Abstract
Background
Since novel coronavirus disease (COVID-19) emerged, various clinical features of COVID-19 have been reported.
Methods
We conducted a systematic review of published studies reporting the clinical features of COVID-19. Two investigators independently searched PubMed (December 2019–February 2020) for eligible articles. A meta-analysis was performed to measure the frequencies of clinical outcomes and symptoms of COVID-19. A stratified analysis was conducted according to the timeline of outbreak and exposure histories: Group I, most patients were exposed to the Hunan seafood wholesale market and lived in Wuhan, Hubei province; Group II, patients lived in Hubei province but were not directly exposed to the market; and Group III, patients lived outside Hubei.
Results
Thirteen studies, all from China, were eligible. The estimated mortality rate among all studies was 2.12%, but that in Group I was 8.66%. The incidence of acute respiratory distress syndrome in Group I was 20.00%. Both fever and cough were major symptoms, and their frequencies were higher in Group I than in Groups II and III, while the frequency of diarrhea in Group I was lower than that in Group III. The estimated frequency of dyspnea in Group I was 37.18%, while those in Groups II and III were 16.95% and 7.03%, respectively.
Conclusions
The trends in the clinical features of COVID-19 changed from December 2019 to February 2020. During this observation period, as the infection continued to spread, the clinical conditions for majority of patients became less severe with the changes in the route of transmission.
Keywords: COVID-19, SARS-CoV-2, Transmission, Exposure history, Timeline
Abbreviations: COVID-19, novel coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus; WHO, World Health Organization
1. Introduction
In December 2019, an acute respiratory illness, now known to be caused by a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), occurred in Wuhan, the capital city of Hubei province, China [[1], [2], [3]]. The disease caused by the virus was termed COVID-19, and it rapidly spread from Wuhan to the rest of China and other countries. On March 11, 2020, the World Health Organization (WHO) declared that COVID-19 can be characterized as a pandemic [4]. The WHO-China Joint Mission recently summarized the prognosis, signs, and symptoms of COVID-19 and indicated that 13.8% of patients had severe disease, while approximately 80.0% of patients had mild to moderate disease [5]. However, in the early stage of the COVID-19 outbreak in China, serious pneumonia that led to acute respiratory distress syndrome (ARDS) and death was reported [2,6]. Most of these patients had an epidemiological link to the Hunan seafood wholesale market in Wuhan city [2,6]. The disease thereafter spread within and beyond China, and both mild and asymptomatic cases have since been reported [7], as have various transmission routes including sustained human-to-human transmission [[8], [9], [10]], transmission from an asymptomatic patient [11], and transmission from a presymptomatic patient [12]. In addition, a study on the pneumonia presenting with COVID-19 in Wuhan indicated that the differences in exposure risks depended on time points between December and January [13]. Currently, the number of countries and individual people affected by COVID-19 have been rapidly increasing worldwide [14]. We hypothesized that changes in the exposure risks and timeline of the outbreak influence the clinical features of COVID-19, including the disease severity. Elucidation of the patterns of change in the disease features of COVID-19 is crucial for discussing the clinical response to and control measures for COVID-19.
Thus, the purpose of the present study was to elucidate the clinical features of COVID-19 using a meta-analysis with a stratified analysis according to the timeline and the changes in the exposure risks before the disease was declared as a pandemic.
2. Materials and methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the statement by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group [15,16].
2.1. Search strategy
Two investigators (T.M. and H.A.) independently searched for eligible studies in PubMed published from December 2019 to March 1, 2020. We used the following key words: “novel coronavirus” OR “new coronavirus” OR “emerging coronavirus” OR “2019-nCoV” OR “COVID-19” OR “SARS-CoV-2.” The search was limited to studies written in English. We also reviewed the reference lists of eligible studies using Google Scholar and performed a manual search to ensure that all appropriate studies were included.
2.2. Eligibility criteria and outcome measures
Studies fulfilling the following selection criteria were included in the meta-analysis: (1) study design and language: cohort studies and case series involving >20 patients written in English; (2) population: patients with SARS-CoV-2 infection who were hospitalized or treated in clinics; (3) primary outcome variables: distributions of clinical outcomes, including death, ARDS, acute cardiac injury, and acute renal injury in patients with COVID-19; and (4) secondary outcome variables: distributions of sex, comorbidities, symptoms, and signs. Studies were excluded based on the following criteria: (1) reporting of surveillance data, (2) no reporting of outcome variables, (3) no clear determination of the epidemiological exposure history of patients, and (4) ≤20 patients.
