Summary
The elevated circulating levels of cytokines associated with a variety of infectious and immune-mediated conditions are frequently termed a cytokine storm. Here, we explain the protective functions of cytokines in “ideal” responses; the multi-factorial origins that can drive these responses to become pathological; and how this ultimately leads to vascular damage, immunopathology, and worsening clinical outcomes.
The elevated circulating levels of cytokines associated with a variety of infectious and immune-mediated conditions are frequently termed a cytokine storm. Here, we explain the protective functions of cytokines in “ideal” responses; the multi-factorial origins that can drive these responses to become pathological; and how this ultimately leads to vascular damage, immunopathology, and worsening clinical outcomes.
Main Text
Defining Cytokine Storm: Basic Concepts in the Cytokine Response
Recent reports of elevated serum cytokine levels associated with coronavirus disease 2019 (COVID-19) have raised questions about the relationship between cytokine storms and the severe complications associated with this infection. The goal of this primer is to provide an introduction into the current concepts, largely through the lens of sepsis (Hotchkiss et al., 2016), that help understand the underlying causes of cytokine storms and how they interact with coagulation and vascular health and to briefly discuss how this may help understand the pathophysiology of COVID-19. Historically, the recognition of sepsis as a clinical condition was associated with the presence of bacterial infections in the blood. This definition has expanded to include all infections or suspected infections that result in immune dysregulation characterized by systemic inflammation and remote organ injury. We will discuss how elevated levels of inflammatory cytokines (most notably interleukin [IL]-1, IL-2, IL-6, granulocyte-macrophage colony-stimulating factor [GM-CSF], interferon [IFN]γ, and tumor necrosis factor [TNF]) interact with the complement and coagulation systems to induce disseminated intravascular coagulation (DIC), respiratory failure (acute respiratory distress syndrome [ARDS]), hemophagocytic lymphohistiocytosis (HLH; histiocyte is another term for macrophage), and multi-organ failure. Finally, given the current COVID-19 pandemic, the potential contribution of different cytokines to viral sepsis is a topic of interest that may provide an opportunity for interventions.
One conceptual framework for how the immune system functions is the idea that the innate ability to recognize an invading organism provides signals that condition cells of the immune system to respond appropriately. This is associated with an amplification of the protective response that is proportionate to pathogen burden but is also influenced by regulatory mechanisms that limit immune hyperactivity. As infection is controlled, there is typically an inflection point associated with entry into a phase of resolution and repair that allows a return to homeostasis (Figure 1 A). Cytokines have a direct role in the activation of anti-microbial effector functions but also provide the regulatory signals that specify, amplify, and resolve the immune response. A cardinal feature of these secreted proteins is that they have short half-lives, which will typically restrict communication to those cell types within lymphoid tissues and at sites of inflammation. At high-enough levels, cytokines can also have systemic activities, and the colony stimulating factors produced at sites of microbial invasion can promote emergency granulopoiesis in the bone marrow associated with increased production and mobilization of neutrophils and monocytes. For those infections with systemic involvement, the presence of increased levels of cytokines will impact a wide range of physiological processes. Consequently, under some circumstances, enhanced innate recognition, elevated T cell responses, or a failure to resolve can manifest as levels of cytokines in the circulation that exceed normal thresholds and that result in collateral damage. There are many experimental and clinical conditions that illustrate this state (Figure 1), and the terms cytokine storm, cytokine release syndrome (CRS), or hypercytokinaemia have been used to describe a variety of conditions that have diverse etiologies and outcomes.
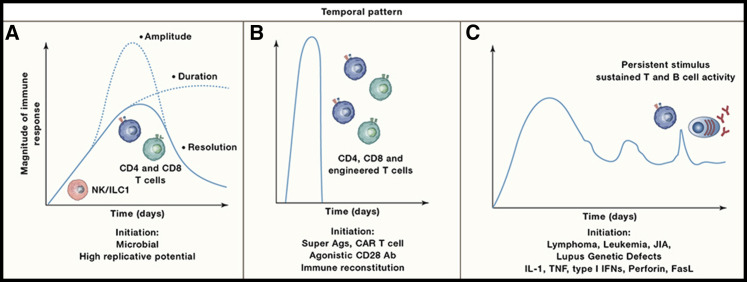
Figure 1.
