B, Schuyler M. et al. Body mass index is a stronger predictor than the metabolic syndrome for future asthma in women the longitudinal cardia study. Am J Respir Crit Care Med. 2013;188(3):319–26. doi: 10.1164/rccm.201303-0457OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forno E, Han Y-Y, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136(2):304–11 e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydin M, Koca C, Ozol D, Uysal S, Yildirim Z, Kavakli HS. et al. Interaction of metabolic syndrome with asthma in postmenopausal women: role of adipokines. Inflammation. 2013;36(6):1232–8. doi: 10.1007/s10753-013-9660-9. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed N, Kazim SM, Gillani SY. Frequency of metabolic syndrome in asthmatic patients of hazara division. J Ayub Med Coll Abbottabad. 2016;28(4):762–5. [PubMed] [Google Scholar]
- 32.Uzunlulu M, Oguz A, Gedik C, Aslan G, Arik S. Is prevalence of metabolic syndrome high in patients with asthma? Acta Clinica Belgica. 2011;66(1):49–52. doi: 10.2143/ACB.66.1.2062514. [DOI] [PubMed] [Google Scholar]
- 33.Pantoja-Alcantar JM, Segura-Méndez NH, Vargas-Ortega G, González-Virla JBG. Association of metabolic syndrome and asthma severity. Revista Alergia México. 2012;59(1):3–8. [PubMed] [Google Scholar]
- 34.Singh M, Gupta N, Kumar R. Effect of obesity and metabolic syndrome on severity, quality of life, sleep quality and inflammatory markers in patients of asthma in India. Adv Respir Med. 2016;84(5):258–64. doi: 10.5603/PiAP.2016.0032. [DOI] [PubMed] [Google Scholar]
- 35.Del-Rio-Navarro BE, Castro-Rodriguez JA, Garibay Nieto N, Berber A, Toussaint G, Sienra-Monge JJ. et al. Higher metabolic syndrome in obese asthmatic compared to obese nonasthmatic adolescent males. J Asthma. 2010;47(5):501–6. doi: 10.3109/02770901003702808. [DOI] [PubMed] [Google Scholar]
- 36.Kuschnir FC, Felix MMR, Kuschnir MCC, Bloch KV, Jordão EAdOC, Solé D. et al. Severe asthma is associated with metabolic syndrome in Brazilian adolescents. J Allergy Clin Immunol. 2018;141(5):1947–9 e4. doi: 10.1016/j.jaci.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Brumpton BM, Camargo CA, Romundstad PR, Langhammer A, Chen Y, Mai X-M. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42(6):1495–502. doi: 10.1183/09031936.00046013. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging Interface between metabolic syndrome and asthma. Am J Respir Cell Mol Biol. 2011;44(3):270–5. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 39.Lugogo Nl, Bappanad D, Kraft M. Obesity, metabolic dysregulation and oxidative stress in asthma. Biochim Biophys Acta. 2011;1810(11):1120–6. doi: 10.1016/J.bbagen.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canöz M, Erdenen F, Uzun H, Müderrisoglu C, Aydin S. The relationship of inflammatory cytokines with asthma and obesity. Clin Invest Med. 2008;31(6):373–9. doi: 10.25011/cim.v31i6.4924. [DOI] [PubMed] [Google Scholar]
- 41.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174(2):112–9. doi: 10.1164/rccm.200602-231Pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arshi M, Cardinal J, Hill RJ, Davies PS, Wainwright C. Asthma and insulin resistance in children. Respirology. 2010;15(5):779–84. doi: 10.1111/J.1440-1843.2010.01767.X. [DOI] [PubMed] [Google Scholar]
- 43.Lazarus R, Gore CJ, Booth M, Owen N. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr. 1998;68(1):35–41. doi: 10.1093/ajcn/68.1.35. [DOI] [PubMed] [Google Scholar]
- 44.Steffes MW, Gross MD, Lee DH, Schreiner PJ, Jacobs DR Jr. Adiponectin, visceral fat, oxidative stress, and early macrovascular disease: the coronary artery risk development in young adults study. Obesity (Silver Spring) 2006;14(2):319–26. doi: 10.1038/oby.2006.41. [DOI] [PubMed] [Google Scholar]
- 45.Dut R, Dizdar EA, Birben E, Sackesen C, Soyer OU, Besler T. et al. Oxidative stress and its determinants in the airways of children with asthma. Allergy. 2008;63(12):1605–9. doi: 10.1111/j.1398-9995.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- 46.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183(4):441–8. doi: 10.1164/rccm.201004-0603oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leone N, Courbon D, Berr C, Barberger-Gateau P, Tzourio C, Alperovitch A. et al. Abdominal obesity and late-onset asthma: cross-sectional and longitudinal results: the 3C study. Obesity (Silver Spring) 2012;20(3):628–35. doi: 10.1038/oby.2011.308. [DOI] [PubMed] [Google Scholar]
