Abstract
The pandemic of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is spreading rapidly across the world. Currently, the COVID-19 pandemic is affecting the continuity of essential routine healthcare services and procedures, including chimeric antigen receptor T-cell (CAR-T) therapy, a life-saving option for patients with relapsed/refractory (R/R) hematologic malignancies. Due to the rapid disease progression of hematological malignancies, there is an urgent need to manufacture and utilize CAR T-cells. However, CAR-T treatment has become extraordinarily challenging during this COVID-19 pandemic. Thus, many medical and technical factors must now be taken into consideration before, during, and after CAR-T therapy. The purpose of this review is to provide brief suggestions for rational decision-making strategies in evaluating and selecting CAR T-cell treatment and appropriate CAR T-cell products, and protective strategies for medical staff and patients to prevent infection in the midst of the current COVID-19 pandemic.
Keywords: Chimeric antigen receptor T-cells, COVID-19, Relapsed/refractory hematological malignancies, Immunocompromised, Cytokine release syndrome
1. Background
Since December 2019, the emergence of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is rapidly spreading worldwide [1]. As of April 15, 2020, cumulatively, more than 1,354,400 cases and 127,400 deaths were reported globally in more than 195 countries. In order to fight the COVID-19 pandemic, a focus has been building a global community to allow for a shared future for humankind, strengthening international cooperation and fostering more significant synergistic relationships between countries.
As this novel coronavirus continues to spread across the world, healthcare systems are under unprecedented pressure. As healthcare systems become overwhelmed, treatment for patients with relapsed/refractory (R/R) malignant diseases might be inevitably affected. Chimeric antigen receptor T-cell (CAR-T) therapy has proven to be a successful therapeutic strategy for improving clinical outcomes of patients with R/R hematologic malignancies [2,3]. Empirically, patients requiring CAR-T therapy are those with highly aggressive or progressive diseases, and, to a certain extent, CAR-T therapy is the sole option for those with high-risk hematologic malignancies and rapid disease progression requires the timely manufacturing and infusion of CAR-T cells into these patients.
Unfortunately, CAR-T treatment strategies are encountering unexpected complications and challenges amid the COVID-19 pandemic. Hence, weighing the risks and benefits of CAR-T therapy and improving the practical efficiency of treatment during this critical period is of utmost importance. In this review, we aimed to summarize the status quo of CAR-T therapy and to provide suggestions for the administration of CAR-T treatment during the COVID-19 pandemic based on our clinical experience.
2. CAR-T treatment and the COVID-19 pandemic
2.1. CAR-T treatment
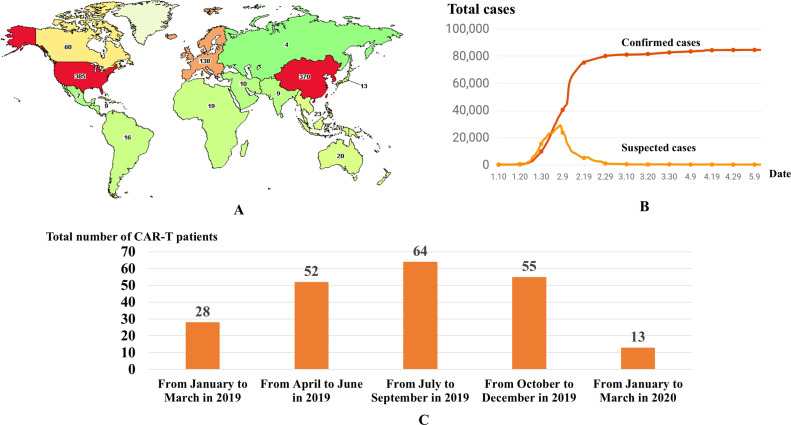
Currently, the Food and Drug Administration (FDA) of the United States has approved two CD19-targeted CAR T-cell products, tisagenlecleucel and axicabtagene ciloleucel, for the treatment of B-cell malignancies. In addition to these two FDA-approved products, other CAR-T products are under investigation in ongoing clinical trials. As of May 29, 2020, 1015 clinical trials of CAR-T therapy have been registered at clinicaltrials.gov. Meanwhile, there are currently 370 Chinese clinical trials underway (Fig. 1 A). The surge of CAR-T studies undoubtedly represents the rapid development of this novel treatment strategy in China.
Fig. 1.
Activities of CAR-T treatment worldwide (A) and confirmed/suspected cases of COVID-19 in China (B). The number of patients receiving CAR-T therapy in Wuhan city quarterly (from January 2019 to March 2020) (C).
2.2. COVID-19 epidemic in China
Since December 2019, SARS-CoV-2 has been transmitted rapidly throughout central China, with a total of 84,548 confirmed cases and 4645 deaths recorded across China as of May 29, 2020. The cumulative numbers of confirmed cases are listed in Fig. 1B.