Kinetics of Cytokine Storms
Cytokine storms have many different underlying causes that can manifest with different kinetics.
(A) The solid line depicts the natural arc of an immune response to infection over a period of days to weeks that transitions to a resolution phase as a pathogen is controlled. For microorganisms with a high replicative potential, changes in the magnitude and duration of the immune response can result in systemic immune pathology. The two dotted lines illustrate different arcs associated with a cytokine storm through either an increased amplitude or a failure to enter the resolution phase.
(B) The rapid and widespread engagement of adaptive responses by bacterially derived superantigens or therapeutic interventions can lead to a rapid surge in immune activity (hour-days) associated with supra-physiological levels of circulating cytokines.
(C) Certain cancers that have a systemic component can lead to sustained (weeks to months) responses associated with elevated cytokine production. Likewise, chronic autoimmune diseases such as juvenile idiopathic arthritis (JIA) and lupus can have flares associated with increased cytokine production. There are also genetic defects closely linked to aberrant cytokine production, enhanced signaling, or a failure to fully control certain viral infections, which can cause periodic spikes in immune hyperactivity.
Guiding Principles
I. Inflammation Is Protective
Under ideal circumstances, the immune system will provide a proportionate and appropriate response that mediates resistance to invading microorganisms and allows the host to survive infection. For many infections, the coordinated production of sustained circulating levels of proinflammatory cytokines is a natural consequence of an appropriate innate and adaptive response critical for pathogen control.
II. Protective Inflammation Can Be Pathological
Many of the cytokines associated with different forms of sepsis can cause fever, induce cell death, and impact vascular physiology and coagulation. As a result, they have the potential to cause significant and widespread collateral tissue damage. Thus, the benefits of sustained levels of circulating cytokines that promote microbial control are balanced by the need to limit immune-mediated damage to the host. From an experimental and clinical perspective, perhaps the most easily recognized form of a cytokine storm is the presence of supra-normal levels of cytokines associated with tissue damage beyond what could be attributed to the pathogen (Figure 1A). There are other forms of cytokines storms that vary in magnitude and kinetics and that do not necessarily have an underlying infectious cause (described below; see Figures 1B and 1C). Nevertheless, in any of these circumstances, the ability to neutralize cytokines to mitigate tissue damage and allow disease resolution highlights that these high levels are not just markers of inflammation but also key contributors to disease.
III. The Immune Response Is Not Unfettered
One of the major themes in immunology over the last 30 years is the increased emphasis on the checks and balances that limit every facet of innate and adaptive cell-mediated and humoral immunity. These include specialized regulatory cell types imbued with anti-inflammatory properties as well as multiple mechanisms that mitigate the deleterious effects of cytokines. Since the overproduction of cytokines represents a major challenge to host health, the presence of these regulatory pathways suggests that they may result from evolutionary pressure to balance pathogen control and tolerance of collateral damage in order to survive infection. For example, the receptor-ligand systems for cytokines are characterized by the presence of soluble receptors, receptor antagonists, and decoy receptors expressed at high levels in the tissues and serum. These antagonists can buffer surges in cytokine activity and limit immunopathology. In addition, IL-10 exemplifies the ability of cytokines to antagonize inflammatory cell populations and prevent immune hyperactivity. Nevertheless, these protective mechanisms have their limits, and excessive or sustained production of cytokines can override these regulatory mechanisms.
