Abstract
In time of SARS-Cov2 pandemic, neurologists need to be vigilant for cerebrovascular complications of Covid-19. We present a case of bilateral occipito-temporal infarction revealed by a sudden cortical blindness with haemorrhagic transformation after intravenous thrombolysis in a diabetic patient infected by Covid-19. Differential diagnoses are discussed in front of this unusual presentation and evolution.
Keywords: Infarction, MR perfusion, COVID-19, Visual loss, SARS-Cov2
Case presentation
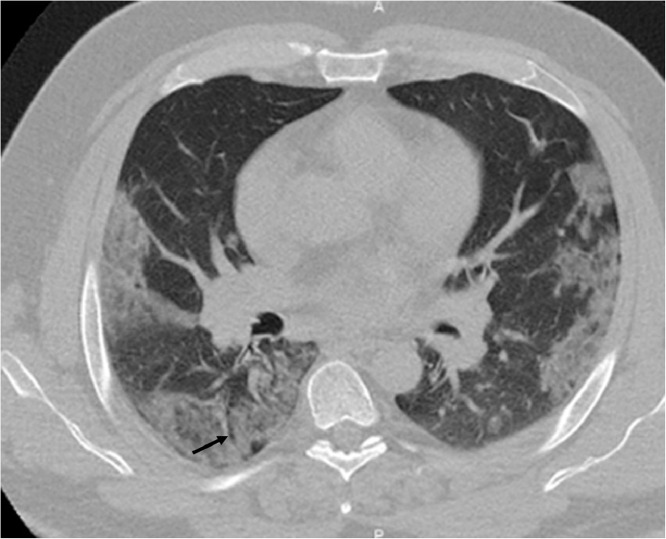
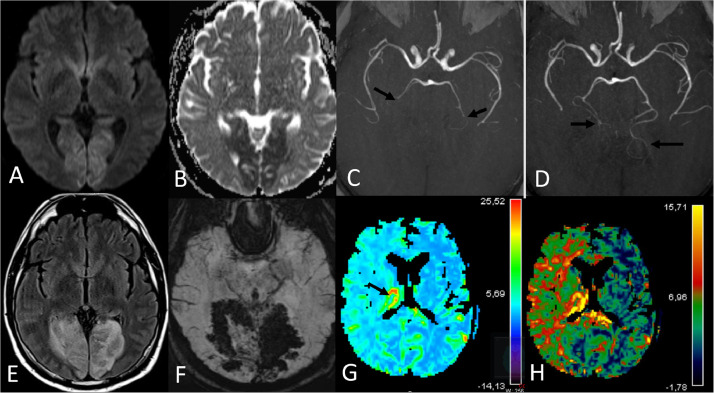
A 51-year-old-man presented with one-week history of cough, dysgeusia and diarrhea. The patient complained of moderate headache without fever. The patient had a history of diabetes mellitus, hypertension and obesity. Blood investigation showed a lymphocytopenia (1.08 giga/L), an increased fibrinogen (5.1 g/L), ferritin (1190 µg/l), creatin-kinase (749 UI/L), C-reactive protein (71 g/L), ASAT (54 UI/L) and glycated hemoglobin (9.1%) concentrations. Prothrombin Ratio was low (66%). Antiphospholipid, platelets and partial thromboplastin time were normal. The RT-PCR for SARS-Cov-2 using a nasopharyngeal swab was positive. A chest scan showed bilateral ground glass opacities concerning more than 50% of the parenchymal lung (Fig. 1 ). Six days after arrival and 30 min after the fourth injection of remdesivir (loading dose: 200 mg IV, 100 mg IV per day thereafter), he presented an abrupt cortical blindness and disorientation (NIHSS score: 4). An atrial fibrillation (AF) was recorded. A first brain MRI performed one hour after clinical onset showed bilateral and asymmetric acute occipito-temporal infarction without visibility of the P3 segments of the posterior cerebral arteries (PCA) (Fig. 2 A to C). Fluid-attenuated inversion recovery (FLAIR), T2*, MR venography and MR angiography of the supra-aortic trunks were normal. No pathological enhancement in leptomeningeal spaces was observed. Alteplase was injected 128 min after symptom onset. The following morning, blindness was unchanged and anterograde memory disorders with anosognosia were noticed. The 24 h control multimodality MRI showed a haemorrhagic transformation of the previous lesions (Fig. 2E-F). Dynamic susceptibility-weighted contrast-enhanced magnetic resonance perfusion imaging (DSC-MRI perfusion) showed an increase of cerebral blood volume (CBV) and flow (CBF) in the right thalamus and an increase of the mean transit time (MTT) and CBV in the right hemisphere (Fig. 1H). Distal segments of the PCA were permeable (Fig. 2D). Nine hours later, the patient died due to a rapid respiratory breakdown, without neurological worsening.