2.3. Administration of CAR-T treatment during the COVID-19 pandemic
Despite the challenges, the administration and execution of CAR-T treatments have not been significantly disrupted during the COVID-19 pandemic. For instance, twenty patients received CAR-T treatment in the First Affiliated Hospital, Zhejiang University; among whom, six patients with R/R multiple myeloma (R/R MM) received B-cell maturation antigen (BCMA) CAR-T therapy, and nine patients with R/R acute lymphocytic leukemia (R/R ALL) and five patients with R/R lymphoma received CD19 CAR-T therapy. Indeed, CAR-T therapy was widely administered in hospitals in many countries, with 11, 8, 4, 6, 1, 7, 11 and 11 patients treated in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology; Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Science & Peking Union Medical College; the First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital; Xinqiao Hospital, Army Medical University; Shanghai Tongji Hospital; Peking University Third Hospital; Zhujiang Hospital of Southern Medical University; Chaim Sheba Medical Center, Israel; and Institut Paoli-Calmettes, Marseille, France, respectively. Detailed information about these treatments is shown in Table 1 . It is worth mentioning that none of the patients or medical staff in these trials contracted SARS-CoV-2. However, in Wuhan, the center of China's coronavirus outbreak, the CAR-T treatments have been severely affected. In Fig. 1C, we summarized the number of patients receiving CAR-T therapies in various CAR-T centers of Wuhan from January 2019 to March 2020. From the chart, it depicted the drastic reduction of CAR-T administration in the first quarter of 2020. The sharp decline of the CAR-T administration coincided with the COVID-19 outbreak period in Wuhan city.
Table 1.
CAR-T Treatment in Representative Regions (From January 2020 to March 2020).
| No. | Centers | CAR-T type (Number) | Disease type (Number) |
|---|---|---|---|
| 1 | The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China | CD19 CAR-T (12) | ALL (9) |
| BCMA CAR-T (6) | MM (6) | ||
| CD20/CD22 dual target CAR-T (2) | NHL (5) | ||
| 2 | Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China | C19 CAR-T (60) | ALL (4) |
| BCMA CAR-T (8) | MM (0) | ||
| CD30 CAR-T (10) | NHL (7) | ||
| 3 | Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Tianjin, China | C19 CAR-T (5) | ALL (1) |
| BCMA CAR-T (3) | MM (3) | ||
| NHL (4) | |||
| 4 | Xinqiao Hospital, Army Medical University, Chongqing, China | C19 CAR-T (3) | ALL (4) |
| CD7 CAR-T (1) | |||
| 5 | Shanghai Tongji Hospital, Shanghai, China | CD19 CAR-T (5) | MM (1) |
| BCMA CAR-T (1) | NHL (5) | ||
| 6 | Peking University Third Hospital, Beijing, China | BCMA CAR-T (1) | MM (1) |
| 7 | Zhujiang Hospital of Southern Medical University, Guangzhou, China | CD19 CAR-T (7) | ALL (6) |
| NHL (1) | |||
| 8 | Chaim Sheba Medical Center, Isarel | CD19 CAR-T (11) | |
| BCMA CAR-T (0) | NHL(11) | ||
| 9 | Institut Paoli-Calmettes, Aix Marseille Univ, CNRS, INSERM, CRCM, Marseille, France | CD19 CAR-T (9) | NHL (9) |
3. Considerations for evaluation prior to initiation of CAR-T therapy
3.1. Clinical characteristics of CAR-T therapy candidates
Following the initial success of CAR-T therapy in treating R/R ALL, its indication was expanded to treat multiple hematologic malignancies, including B-cell non-Hodgkin's lymphoma (B-NHL) and MM. Patients with R/R ALL often develop cytopenia, as well as elevated levels of C-reactive protein (CRP), enzymes including alanine aminotransferase, aspartate aminotransferase, and creatine kinase, and D-dimer, a fibrin degradation product. These conditions are quite similar to the clinical laboratory manifestations of SARS-CoV-2 infection. Furthermore, R/R ALL patients usually present with a high tumor burden, leukopenia, and suppression of normal myelopoiesis by proliferating lymphoblasts. These clinical presentations pose significant challenges in the diagnosis of comorbid hematological malignancies and SARS-CoV-2 infection. Moreover, patients with R/R B-NHL and MM often manifest fever of unknown origin. Since patients with R/R hematological malignancies are mainly immunocompromised with a high risk of viral infection, they would present with clinical signs and symptoms such as fever, ground-glass opacity in the lungs, or patchy bilateral shadows under chest computed tomography (CT) scans. In addition, there is a possibility of overlapping of chest radiographic findings in COVID-19 and lymphoma patients. For instance, the chest imaging of the COVID-19 patient might be misinterpreted as tumor progression. It should be noted that some regions have a limited capability of conducting CAR-T therapy. So, CAR-T candidates from other hospitals might be a source of inter-hospital transmission of COVID-19. This approach would increase the difficulties of COVID-19 prevention and complicate the identification of coexisting SARS-CoV-2 infection from hematological malignancies. Thus, prior SARS-CoV-2 screening tests are compulsory when transferring patients to designated hospitals or CAR-T centers to avoid cross-infection. The patients must be tested negative for COVID-19 before admitting to the hospitals for further treatment. To date, there are only dispersed data regarding the impact of COVID-19 in these vulnerable patients.