- 48.Lee Ej, In Kh, Ha Es, Lee Kj, Hur Gy, Kang Eh. et al. Asthma-Like Symptoms Are Increased In The Metabolic Syndrome. J Asthma. 2009;46(4):339–42. doi: 10.1080/02770900802660931. [DOI] [PubMed] [Google Scholar]
- 49.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol. 2010;126(4):754–62 e1. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Zeki A, J Kenyon N, Goldkorn T. Statin drugs, metabolic pathways, and asthma: a therapeutic opportunity needing further research. Drug Metab Lett. 2011;5(1):40–4. doi: 10.2174/187231211794455217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ. et al. Statin use in patients with asthma–a nationwide population‐based study. Eur J Clin Invest. 2011;41(5):507–12. doi: 10.1111/j.1365-2362.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 52.Yuan C, Zhou L, Cheng J, Zhang J, Teng Y, Huang M. et al. Statins as potential therapeutic drug for asthma? Respir Res. 2012;13(1):108. doi: 10.1186/1465-9921-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva D, Couto M, Delgado L, Moreira A. A systematic review of statin efficacy in asthma. J Asthma. 2012;49(9):885–94. doi: 10.3109/02770903.2012.721433. [DOI] [PubMed] [Google Scholar]
- 54.Moini A, Azimi G, Farivar A. Evaluation of atorvastatin for the treatment of patients with asthma: a double-blind randomized clinical trial. Allergy Asthma Immunol Res. 2012;4(5):290–4. doi: 10.4168/aair.2012.4.5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Si X-B, Zhang S, Huo L-Y, Dai W-L, Wang H-L. Statin therapy does not improve lung function in asthma: a meta-analysis of randomized controlled trials. J Int Med Res. 2013;41(2):276–83. doi: 10.1177/0300060513477005. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki O, Imamura M, Yamazumi Y, Harada H, Matsumoto T, Okunishi K. et al. Alendronate attenuates eosinophilic airway inflammation associated with suppression of th2 cytokines, th17 cytokines, and eotaxin-2. J Immunol. 2013;191(6):2879–89. doi: 10.4049/jimmunol.1300460. [DOI] [PubMed] [Google Scholar]
- 57.Dandona P, Ghanim H, Monte SV, Caruana JA, Green K, Abuaysheh S. et al. Increase in the mediators of asthma in obesity and obesity with type 2 diabetes: reduction with weight loss. Obesity (Silver Spring) 2014;22(2):356–62. doi: 10.1002/oby.20524. [DOI] [PubMed] [Google Scholar]
- 58.Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T. et al. Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice. Hepatology. 2012;56(4):1319–30. doi: 10.1002/hep.25798. [DOI] [PubMed] [Google Scholar]
- 59.Mcilroy GD, Delibegovic M, Owen C, Stoney PN, Shearer KD, Mccaffery PJ. et al. Fenretinide Treatment Prevents Diet-Induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes. 2013;62(3):825–36. doi: 10.2337/db12-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arron JR, Scheerens H, Matthews JG. Redefining approaches to asthma: developing targeted biologic therapies. Adv Pharmacol. 2013;66:1–49. doi: 10.1016/B978-0-12-404717-4.00001-9. [DOI] [PubMed] [Google Scholar]
- 61.Garcia G, Taille C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Anti-interleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22(129):251–7. doi: 10.1183/09059180.00004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab J. 2013;37(3):165–72. doi: 10.4093/dmj.2013.37.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malagon M, Díaz-Ruiz A, Guzman-Ruiz R, Jimenez-Gomez Y, R Moreno N, Garcia-Navarro S. et al. Adipobiology for novel therapeutic approaches in metabolic syndrome. Curr Vasc Pharmacol. 2013;11(6):954–67. doi: 10.2174/15701611113116660170. [DOI] [PubMed] [Google Scholar]
- 64.Linderholm AL, Bratt JM, Schuster GU, Zeki AA, Kenyon NJ. Novel therapeutic strategies for adult obese asthmatics. Immunol Allergy Clin North Am. 2014;34(4):809–23. doi: 10.1016/j.iac.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