A stratified analysis was conducted according to the exposure history and the timeline of the COVID-19 outbreak: Group I, most patients were exposed to the Hunan seafood wholesale market in the initial stage of the outbreak; Group II, patients lived in Hubei province but could not be linked to the Hunan seafood wholesale market or community exposure after 1 January; and Group III, patients lived outside of Hubei province.
2.3. Data extraction
Two reviewers extracted the data independently. Articles retrieved in the search were stored in a citation manager (EndNote X8; Thomson Reuters, New York, NY, USA). After removing redundant articles, titles and abstracts and then full-text articles were investigated. We extracted the following data: study design, observational period, study site, and inclusion/exclusion criteria of each study. Outcome variables were extracted into predesigned data collection forms. We verified data accuracy by comparing the collection forms of each investigator; any discrepancies were resolved through discussion together with other authors.
2.4. Data analysis
Throughout the meta-analysis, we calculated the prevalence of each outcome variable with 95% confidence intervals (CIs) using a random-effects model (generic inverse variance method). To assess the prevalence of each variable among patients with COVID-19, the standard error was calculated using the Agresti–Coull method [17]. Heterogeneity among the original studies was evaluated using the I2 statistic [18]. Publication bias was examined using a funnel plot. For all analyses, significance levels were two-tailed, and p < 0.05 was considered significant. All statistical tests were performed using Review Manager (RevMan) ver. 5.3.5 (Cochrane Collaboration, Copenhagen, Denmark) [19].
3. Results
3.1. Study selection and characteristics
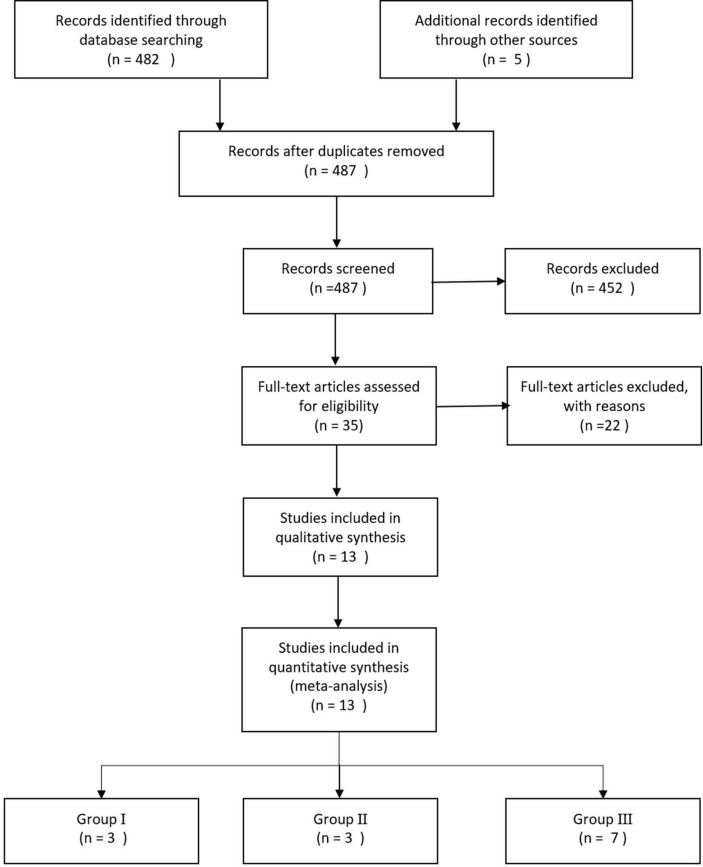
Of the 482 references screened, 13 studies [2,6,7,[20], [21], [22], [23], [24], [25], [26], [27], [28], [29]] reported the outcome variables (Fig. 1 ). In these 13 studies, we identified 2397 patients with COVID-19, and all eligible studies were reports from China. Among the 13 eligible studies, 3 [2,6,20] were identified as Group I (n = 279), 3 [[21], [22], [23]] were identified as Group II (n = 1356), and 7 [7,[24], [25], [26], [27], [28], [29]] were identified as Group III (n = 743) (Table 1 ).
Fig. 1.
Systematic review flow diagram. n is the number of articles.
Table 1.