3.2. Evaluation of CAR-T treatment in accordance with epidemic prevalence
Patients requiring CAR-T cell therapy are usually those with extremely aggressive diseases and at high risk of rapid disease progression. Hence, the manufacture and infusion of CAR-T cells in these patients requires a tight and precise manner, as timeliness could eventually affect the treatment response rate. During the COVID-19 pandemic, constrained transportation resources are the most critical challenges for the delivery of CAR-T products, and accessibility to this therapy has sharply decreased. Therefore, local medical facilities and available resources should be adequately evaluated before commencing CAR-T treatment. If a patient is in a country, region, or community with a high prevalence of COVID-19 and local transportation is wholly blocked, CAR-T treatment should be deferred or replaced with an alternative treatment to reconcile with available healthcare system resources. Nonetheless, if the local prevalence of COVID-19 is low and the transportation system is not blocked, the CAR-T therapy or clinical trials should proceed in large university hospitals or CAR-T centers where all activities such as the manufacturing and CAR-T cell infusions take place on the same site. Resources, such as blood product supplies and intensive care units, should be prioritized for COVID-19 patients at this critical time. In unaffected areas or areas of the low prevalence of COVID-19, high-risk areas with sufficient relevant medical resources, areas where COVID-19 outbreak has been controlled, and/or areas where the government has lifted social confinement or traffic restrictions, the timing of CAR T-cell infusion should be evaluated on a case-by-case basis.
3.3. Evaluation of CAR-T treatment by screening epidemiological history
As recommended by the European Society for Blood and Marrow Transplantation (EBMT) [4] and the American Society for Transplantation and Cellular Therapy [5], in case of close contact with a person diagnosed with COVID-19, transplant procedures shall not be performed within at least 14, but preferably 21, days from the last contact. Moreover, patients must be tested negative in PCR tests prior to any transplant procedures and should be kept under close observation for the presence of COVID-19. We also suggest that for CAR-T therapy candidates who have been in close contact with individuals who are infected with or suspected to be infected with COVID-19, as well as those who have traveled to high-risk areas, medical procedures, including lymphocyte apheresis and lymphodepletion should be deferred for at least 14 days. In addition, patients should be quarantined, physical distancing should be maintained, and they should be closely monitored. For those with two consecutive negative results based on reverse transcription-polymerase chain reaction (RT-PCR) testing or genomic sequencing for SARS-CoV-2 from nasopharyngeal swabs, sputum, or other samples (taken approximately one week apart), COVID-19 infection in these patients can be ruled out entirely.
3.4. Evaluation of CAR-T treatment based on clinical manifestations
Clinical conditions should be further evaluated to determine whether the patient is an appropriate candidate for CAR-T treatment. During the COVID-19 pandemic, CAR-T treatment might be reconsidered for advanced patients at high risk of complications due to healthcare resource scarcity, such as situations where intensive care units (ICU), dialysis machines, and ventilators are being prioritized for severely ill COVID-19 patients. If patients with hematologic malignancies are suffering from relapsed or refractory diseases and require urgent treatment, timely CAR-T treatment might be beneficial if the epidemiological and clinical assessments rule out the possibility of SARS-CoV-2 infection. Presently, viral nucleic acid testing is a preferred method for confirmation of SARS-CoV-2 infection; however, among patients who have tested positive based on samples collected from the respiratory tract, approximately only 30 %–40 % of patients will have detectable levels of viral nucleic acids in blood samples [6,7]. Furthermore, patients with mild symptoms or asymptomatic cases remain significant challenges for mass screening. Due to the possibility of false negatives in nucleic acid testing and the existence of an infected but asymptomatic population, combined testing of different types of specimens (sputum, nasopharyngeal swab, fecal specimens, or lower respiratory tract secretion), serum immunoglobulin M (IgM) and immunoglobulin G (IgG) against SARS-CoV-2, along with lung CT scans should aid in improving the diagnostic sensitivity of suspected cases [5]. Expert consultation would be warranted in those conditions.
