Abstract
Objectives
The main objective was to demonstrate the feasibility of percutaneous tracheostomy performed under difficult conditions by military ENT physicians during their deployment in the military intensive care field hospital of the French Military Medical Service in Mulhouse to confront the exceptional COVID-19 pandemic. The secondary objective was to assess reliability and safety for patient and caregivers, with a risk of iatrogenic viral contamination.
Material and methods
A single-center retrospective study was conducted between March 25 and April 25, 2020, in 47 COVID-19 patients requiring prolonged mechanical ventilation. The inclusion criterion was having undergone percutaneous tracheostomy.
Results
Eighteen consecutively included patients had successfully undergone percutaneous tracheostomy despite unfavorable anatomical conditions (short neck: 83.3%, overweight or obese: 88.9%). Median time to completion was 11 days after intubation, with an average duration of 7 minutes. The procedure was technically compliant in 83.3% of cases, and considered easy (on self-assessment) in 72.2%, with 2 minor per-procedural complications. No crossover to surgery was required. There was only 1 major post-procedural complication (late hemorrhage).
Conclusion
This study showed the feasibility of percutaneous tracheostomy by an ENT physician under COVID-19 biohazard conditions. The technique was fast, easy and safe and met safety requirements for patient and staff.
Keywords: Percutaneous tracheostomy, SARS-CoV-2, COVID-19, Military, ENT physician
1. Introduction
In the unprecedented health crisis caused by the COVID-19 pandemic, with massive saturating influx of patients requiring prolonged mechanical ventilation, some hospitals quickly exceeded capacity, especially in intensive care. On March 16, 2020, as part of “Operation Resilience”, the French President, Emmanuel Macron, deployed a field hospital to back up the Émile Muller hospital in Mulhouse. The Military Intensive Care Unit of the Military Health Service (EMRSSA) increased ICU capacity with 30 beds in military tents. Medical teams adopted unusual collective indication strategies for early tracheostomy, going beyond the usual individual indications, so as to achieve earlier withdrawal of mechanical ventilation, shorter intensive care stay and optimal management of patient flow [1].
In Covid-19 patients, tracheostomy and cannula care incur a high risk of aerosolization and iatrogenic viral contamination for care-givers. Bedside percutaneous dilatational tracheostomy (PDT) is associated with a better risk/benefit trade-off in terms of speed and safety, in line with the guidelines of the French Society of Anesthesia and Intensive Care Medicine (SFAR) [2] and French Society of Otorhinolaryngology (SFORL) [3]. To secure the procedure and ensure that the EMRSSA could handle crossover to surgery without patient transportation, a ENT physician (the EMRSSA's only surgeon) and an operating room (OR) nurse were attached to the EMRSSA specifically for tracheostomy.
The main objective of the present study was to demonstrate the feasibility of PDT performed by an ENT physician under exceptional conditions. The secondary objective was to assess reliability and safety for patient and staff, with a risk of iatrogenic viral contamination.
2. Material and method
A single-center retrospective study included adult Covid-19 patients managed in the military intensive care field hospital of the French Military Medical Service (EMRSSA) of Mulhouse for severe acute respiratory distress syndrome requiring mechanical ventilation, between March 25 and April 25, 2020. Analyses were based on the usual data in patients’ computerized files.
All patients with PDT performed by the ENT physician in the EMRSSA were consecutively included. Patients or their designated person of trust refusing to participate in the study were excluded. Indications and technique were decided on jointly by the intensive care team and ENT physician.
Clinical data (age, gender, weight, body-mass index, short neck), diathesis (history, allergy, smoking status), Covid-19 diagnosis modalities and the SAPS2 (Simplified Acute Physiology Score) were collated.