Backgrounds of patients with COVID-19 in the eligible studies.
| Study (publication date) | Province in China | Observational period | No. of patients | Exposure history to Hunan Seafood Wholesale Market in Wuhan, n (%) | Sex-male, n (%) | Age, median (IQR), yr. | |
|---|---|---|---|---|---|---|---|
| Group 1 | Huang C [2] (Jan 24, 2020) | Hubei | Dec 16, 2019–Jan 2, 2020 | 41 | 27 (66) | 30 (73.1) | 49.0 (41.0–58.0) |
| Chen N [6] (Jan 29, 2020) | Hubei | Jan 1, 2020–Jan 20, 2020 | 99 | 49 (49) | 67 (67.7) | 55.5 (13.1)a | |
| Wang D [20] (Feb 7, 2020) | Hubei | Jan 1, 2020–Jan 28, 2020 | 138 | 12 (8.7 | 75 (54.3) | 56 (42–68) | |
| Group 2 | Liu K ]21] (Feb 7, 2020) | Hubei | Dec 30, 2019–Jan 24, 2020 | 137 | None | 61 (44.5) | 57 (range, 20–83) |
| Zhang JJ [22] (Feb 19, 2020) | Hubei | Jan 16, 2020–Feb. 3, 2020 | 140 | Living in Wuhan | 71 (50.7) | 57 (25–87) | |
| Guan W [23] (Feb 28, 2020) | 30 provinces | Dec 11, 2019–Jan 31, 2020 | 1099 | Living in Wuhan, 489 (44.5) Recently visited Wuhan, 193/616 (31.3) | 637 (58.1) | 47 (35–58) | |
| Group 3 | Song F et al. [24] (Feb 6, 2020) | Shanghai | Jan 20, 2020–Jan 27, 2020 | 50 | 50 (98) had some contact with individuals from Wuhan | 25 (49) | 49 (16)a |
| Xu XW [25] (Feb 19, 2020) | Zhejiang | Jan 10, 2020–Jan 26, 2020 | 62 | Exposure history in Wuhan > 2 weeks, 23 (37) | 35 (56) | ≤18, 2 (3) | |
| 19-40.25 (40) | |||||||
| 41-65, 33 (53) | |||||||
| ≥66, 2 (3) | |||||||
| Xu YH [26] (Feb 25, 2020 | Hebei | January and February 2020 | 50 | Wuhan or nearby, 30 (60) | 29 (58) | <18, 5 (10) 18-50.30 (60) >50, 15 (30) | |
| Close contact, 18 (36) | |||||||
| Tian S [7] (Feb 26, 2020 | Beijing | Jan 20, 2020–Feb 10, 2020 | 262 | Had been to Wuhan in 14 days, 106 (40.5) | 127 (48.5) | 47.5 (range, 1–04) | |
| Contacted with a symptomatic case in 14 days, 129 (49.2) | |||||||
| Yang W [27] (Feb 27, 2020 | Zhejiang | Jan 17, 2020–Feb 10, 2020 | 149 | Stayed in Wuhan, 80 (53.7); stayed in provinces other than Wuhan 5 (3.4); contacted with people in Hubei, 49 (32.9); no relation with Hubei | 81 (54.4) | 45.11 (13.35)a | |
| Xu X [28] (Feb 28, 2020 | Guangdong | Jan 23, 2019–Feb 4, 2020 | 90 | Wuhan or infected patient | 39 (43) | 50 (18–86) | |
| Wu J [29] (Feb 29, 2020 | Jiangsu | Jan 22, 2020–Feb 14, 2020 | 80 | None | 39 (48.6) | 46.10 (15.42)a |
Mean (standard deviation); IQR, interquartile range.
Table 1 shows the characteristics of the included studies. The observational period (initial to end of observation date) in the eligible studies for each group was December 19, 2019 to January 28, 2020 in Group I, December 30, 2019 to January 31, 2020 in Group II, and January 17, 2020 to February 14, 2020 in Group III. The age distribution was similar in all studies, and the median or mean age was around 50 years. The frequency of male patients among all groups or each group was estimated using a meta-analysis. The estimated frequency of male patients in all 13 eligible studies was 51.91% (95% CI, 48.08–55.75); however, the subgroup analysis showed that the estimated frequency of male patients decreased from Group Ⅰ (64.30%; 95% CI, 53.11–75.49) to Group III (52.12%; 95% CI, 48.75–55.52) (Supplementary Fig. S1).
The frequency of comorbidities in patients with COVID-19 was estimated and compared among the three groups (Table 2 ; Supplementary Fig. S2i - S2ⅷ). Hypertension (17.08%) showed the highest frequency among observed comorbidities, followed by cerebrovascular disease (8.26%), diabetes (7.98%), and cardiovascular disease (7.69%). The frequencies of hypertension, cardiovascular disease, and diabetes in Group I was higher than those in Groups II and III, while the estimated frequencies of cerebrovascular disease (16.79%) and chronic liver disease (2.81%) in Group Ⅰ were lower than those in Groups II and III.
