Abstract
Background
Coronavirus disease 2019 (COVID-19) is a new infectious disease that emerged in China in late 2019 and is now spreading around the world. Social distancing measures were needed to reduce transmission, and lockdown included restricted access to health care facilities. The impact of COVID-19 on transplant recipients is unknown, but considering their immunosuppression status and associated comorbidities, they should be considered a high-risk population.
Methods
A kidney transplant center in Central Italy implemented a strategy to maintain follow-up of kidney transplant recipients by phone and e-mail during lockdown. Telephone interviews were used to administer a clinical questionnaire to patients, and e-mail was used to receive the results of diagnostic tests conducted in outpatient settings.
Results
From March 17 to April 23, 2020, a total of 143 kidney transplant recipients were contacted. Twenty-eight patients needed in-hospital consultation for problems unrelated to COVID-19, 3 of whom needed hospitalization. Eleven patients were managed at home for mild urinary or respiratory diseases, and 1 was referred to the hematologist. We identified 2 suspected cases of COVID-19 infection, and the patients were referred to hospital care. Immunosuppressive therapy was modulated, and intravenous corticosteroids and potentially effective antiviral therapy were administered with a favorable outcome.
Conclusions
In the context of a lockdown, such as that occurring in response to COVID-19, we suggest implementing remote surveillance programs in kidney transplant recipients with the help of any available technology and offering medical consulting and logistic support as needed.
Highlights
-
•
Lockdown has helped contain the transmission of coronavirus disease 2019 (COVID-19) in Italy.
-
•
Lockdown measures include reduced access to medical care for chronic conditions.
-
•
Kidney transplant recipients are fragile (immunosuppression and comorbidities).
-
•
Remote surveillance programs in fragile populations are needed.
-
•
Immunosuppression modulation is advised for COVID-19 in kidney transplant patients.
The coronavirus disease 2019 (COVID-19) is a new infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that had its first outbreak in China in late 2019 and quickly spread around the entire world. As of April 23, 2020, in Italy there were 177,143 confirmed cases of COVID-19 and 23,188 related deaths, with a case fatality rate of about 13% [1]. Despite being affected by the choice to perform the diagnostic nasopharyngeal swab only on symptomatic patients, these data still reflect the aggressiveness of the virus. We know that age and comorbidities are predictors of a fatal outcome. Moreover, chronic immunosuppression in solid organ transplant recipients increases the risk of a potentially unfavorable outcome.
Social distancing and lockdown measures, including restricted access to health care facilities, are essential components of the health authorities’ response to the epidemic. However, follow-up of transplant patients should be maintained to enable identification of changes in clinical condition requiring examination and/or treatment during the lockdown period.
Data on COVID-19 infection in kidney transplant (KT) recipients are scarce and mostly concern single-case reports or small case series. In this article, we report the strategy implemented by our KT transplant center in Central Italy to maintain follow-up of KT recipients during the lockdown response to COVID-19. We also describe 2 cases of COVID-19 pneumonia identified using this strategy.
Materials and Methods
We report the experience of a KT center located in L’Aquila, Abruzzo. The center mainly follows patients from Abruzzo and Molise (Central Italy), where the cumulative incidence of COVID-19 is currently 203.57 and 92.93 per 100,000 inhabitants, respectively [1].
About 500 KT recipients are usually followed at our KT center clinic. Since March 17, 2020, the lockdown imposed the minimization of outpatient visits. From March 17 to April 23, 2020, we proposed that all patients previously booked for a visit participate in a system of phone interviews based on a preset questionnaire so as to not interrupt the follow-up and to enable the identification of significant clinical variations. The questionnaire (Table 1 ) included questions about epidemiologic COVID-19 risks, general health, respiratory, urinary and intestinal symptoms, and previous illnesses. We used the hospital e-mail service to obtain the results of any tests performed in outpatient settings and provided continuous on-call availability for subsequent contacts, if necessary. The aim was to identify any alteration regarding the graft function, any change in general health, and any suspicious symptom of coronavirus infection. This information was integrated with clinical data regarding comorbidities and therapies from the outpatient medical records. When indicated, patients were referred for in-hospital consulting or treatment.
Table 1.