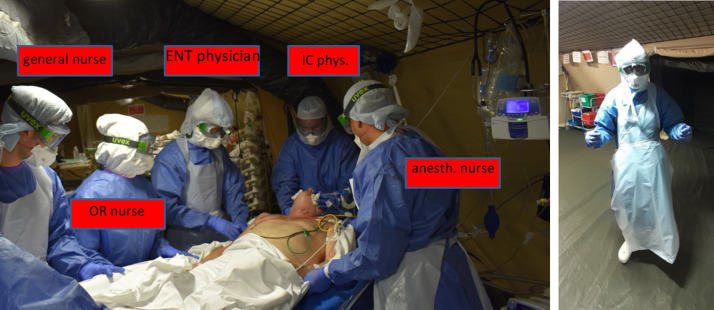
The dedicated specifically trained teams comprised 1 intensive care physician, 1 ENT physician, 1 anesthesia nurse, 1OR nurse and 1 general nurse (Fig. 1 ). In the week before deployment, the 2 senior ENT physiciansunderwent theoretic and practical training in PDT under the supervision of 2 intensive care physicians under conditions similar to those of Covid-19 intensive care, within their own hospital. All the intensive care physicians of the EMRSSA were senior, experienced in PDT. The 2 surgeons worked successively, for 15 and 17 days, with 10 and 8 tracheostomies, respectively; both were experienced ENT physicians, seniors for 7 and 4 years respectively.
Fig. 1.
Composition of percutaneous tracheostomy team (left); ENT physician's clothing (right).
The Ciaglia Blue Rhino® technique [4], [5] was used, with tracheal catheterization, guide introduction, predilation then dilation before introducing the tracheostomy cannula (Tracoe® Experc Kit, Pouret Medical, Clichy, France: n° 8 double-chamber cannula in all cases).
PDT was performed at the bedside under the EMRSSA tents, under continuous control by a single-use bronchoscope (aScope™ 4 Broncho Regular 5.0/2.2, Ambu®, Ballerup, Denmark) connected to a compact portable high-resolution video monitor (aView™, Ambu®) for the intensive care and ENT physicians to control the tracheal lumen. The bronchoscope was introduced by the intensive care physician in the orotracheal cannula via an angled Mount fitting with valved port to keep the seal and ventilation circuit closed. Patients were under deep intravenous sedation with curarization and controlled assisted ventilation. At end of procedure, the bronchoscope was introduced in the circuit connected to the cannula to confirm positioning and aspirate blood and tracheobronchial secretions.
A systematic check-list was taken: identity, consent, hemorrhage risk control (INR < 1.8; aPTT < 1.6; thrombopenia > 80,000/m3; withdrawal of heparin and/or anti-platelets), gastric aspiration, withdrawal of enteral feeding. The intensive care physician performed systematic cervical ultrasound to confirm cervical trachea level and depth, and check absence of thick thyroid isthmus and of at-risk midline vascularization.
Feasibility and safety criteria comprised: admission-to-tracheostomy time and intubation-to-tracheostomy time, technical difficulties encountered, ENT self-assessment score and difficulty score (1: easy; 2: moderately easy; 3: difficult), per-procedural complications (procedure compliant if without problems or complications; any crossover to surgery was noted), and procedure time (min), from skin incision to respirator connection onto cannula, with balloon inflated.
Post-procedural complications were categorized following Petiot [6] by onset (acute: ≤ 48 h; early: D3-12) and severity (minor: without impact on patient; moderate: potentially harmful; major: harmful with indication for emergency treatment) (Table 1 ). Safety with respect to team contamination risk was assessed in terms of onset of probable or confirmed Covid-19 infection according to Health Ministry criteria: “probable” in case of clinical signs of acute respiratory infection within 14 days of close contact with a confirmed case or clinical signs of acute respiratory infection and visible thoracic CT signs suggesting Covid-19, and “confirmed” in case of test confirming SARS-CoV-2 infection, whether symptomatic or not.
Table 1.