Table 2.
Estimated frequencies of comorbidities in patients with COVID-19.
| Total |
Group I |
Group II |
Group III |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) | |
| Cerebrovascular disease | 6 | 1668 | 8.26% (3.19–13.32) | 1 | 138 | 5.10% (1.18–9.02) | 2 | 1239 | 1.45% (0.69–2.20) | 3 | 291 | 16.79% (0.37–33.21) |
| Cardiovascular disease | 6 | 596 | 7.69 (3.87–11.50) | 2 | 178 | 14.52% (9.32–19.72) | 2 | 277 | 7.90% (4.58–11.21) | 2 | 140 | 2.84% (−0.78–6.46) |
| Hypertension | 8 | 1757 | 17.08% (11.71–22.45) | 2 | 179 | 23.42% (7.18–39.66) | 3 | 1376 | 17.58% (9.07–26.08) | 3 | 202 | 12.32 (5.62–19.01) |
| Diabetes | 8 | 1757 | 7.98% (5.46–10.51) | 2 | 179 | 13.44% (4.52–21.76) | 3 | 1376 | 8.90% (6.06–11.75) | 3 | 202 | 3.91% (0.66–7.16) |
| Malignancy | 8 | 1833 | 1.33% (0.45–2.20) | 3 | 278 | 3.46 (−0.79–7.71) | 2 | 1236 | 0.93% (0.35–1.50) | 3 | 319 | 1.49% (−0.41–3.39) |
| COPD | 8 | 1757 | 1.20% (0.65–1.74) | 2 | 179 | 2.81% (−0.21–5.84) | 3 | 1376 | 1.13% (0.57–1.69) | 3 | 202 | 1.40% (−1.19–3.99) |
| Chronic liver disease | 5 | 371 | 2.94% (0.49–5.38) | 2 | 179 | 2.81% (−0.21–5.84) | – | – | – | 3 | 172 | 3.90% (−1.31–9.11) |
| Chronic renal disease | 5 | 1519 | 0.81% (0.26–1.37) | 1 | 138 | 2.90% (−0.43–1.31) | 2 | 1239 | 0.73% (0.16–1.31) | 2 | 142 | 1.42% (−1.64–4.48) |
COPD, Chronic obstructive pulmonary disease; CI, confidence interval.
3.2. Frequency of clinical outcomes among patients with COVID-19
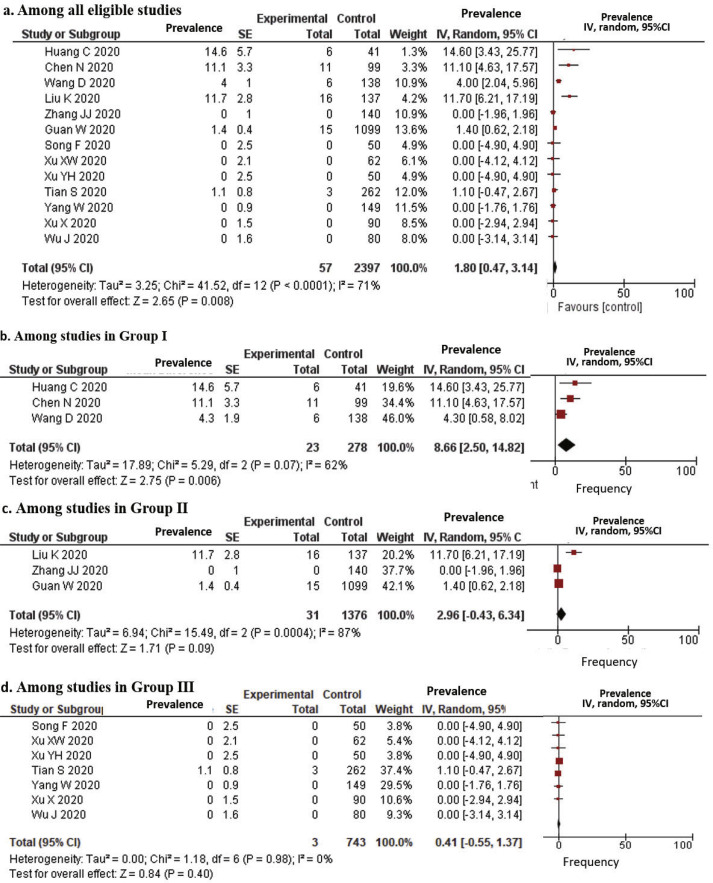
Among the studies that reported patients’ clinical outcomes, more than half of the patients remained in hospitals at the end of the observational period, except one study in Group I in which 14.6% of patients died and 68.3% were discharged [2]. The estimated case fatality rate in all 13 eligible studies was 1.80% (95% CI, 0.47–3.14), while the estimated case fatality rates in Groups I and II were 8.66% and 2.96%, respectively (Fig. 2 ). In Group III, the mortality was reported in only one study (3 cases among 262 patients, 1.15%) [7].
