Abstract
Very few economical and process engineering studies have been made concerning the scale-up and implementation of nanomagnetic particle manufacturing into a full-scale plant, and determination of its viability. In this work we describe such a study for two types of industrial plants, one for manufacturing magnetic particles for applications in the environmental area, and the other for manufacturing nanomagnetic particles for applications in the biotechnology area; the two different applications are compared. The following methodology was followed: establish the manufacturing process for each application; determine the market demand of the product (magnetic nanoparticles) for both applications; determine the production capacity of each plant; engineer all the manufacturing process, determining all the process units and performing all the mass and energy balances for both plants; scale-up the main equipment; and determine the global economic impact and profitability. At the end both plants are found to be technologically and economically viable, the characteristics of the final products being, however, quite different, as well as the process engineering, economic analysis, and scale-up.
Keywords: economic analysis, plant design and process engineering, nanomagnetic particles
1. Introduction
A large amount of literature has been devoted to the research on the manufacture of magnetic nanoparticles [1,2,3,4,5]. The potential applications of magnetic nanoparticles have been identified and represent a broad window of research areas [6,7,8,9,10,11,12]. Examples of some existing magnetic nanoparticle products in the market for environmental and biotechnological applications are: 3320DX-SkySpring Nanomaterials, and 900062-SigmaAldrich. Nonetheless, the large majority of the manufacturing methods described in literature and their applications have not reached yet large-scale production. The main reason behind this failure is that usually when reaching industrial scale, most of the methods are simply not economical or technological viable. It is also important to notice that among all this literature almost no economic and technological study has been published considering the possible adaptation of the laboratorial manufacturing processes into a larger-scale production. It is, therefore, vital, at this stage of scientific and technological development, to analyze the economic and technological viability and adaptation of the proposed manufacturing processes in order to determine if and how they may be actually applied into industrial applications [13].
In this paper we present a study of the economic and technological viability of selected magnetic nanoparticle manufacturing processes for environmental and biotechnological applications. Results and differences between both are presented and analyzed.
2. Materials and Methods
The following methodology was applied: (a) choose the manufacturing process for each application; (b) proceed with a market study to determine the demand of the product (magnetic nanoparticles) for both applications; (c) determine the size of each plant; (d) analyze all the process steps, choose all the process units and perform all the mass and energy balances required in both plants; (e) detail scale-up design of the main equipment; and (f) analyze the global economic impact and profitability.
3. Industrial Design-Economic and Technological Viability
For a good estimation of economic and technological viability it is important to follow a determined thread-line [13]: First, the selection of the manufacturing process, considering the particular application, must be done; then a good study of the market will define the size of the plant; the size of the plant will define the required mass flows; these will then be the basis for the design of the equipment, the mass and energy balances, and the overall process flowsheet, leading to conclusions on the technological viability of the process; finally the overall economic analysis of the full industrial process is done and economic viability determined.
In this section we will follow this thread-line comparing the results obtained for the plant producing magnetic nanoparticles for environmental applications with the one for biotechnological applications.
It is important to be aware that making an overall economic estimation is always risky as it depends on many factors. However, to assure the accuracy of the estimations, we have based our calculations on well recognized and established economic analysis published in reference books such as [14,15].
3.1. Process Selection
The selection of the manufacturing process for the magnetic nanoparticles is dependent of their future application. In the cases under study, for biotechnological applications usually monodispersed particles are required with same shape and size, and nanosize is a requirement (so they behave superparamagnetically), for environmental applications polydispersed particles are not a drawback and large sizes are usually required for cheaper and more efficient real treatment processes [16].
As indicated in the introduction, many methods have been developed for the manufacturing of magnetic nanoparticles; these include methods like co-precipitation [17], hydrothermal [18], microemulsion [19], thermal decomposition [20], sol-gel [21], laser pyrolysis [22], etc. After a preselection based on applicability level, we have reduced the proposed methods to the ones presented in Table 1, where a comparison on their characteristics is given.
Table 1.
| Manufacturing Method | Synthesis | Reaction Temp. (°C) | Reaction time | Solvent | Surface-Capping Agents | Size Distribution | Shape Control | Yield |
|---|---|---|---|---|---|---|---|---|
| Co-precipitation | Very simple | 20–90 | Minutes | Water | During/after reaction | Relatively narrow | Not good | High |
| Microemulsion | Complicated | 20–50 | Hours | Organic agents | During reaction | Relatively narrow | Good | Low |
| Thermal decomposition | Complicated | 100–320 | Hours-days | Organic agents | During reaction | Very narrow | Very good | High |
| Hydrothermal | Simple | 200–250 | Hours-days | Water-ethanol | During reaction | Very narrow | Very good | Medium |
3.1.1. Environmental Applications
For the applications in sight (adsorption, Fenton reaction, catalysis, etc.) [11,27] the main characteristics of the chosen process must be: easiness of synthesis; low-cost particles; easiness of processing; easiness of scale-up; production of large quantities of particles; high flow throughputs; and monodispersity is not a requirement. Considering these factors and the information on Table 1, the choice fell on the co-precipitation method.