Questionnaire Administered by Telephone to Kidney Transplant Patients
| Name | ||
| Date of birth | ||
| Transplant date | ||
| Behavior | Yes/no | Start date |
| Preventive social distancing rules | ||
| Contact with suspected or confirmed case of COVID-19 | ||
| Symptoms | Yes/no | Start date |
| Fever >37.5°C | ||
| Chills | ||
| Taking antipyretics | ||
| Taking antibiotics | ||
| Asthenia | ||
| Arthralgia/myalgia | ||
| Headache | ||
| Eyes discomfort | ||
| Sore throat | ||
| Cough | ||
| Difficulty in breathing | ||
| Anosmia | ||
| Dysgeusia | ||
| Vomiting | ||
| Diarrhea | ||
| Urinary disorders | ||
| Abdominal pain | ||
| Other symptoms | ||
| Comorbidity | Yes/no | |
| Hypertension | ||
| Cardiovascular disease | ||
| Diabetes mellitus | ||
| Chronic lung disease | ||
| Hyperlipidemia | ||
| Neoplastic diseases | ||
| Other | ||
Abbreviation: COVID-19, coronavirus disease 2019.
Results
A total of 143 KT recipients were contacted (Table 2 ), which included 94 men (65.7%) and 49 women (34.3%); mean age was 57 years (range of 22-78 years), and the mean time from transplant was 101 months (range of 3-374 months). Eight (5.6%) transplants were from living donors and 135 (94.4%) from deceased donors. Most patients had 1 or more comorbidities: 6.3% (9) did not experience any comorbidities, while 43.4% (62) had 1 comorbidity, 35% (50) had 2 comorbidities, and 15.4% (22) had 3 or more comorbidities that were considered to be related to an increased risk of major complications from SARS-CoV-2 (ie, hypertension, cardiovascular disease other than hypertension, diabetes mellitus, hyperlipidemia, chronic lung disease, and cancer). We received and analyzed 59 e-mails or faxes containing blood test results, radiologic examinations, or specialist advice.
Table 2.
Demographic Description of Kidney Transplant Recipients
| Total, N | 143 |
|---|---|
| Age, years (mean; range) | 57 (22-78) |
| Male, n (%) | 94 (65.7) |
| Female, n (%) | 49 (34.3) |
| Donor, n (%) | |
| Living | 8 (5.6) |
| Deceased | 135 (94.4) |
| Time from transplant, mean (range), mo | 101 (3-374) |
| Comorbidity, n (%) | |
| Hypertension | 128 (89.5) |
| Cardiovascular disease other than hypertension | 21 (14.7) |
| Diabetes mellitus | 20 (14) |
| Hyperlipidemia | 69 (48.3) |
| Cancer | 6 (4.2) |
| Chronic lung disease | 7 (4.9) |
All patients confirmed that they were abiding by the social distancing rules suggested by the Istituto Superiore di Sanità (the Italian Higher Institute of Health). Only 1 patient reported contact with a suspected case of COVID-19 (subsequently unconfirmed), with the need for 14 days of mandatory quarantine.
Hospital Consultations and Hospitalizations Unrelated to COVID-19
During the study, 28 patients were referred for in-hospital consultation because of the logistical impossibility of evaluating them elsewhere (unavailability of some peripheral hospitals) or because of the presence of relevant symptoms; of these, 3 patients were hospitalized. A patient admitted for dehydration and vomiting needed intravenous hydration and investigation, and during hospitalization, cytomegalovirus DNA (25,400 copies/mL) positivity and an intestinal parasitic infestation from Strongyloides stercoralis were found and treated, with subsequent relief of symptoms. A patient was admitted for deteriorating kidney function requiring a graft biopsy, which resulted in T-cell acute rejection (treated after the end of the study). A patient with fever, voiding disturbance, and deteriorating renal function was hospitalized for complicated urinary tract infection (UTI). All other patients were managed at home for mild urinary or respiratory diseases.
Altogether, we identified and remotely treated 6 patients with UTI and 5 patients with a mild infection of the upper respiratory tract who did not require hospitalization; in addition, 1 patient was referred to the hematologist and started on a new cycle of rituximab for increased Epstein-Barr virus (>30,000 copies/mL) viral load. The patient was followed-up for a recent Epstein-Barr virus–associated post-transplant lymphoproliferative disease.
COVID-19–related Hospitalizations
Two patients reporting fever and respiratory symptoms suggestive of COVID-19 infection were hospitalized. SARS-CoV-2 infection with pneumonia was confirmed during hospitalization in both of these patients.