Fig. 2.
Forrest plots for case fatality among patients with COVID-19. SE: standard error; IV: inverse variance method; 95% CI: 95% confidence interval.
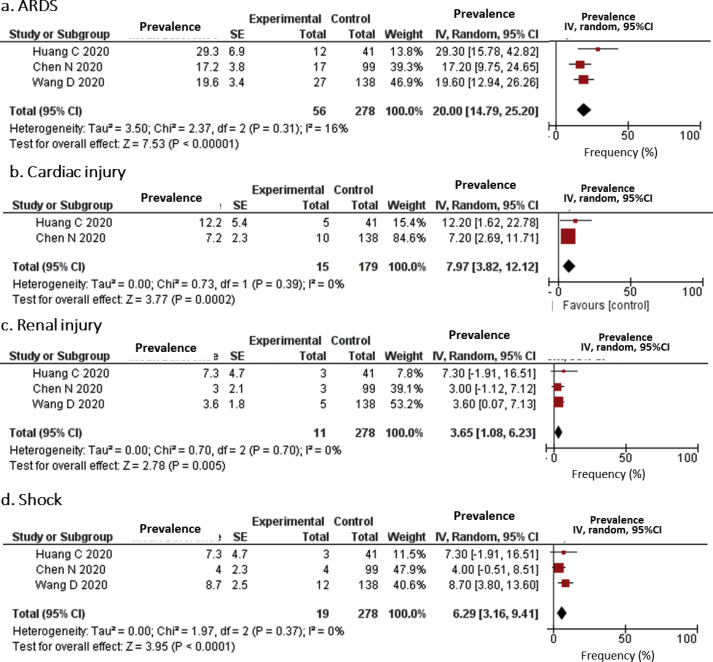
With respect to complications, the frequencies of ARDS, cardiac injury, renal injury, and shock were estimated and are shown in Fig. 3 . These complications were not reported from studies in Group III. ARDS was reported in all three studies in Group I and one study in Group III. The estimated frequency of ARDS in Group I was 20.0% (95% CI, 14.79–25.20), while the prevalence of ARDS in a study in Group II was 3.37% [23] and no studies in Group I (Fig. 3). The estimated frequencies of cardiac injury, renal injury, and shock were reported only in Group I (estimated frequencies of 7.97%, 3.65%, and 6.29%, respectively), except one study in Group II for shock (1.09%) [23].
Fig. 3.
Forrest plots for complications among patients with COVID-19. SE: standard error; IV: inverse variance method; 95% CI: 95% confidence interval.
3.3. Frequency of symptoms among patients with COVID-19
The estimated frequencies of symptoms among all available studies and stratified groups are shown in Table 3 and Supplementary Fig. S3i-3ⅸ. Fever and cough were the major symptoms and were reported in all eligible studies. The estimated frequency of fever was 82.46% in 13 eligible studies, and the frequency in Group I was 93.39% and was the highest among the three groups (Table 3; Fig. S3i). The estimated frequency of cough was 62.36% in all 13 eligible studies, and those in Groups I and III were 72.14% and 57.36%, respectively (Table 3; Fig. S3ii). Dyspnea was reported in all three studies in Group I and two studies each in Groups II and III. The estimated prevalence of dyspnea was higher in Group I (37.18%) than in Group II (24.12%) and Group III (6.16%) (Table 3; Fig. S3ⅲ). The prevalence of myalgia varied among the studies; the estimated frequency was similar in Group I (22.84%) and Group III (20.82%) (Table 3; Fig. S3ⅳ). The estimated frequency of fatigue was quite high, especially in Groups I (69.60%) and II (56.37%); however, the frequency in Group III was 22.44% (Table 3; Fig. S3ⅴ). The estimated frequencies of headache, sore throat, and diarrhea were lower in Group I (7.17%, 11.09%, and 4.91%, respectively) than in Group III (11.13%, 13.32%, and 6.06%, respectively) (Table 3; Fig. S3ⅵ, S3ⅶ, S3ⅸ). The estimated frequency of expectoration in Group III (29.21%) was the third highest among the observed symptoms in Group III and higher than the estimated frequency of expectoration in Group I (7.11%) (Table 3; Fig. S3ⅷ).