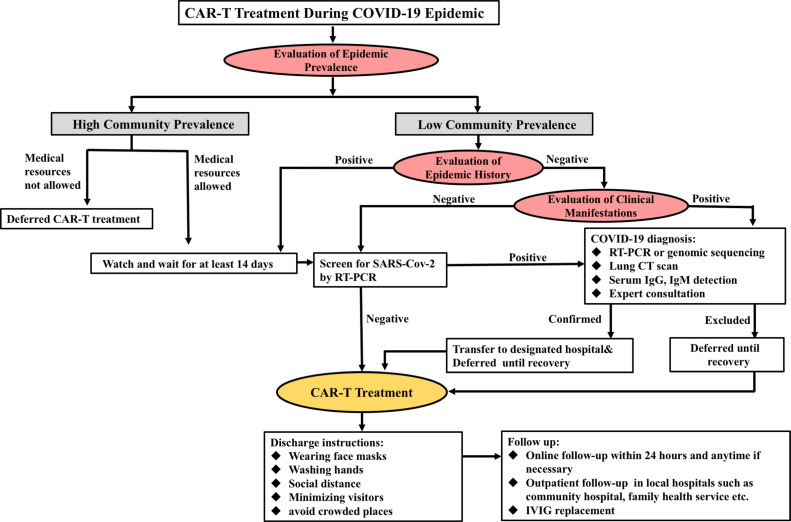
In situations where a patient has symptoms such as fever, fatigue, dry cough, or gastrointestinal manifestations, an extensive and thorough test should be conducted immediately to exclude the possibility of SARS-CoV-2 infection as soon as possible. Since specific lung CT imaging has shown various features of COVID-19 pneumonia, screening for COVID-19 is necessary when lung CT suggests typical changes associated with viral pneumonia, such as multiple small patches, interstitial changes, and ground-glass opacities. However, in some countries, CT is used as the diagnostic method for COVID-19 patients. Additionally, many patients were diagnosed incidentally based on CT scans without any clinical symptoms. Hence, CT scans should be done on every patient with hematological malignancies to rule out SARS-CoV-2 infection before hospital admission. In summary, CAR-T treatment should be either reconsidered or delayed in cases of equivocal imaging findings, as the sensitivity of the nucleic assay has yet to be clearly defined. The detailed admission procedure for receiving CAR-T treatment during the COVID-19 pandemic is displayed in Fig. 2 .
Fig. 2.
The admission procedures for CAR-T treatment during the COVID-19 pandemic. (Notes: Negative SARS-CoV-2 detection: both tests at an interval of more than 24 h are negative. Positive SARS-CoV-2 detection: any positive results in either sputum, nasopharyngeal swab, fecal specimens, or lower respiratory tract secretion test.).
4. Selection of appropriate CAR-T cell products
Genetically engineered CAR-T products are mostly mass-produced in good manufacturing practices (GMP) laboratories of local hospitals or related facilities, while others are manufactured in centralized laboratories, requiring a transfer from standardized laboratories to hospitals. Currently, most patients undergoing CAR-T therapy receive CAR T-cells derived from autologous T-cells, whereas those patients who have received allogeneic transplantation (allo-HSCT) may receive infusions of CAR-T cells derived from allogeneic donors. During the COVID-19 pandemic, restricted transportation and travel bans have resulted in enormous challenges for the timely delivery of manufactured CAR-T products and have been the main factors limiting the accessibility to this cellular therapy. Consequently, pre-emptive cryopreservation of manufactured CAR-T products prior to CAR-T cell infusion is a promising solution to help ensure timely administration of scheduled CAR-T infusions after the completion of lymphodepletion conditioning chemotherapy, without any delay in treatment.
However, for patients receiving donor-derived CAR-T cells, the donors' possible COVID-19 exposure statuses may affect the safety profiles of the CAR-T products. Recently, SARS-CoV-2 has been proven to be transmitted through the respiratory tract and close contacts [8]. Although RNA of SARS-CoV-2 can be detected in blood samples of infected patients, it remains unclear whether SARS-CoV-2 can be transmitted through blood transfusion or whether its viremia period could be a threat to CAR-T recipients [9]. Based on the US-FDA recommendation for human cells, tissues, or cellular or tissue-based products (HCT/P), donors with a confirmed or suspected COVID-19 infection are advised to delay T-cell donation for at least 28 days [10], so we suggest deferring cell donation for at least 28 days for donors who have a history of traveling to coronavirus-affected areas, have had close contact with suspected COVID-19 cases, and, of course, for individuals with COVID-19 infection. Nonetheless, concerning some patients might be in urgent need of treatment, and the treatments could not be deferred for 28 days, autologous T-cells CAR-T therapy is suggested. On the contrary, for those patients whose treatments could be deferred for 28 days, allogeneic CAR-T cell treatment is suggested. For axicabtagene ciloleucel immunotherapy products, fresh leukapheresis is shipped from Europe and other parts of the world to America for manufacturing, while for another commercial product, KYMRIAH, the leukapheresis is cryopreserved before shipping. Thus, we recommend KYMRIAH as the preferred choice during the COVID-19 period.