Classification of complications.
| Minor complications | Moderate complications | Major complications |
|---|---|---|
| Hypoxemia with SpO2 < 90% for <5 minutes (SpO2 = oxygen saturation on pulse oximetry) | Posterior tracheal wall lesion without surgical repair | Posterior tracheal wall lesion requiring surgical repair |
| Difficulty puncturing: > 3 attempts | Inflammatory granuloma | Esophageal lesion, mediastinitis |
| Puncture against cannula | Subglottic stenosis | Subcutaneous emphysema, pneumothorax |
| Peri-cannula infection not requiring antibiotic therapy | False submucosal guide route or pre- or para-tracheal cannula insertion | Peri-cannula infection requiring local care and/or antibiotic therapy |
| Hemorrhage not requiring compression or packed red blood cell transfusion | Hemorrhage requiring compression without packed red blood cell transfusion | Hemorrhage requiring compression and packed red blood cell transfusion |
| Crossover to surgery | ||
| Loss of airway control–accidental extubation | ||
| Cardiac arrest | ||
| Death |
EMRSSA staff underwent mandatory twice-daily checks on temperature and onset of clinical signs of acute respiratory infection up to 14 days after the last tracheostomy performed. In case of suspicion, a nasopharyngeal sample was taken for RT-PCR in the Emile Muller hospital.
3. Results
Eighteen of the 47 patients had PDT and were included in the study: 14 male, 4 female; median age, 64 years (range, 31-76 years). Table 2 shows clinical data.
Table 2.
Clinical data.
| Patient | Gender | Age | BMI | Short neck | History | Allergy | Smoking | SAPS2 score | Covid-19 diagnosis | Covid-19 PCR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 67 | 25 | No | HBP, cardiac arrhythmia | No | No | 34 | PCR | POS |
| 2 | M | 31 | 39 | Yes | OSAHS (equipped) | No | No | 28 | PCR + radio | POS |
| 3 | M | 46 | 34 | Yes | HBP | Yes | No | 24 | PCR + radio | POS |
| 4 | M | 67 | 30 | Yes | Dyslipidemia, HBP, type-2 diabetes, stroke, cardiac arrhythmia, prostatectomy | No | No | 45 | Radio | NP |
| 5 | M | 47 | 33 | Yes | HBP, type-2 diabetes | No | No | 19 | PCR + radio | POS |
| 6 | F | 56 | 25 | No | / | Yes | Yes | 31 | PCR + CT | POS |
| 7 | M | 60 | 22 | Yes | / | No | No | 37 | CT | NEG |
| 8 | M | 66 | 25 | Yes | Hemochromatosis, type-2 diabetes | No | No | 40 | PCR + radio | POS |
| 9 | F | 70 | 35 | Yes | Cardiopathy, HBP, dyslipidemia, type-2 diabetes, gastric ulcer, asthma | Yes | No | 35 | PCR + radio | POS |
| 10 | F | 64 | 36 | Yes | Hypothyroidism, spine fixation | No | No | 41 | PCR + radio | POS |
| 11 | M | 73 | 30 | Yes | HBP, OSAHS (equipped), asthma, sigmoiditis, Forestier's disease | No | No | 32 | PCR + radio | POS |
| 12 | M | 65 | 30 | Yes | HBP, Gout | No | Ceased | 50 | PCR + radio | POS |
| 13 | M | 67 | 32 | Yes | HBP, Gout, testicular cancer | No | No | 46 | PCR + radio | POS |
| 14 | M | 52 | 27 | Yes | Lumbar fusion | No | Yes | 34 | PCR + radio | POS |
| 15 | M | 76 | 24 | No | HBP, multi-stented ischemic cardiomyopathy, OLLA (bypassed), TIA endarteriectomy | No | Ceased | 75 | PCR + CT | POS |
| 16 | F | 56 | 36 | Yes | Type-2 diabetes, schizophrenia, dyslipidemia, HBP | No | Yes | 35 | PCR + radio | POS |
| 17 | M | 68 | 26 | Yes | Large goiter, chronic respiratory failure | No | Ceased | 33 | PCR + CT | POS |
| 18 | M | 60 | 29 | Yes | / | No | Ceased | 31 | PCR + CT | POS |
BMI: body-mass index; SAPS2: simplified acute physiology score; M: male; F: female; POS: positive; NEG: negative; NP: not performed; HBP: high blood pressure; OSAHS: obstructive sleep apnea/hypopnea syndrome; OLLA: obstructive lower-limb arteriopathy; TIA transient ischemic attack.
All procedures were successful, with zero mortality (Table 3 ). Median time to tracheostomy was 13 days (range 7-25 days) after admission and 11 days (range, 6-25 days) after orotracheal intubation.