IV. The Host-Microbe Interaction Matters
The successful response to an infection is invariably accompanied by some level of inflammation, and this is apparent by the elevated production of cytokines and/or clinical symptoms. Ideally, the magnitude of the response is related to pathogen burden and is restricted to the organ systems that are involved. Microbial pathogenesis is a critical determinant of these events, whereby variables such as the replicative potential of the microorganism, cell and tissue tropism, production of toxins, and immune evasion strategies will influence the duration and magnitude of the immune response, whether there is local versus systemic involvement, and the potential for sequelae. These principles are of obvious relevance to many primary microbial challenges, but all of humanity is persistently infected with a range of potentially lethal pathogens, which are generally restrained in the immune competent host but can cause sepsis in the immunocompromised host. This reality illustrates the concept that hosts need to be able to tolerate the presence of infections that can be life threatening with the production of cytokines that can be damaging. The idea that resilience in the face of infection is a balance between protective and pathological responses is not new and is discussed here (Ayres, 2020).
Clinical Impact
Before describing the diverse events that can drive the innate and adaptive responses that are the basis of a cytokine storm, it is helpful to consider the clinical consequences of high levels of cytokines (Figure 2 ). These can converge on profound changes to the target tissues and the physiology of the host, where not a single organ is spared and host survival is threatened. A hallmark of a cytokine storm is persistent fever and non-specific constitutional symptoms (weight loss, joint and muscle pain, fatigue, headache). Progressive widespread systemic inflammation leads to a loss of vascular tone that is manifested as a drop in blood pressure, vasodilatory shock, and progressive organ failure. In this context, respiratory failure is the most prominent but will also impact the heart, central nervous system, and kidneys. Some of the clinical manifestations have been associated to specific cytokines: IL-6 and TNF are linked with fever and with constitutional symptoms. Capillary leak syndrome, which refers to an increase in capillary permeability to proteins and is manifested clinically by hypotension, edema, acute respiratory failure, and kidney injury is thought to be driven by IL-2. In patients treated with IL-2 or a monoclonal antibody (OKT3) that targets the CD3 complex on T cells, thereby inducing IL-2, this can be a significant clinical problem. Abnormalities revealed by clinical laboratory assays are often driven by the underlying driver of CRS. In patients with concomitant HLH, pancytopenia (reduced red blood cells [RBCs], white blood cells [WBCs], and platelets), elevated triglycerides and ferritin predominate with accompanying hepatosplenomegaly. Patients with concurrent tumor lysis exhibit elevated uric acid, LDH, lactate, and acute kidney injury. Hemostatic imbalance manifest by DIC and thrombocytopenia (reduced platelet numbers) can be observed in almost all clinical syndromes associated with CRS. The levels of these endogenous molecules are important prognostic indicators of secondary complications and organ damage: elevated triglycerides may cause pancreatitis, uric acid can induce kidney failure, and spontaneous intracranial hemorrhages are observed with profound thrombocytopenia. Thus, treating the underlying driver of CRS is a cornerstone of management to avoid further organ injury.
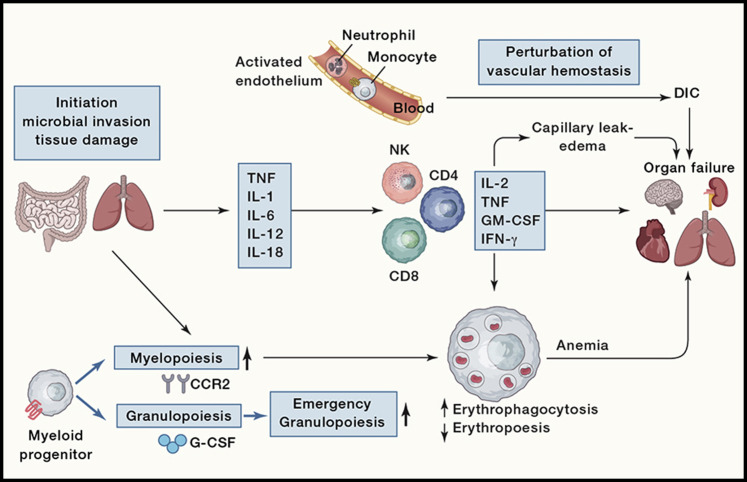
Figure 2.