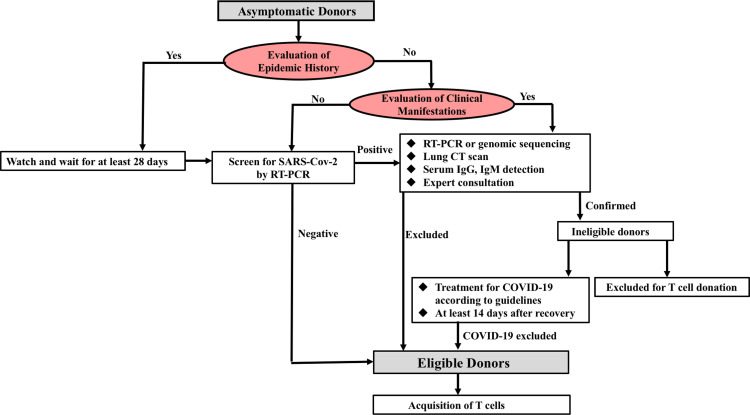
Considering the vast majority of patients who might be infected with SARS-CoV-2 who remain asymptomatic [11], it is necessary to prudently establish screening of the epidemiological history of the potential donors and to perform SARS-CoV-2 testing before apheresis. Also, the donor's body temperature should be closely monitored before and after apheresis to exclude donors with pre-symptomatic or asymptomatic infection. Next, to assure the safety profile of CAR-T products, antibody detection for IgM/IgG against SARS-CoV-2 should be conducted, if available. The screening procedures of CAR-T donors are shown in Fig. 3 .
Fig. 3.
The screening procedures of CAR-T cell donors.
In this regard, ongoing clinical trials with universal "off-the-shelf" CAR T-cell (UCAR-T) products, manufactured from third-party donors, are emerging as an attractive new concept, and it may be an ultimate encouraging solution [12]. This allogeneic, ready-to-use CAR-T product holds great promise in providing a more readily available choice, which as well as shortening manufacturing turnaround times, would bypass complicated donor screening procedures during the COVID-19 pandemic.
5. Protective strategies for medical staff and patients during CAR-T treatment
Based on previous experiences, patients with hematologic malignancies could be severely affected by SARS-CoV-2 infection mainly because of immunosuppression [13]. For immunosuppressed CAR-T patients, standardized management procedures and strict infection control measures must be implemented to minimize the risk of nosocomial infections and to ensure zero transmissions among patients and medical staff. Moreover, patients should receive CAR-T infusion in a ward equipped with laminar airflow facilities. All medical staff must adhere to strict preventive measures and rules during ward rounds to avoid cross-infection, and the use of personal protective equipment (PPE), such as disposable caps, surgical masks, and other garments, is imperative. Apart from PPE, proper handwashing methods and disinfection with alcohol or hydrogen peroxide should also be practiced by every staff member and patients [1].
It is also necessary to conduct health monitoring, including body temperature and respiratory symptoms in all medical workers who are involved in the administration of CAR-T treatments. If anyone shows any relevant signs or symptoms of infection such as fever, cough, fatigue, nasal congestion, runny nose, or diarrhea, social distancing should begin immediately and undergo nucleic acid testing should be conducted. During this pandemic, it is crucial to minimize the number of ward visits, and patients and caregivers should not be allowed to leave the ward during hospitalization. Like medical workers, patients’ vital signs, like body temperature, should also be closely monitored [14]. It is recommended that for some departments, the ward rounds and patient visits should be held via remote electronic communicating devices and software such as Zoom or Skype. For example, in some departments, only two physicians (a senior and a junior doctor) and a nurse should make up the group conducting rounds, and all doctors and nurses should be divided into a few groups, working different shifts to minimize person-to-person contact; this would minimize the likelihood of a doctor or nurse becoming infected, which would require the whole department being quarantined and leaving vulnerable patients without medical care.
6. Differential diagnosis of CAR-T-related toxicities and COVID-19 infection
The most common adverse effect of CAR-T therapy is cytokine release syndrome (CRS), which is triggered by the binding of chimeric antigen receptors with their target antigens [[15], [16], [17]]. Generally, CRS is reversible and only requires supportive therapy, but in some extreme cases, it can be life-threatening [18]. CRS is characterized by the activation of epithelial cells, which may result in pulmonary edema, eventually leading to acute respiratory failure in severe cases [19]. Previous studies have explored the correlation between cytokine profiles and the severity of CRS after CAR T-cell infusion. Early elevated serum levels of multiple cytokines, including interleukins IL-6, IL-8, and IL-10, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), and interferon-γ (IFN-γ) are associated with the development of severe CRS [20,21].