Table 3.
Estimated frequencies of symptoms among patients with COVID-19.
| Total |
Group I |
Group II |
Group III |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) | |
| Fever | 13 | 2377 | 82.46% (69.62–95.30) | 3 | 278 | 93.39% (84.25–102.53) | 3 | 1356 | 72.10% (38.02–106.18) | 7 | 743 | 82.64% (76.45–88.71) |
| Cough | 13 | 2377 | 62.36% (55.67–69.05) | 3 | 278 | 72.14% (57.05–87.23) | 3 | 1356 | 63.92% (51.58–76.26) | 7 | 743 | 57.36% (47.38–67.33) |
| Dyspnea | 10 | 2145 | 21.06% (13.41–28.71) | 3 | 278 | 37.18% (25.69–48.67) | 3 | 1356 | 24.12% (14.77–33.47) | 4 | 511 | 6.16% (1.38–10.95) |
| Myalgia | 7 | 1705 | 20.10% (12.08–28.11) | 2 | 237 | 22.84% (−0.38-46.07) | 1 | 1099 | 14.90% (12.74–17.06) | 4 | 369 | 20.82% (3.82–37.83) |
| Fatigue | 6 | 1759 | 41.11% (25.08–57.13) | 1 | 138 | 69.60% (61.96–77.24) | 2 | 1219 | 56.37% (20.21–92.53) | 3 | 402 | 22.44% (16.63–28.25) |
| Headache | 12 | 2254 | 10.05% (6.72–13.39) | 3 | 275 | 7.17% (3.93–10.42) | 2 | 1236 | 12.25% (8.48–16.03) | 7 | 856 | 11.13% (5.87–16.39) |
| Sore throat | 8 | 1755 | 12.71% (8.95–16.47) | 2 | 237 | 11.09 (−0.96–23.14) | 1 | 1099 | 13.90% (11.94–15.86) | 5 | 419 | 13.32% (7.40–19.23) |
| Expectoration | 8 | 1764 | 26.09% (14.68–37.51) | 2 | 177 | 27.11% (20.69–33.52) | 2 | 1236 | 19.08% (−9.64–47.79) | 4 | 351 | 29.21% (12.76–45.67) |
| Diarrhea | 10 | 2000 | 6.25% (4.06–8.44) | 3 | 164 | 2.12% (−1.23–5.46) | 3 | 1375 | 7.68% (2.32–13.04) | 4 | 351 | 6.60% (3.74–9.45) |
CI, confidence interval.
4. Discussion
The present systematic review and meta-analysis revealed that the trend of clinical features of COVID-19 in China changed from December 2019 to the end of February 2020. The estimated frequencies of death and complications of ARDS, cardiac injury, and renal injury as well as symptoms of dyspnea in the initial stage of the outbreak were higher than those in the later times. These results indicated that at the time of the outbreak, the disease severity was greater in the initial phase than at later times.
The previous studies mentioned that most affected patients were thought to be workers in the Hunan seafood wholesale market in the initial time of the outbreak [2,6]. In the present study, although none of the studies mentioned the prevalence of workers in the Hunan seafood wholesale market, the estimated frequency of male patients in Group I was higher than that in Groups II and III. It seemed reasonable that the patients with COVID-19 during the initial period of the outbreak included many workers in the Hunan seafood wholesale market. After that initial phase, most affected patients were no longer only linked to the Hunan seafood wholesale market, and the proportions of males and females became similar. More importantly, the patients who had an exposure history to the Hunan seafood wholesale market were not all of the patients in Group Ⅰ (Table 1). In particular, Wang et al. [20] found that only 8.7% of patients were exposed to the Hunan seafood wholesale market. The results indicated that human-to-human transmission was presumed to have occurred even at the initial phase of the outbreak in China. Notably, our evaluation indicated that the estimated case fatality was 8.66%, which is much higher than the crude fatality ratio in China (3.6%) reported by the WHO as of March 1, 2020 [4], and the estimated prevalence of ARDS decreased over time from Groups I to III. These results indicated that the disease prognosis was worse in the initial period than at the later phases of the COVID-19 outbreak in China. A study reported that angiotensin converting enzyme 2 (ACE2) is the receptor for SARS-CoV-2 [30], and men had a higher ACE level in their alveolar cells than women [31,32]. In addition to the exposure risk, it may be possible that the cause of the higher prevalence in male patients in the early phase of the outbreak in China was that male sex contributed to the severity of COVID-19.