Pathophysiology of a Cytokine Storm
An infectious or non-infectious stimulus in barrier sites such as the gut or lungs that leads to tissue damage initiates a complex series of events. In circumstances that leads to vascular damage, the coagulation system is critical for tissue repair, but this can progress to the development of DIC. The early response to microbial invasion or tissue damage is characterized by the innate production of cytokines and the induction of emergency granulopoiesis that leads to the mobilization of neutrophils and monocytes. These events will engage and amplify NK and T cell production of proinflammatory cytokines. These can promote capillary leak syndrome and thrombus formation that can progress to DIC. High circulating levels of these cytokines can cause cell death and tissue damage, while their ability to activate macrophages can lead to erythro-phagocytosis and anemia. The combination of anemia, alterations in vascular hemostasis, and cytokine-mediated damage can result in multi-organ failure.
Innate and Adaptive Immune Compartments Contribute to Cytokine Storms
The increased production (myelopoiesis) and mobilization of monocyte and neutrophil populations from the bone marrow is a response to many acute infections and cytokines (Figure 2). These populations are typically considered proinflammatory, are imbued with a range of antimicrobial activities, and are recruited to sites of inflammation where they can respond to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) by producing IL-1, IL-6, IL-12, and TNF (Table 1 ). In many experimental models, such as the injection of high doses of lipopolysaccharides (LPS) to mimic Gram-negative infections or cecal ligation and puncture to model bacterial peritonitis, these innate responses are dominated by neutrophils and monocytes and are sufficient to induce a cytokine storm that eschews the normal kinetics of an immune response (Figure 1). There are also examples where primary genetic defects associated with inappropriate activation of the inflammasome can lead to sustained production of IL-1 by macrophages that results in a relapsing-remitting disease associated with periodic fevers (Figure 1C). A major function of macrophages in the red pulp of the spleen is to remove senescent or damaged RBCs (erythrophagocytosis), and for some pathogens or erythrocytes, this process is critical for the clearance of infected cells. However, the sustained production of IFNγ and TNF can lead to macrophage activation syndrome associated with HLH, which contributes to the anemia that is characteristic of sepsis and almost all systemic infections (Al-Samkari and Berliner, 2018).
Table 1.
Cytokines Associated with Systemic Disease
| Cytokine (Sources) | Regulation | Impact |
|---|---|---|
| IL-1 (macrophages, DCs, endothelium) | Produced in response to microbial stimuli and released by dying cells. The IL-1RA blocks IL-1 binding to its receptor. | Fever, emergency hematopoiesis, monocyte, and neutrophil activation. Periodic fevers and systemic inflammation linked to genetic disorders associated with IL-1 production can be treated with IL-1RA. |
| IL-18 (epithelia, neurons, myocytes) |
The IL-18 binding protein is an inducible negative regulator of IL-18 that is induced by IFNγ and limits IL-18 activity. | NK and T cell activation and production of IFNγ. Genetic defects in IL-18bp are associated with elevated NK activity. |
| IL-6 (macrophages, myocytes) | Produced in response to microbial stimuli. The low-affinity receptor chain gp130 is expressed by immune and non-immune cells. High levels of soluble gp130 in serum and tissues may buffer the effects of IL-6. | Fever, granulopoiesis, hematopoiesis, and the accumulation of neutrophils at sites of infection or trauma. Notable player in Casteleman’s disease and juvenile idiopathic arthritis and association with fever. |
| IL-12 (macrophages, DCs, B cells) | Composed of two subunits, IL-12p40 and IL-12-p35, this heterodimer is produced in response to microbial stimuli. IL-12-p40 homodimers are 10- to 100-fold in excess of the heterodimer and antagonize IL-12 signaling. | Drives NK and T cell production of IFNγ, which promotes cell-mediated immunity to intracellular microorganisms. Toxicity noted in clinical trials for cancer. |
| IL-2 (CD4+ T cells) | Production governed by TCR activation. Complex trimeric cytokine receptor but increased levels of the IL-2Rα chain in the circulation can act as a decoy receptor. | Growth factor for regulatory T cells and effector T cell populations and can promote NK cell activities. High doses of IL-2 result in flu-like symptoms and capillary leak syndrome. |
| IFNγ (ILC, T cells) | Produced in response to TCR and cytokine signals. The heterodimeric IFNγR is present on most cells, and soluble levels of the IFNγR are associated with inflammation and may act as a decoy receptor. | Promotes accessory cell functions that amplify adaptive response. Prominent role in the induction of macrophage anti-microbial activities but also leads to erythrophagocytosis. |
| TNF (macrophages, DCs, endothelium, lymphocytes, myocytes) | TNFR-I and -II are widely expressed and increased soluble levels are associated with disease. A fusion protein of soluble TNF-R2 is used to block TNF in chronic inflammation. | Fever and wasting. TNF can also mediate cell death and its effects on the vasculature intersects with coagulation and capillary leak syndrome. Genetic defects in TNFR-I associated with recurrent fevers. |
IL, interleukin; TCR, T cell receptor; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor; DC, dendritic cell; ILC, innate lymphoid cell; NK, natural killer.