Among all co-precipitation methods, the inverse co-precipitation is preferred [28,29,30,31,32,33] due to its superior process control and characteristics of the obtained particles. In order to choose the best values for the process variables (drying temperature, type of alkaline base, concentration of precursor solution, type of surface agent, etc.) a laboratorial study was done and is presented in [28]. Based in this article the characteristics of the process to be used in large-scale production are: use NH4OH as alkaline base, no surfactant required, concentration of salt equal to 0.2 M and drying temperature of 90 °C, while other variables are as detailed in [28] with the modification of using ambient atmosphere instead of inert atmosphere so we could shorten the reaction time and increase production capability, obtaining a mixture of micron and nano-sized particles [28]. The global reaction (1) for this process is:
| 3FeSO4 + 6NH4OH + ½ O2 → Fe3O4 + 3H2O + 3(NH4)2SO4 | (1) |
3.1.2. Biotechnological Applications
For the applications in sight (drug delivery, magnetic hyperthermia, etc.) [27,34,35] the main characteristics of the chosen process must be: easiness of synthesis; monodispersity; easiness to scale-up; production of moderate quantities; low flow-throughputs; good shape control and crystallinity and low nanosizes (particles should behave supermagnetically). Considering these factors and Table 1, the choice fell on the hydrothermal method.
The final particles will be delivered with a carbon layer at their surface, so they can be further functionalized according to each specific need.
The method we have chosen to upscale will be the one followed by [36], choosing to use as reactants the iron salt, starch and sodium acetate, and as reaction conditions a temperature of 80 °C during 20 min and then a hydrothermal treatment of 20 h. The involved reactions (2)–(4) are [37,38,39,40]:
| FeCl2·4H2O + 2CH3COONa → Fe(OH)2 + 2NaCl + 2CH3COOH + 2H2O | (2) |
| 2Fe(OH)2 + ½ O2 → 2FeOOH + H2O | (3) |
| Fe(OH)2 + 2FeOOH → Fe3O4 + 2H2O | (4) |
Global reaction between (3) and (4):
| 3Fe(OH)2 + ½ O2 → Fe3O4 + 3H2O | (5) |
Global general reaction (6):
| FeCl2·4H2O + 2CH3COONa + 2Fe(OH)2 + ½ O2 → 2NaCl + 2CH3COOH + Fe3O4 + 5H2O | (6) |
3.2. Market Study
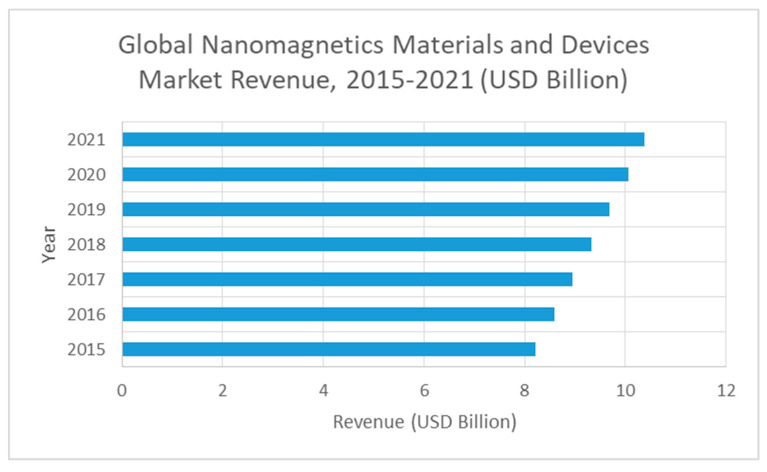
Nanomagnetic materials are a very recent commodity. Therefore, market data is very limited and still restricted. Spanish internal market is not big enough and therefore the global market must be analyzed. It is important to notice that in Europe nanomaterials and nanotechnologies are considered a key enabling technology. The global market value of products that incorporate nanotechnologies as their key component was estimated at € 700 billion in 2015 and is estimated at € 2 trillion by 2020 (generating, at the same time, between 2 and 6 million jobs, respectively) [41]. According to various reports, a large increase is expected in the global nanomaterials market, at least, until 2021, as shown in Figure 1.