Table 3.
Percutaneous dilatational tracheostomy procedural characteristics.
| Patient number/ENT A or B | Admission/intubation to tracheotomy time (days) | Duration (minutes) | Compliance | Self-assessed difficulty | Per-procedural complication (C) or technical problem (T) | Post-procedural complication |
|---|---|---|---|---|---|---|
| 1/A | 12/11 | 12 | Compliant | 1 | / | / |
| 2/A | 20/19 | 9 | Compliant | 1 | / | / |
| 3/A | 7/6 | 8 | Compliant | 1 | / | / |
| 4/A | 16/14 | 21 | Problem | 2 | (C) Minor: hypoxia | / |
| 5/A | 9/8 | 10 | Problem | 2 | (T) Exchange of obstructed cannula | Acute minor: infection |
| 6/A | 7/7 | 6 | Compliant | 1 | / | / |
| 7/A | 12/10 | 6 | Compliant | 1 | / | / |
| 8/A | 15/15 | 7 | Compliant | 1 | / | / |
| 9/A | 12/11 | 4 | Compliant | 1 | / | Acute minor: hemoptysis |
| 10/A | 10/10 | 4 | Compliant | 1 | / | / |
| 11/B | 16/14 | 8 | Compliant | 2 | / | Acute major: hemoptysis |
| 12/B | 15/14 | 5 | Compliant | 1 | / | / |
| 13/B | 27/25 | 4 | Compliant | 1 | / | / |
| 14/B | 13/12 | 6 | Compliant | 1 | / | / |
| 15/B | 15/10 | 3 | Compliant | 1 | / | / |
| 16/B | 12/11 | 3 | Compliant | 1 | / | Acute minor: hemoptysis |
| 17/B | 14/13 | 4 | Compliant | 2 | / | / |
| 18/B | 16/14 | 14 | Problem | 2 | (C) Minor: difficulty puncturing trachea | / |
Mean procedure time was 7 minutes (range, 3-21 min), and < 12 minutes in 88.9% of cases, counting from each surgeon's first procedure.
The procedure was technically compliant in 83.3% of cases, with a technical difficulty (cannula obstruction by thick secretion, requiring exchange on guide ahead of PDT) in 1 case and minor per-procedural complications in 2 (respectively, <1 min sudden oxygen desaturation up to 40% SpO2, without cardiac arrest, requiring endoscope withdrawal with repeated pauses during the procedure to optimize oxygenation, and difficulty in locating the trachea, with iterative puncture). Self-assessed difficulty was “easy” in 72.2% of cases, “moderately easy” in 27.8% and “difficult” in none.
There were no cases of emergency surgical crossover for pre-procedural complications. In 1 case, immediate peri-cannula air leak with risk of staff contamination required peri-cannula packing with Surgicel®.
There were 4 post-procedural complications (22.2%): 3 acute (75%), 1 early (25%); 3 minor (75%) (1 peri-cannula infection, 2 cases of mild hemoptysis exteriorized by the cannula in patients with hypertension treated by curative-dose heparin with dose increment and tranexamic acid aerosol), 1 major (severe hemoptysis exteriorized orally, on post-procedure day 10 during a hypertension episode in a patient under anticoagulation-dose heparin, controlled by 48 hours’ transoral subglottic packing and requiring transfusion and cauterization under suspension laryngoscopy of glottic and subglottic mucosal lesions). No patients had multiple per- and post-procedural complications.
There were no probable, suspect or possible Covid-19 cases in EMRSSA staff involved in PDT.
4. Discussion
The present study, conducted in the context of the Covid-19 pandemic, confirmed the feasibility and safety of percutaneous tracheostomy for patient and staff.
In the Covid-19 context, PDT protects both patient and caregivers [3], [7]. It is usually (74% of cases) performed by one or more intensive care physicians to allow withdrawal of prolonged mechanical ventilation in intensive care patients between days 7 and 15, with a 53.7% rate of indications according to Vargas's international study [8]. It is relatively non-invasive, quick and safe and can be performed at the bedside and, in the present context, ensures peri-cannula tightness, reducing the risk of contaminating aerosol for staff managing the cannula. In the present series, tightness was systematic and no caregiver contamination occurred.