Coronavirus mainly causes respiratory tract infections, and some strains have high infectivity and mortality [33]. SARS-CoV-2 has at least 70% similarity in its genetic sequence to SARS-CoV [28]. One report stated that the severity of some cases of COVID-19 mimicked that of SARS [23], which affected 8098 people in 26 countries and caused 774 deaths from 2002 to 2003 [34]. Besides the pathogenicity of coronavirus, one cause of the high severity and mortality during the initial outbreak in China may have been the lack of knowledge and recognition of the disease among residents, clinicians, and policymakers in Wuhan city, since Wuhan was the place where the infection initially occurred. In the present study, the symptoms with the highest overall estimated frequencies were fever and cough in all three groups, while the characteristic symptoms of COVID-19, such as olfactory and taste disorders, are reported after the disease spread globally [35]. These flu-like symptoms may not have prompted people to seek health care. However, with the development of more serious symptoms, such as dyspnea, the patients went to the hospital and underwent delayed initiation of treatment. The same phenomenon occurred in the early stage of the 2009 influenza pandemic in Mexico, where the first patient with H1N1pdm09 virus infection was identified [36]. Delayed access to health care and delayed initiation of proper treatments for COVID-19 may also lead to greater disease severity. Additionally, one study indicated that the viral load of SARS-CoV in upper respiratory tract specimens was low during the first week of illness, peaking around 10 days after illness onset [37]. This reflected in the fact that the first patient with COVID-19 in Korea had no clinical features suggesting pneumonia during the first 3 days [38]. Our evaluation suggested that the estimated frequency of atypical flu-like symptoms such as diarrhea was higher in Group III than Group I. Although the pathophysiology of SARS-CoV-2 may not be the same as that of SARS-CoV, the characteristics of the virus may contribute to delayed diagnosis leading to delayed early initiation of treatments, especially for initially mild cases. In addition, after the spreading outside Hubei province, it can be presumed that the availability of sufficient medical facilities can contribute to reduce the severity of disease [26].
As the timeline progressed from Group I to Groups II and III, the proportion of mild cases increased, especially in Group III, in which most patients lived outside of Hubei province, China, and none of the patients had a history of contact with the Hunan seafood wholesale market. This manner of spread is different from that of avian influenza (H7N9) virus infection, the reported cases of which were only concentrated in the mainland of China even over 1500 human cases had been reported for the 5 years following 2013 [39]. This suggests that, in COVID-19, human-to-human transmission or ongoing transmission from a market or other primary sources likely existed. Another aspect from the etiological point-of-view for a pandemic disease is that, in general, pathogens tend to reduce their virulence in order to maximize their between-host transmission [40], and hosts are selected to reduce the harm caused by the pathogen [41,42]. The route of transmission or source of exposure might influence the disease severity of COVID-19. In addition to the virulence factors of the pathogen, the host's immune status is another important factor preventing infection and reducing severity [43].
In terms of comorbidities, the results of the present study indicated that hypertension, cardiovascular disease, and diabetes can be risk factors for the severity of COVID-19. A study among critically ill patients with COVID-19 in the US reported that the prevalence of diabetes mellitus (58%) was the highest among those showing comorbidities, followed by chronic kidney disease (21%) [44]. These heterogenetic results suggested that comorbidities of patients with COVID-19, as the risks for severe conditions, may be different according to the characteristics of patients in each country and region, and any comorbidities in patients with COVID-19 yielded poor clinical outcomes than those without. Additionally, most of the reported comorbidities in these previous studies were collected from the patients’ self-report, and it is not clear if their comorbidities were controlled or treated. Considering the presence of various risk factors relating to severity of COVID-19, comorbidities would not be considered the only factor related to poor outcomes of COVID-19.
Our study has some limitations, including those inherent to the nature of systematic reviews and meta-analyses. The study was limited to articles written in English. We collected data on the clinical features of patients with COVID-19 from previously published studies. Because the reported symptoms, signs, and clinical outcomes varied among the studies, the number of studies focusing on each clinical feature varied in this meta-analysis. Unreported symptoms, comorbidities, and complications in each study may exist due to the low prevalence. The prevalence of various manifestations of disease severity, including ARDS, may be influenced by the number of people in whom the infection was confirmed using PCR. This study was not a meta-analysis using individual patients’ data, so we were unable to obtain data for the number of patients with multiple symptoms or comorbidities. Some patients in each study remained in the hospitals at the end of the observational period. Therefore, the clinical outcomes, such as the number of patients who died or were discharged, may have differed at the final outcomes.