While the ability to engage innate responses can be sufficient for the development of cytokine storms, the activation of T cells and their ability to produce high amounts of effector cytokines (IL-2, IFNγ, and TNF) are also important. Two of the best examples that illustrate how the rapid engagement of T cells can result in cytokine storms come from the development of therapies to enhance T cell function for the treatment of cancer. The clinical trial of an agonistic antibody to the costimulatory molecule CD28 present on T cells provides a notable illustration of how the simultaneous activation of a large number of T cells results in the rapid induction of a cytokine storm (Suntharalingam et al., 2006). The authors description of these events is sobering: “Within 90 minutes after receiving a single intravenous dose of the drug, all six volunteers had a systemic inflammatory response characterized by a rapid induction of TNF-α followed by IL-2, IL-6, and IL-10 and IFNγ accompanied by headache, myalgias, nausea, diarrhea, erythema, vasodilatation, and hypotension that progressed to pulmonary infiltrates and lung injury, renal failure, and DIC. Reduced lymphocytes and monocyte counts occurred that correlated with the peak cytokine levels.” A frequent observation associated with experimental and clinical examples of cytokine storms is a transient reduction in peripheral blood T cell counts. This has been perceived as an indicator of immunosuppression and attributed to death of immune cells, but the sequestration of WBCs in affected tissues, which includes adherence to vascular endothelium, may account for this apparent loss of peripheral WBCs. Another example occurred with patients with acute lymphoid leukemia, in which this B cell lymphoma expressed CD19. In these two patients, their T cells were genetically modified to express a chimeric antigen receptor (CAR) that recognized CD19 and that would allow these modified T cells to eliminate the tumor cells. After infusion of the CAR T cells, there was a rapid onset of a CRS characterized by 100- to 1,000-fold increase in levels of IFNγ and IL-6 that were temporally correlated with systemic inflammation and fever and evolved into the macrophage activation syndrome (Grupp et al., 2013). These examples, from therapeutic interventions, highlight that the rapid, widespread activation of T cells can bypass many of the natural negative cell-extrinsic checkpoints and have a systemic impact on patient physiology.
There are other more natural examples where microbial context is important to understand the basis for T cell hyperactivation. This is illustrated by the ability of bacterial toxins, such as superantigens associated with Streptococcus and Staphylococcus aureus, to crosslink the major histocompatibility complex (MHC) and TCR, thus leading to polyclonal activation of T cells, high levels of cytokine secretion, and the development of toxic shock syndrome. Another example is the mosquito-transmitted Dengue virus, in which secondary challenge with a heterologous virus can result in Dengue hemorrhagic fever characterized by high levels of cytokines, alterations in vascular permeability, and DIC. Surprisingly, analysis of postmortem tissues highlighted the presence of edema in the lungs but relative absence of tissue inflammation (Srikiatkhachorn et al., 2017). This lack of overt cellular infiltration can be disconcerting to cellular immunologists trying to understand how cytokines mediate tissue damage. The last major influenza pandemic in 2009 was caused by the H1N1 strain and in some patients was associated with the presence of coagulopathies and haemophagocytosis. Analysis of a cohort of fatal H1N1 infections highlighted that while these patients did not have any overt preexisting immune deficiencies, many had gene mutations that had previously been associated with the development of HLH, indicating their possible role as risk factors for mortality (Schulert et al., 2016). Similarly, in patients that lack perforin, the inability to clear the herpesviruses Epstein-Barr virus (EBV) and cytomegalovirus (CMV) results in sustained T cell production of IFNγ and TNF that results in macrophage activation and HLH. These types of more persistent situations where the underlying causes may not be resolved are not restricted to infectious settings but can manifest in other conditions. These include malignancies and autoimmune diseases as well as in the context of transplantation (Figure 1C), and in these examples, an overt cytokine storm is not apparent, but flares in disease can result in CRS.