Flexible endoscopic control of the tracheal lumen is now the gold-standard means of minimizing complications and ensuring effective mechanical ventilation by keeping the system hermetically closed [4]. If control is hampered by secretions obstructing the intubation cannula, as in 1 of the present cases, we recommend exchange on a guide ahead of PDT. It is essential to withdraw the cannula carefully, as the risk of accidental extubation is reported to be 1.4% [9]. This did not arise in the present series, but equipment for difficult intubation was always kept at hand.
The PDT technique and its principles and the numerous specific kits and associated complications are all well-known [8], [9], [10]. Over and above the technique as such, however, team experience, communication between the intensive care and ENT physicians and perfect management of the ventilation equipment are key issues in the Covid-19 context (with risk of hypoxemia and aerosolization).
Well-codified stepwise acquisition of the PDT technique is quick and easy for an experienced ENT physician mastering surgical technique. The present exceptional study of 2 ENT physicians without prior experience in PDT demonstrated feasibility despite difficult conditions. The difficult conditions were 3-fold: risk of care-team Covid-19 contamination; execution in a military tent at the bedside, with personal protective equipment; and predominantly unfavorable anatomic conditions, with 83.3% short neck and 88.9% obesity.
Execution was quick and simple, with a mean duration of 7 minutes, as in Petiot's study where mean duration stabilized for intensive-care physicians at 11 minutes after a learning curve of 65 procedures [6]. Except for 2 cases with duration up to 21 minutes, due to sudden oxygen desaturation in 1 case and difficult tracheal puncture in the other, procedure time was < 12 minutes in 88.9% of cases counting from each surgeon's first procedure.
Tracheostomy proved reliable, with no failures, major per-procedural complications or crossover despite predominantly difficult anatomic conditions. Ultrasound is the gold-standard to guide choice of technique [6]. As conditions for bedside execution were difficult, patient positioning and equipment set-up had to be rigorous. A horizontal surgical cushion under the scapula optimized cervical trachea position. The double team would allow safe and immediate crossover to cervicotomy if necessary; equipment for such surgical crossover was always nearby, on an identified trolley. Most complications were minor; the cases of hemoptysis were partly due to the patients’ clinical condition, with hypertension episodes and curative anticoagulation therapy.
The present series of PDT in the exceptional Covid-19 context highlighted two main risks.
The first was related to the frailty of Covid-19 patients, with risk of sudden hypoxia, within a matter of seconds (patient 4), liable to induce cardiac arrest [11]. This necessitates 15 minutes’ prior oxygenation at 100% FiO2. Moreover, all maneuvers disconnecting the respirator are liable to reduce alveolar recruitment, inducing acute hypoxemia, and are therefore to be avoided or at least kept to a minimum; this means keeping the circuit closed and adapting the respirator settings to the increased resistance caused by the endoscope inside the cannula by increasing the maximum insufflation pressure, and using an angled Mount connector with tight check-valve to introduce the fiberoptic bronchoscope without loss of tracheal pressure. Endoscopic control is essential and should be implemented as quickly as possible, by experienced senior operators, with close teamwork between intensive care and ENT physicians so as to enable introduction, withdrawal and iterative dilatation maneuvers when clinical status allows the respirator to be paused.
The second risk is team contamination during the phases at high risk of aerosolization [12], [13], [14]. Like with SARS (severe acute respiratory syndrome) in 2003 and MERS (Middle East respiratory syndrome), both implicating coronaviruses, SARS-CoV-2 is transmitted by droplet aerosolization and surface contact, and the hygiene rules are similar [15]. Hand disinfection and PPE (personal protective equipment) are indispensable [16]. All team members must adhere strictly to the hygiene rules and clothing validated by the local health and safety team: goggles, hood, FFP3 mask, coat, boots, gloves; plus sterile apron and double sterile gloves for the intensive care physician, OR nurse and ENT physician, who also wears a cold-light helmet (Fig. 1). Two military supervisors in were in charge of helping with and checking this protection in a vestibule.