5. Conclusion
The disease severity and the frequencies of clinical outcomes and symptoms of COVID-19 changed from December 2019 to February 2020 in accordance with increasing variations in both the transmission route and the risk of exposure. After the disease spread globally, the disease severity for the majority of patients with COVID-19 became less severe in China, while explosive outbreaks with many fatal cases occurred in countries such as the US, Italy, Spain and the UK. More importantly, even though patients with COVID-19 initially showed mild conditions, some patients developed severe conditions later. Further studies are needed with patients whose final outcome can be determined, especially after the spread of COVID-19 as a pandemic.
Financial disclosure
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
T.M. and K.K. conceived the study. T.M. and H.A. conducted the literature search, compiled and screened the list of articles, selected the relevant documents, reviewed the eligible articles, and extracted the data. T.M. analyzed the data. All authors interpreted the study results. T.M. wrote the initial draft of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
Acknowledgment
The authors thank Serika Nakamura for her general assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resinv.2020.05.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 11 March 2020. WHO Director-General’s opening remarks at the media briefing on COVID-19.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at: [Google Scholar]
- 5.World Health Organization. reportReport of the WHO-China Joint mission on coronavirus disease 2019 (COVID-19). Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. (Accessed March 3, 2020).
- 6.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong Z.D., Tang A., Li K.F., Li P., Wang H.L., Yi J.P. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang province, China, 2020. Emerg Infect Dis. 2020;26(5) doi: 10.3201/eid2605.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. reportCoronavirus disease (COVID-19) situation Reports. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200301-sitrep-41-covid-19.pdf?sfvrsn=6768306d_2 (Accessed March 3, 2020).
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J Am Med Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Agresti A., Coull B.A. Approximate is better than “exact” for interval estimation of binomial proportions. Am Statistician. 1998;52:119–126. [Google Scholar]
- 18.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Review manager (RevMan) [computer program] version 5.3 copenhagen: the nordic cochrane center. The Cochrane Collaboration; 2014. [Google Scholar]
- 20.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 23.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X.-W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y.H., Dong J.H., An W.M., Lv X.Y., Yin X.P., Zhang J.Z. Clinical and computed tomographic imaging features of Novel Coronavirus Pneumonia caused by SARS-CoV-2. J Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imag. 2020 Feb 28 doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020 Feb 29 doi: 10.1093/cid/ciaa199. pii: ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020 Feb 25 doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gracia Gutiérrez A., Poblador-Plou B., Prados-Torres A., Ruiz Laiglesia F.J., Gimeno-Miguel A. Sex differences in comorbidity, therapy, and health services' use of heart failure in Spain: evidence from real-world data. Int J Environ Res Publ Health. 2020;17(6) doi: 10.3390/ijerph17062136. pii: E2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2) doi: 10.3390/v12020135. pii: E135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . October 2004. WHO SARS risk assessment and preparedness framework.https://www.who.int/csr/resources/publications/WHO_CDS_CSR_ARO_2004_2/en/ Available at: [Google Scholar]
- 35.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 Mar 26 doi: 10.1093/cid/ciaa330. pii: ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higuera Iglesias A.L., Kudo K., Manabe T., Corcho Berdugo A.E., Corrales Baeza A., Alfaro Ramos L. Reducing occurrence and severity of pneumonia due to pandemic H1N1 2009 by early oseltamivir administration: a retrospective study in Mexico. PloS One. 2011;6(7):e21838. doi: 10.1371/journal.pone.0021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keyaerts E., Vijgen L., Maes P., Duson G., Neyts J., Van Ranst M. Viral load quantitation of SARS-coronavirus RNA using a one-step real-time RT-PCR. Int J Infect Dis. 2006;10(1):32–37. doi: 10.1016/j.ijid.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.Y., Choe P.G., Oh Y., Oh K.J., Kim J., Park S.J. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Kor Med Sci. 2020;35(5):e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Emergencies preparedness, response. Human infection with avian influenza (H7N9) virus – China: Update. Available at: https://www.who.int/csr/don/05-september-2018-ah7n9-china/en/. Accessed March 3, 2020.
- 40.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolhouse M.E., Haydon D.T., Antia R. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol Evol. 2005;20(5):238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Roode J.C., de Castillejo C.L., Faits T., Alizon S. Virulence evolution in response to anti-infection resistance: toxic food plants can select for virulent parasites of monarch butterflies. J Evol Biol. 2011;24(4):712–722. doi: 10.1111/j.1420-9101.2010.02213.x. [DOI] [PubMed] [Google Scholar]
- 43.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. 2020 Mar 30 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.