The Vascular Compartment, Coagulation, and Complement
As discussed above, some of the prominent clinical features of cytokine storms are characterized by DIC, capillary leak syndrome, and loss of blood pressure, which indicates the crosstalk between cytokines and hemostasis. In the absence of systemic inflammation, the vascular endothelium employs numerous mechanisms to maintain barrier function and prevent aberrant coagulation. A common feature of many systemic infections, however, is endothelial cell death (a consequence of cytolytic infection or immune-mediated pathways), which gives rise to vascular damage and the development of capillary leak syndrome. As endothelial cells would be exposed to circulating cytokines and other immune mediators, there are multiple systems to maintain their quiescence under inflammatory duress. An evolutionary conserved component of the hemostatic response to tissue damage and to systemic infections includes the development of coagulation to prevent blood loss. As part of this process, fibrin deposition and the recruitment of platelets can form clots to seal areas of trauma. In many settings, elevated systemic cytokine levels are associated with a transient drop in platelet numbers in the circulation, likely reflecting their consumption. Although there is continuous basal production of neutrophils from the bone marrow, systemic cytokines can initiate emergency granulopoesis and increased recruitment of these cells into circulation. There, inflammatory mediators will promote neutrophil release of nuclear DNA to form neutrophil extracellular traps (NETs) which can snare pathogens but also contribute to thrombi formation. This process, termed immuno-thrombosis (Engelmann and Massberg, 2013), can also amplify the production of cytokines and is exemplified by links of thrombin with inflammasome activation and production of IL-1. While these events are essentially host protective, systemic inflammation can lead to widespread thrombus formation associated with vascular occlusion, tissue damage, DIC, and death of the host. If these events are sustained, one consequence is the depletion of clotting factors, which renders patients more susceptible to catastrophic bleeding into critical spaces like the brain or thorax. Thus, thrombi formation has an important role in host protection but needs to be carefully controlled to prevent aberrant pathology.
Another evolutionary conserved line of defense against invading pathogens that intersects with cytokine biology and coagulation is the complement system. This arm of the innate response is composed of soluble proteins that can recognize and lyse pathogens but can also act to amplify cellular responses. The complex crosstalk between cytokines and the complement system is illustrated by the ability of cytokines to enhance production of components of the complement cascade, which in turn can enhance or inhibit cytokine production. Although complement-mediated opsonization and formation of lytic complexes are critical processes necessary for pathogen elimination, each of these effector functions can be injurious to bystander host cells. Given the importance of the endothelium in barrier function, vascular tone, and hemostasis, there are several safeguards, such as surface-bound and soluble complement regulatory proteins, to prevent excessive complement-mediated injury to the normally quiescent endothelium. Failure of these safeguards or excessive complement activation result in endothelial injury that manifest as dysregulated coagulation and hemostasis, loss of barrier function, and vascular tone. The vascular endothelium of the lung and kidneys are recognized as particularly vulnerable sites to complement-mediated injury and the clinical implications of widespread endothelial dysfunction, as discussed above.