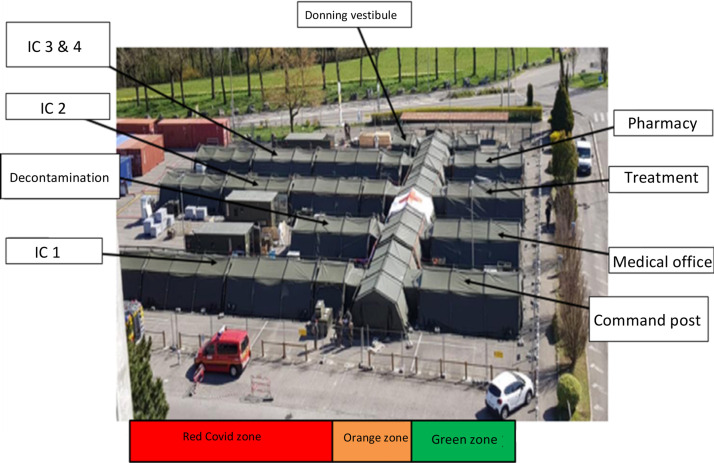
In this exceptional home mission of the French Army Health Service, the EMRSSA's health rules, design and logistics were based on the operational experience of the Service, especially during the deployment of a field laboratory in Guinea in 2015 in the fight against the Ebola virus [17]. The EMRSSA was structured in 3 distinct hygiene zones (Fig. 2 ): a “clean” green zone containing the medical and technical sectors, an orange buffer zone, and a contaminated red zone with Covid-19 patients. The unit included a donning/doffing sector, transfer vestibules at the entrance to the intensive care wings, and a one-way “forward motion” system to optimize and reinforce hygiene and reduce staff contamination risk. Tracheostomy was performed at the bedside inside the red zone to avoid transportation to an operating room in the hospital. Curarization was systematic, to reduce risk of coughing during tracheal opening [18].
Fig. 2.
Aerial view of EMRSSA, with 3 contamination risk zones.
Biocleansing, decontamination and reconditioning of material were performed immediately. The disinfectant detergent met NF14476 standards (Covid-19 virucide). Waste management followed the procedure for infectious waste.
Staff handling the cannula wore PPE with FFP3 mask and underwent specific training by the ENT physician in cannula management under Covid-19. A double-chamber non-fenestrated cannula was used from the start, to avoid early cannula exchange and allow fast exchange of the inner tube in case of obstruction by secretion (frequent and intense in Covid-19 patients).
The present study has certain limitations, being retrospective, single-center, with a small patient sample, over a period of just 1 month. On the other hand, it was performed in a unique situation related to the Covid-19 pandemic, which was severe in Mulhouse, in a special structure (military intensive care field hospital) deployed on the home front for the first time in the history of the French Army Health Service.
5. Conclusion
The present study, conducted during a unique military ENT mission in response to an exceptional health emergency, showed the feasibility of percutaneous dilatational tracheostomy performed by experienced ENT physicians using a quick, easy and safe technique, under difficult conditions.
The technique met the safety requirements for bedside execution in a Covid-19 intensive care unit. Specific training, associating percutaneous tracheostomy technique and infection risk control may be advisable.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The authors express especial thanks to Inspector General Jacques Escarment, Director of the military intensive care field hospital for his expertise in percutaneous tracheostomy and advice, and to all the military and reserve personnel involved in patient management in the EMRSSA.
They also thank the civilian staff of the Émile Muller hospital, and in particular their ENT colleague, Dr Emilien Lemaire.