Figure 1.
Global market for nanomaterials (based on data from [42]).
The largest difference between environmental and biotechnological applications are their market and flow quantities that are handled. In fact, environment tends to be a low-cost market with high-throughputs, while biotechnology is usually a high-cost market with low-throughputs. This marks a very distinctive difference between the demand of each market: environmental applications require simple (including microsized particles to handle large flows) and low-cost particles (no need for good shape and functionalized particles as they would represent an unbearable cost when comparing with competitive technologies), while biotechnological applications require more complicated (small nanosized particles) and functionalized and well-shaped (thus more expensive) particles.
3.2.1. Environmental Applications
The majority of the current nanomagnetic particles in the market are directed towards the pharmaceutical, biotechnological and biomedical applications. Concerning environmental applications, the market share is low and almost no data is available. From the world market economy values presented in Figure 1, we may conclude that this increasing market represents a good opportunity for well-planned business plants, and not only for biotech applications. However, due to the low market share corresponding to environmental applications and to the low-level of competition we choose a conservative figure of about 0.5% of the worldwide nanomagnetic particle production for our plant production. This represents 40 million euros in annual production. The next step is to settle the price for the main product of our plant (nanomagnetic particles). By consulting the literature, we see that the price of magnetic nanoparticles varies depending on their utility, characteristics and form of presentation. For commercial nanoparticles without any functionalization, the cost can range from $380/kg (for iron oxides, both magnetite and maghemite) to $2255/kg (for nZVI) [43]. Other authors indicate that the price of 1 kg of maghemite nanoparticles varies between $200 and $400 depending, as already mentioned, on the size of the particles, the purity and the method of synthesis [44]. In Table 2 we present the current prices practiced by the company Skyspring Nanomaterials Inc.
Table 2.
Prices of iron oxide nanomagnetic particles (Skyspring Nanomaterials Inc, 2020, [45]).
| Product | Price |
|---|---|
| Iron oxide nanopowder/nanoparticles (alpha-Fe2O3, 99%, 20–40 nm) | $98/100 g $262/500 g $380/1000 g |
| Iron oxide nanopowder/nanoparticles (gamma-Fe2O3, 99%, 20–40 nm) | $98/100 g $262/500 g $380/1 kg |
| Iron oxide nanopowder/nanoparticles (Fe3O4, 98 + %, 20–30 nm) | $98/100 g $262/500 g $340/1 kg |
For our study we will consider a price of 380 €/kg, which corresponds to a production of about 100 metric ton/year.
3.2.2. Biotechnological Applications
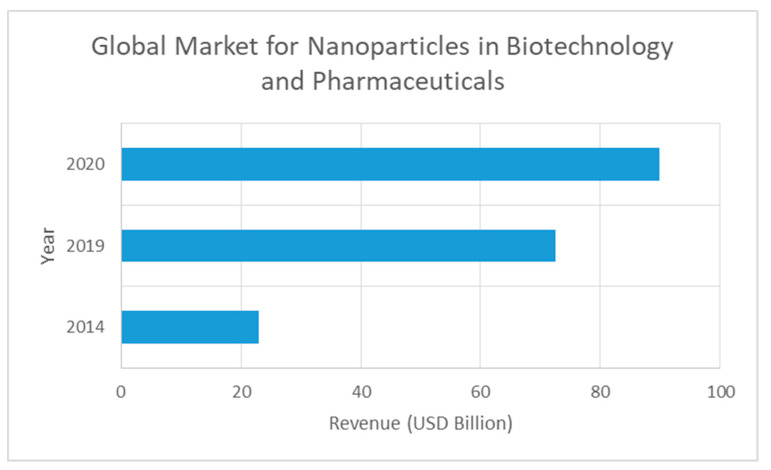
Biotechnological applications are responsible for a large share of today’s market of nanomagnetic particles. It is a novel and open business opportunity. Nonetheless, few data exist (concerning markets) and many fierce competitors are in the market. In order to determine the market share, besides the previous detailed factors and Figure 1, it must be also taken into account the numbers indicated in Figure 2 (estimated demand of 89.84 billion US$ for 2020). Therefore, and considering the special high-level competitivity and high-value product, we consider that, as a conservative value, we will be able to supply about 0.10% (0.0915% updated to 2020 figures) of the world market with our plant. This corresponds to about 200 million euros.
Figure 2.
Global market for nanoparticles in biotechnology and pharmaceuticals (based on data from [46,47]).