As IL-6 plays a vital role in CRS, the FDA has approved tocilizumab, an IL-6/IL-6R (interleukin-6 receptor) inhibitor, for the treatment of CRS in CAR-T patients. CRS also occurs in many patients with severe COVID-19 symptoms [21], resulting from the infiltration of SARS-CoV-2 in alveolar epithelial cells through activation of the angiotensin-converting enzyme 2 (ACE2) receptor. Interactions between these factors might induce activation of immune responses and the release of many pro-inflammatory cytokines. According to a multicenter retrospective study, some patients with SARS-CoV-2 infection showed higher levels of IL-1β, interleukin-1 receptor antagonist (IL-1RA), IL-7, IL-8, IL-9, IL-10, IFN-γ, macrophage inflammatory protein 1 alpha (MIP-1α), tumor necrosis factor-alpha (TNFα), and MCP-1 than those in the normal, healthy population [[21], [22], [23]]. In addition, the concentration of IL-6 in COVID-19 patients was reported to be significantly higher in non-survivors than survivors [8]. Therefore, early monitoring of IL-6 and IL-10 concentrations in serum would help identify critical cases and provide a basis for potential treatment strategies.
Some shreds of evidence suggest that early control of the cytokine storm might benefit severe cases. For instance, a multicenter trial is evaluating the efficacy of tocilizumab in patients with COVID-19-related pneumonia and elevated IL-6 concentrations (ChiCTR2000029765). In the latest analysis, this study has used tocilizumab to treat 21 severe or critical COVID-19 patients with a baseline IL-6 concentration of 132.38 ± 278.54 pg/mL. Treatment with tocilizumab has significantly improved both the symptoms and imaging manifestations of these patients [9]. Recently, Zhang et al. reported successful treatment with tocilizumab in a patient with MM complicated by a severe COVID-19 infection [24]. Nonetheless, further data from large-scale randomized controlled trials are urgently needed to confirm the efficacy of tocilizumab in these settings.
Intriguingly, an artificial liver treatment is recommended to inhibit pro-inflammatory cytokine cascade in these critically ill patients. The indication for an artificial liver support system is based on the concentration of inflammatory factors in the blood; for example, an IL-6 concentration ≥ 5 times the upper limit of normal or an increase in concentrations occurring at least once per day [10]. As for patients with respiratory symptoms or abnormalities on lung imaging after CAR T-cell infusion, it is crucial to differentiate COVID-19 pneumonia from CAR-T-related pulmonary changes. Rapid and repeated COVID-19 RT-PCR testing should be conducted in suspected cases of COVID-19. Considering that both CAR-T therapy-related CRS and COVID-19-related CRS would benefit from immunosuppressive interventions, the pre-emptive use of tocilizumab might serve as an effective therapeutic option for patients with elevated IL-6 concentrations before a final diagnosis is confirmed.
7. Follow-up and patient education after hospital discharge
Before discharging patients from the hospital, adequate verbal instructions must be provided in-person to educate the individuals on COVID-19 prevention strategies. Pamphlets with clear instructions and steps showing how to wear a face mask properly along with correct handwashing methods should be distributed. Patients should also be reminded to practice social distancing and avoid gathering in crowded places. With the rapid evolution of the COVID-19 pandemic, telemedicine is now becoming a safe strategy, as it reduces the frequency of close contact and the potential risk of infection, especially in immunocompromised populations. As an indispensable communication platform in China, WeChat allows for smooth online video calling at affordable prices, while WhatsApp and Zoom are commonly used for multidisciplinary meetings in Israel and other countries. These social media platforms serve as a valuable alternative to replace in-person patient follow-ups [25]. Therefore, we recommend routine online follow-ups for outpatients after CAR-T therapy, if accessible. Nevertheless, if the patients are presented with acute or worsening symptoms, they should seek medical care from nearby hospitals or non-COVID-19 hospitals.
For each discharged patient, the first online follow-up should be arranged within 48 h by the primary doctor, who should be available at any time, if necessary. The outpatient follow-ups should be performed at two weeks, one month, two months, three months, six months, and twelve months after hospital discharge. To reduce the risks of public transportation, patients can complete follow-ups with physical examinations and blood tests at local hospitals, such as community hospitals and family health services. In addition to routine examinations, SARS-CoV-2 RT-PCR testing of sputum and fecal specimens, pulmonary function tests, and lung CT scans are also required.
It should be noted that after CAR-T treatment, patients might experience prolonged B-cell or plasma-cell aplasia and concomitant hypoimmunoglobulinemia. If applicable, intravenous immunoglobulin (IVIG) replacement should be administered to these patients every two weeks at local hospitals.