References
- 1.SRLF-SFAR-SFMU-GFRUP-SPILF-SPLF. 2020. Recommandations d’experts portant sur la prise en charge en réanimation des patients en période d’épidémie à SARS-CoV2 SRLF-SFAR-SFMU-GFRUP-SPILF-SPLF. [Google Scholar]
- 2.Trouillet J.-L., Collange O., Belafia F. Tracheotomy in the intensive care unit: Guidelines from a French expert panel: The French Intensive Care Society and the French Society of Anaesthesia and Intensive Care Medicine. Anaesth Crit Care Pain Med. 2018;37(3):281–294. doi: 10.1016/j.accpm.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Schultz P., Morvan J.-B., Fakhry N. French consensus regarding precautions during tracheostomy and post-tracheostomy care in the context of COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137(3):167–169. doi: 10.1016/j.anorl.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kost K.M. Endoscopic percutaneous dilatational tracheotomy: a prospective evaluation of 500 consecutive cases. Laryngoscope. 2005;115(10 Pt 2):1–30. doi: 10.1097/01.MLG.0000163744.89688.E8. [DOI] [PubMed] [Google Scholar]
- 5.Blondonnet R., Chabanne R., Godet T. Tracheostomy in French ICUs and patient outcome: national opinion survey. Ann Fr Anesth Reanim. 2014;33(4):227–231. doi: 10.1016/j.annfar.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Petiot S., Guinot P.-G., Diouf M., Zogheib E., Dupont H. Learning curve for real-time ultrasound-guided percutaneous tracheostomy. Anaesth Crit Care Pain Med. 2017;36(5):279–283. doi: 10.1016/j.accpm.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Mattioli F., Fermi M., Ghirelli M. Tracheostomy in the COVID-19 pandemic [published online ahead of print, 2020 Apr 22] Eur Arch Otorhinolaryngol. 2020:1–3. doi: 10.1007/s00405-020-05982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vargas M., Sutherasan Y., Antonelli M. Tracheostomy procedures in the intensive care unit: an international survey. Crit Care. 2015;19(1):291. doi: 10.1186/s13054-015-1013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser E., Cantais E., Goutorbe P., Salinier L., Palmier B. Prospective randomized comparison of progressive dilational vs forceps dilational percutaneous tracheostomy. Anaesth Intensive Care. 2006;34(1):51–54. doi: 10.1177/0310057X0603400119. [DOI] [PubMed] [Google Scholar]
- 10.Massick D.D., Powell D.M., Price P.D. Quantification of the learning curve for percutaneous dilatational tracheotomy. Laryngoscope. 2000;110(2 Pt 1):222–228. doi: 10.1097/00005537-200002010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Wei W.I., Tuen H.H., Ng R.W., Lam L.K. Safe tracheostomy for patients with severe acute respiratory syndrome. Laryngoscope. 2003;113(10):1777–1779. doi: 10.1097/00005537-200310000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer D.D., Engels P.T., Weitzel E.K. Recommendations from the CSO-HNS taskforce on performance of tracheotomy during the COVID-19 pandemic. J Otolaryngol Head Neck Surg. 2020;49(1):23. doi: 10.1186/s40463-020-00414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiba T., Ghazizadeh S., Chhetri D., St John M., Long J. Tracheostomy Considerations during the COVID-19 Pandemic. OTO Open. 2020;4(2) doi: 10.1177/2473974X20922528. [2473974X20922528] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattei A., Amy de la Bretèque B., Crestani S. Guidelines of clinical practice for the management of swallowing disorders and recent dysphonia in the context of the COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137(3):173–175. doi: 10.1016/j.anorl.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan A., Fok W.G., Law K.I., Lam S.H. Tracheostomy in a patient with severe acute respiratory syndrome. Br J Anaesth. 2004;92(2):280–282. doi: 10.1093/bja/aeh035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benge C.D., Barwise J.A. Aerosolization of COVID-19 and Contamination Risks During Respiratory Treatments. Fed Pract. 2020;37(4):160–163. [PMC free article] [PubMed] [Google Scholar]
- 17.Janvier F, Foissaud V, Delaune D, et al. Deployment of the French Military Field Laboratory Dedicated to Ebola Virus Infected Patients in Guinea, January-July 2015 [published correction appears in J Infect Dis. 2016; 213(12):2023]. J Infect Dis. [DOI] [PubMed]
- 18.Tay J.K., Khoo M.L., Loh W.S. Surgical Considerations for Tracheostomy During the COVID-19 Pandemic: Lessons Learned From the Severe Acute Respiratory Syndrome Outbreak [published online ahead of print, 2020 Mar 31] JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0764. [DOI] [PubMed] [Google Scholar]