To settle a price for the produced particles we analyze again Table 2 noting that in the case of our plant the particles to be produced will be delivered covered with a carbon layer (prepared for further functionalization). Therefore, we have analyzed prices of particles produced by other suppliers, like Sigma-Aldrich, and observed that particles with more or less similar properties to the ones being produced in our plant, for example, those containing PEG layer, are sold at a price of 316 €/10 mL [48]. In order to be more competitive, we will opt for a lower price for the final particles produced at our plant: 118.50 €/10 mL. Hence, a total of 6300 kg/year of nanomagnetic particles covered with carbon for biotechnological applications should be produced at the plant.
3.3. Size of the Plants
To estimate the size of the plant is required to balance between profits (in this case the income due to the selling of the particles) and the costs (implementation and production costs). This step only constitutes a first approach in order to determine the minimum profitable production size, as the full economical balance that is presented later in this article is only made after we have previously established all the process engineering [13]. For the calculation details the reader is addressed to the Supplementary Material.
3.3.1. Environmental Applications
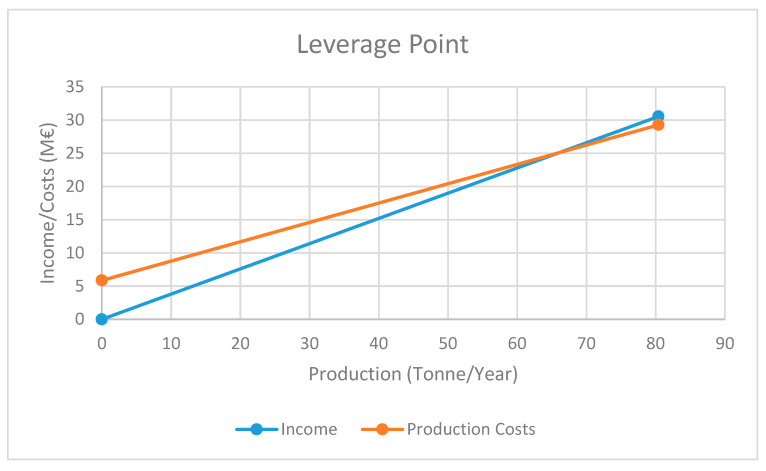
Balancing between the income and the costs (details presented in Supplementary Material) we obtain the graphical depiction represented in Figure 3. As we see, the income line (Y = 0.38X, where Y are the income/costs measured in M€/year, and X the production capacity measured in tonnes/year) and the cost line (Y = 0.2914X + 5.8578, where the fixed costs were assumed to be 20% of the maximum production costs) intersect at 66.1 tonnes/year, which represents the minimum capacity value of the plant in order to get a balance between income and costs (zero profit), knowing that working at higher production capacities will always lead to positive profit values. For our plant, in this case, the maximum capacity will be 80.4 tonnes/year (the value we chose for our total production), above the minimum profitable production amount.
Figure 3.
Minimum production capacity of the plant for profit (environmental applications).
3.3.2. Biotechnological Applications
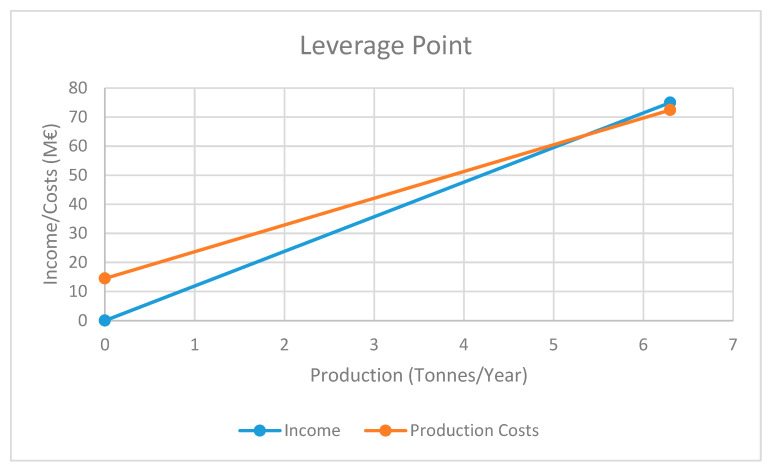
By making the same balance between profit and costs, we obtain the curves represented in Figure 4. The intersection of the income line (Y = 11.905X) and cost line (Y = 0.1981X + 14.487) is at 5.35 tonnes/year, which represents the leverage point between Income and Costs, and represents about 85% of the total maximum production defined for our plant (6.3 tonnes/year).
Figure 4.
Minimum production capacity of the plant for profit (biotechnological applications).