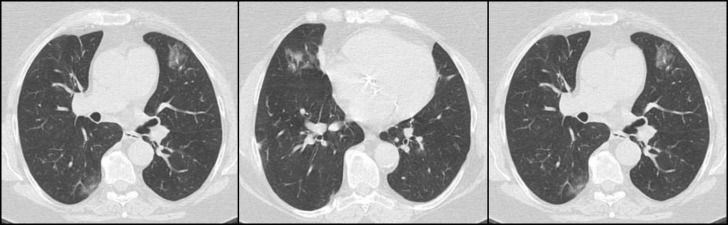
Case 1 was a 63-year-old woman who underwent KT from a deceased donor in 2004. She received triple maintenance therapy with cyclosporine, mycophenolate mofetil (MMF), and prednisone. She was a former smoker with a history of mild chronic lung disease, diabetes mellitus, hypertension, mixed dyslipidemia, and body weight excess. She reported exposure to a confirmed COVID-19 patient. On March 18, she was admitted to the infectious disease ward in L’Aquila (Abruzzo) because of the onset of diarrhea, dry cough, and difficulty breathing (7 days before admission), and fever (1 day before admission). MMF had been precautionarily suspended 2 days before admission. Physical examination revealed a body temperature of 37.5°C, normal blood pressure and pulse rate, and blood oxygen saturation of 96% on room air. Relevant blood tests showed lymphopenia (lymphocyte count 0.98 × 109 U/L), D-dimer 3900 ng/mL, C-reactive protein 7.85 mg/dL, procalcitonin 0.052 ng/mL, and ferritin 355 ng/mL. The arterial blood gas analysis showed hypoxemia, with a PaO2/FiO2 ratio of 314, and respiratory alkalosis. Chest computed tomography (CT) showed multiple bilateral patchy ground glass opacity, highly suggestive of bilateral interstitial pneumonia (Fig 1 ). Nine days after the onset of the symptoms, SARS-CoV-2 was identified by real-time reverse transcription-polymerase chain reaction on nasopharyngeal swab. The same day, cyclosporine was suspended and the corticosteroid increased to methylprednisolone 20 mg/d (maintenance dose prednisone 2.5 mg/d); an antiviral therapy with oral protease inhibitor darunavir 800 mg plus ritonavir 100 mg/d was started. Hydroxychloroquine was contraindicated because of an elevated QT interval and was not administered. Sodium enoxaparin was started. After 72 hours the patient’s respiratory symptoms deteriorated, with tachypnea, fever, and an oxygen saturation of 85% (PaO2/FiO2 ratio 223) on room air, which required flow oxygen supplementation (oxygen flow rate 4 L/min). The appearance of hyperglycemic and hypertensive peaks required a temporary reduction of the steroid dose. Two weeks after the onset of symptoms, the patient started to improve, with the gradual disappearance of fever and dry cough, while diarrhea disappeared soon thereafter. After 2 consecutive negative SARS-CoV-2 nasopharyngeal swab tests, the patient was discharged. Cyclosporine was reintroduced the same day, together with the oral prednisone; corticosteroid was gradually tapered to baseline levels. Kidney function remained stable during hospitalization, with a serum creatinine level of 1.5 mg/dL and an estimated glomerular filtration rate of 36 mL/min/1.73 m2.
Fig 1.
Chest computed tomography images of coronavirus disease 2019 (COVID-19) case 1, taken on March 18, 2020, showing multiple ground glass opacity in the bilateral lung on illness day 7.