Calming the Storm
Given the potential for cytokines to cause tissue damage, there is a need to mitigate the effects of high levels of cytokines and promote the transition into the resolution phase of an immune response. There are numerous natural receptor and cytokine antagonists, such as the IL-1 receptor antagonist (IL-1RA), that are a feature of cytokine signaling systems that buffer systemic off-target effects and restrict immune activities to local sites of cytokine release. Other cytokines and regulatory pathways can act more directly to limit the magnitude of the immune response. For example, IL-10 is produced by many cell types (subsets of monocytes as well as natural killer [NK], T and B cells) and inhibits the ability of macrophage and dendritic cell (DC) populations to produce TNF, IL-1, IL-6, and IL-12 and decreases the ability of these cells to present antigen and provide costimulation. Thus, IL-10 is a key negative regulator of the accessory functions required for T cell mediated responses, and there are numerous examples where infection of mice that lack IL-10 is characterized by elevated, sustained circulating levels of IL-12, TNF, and IFNγ (i.e., a bona fide cytokine storm) (Couper et al., 2008). Paradoxically, increased circulating levels of IL-10 are characteristic of the cytokine storms in humans, and the assumption is that these increased levels reflect the presence of a negative feedback loop to control the magnitude of the response and to help with the resolution and repair phase of the response.
The existence of immune-regulatory networks to limit aberrant cytokine responses is critical to survive many infectious challenges, but these do have a cost. Many of these pathways block the development of sterile immunity to infection and promote pathogen persistence. In addition, many patients who survive sepsis have long-term sequela that include profound immunosuppression and susceptibility to secondary infections. There is a poor understanding of how this is mediated, and one explanation is that the anti-inflammatory regulatory networks do not returns to pre-crisis levels of function and instead operate at a heightened state. Alternatively, the systemic action of cytokines can result in remarkable changes in the cellular composition and organization of lymphoid structures, such as the bone marrow, thymus, and spleen. Whether this disruption or loss of immunological infrastructure contributes to the long-term dysregulation is not understood.
COVID-19 and Cytokine Storms
The reports of elevated serum cytokines (IFNγ, IL-6, and GM-CSF) associated with the coronaviruses severe acute respiratory syndrome coronavirus (SARS-CoV-1), and Middle East respiratory syndrome coronavirus (MERS-CoV) combined with ARDS have led to relevant questions about whether the subset of patients that develop life-threatening complications are a result of a cytokine storm (Min et al., 2016; Zhang et al., 2004). At the time of writing, a consensus has not yet emerged as to whether the pneumonia and ARDS associated with COVID-19 caused by SARS-CoV-2 is a consequence of sustained virus replication, whether there is aberrant recruitment of macrophages and neutrophils into the lungs, or how the vascular compartment may be involved. The kinetics of the response to SARS-CoV-2 fits with models of the induction of conventional antiviral immunity and with a crisis that correlates with the likely peak phase of T cell responses. Nevertheless, it is unclear if immune hyperactivity or a failure to resolve an inflammatory response because of ongoing viral replication or immune dysregulation underlies severe disease. Nevertheless, the reports of increased levels of thrombi formation and endothelial cell death in COVID-19 patients would indicate damage to the vascular endothelium and the involvement of cytokines and immunothrombosis (Ackermann et al., 2020; Fox et al., 2020).