Fig. 1.
Axial CT scanner shows focal subpleural ground-glass opacities in the left and right lobes. The right lower lobe lesion is accompanied by air bronchogram (arrow).
Fig. 2.
First MRI (A to C). Diffusion-Weighted MRI (DWI) shows a high signal on b 1000 (A) with low Apparent Diffusion Coefficient (ADC) in the occipital lobes (B). Time of Flight (TOF) (C) shows P3 segments of posterior cerebral arteries (PCA) bilateral occlusion (arrows) (C). Second MRI (D to F). TOF shows better visualisation of distal segments of bilateral PCA (arrows) (D). Fluid–attenuated inversion recovery (FLAIR) shows a hypersignal in the initial ischemic lesions (initial FLAIR was normal) (E). Susceptibility-Weighted imaging (SWI) shows hypo-intensity (haemorrhage) concerning the totality of the ischemic lesion (F). MRI perfusion shows an increase cerebral blood volume (CBV) in the right thalamus (arrow) (G) and an increase of MTT in the right hemisphere (H).
Discussion
This bilateral cerebral posterior stroke may be secondary to an embolic event (AF). Stroke could also be explained by the state of hypercoagulability induced by SARS-Cov-2 infection1. Severe patients are more likely to have neurologic symptoms2 and bilateral frontotemporal hypoperfusion3 has been reported. In our case, MTT and CBV were increased in the right hemisphere which may reflect reduced cerebral perfusion pressure. The increase value of the CBF in the right thalamus may correspond to a post-recanalization hyperperfusion4.
Other stroke mechanisms can be suggested. Infection may have induced cerebral vasculitis, explaining the stroke and the perfusion's anomalies. Severe reversible cerebral vasoconstriction syndrome (RCVS) cases with cerebral infarction and intracranial haemorrhage have been reported but the absence of thunderclap headache is unusual in RCVS5. The absence of rapid increase in blood pressure and the presence of an initial cytotoxic oedema instead of vasogenic is less in favour of Posterior Reversible Encephalopathy Syndrome (PRES)6. An adverse effect of remdesivir is also to be discussed, but no neurological adverse effect potentially related to remdesivir have been reported7. Finally, the absence of thalamus involvement makes the diagnosis of acute necrotizing encephalitis unlikely8.
In conclusion, the origin of the stroke is probably multifactorial: the cytokine storm syndrome and hypercoagulability may have induced blood flow dysregulation, associated with an embolic event that may finally induce arterial thrombosis. A cerebral artery vasculitis or a RVCS are not excluded. This unusual case confirms the increased risk of thrombotic events in SARS-Cov2 infected patients.
Declaration of Competing Interest
The authors report no disclosures. Informed consent for publication has been signed by the wife of the patient.
References
- 1.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. Epub. 2020 Apr 3 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan. China. JAMA Neurol Epub. 2020 Apr 10 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J, Kremer S, Merdji H. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. Epub. 2020 Apr 15 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidwell CS, Saver JL, Mattiello J. Diffusion-perfusion MRI characterization of post-recanalization hyperperfusion in humans. Neurology. 2001;57:2015–2021. doi: 10.1212/wnl.57.11.2015. [DOI] [PubMed] [Google Scholar]
- 5.Yamada H, Kikuchi R, Nakamura A, Miyazaki H. Severe reversible cerebral vasoconstriction syndrome with large posterior cerebral infarction. J Stroke Cerebrovasc Dis. 2018;27:3043–3045. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Pilato F, Distefano M, Calandrelli R. Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome: clinical and radiological considerations. Front Neurol. 2020;11:34. doi: 10.3389/fneur.2020.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulangu S, Dodd LE, Davey RT. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. radiology. Epub. 2020 Mar 31 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]