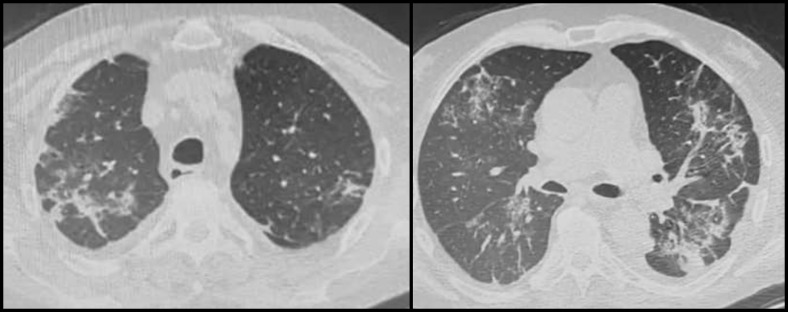
Case 2 was a 70-year-old man who underwent KT from a deceased donor in 2007, initially undergoing triple immunosuppressive maintenance therapy with MMF, cyclosporine, and prednisone, he was subsequently switched from MMF to everolimus (in 2014) because of native renal cell carcinoma. He was a former smoker with hypertensive heart disease, previous deep vein thrombosis, and dyslipidemia. On March 18, he was admitted to the internal medicine department in Chieti (Abruzzo) because of low-grade fever and cough, which had persisted for 10 days. Physical examination revealed a body temperature of 37.8°C, a normal blood pressure and pulse rate, and a blood oxygen saturation of 97% on room air. Relevant blood tests showed lymphopenia (lymphocyte count 0.88 × 109/L), D-dimer (4920 ng/mL), C-reactive protein (15.9 mg/dL), and ferritin (1000 ng/mL) elevation and procalcitonin within range. Chest CT scan was consistent with bilateral interstitial pneumonia (Fig 2 ). Two consecutive SARS-CoV-2 nasopharyngeal swab tests were negative. Cytomegalovirus DNA and Pneumocystis jirovecii tests were negative, too. The patient was nevertheless classified and treated as COVID-19 positive, in relation to the clinical and radiological findings. In view of an initially favorable clinical course, only everolimus was stopped. Hydroxychloroquine and sodium enoxaparin were initiated. Two weeks after admission, the patient exhibited deteriorating respiratory symptoms and hypoxia (PaO2/FiO2 ratio 220). He started an antiviral therapy with lopinavir/ritonavir 200 mg/50 mg twice daily and flow oxygen supplementation (oxygen flow rate 4 L/min). He stopped cyclosporine and increased the corticosteroid dose to methylprednisolone 40 mg/d (prednisone maintenance dose 5 mg/d). One week later, he gradually improved. On April 14, the SARS-CoV-2 nasopharyngeal swab test was positive. At the time of writing, the patient was breathing without the need for oxygen supplementation and, after a double negative SARS-CoV-2 nasopharyngeal swab test, cyclosporine and everolimus had been reintroduced (April 22) at half the initial dose. A transient decline in kidney function was reversed, reaching baseline values (serum creatinine level 1.2 mg/dL, estimated glomerular filtration rate 61 mL/min/1.73 m2).
Fig 2.
Chest computed tomography images of coronavirus disease 2019 (COVID-19) case 2, taken on March 20, 2020, showing multiple ground glass opacity and consolidation shadows in the bilateral lung on illness day 12
Discussion
Social distancing measures are essential components of the public health response to the COVID-19 pandemic. The objective of these measures is to decrease transmission by reducing the absolute number of infected people and by spreading cases over a longer time to relieve pressure on the health care system [2,3]. Nonetheless, these measures, especially the suspension of scheduled outpatient medical visits, can expose fragile populations to the failure to recognize the appearance of new conditions or the worsening of previous ones. This, in addition to the massive amount of information circulating with respect to the contagious and dangerous nature of the virus, especially in the hospital setting, can trigger fear of accessing medical care even in the presence of significant symptoms, including those related to COVID-19.
It has been reported in the literature that, aside from age, comorbidities such as cardiovascular diseases (10.5% mortality), diabetes mellitus (7.3% mortality), chronic respiratory diseases (6.3% mortality), high blood pressure (6% mortality), and cancer (5.6% mortality) represent the greatest risk factors for a negative evolution of COVID-19 [4].
Solid organ recipients have to be considered a fragile population because of the chronic immunosuppressive therapy necessary for the prevention of rejection, as well as the high prevalence of comorbidities. Specifically, chronic kidney disease is burdened by a high prevalence of cardiovascular diseases, which are only partially resolved or mitigated by transplantation. Moreover, a significant proportion of patients have diabetes mellitus as a cause of kidney disease or as a consequence of immunosuppressive therapy with calcineurin inhibitors (CNIs) [5]. Immunosuppressed patients also have a higher incidence of cancer than the general population, with particular reference to skin cancers and lymphoproliferative diseases.
Little is reported in the literature about specific surveillance programs of KT recipients during a quarantine or lockdown period. To limit the risk of medical care suspension during COVID-19 lockdown, we activated “crisis” hotlines to follow up and support our solid organ recipients. We employed phone interviews, hospital e-mail service, and fax to communicate with the patients and to obtain the results of tests performed in outpatient settings. This remote observation allowed us to avoid access to hospital care for most patients but also to identify the development of high-risk situations. Of the 143 patients followed in this manner, 28 required an in-hospital consultation for reasons other than related covid symptoms, of whom only 3 needed hospitalization. We have been able to help patients manage mild conditions, including mild UTI and airway diseases, at home without any hospital access.