8. CAR-T treatment for patients after recovery from COVID-19
Most CAR-T patients experience extremely aggressive disease states and are at risk of disease progression, which tends to postpone therapeutic schemes and can significantly affect clinical outcomes of high-risk patient populations. To date, safety profiles of CAR-T treatment in those recovering from a COVID-19 infection have not yet been confirmed. However, the risk of conservative suspension of CAR T-cell therapy to avoid the theoretical risk of viral reactivation outweighs any advantages. Therefore, we recommend that CAR T-cell therapy should commence once patients have clinically recovered from COVID-19. These patients must have no symptoms (symptom remission) for at least 14 days, show noticeable improvement in lung imaging, improvement in pulmonary function test, and have two consecutive negative results of nucleic acid tests with a sampling interval of more than 24 h. What is worth highlighting is that the treatment of COVID-19 should be prioritized over CAR T-cell therapy under any circumstances, and CAR-T cell therapy should not be administered to a COVID-19 patient or patients with hypoxemia.
9. Patient care during treatment deferment
CAR-T therapy is often the only treatment option for patients with relapsed or refractory hematologic diseases. As recommended by the expert opinion of the EBMT and the American Society for Blood and Marrow Transplantation (ASBMT), bridging chemotherapy should focus on disease control rather than remission induction to minimize organ toxicity or the risk of infections [26]. Furthermore, we also recommend low-dose daily or weekly maintenance chemotherapy for patients under CAR-T deferment, but systemic steroids should be stopped for three days before leukapheresis to preserve functional T-cells. Cytotoxic drugs should also be avoided before leukapheresis, as recommended by experts from the EBMT and ASBMT [26].
10. COVID-19 infection after CAR-T treatment
10.1. Susceptibility to COVID-19 infection
Although there is limited data on the epidemiology and clinical manifestations of COVID-19 in CAR-T patients, malignant comorbidities have been reported as a risk factor for poor clinical outcomes in COVID-19 patients according to a retrospective study4. Furthermore, CAR-T patients are usually immunocompromised due to characteristics of R/R hematologic malignancies per se, previous histories of chemotherapy, other immunosuppressive agents, or allo-HSCT, or prolonged B-cell or plasma-cell aplasia and concomitant hypoimmunoglobulinemia after CAR-T treatment [27]. Hence, it is rational to assume that patients receiving CAR-T treatment are populations that are inherently more vulnerable to SARS-CoV-2 infection.
10.2. Diagnosis of COVID-19 infection in CAR-T patients
If CAR-T patients complain of fever (temperature >37.5 °C), cough, or any other respiratory symptoms after CAR-T treatment, they should be quarantined as soon as possible and be examined for respiratory viral pathogens, including SARS-CoV-2. A lung CT scan is also recommended. Of note, these patients might present with atypical and delayed clinical manifestations, such as mild fever and nonproductive cough, with atypical radiologic patterns [28] and even negative IgM and IgG tests, so conventional SARS-CoV-2 screening should, therefore, be performed in these patients. It might be necessary to repeat nucleic acid tests.
10.3. Treatment of COVID-19 infection in CAR-T patients
If a patient who has completed CAR-T treatment is confirmed to have a COVID-19 infection, we recommend he or she be treated based on well-acknowledged guidelines [4,5] and local medical guidelines for the diagnosis and treatment of COVID-19. On the other hand, high-dose steroids should be avoided as it could disrupt CAR-T cell persistence and affect the outcomes of CAR-T therapy. Also, tocilizumab or artificial liver treatment is recommended for severe COVID-19 cases with elevated cytokine levels.
11. Conclusion
In conclusion, this report is aimed at providing practical strategies for rational decision-making of CAR-T treatment in patients with hematological malignancies amid the COVID-19 pandemic. We advise that the recommendations described above should be updated and refined continuously as additional information about the pandemic become available. Moreover, our recommendations should be applied with flexibility based on local resources, governmental policies, and the severity of the COVID-19 outbreaks.
Declaration of Competing Interest
No competing interests to declare.
Acknowledgments
This work was supported by the grants from the Natural Science Foundation of China (81770201, 81730008) and Key Project of Science and Technology Department of Zhejiang Province (2018C03016-2).
Contributor Information
Yongxian Hu, Email: huyongxian2000@aliyun.com.
Elaine Tan Su Yin, Email: elainetansuyin@outlook.com.
Yingying Yang, Email: yangyingying15@zju.edu.cn.
Hengwei Wu, Email: 457166156@qq.com.
Guoqing Wei, Email: weiguoqing2000@sina.com.
Junwei Su, Email: zjusujunwei@zju.edu.cn.
Qu Cui, Email: cncuiqu@hotmail.com.
Aiyun Jin, Email: ayjin2007@aliyun.com.
Li Yang, Email: yanglizju@163.com.
Shan Fu, Email: fushan0817@163.com.
Jianfeng Zhou, Email: jfzhou@tjh.tjmu.edu.cn.
Lugui Qiu, Email: qiulg@ihcams.ac.cn.
Xi Zhang, Email: zhangxxi@sina.com.
Aibin Liang, Email: lab7182@tongji.edu.cn.
Hongmei Jing, Email: drjinghm@163.com.