Multiple studies deposited on bioRxiv and medRxiv report elevated cytokine levels in severely ill COVID-19 patients and the successful use of cytokine antagonists, reviewed in depth here (Manjili et al., 2020). Some have even been able to compare these samples with those from other forms of sepsis or ARDS. The presence of elevated cytokine levels in any patient with clinically diagnosed COVID-19 should not be a surprise, but whether these cross the threshold from protective to pathological is unclear. Since preexisting conditions that affect vascular health, such as diabetes, hypertension, and cardiovascular disease, appear to be the single largest factor that underlies COVID-19 pathogenesis, it is possible that these comorbidities may decrease resilience and lower the ability to tolerate systemic cytokines. As this literature evolves, it is important that cytokine measurements are standardized to allow comparisons between cohorts to determine whether there is prognostic value to identify patients who develop a bona fide cytokine storm. The application of systems biology approaches that have already been developed to understand other forms of sepsis will help to guide the design of trials to test whether any of the approved antagonists of IL-1, TNF, IL-6, IL-12, IL-17, GM-CSF, and IFNγ would prove useful to manage severe COVID-19 patients. However, it is appropriate to note that anti-cytokine therapies based on the use of neutralizing antibodies or filters to remove circulating proteins have failed to improve mortality in sepsis, and at this stage, it’s unclear why COVID-19 would be the exception. There is also a significant population of patients with immune-mediated conditions who are currently treated with antagonists of TNF, IL-1, IL-12/IL-23, and IL-17 and knowing whether these interventions influence the outcome of SARS-CoV-2 may help to identify those cytokines that are pathological. There are also significant concerns that cytokine neutralization would interfere with antiviral responses or lead to increased complications associated with other respiratory pathogens. Nevertheless, sophisticated approaches to identify disease endotypes may provide new opportunities to revisit when to deploy immunomodulatory therapies, as has been done retrospectively for the use of IL-1RA in sepsis (Shakoory et al., 2016). It may be beneficial to develop strategies to target cytokine-induced injury in the vascular compartment while maintaining cytokine signaling in the tissues that are essential for host defense. It is also too early to understand whether patients who survived severe forms of COVID-19 infection will have long-term sequelae that are similar to those associated with sepsis survivors. The reports of pediatric patients who develop a post-SARS-CoV-2 multi-system inflammatory syndrome that includes vascular inflammation highlights this issue.
Recap
There is a natural arc to the induction and resolution of an immune response, and its magnitude and duration is coordinated by an array of regulatory checks and balances. Disruption of this arc can lead to hyper responses, or a delay in the resolution phase and elevated or sustained cytokine production can be pathological. However, not all cytokine storms are the same, and there are many variables—the nature of the insult, host immune status, tissue affected, crosstalk with immune thrombosis, and complement activation—that influence the magnitude and kinetics of these responses and thus clinical manifestations. Nevertheless, there are numerous mechanisms that allow a host to tolerate, survive, and even thrive in the face of immune stressors, and understanding these natural processes may be useful to design ways to manage distinct forms of CRS. At the time of writing, we still do not know enough to state with confidence that a bona fide cytokine storm underlies COVID-19 pathologies, but the rapid progress in immune phenotyping these patients will help our scientific community understand the complexities of this disease. Perhaps research on COVID-19 will provide impetus to understand the consequences of dysregulated immunity in the vascular compartment and the development of strategies that target cytokines, complement, and coagulation to improve management of other forms of sepsis.
References
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. Published online May 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Samkari H., Berliner N. Hemophagocytic Lymphohistiocytosis. Annu. Rev. Pathol. 2018;13:27–49. doi: 10.1146/annurev-pathol-020117-043625. [DOI] [PubMed] [Google Scholar]
- Ayres J.S. The Biology of Physiological Health. Cell. 2020;181:250–269. doi: 10.1016/j.cell.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., Riley E.M. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30243-5. Published online May 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R.S., Moldawer L.L., Opal S.M., Reinhart K., Turnbull I.R., Vincent J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjili R.H., Zarei M., Habibi M., Manjili M.H. COVID-19 as an Acute Inflammatory Disease. J. Immunol. 2020 doi: 10.4049/jimmunol.2000413. Published online May 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C.K., Cheon S., Ha N.Y., Sohn K.M., Kim Y., Aigerim A., Shin H.M., Choi J.Y., Inn K.S., Kim J.H. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulert G.S., Zhang M., Fall N., Husami A., Kissell D., Hanosh A., Zhang K., Davis K., Jentzen J.M., Napolitano L. Whole-Exome Sequencing Reveals Mutations in Genes Linked to Hemophagocytic Lymphohistiocytosis and Macrophage Activation Syndrome in Fatal Cases of H1N1 Influenza. J. Infect. Dis. 2016;213:1180–1188. doi: 10.1093/infdis/jiv550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A., Cron R.Q., Opal S.M. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikiatkhachorn A., Mathew A., Rothman A.L. Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol. 2017;39:563–574. doi: 10.1007/s00281-017-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D., Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W., Ye L., Xu S., Sun R., Wang Y., Lou J. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]