Interestingly, we report 2 cases of suspected COVID-19 pneumonia that emerged during our phone interviews and were managed in 2 different regional hospitals through a close and constant collaboration with the transplant center. Although there are still limited data on COVID-19 in KT recipients, we could consider that, as a fragile population, clinical course, treatment, and evolution could differ from the general population [6]. When treating pneumonia caused by opportunistic virus or bacterial infection in KT recipients, a common strategy is the reduction/discontinuation of immunosuppressive therapy to allow the patient to restore an adequate immune response. In the absence of validated guidelines, our COVID-19 therapeutic approach was guided by our experience with other infectious diseases and the few specific data reported in the literature [7,8]. Timely supportive treatment, oxygen therapy as needed, and strategies for the prevention of secondary infection were administered. We reduced or interrupted MMF as a first approach in case of persistent fever and/or cough or diarrhea. Everolimus was withdrawn because of the reported additional risk of mammalian target of rapamycin inhibitor–induced lung disease [9,10]. In the presence of moderate-severe symptoms and fever above 38°C, or simply considering the fragility of the recipients, CNI therapy was stopped, and antiviral and hydroxychloroquine therapies (contraindicated in 1 case) were started. The reduction or suppression of CNI therapy was also necessary, because of the known interaction of this drug with antiretroviral drugs, to avoid the onset of renal toxicity in particular.
To date, no antiviral medication or vaccine of proven efficacy exists to treat or prevent the SARS-CoV-2 infection. Considering the structural similarity to SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), antiviral drugs already considered effective for these infections are being tested in the COVID-19 setting. Lopinavir/ritonavir was found to show in vitro activity against SARS-CoV and to inhibit the replication of MERS-CoV in vitro [11]. Some anecdotal reports suggest the safety and efficacy of lopinavir/ritonavir, encouraging its use in this pandemic. Darunavir/ritonavir is a potential alternative, based on the similar mechanism of action and a lower number of adverse events, such as diarrhea, that can increase the gastrointestinal symptoms often found in these patients. Protease inhibitors have the disadvantage of a significant drug-to-drug interaction also with immunosuppressant drugs.
Chloroquine/hydroxychloroquine demonstrated antiviral activity on SARS-CoV and SARS-CoV-2 in vitro [12]. Gao et al [13] have reported preliminary results on more than 100 patients treated in China that seem to demonstrate the superiority of chloroquine over control in the course of COVID-19–associated pneumonia [13]. On April 29 the Italian Medicines Agency states that, in this emergency phase, the use of hydroxychloroquine can be considered in COVID-19 patients of any severity, evaluating the risk/benefit ratio in each individual case. Attention should be paid to identifying patients with long QT syndrome, major arrhythmias, significant electrolyte disorders, and glucose-6-phosphate dehydrogenase deficiency. Attention is also needed to prevent drug interactions [14].
Intravenous corticosteroids have been used with the dual purpose of reducing the risk of acute rejection, in consideration of the suspension of other immunosuppressive treatment, and of exploiting their anti-inflammatory properties. Their extended use in an anti-inflammatory setting in all patients with COVID-19 pneumonia is controversial because of a potential slowing down of viral clearance and a secondary bacterial infection [15]. In any case, the histologic features in COVID-19 are similar to those in acute respiratory distress syndrome, in which corticosteroids are effective [16]. A recent retrospective cohort study in COVID-19 patients showed that early, low-dose, short-term administration of methylprednisolone was associated with better clinical outcomes in patients with severe COVID-19 pneumonia and should be considered before the occurrence of acute respiratory distress syndrome [17].
As reported by Terpos et al [18], coagulation disorders are frequent among COVID-19 patients, and D-dimer elevation is often associated with relatively poor prognosis. In addition to the common risk factors for venous thromboembolism in all acutely ill hospitalized patients, the possibility of endothelial cell activation/damage due to the SARS-CoV-2 may further increase the venous thromboembolism risk, so much so that pharmacologic thromboprophylaxis with low-molecular-weight heparin is recommended [18].