Yuhua Li, Email: liyuhua2011gz@163.com.
Didier Blaise, Email: BLAISED@ipc.unicancer.fr.
Mohamad Mohty, Email: mohamad.mohty@inserm.fr.
Arnon Nagler, Email: Arnon.Nagler@sheba.health.gov.il.
He Huang, Email: huanghe@zju.edu.cn.
References
- 1.World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. [Google Scholar]
- 2.Singh A.K., McGuirk J.P. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21(3):e168–e178. doi: 10.1016/S1470-2045(19)30823-X. [DOI] [PubMed] [Google Scholar]
- 3.Elsallab M., Levine B.L., Wayne A.S., Abou-El-Enein M. CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol. 2020;21(2):e104–e116. doi: 10.1016/S1470-2045(19)30729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Available from: https://www.ebmt.org/sites/default/files/2020-04/EBMT-COVID-19-guidelines_v.6.1%282020-04-07%29.pdf.
- 5.Available from: https://www.astct.org/viewdocument/astct-interim-patient-guidelines-ap?CommunityKey=d3949d84-3440-45f4-814290ea05adb0e5&tab=librarydocuments.
- 6.Wang Ming, Wu Qing, Xu Wanzhou, Qiao Bin, Wang Jingwei, Zheng Hongyun. Clinical diagnosis of 8274 samples with 2019 novel coronavirus in Wuhan. medRxiv. 2020 doi: 10.1101/2020.02.12.20022327. [DOI] [Google Scholar]
- 7.Hong Mei, Fang Yun, Xia Linhui. Recommendations for prophylaxis and prevention of COVID-19 in hematology department. Zhonghua Xue Ye Xue Za Zhi. 2020 doi: 10.3760/cma.j.issn.02532727.2020.0005. published online. March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi Qifang, Wu Yongsheng, Mei Shujiang, Ye Chenfei, Zou Xuan, Zhang Zhen. Epidemiology and transmission of COVID-19 in Shenzhen China: analysis of 391 cases and 1,286 of their close contacts. MedRxiv. 2020 doi: 10.1101/2020.03.03.20028423. 03.03.20028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020 doi: 10.1016/j.tmrv.2020.02.003. pii: S0887-7963(20)30014-30016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Yimin, Yu Liang, Tang Ling Ling, Zhu Mengfei, Jin Yanqi, Wang Zhouhan. A promising anti-cytokine-storm targeted therapy for COVID-19: the artificial-liver blood-purification system. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020 doi: 10.1126/science.abb3221. March 16. pii: eabb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D., Zhao J., Song Y. Engineering switchable and programmable universal CARs for CAR T therapy. J Hematol Oncol. 2019;12(1):69. doi: 10.1186/s13045-019-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z., Xiong H., Li J.X., Li H., Tao F., Yang Y.T. COVID-19 with post-chemotherapy agranulocytosis in childhood acute leukemia: a case report. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E004. doi: 10.3760/cma.j.issn.0253-2727.2020.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozma M.A., Maroufi P., Khodadadi E., Köse Ş, Esposito I., Ganbarov K. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez Med. 2020;28(2):153–165. [PubMed] [Google Scholar]
- 15.Liu D., Zhao J. Cytokine release syndrome: grading, modeling, and new therapy. J Hematol Oncol. 2018;11(1):121. doi: 10.1186/s13045-018-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yakoub-Agha I., Chabannon C., Bader P., Basak G.W., Bonig H., Ciceri F. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) Haematologica. 2020;105(2):297–316. doi: 10.3324/haematol.2019.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohty M., Gautier J., Malard F., Aljurf M., Bazarbachi A., Chabannon C. CD19 chimeric antigen receptor-T cells in B-cell leukemia and lymphoma: current status and perspectives. Leukemia. 2019;33(12):2767–2778. doi: 10.1038/s41375-019-0615-5. [DOI] [PubMed] [Google Scholar]
- 18.Hay K.A., Hanafi L.A., Li D., Gust J., Liles W.C., Wurfel M.M. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(1) doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portnoy J., Waller M., Elliott T. Telemedicine in the era of COVID-19. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.03.008. pii: S2213-2198(20)30249-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kansagra A.J., Frey N.V., Bar M., Laetsch T.W., Carpenter P.A., Savani B.N. Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT) Bone Marrow Transplant. 2019;54(11):1868–1880. doi: 10.1038/s41409-019-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruso H.G., Heimberger A.B., Cooper L.J.N. Steering CAR T cells to distinguish friend from foe. Oncoimmunology. 2018;8(10) doi: 10.1080/2162402X.2016.1271857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Xh, Zheng Ki, Pan Kh, Xie Yp, Zheng Mh. COVID-19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol. 2020;7(4):e351–e352. doi: 10.1016/S2352-3026(20)30074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]