Both of our COVID-19 patients had several risk factors for an unfavorable outcome of the infection: chronic immunosuppression, advanced age, cardiovascular chronic disease, and, in 1 case, diabetes mellitus. Nevertheless, the clinical manifestations and the course of the disease were actually similar to those described in the general population, with a moderate-severe pulmonary involvement, but with only slight impairment of systemic conditions. Timely treatment, with modulation of immunosuppressive therapy and intravenous corticosteroids, together with antiviral therapy and supportive care, eventually facilitated a favorable evolution.
Overall, the number of SARS-CoV-2 infections in our group of patients was low. This was probably due to both a relatively low local incidence and the significant ability of transplant patients to accept the rules of social distancing: we instruct postsurgery transplant recipients to pay particular attention to their personal care and hygiene and to limit their social contacts because of the high level of immunosuppression needed soon after transplantation. COVID-19–related social distancing measures are therefore similar to precautions that transplant patients have already implemented, which significantly increases the likelihood of compliance with government regulations. As previously stated, transplant patients are potentially at risk of refusal to undergo medical examination even when urgently needed. In accordance with this, our patients favorably accepted the phone interviews solution, aimed at minimizing the need for access to health care facilities by identifying only the cases of real need.
As stated by Michaels et al [19], past outbreaks of similar viruses (SARS, MERS) revealed the need to establish plans for the evaluation of solid organ recipients if they become infected and require an evaluation relating to the emerging pathogen, as well as plans for recipients requiring evaluation for other reasons. Moreover, they pointed out the need to communicate with transplant recipients to offer reassurance and provide updated information and recommendations [19]. Consistent with this, we suggest implementing remote surveillance programs in fragile populations, such as transplant recipients, with the help of any available technology (e-mail, phone calls, video calls) as soon as possible and offering medical consulting and logistic support as needed during the COVID-19 pandemic.
Acknowledgments
We thank Joanne Dalton, an independent medical writer, who provided English language editing and journal styling prior to submission on behalf of Springer Healthcare. This assistance was funded by Novartis Farma SpA.
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) situation report ‒ 92. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200421-sitrep-92-COVID-19.pdf?sfvrsn=38e6b06d_4 [accessed 23.04.20]
- 2.Fong M.W., Gao H., Wong J.Y., Xiao J., Shiu E., Ryu Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures. Emerg Infect Dis. 2020;26(5):976–984. doi: 10.3201/eid2605.190995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323:1–9. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia. Italy. Kidney Int Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinsteuber A., Halleck F., Khadzhynov D., Staeck A., Lehner L., Duerrr M. Impact of pre-existing comorbidities on long-term outcomes in kidney transplant recipients. Transplant Proc. 2018;50:3232–3241. doi: 10.1016/j.transproceed.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L., Xu X., Ma K., Yang J., Guan H., Chen S. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Yin Q., Shi H., Du D., Chang S., Ni L. A familial cluster, including a kidney transplant recipient of coronavirus disease 2019 (COVID-19) in Wuhan, China. Am J Transplant. 2020;20:1869–1874. doi: 10.1111/ajt.15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López V., Vázquez T., Alanso-Titos J., Cabello M., Alonso A., Beneyto I. Recomendaciones en el manejo de la pandemia por coronavirus SARS-CoV-2 (COVID-19) in kidney transplant patients. Nefrologia. 2020;40:265–271. doi: 10.1016/j.nefro.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventura-Aguiar P., Campistol J.M., Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin Drug Saf. 2016;15:303–319. doi: 10.1517/14740338.2016.1132698. [DOI] [PubMed] [Google Scholar]
- 10.Guillen E., Pineiro G.J., Revuelta I., Rodriguez D., Bodro M., Moreno A. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-n-CoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 14.Agenzia Italiana del Farmaco Idrossiclorochina nella terapia dei pazeinti adulti con COVID-19. 2020. https://www.aifa.gov.it/documents/20142/1123276/idrossiclorochina_29.04.2020.pdf/386d6ea3-c79b-6437-f457-23d33df74256 [accessed 23.04.20]
- 15.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. 2020. https://apps.who.int/iris/handle/10665/330893 [accessed 23.04.20]
- 16.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., JiangW, He Q., Wang C., Wang B., Zho P. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5:57. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M. Hematological findings and complications of COVID-19. Am J Hematol. 2020;94:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaels M.G., La Hoz R.M., Danziger-Isakov L.A., Blumberg E.A., Kumar D., Green M. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;20:1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]