3.4. Process Engineering and Energy and Mass Balances
In the previous section we determined the operating production capability for both factories. With these numbers defined we have analyzed all the process steps, chosen all the process units and performed all the mass and energy balances required in our plant. Full schematic diagrams are presented in Figure 5 and Figure 6, while in Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8 we present the corresponding nomenclature of the process units and all the mass and heat characteristics of the processing streams.
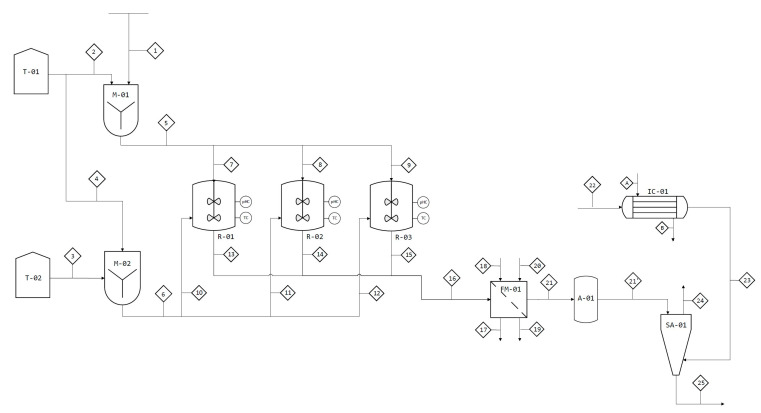
Figure 5.
Environmental applications: Full schematic diagram of the plant (flowsheet).
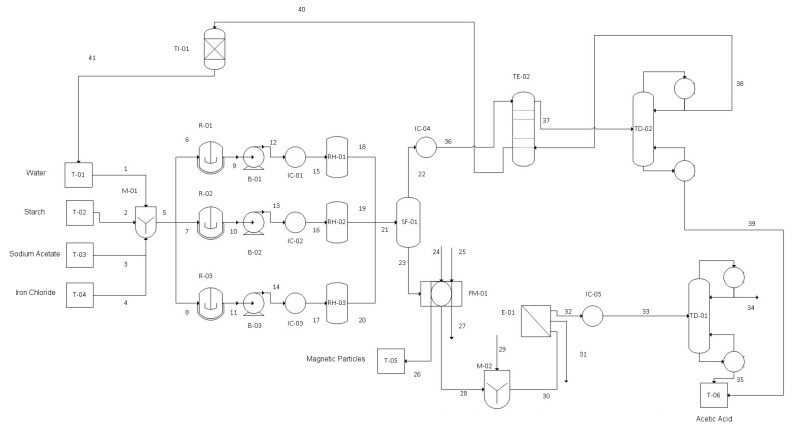
Figure 6.
Biotechnological applications: Full schematic diagram of the plant (flowsheet).
Table 3.
Environmental applications: Streams.
| Materials | Unit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| FeSO4 | kg/h | 19.68 | - | - | - | 19.68 | - | 6.56 | 6.56 | 6.56 | - | - | - | - | - |
| H2O | kg/h | 16.34 | 647.86 | 1297.18 | 3321.00 | 664.20 | 4618.19 | 221.40 | 221.40 | 221.40 | 1539.40 | 1539.40 | 1539.40 | 1540.17 | 1540.17 |
| NH4OH | kg/h | - | - | 1691.72 | - | - | 1691.72 | - | - | - | 563.91 | 563.91 | 563.91 | 560.88 | 560.88 |
| Fe3O4 | kg/h | - | - | - | - | - | - | - | - | - | - | - | - | 3.33 | 3.33 |
| (NH4)2SO4 | kg/h | - | - | - | - | - | - | - | - | - | - | - | - | 5.71 | 5.71 |
| Air | kg/h | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Total | kg/h | 36.02 | 647.86 | 2988.90 | 3321.00 | 683.88 | 6309.91 | 227.96 | 227.96 | 227.96 | 2103.30 | 2103.30 | 2103.30 | 2110.09 | 2110.09 |
| Total | kmol/h | 1.04 | 35.96 | 120.28 | 184.35 | 37.00 | 304.63 | 12.33 | 12.33 | 12.33 | 101.54 | 101.54 | 101.54 | 101.56 | 101.56 |
| T | K | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 |
| P | kPa | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 |
| Enthalpy | kJ/h | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Materials | Unit | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 21ª | 22 | 23 | 24 | 25 | A | B |
| FeSO4 | kg/h | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| H2O | kg/h | 1540.17 | 4620.52 | 4620.52 | 10.00 | 10.00 | 4.29 | 4.29 | 4.29 | 3.12 | 3.12 | 7.10 | 0.31 | 32.14 | 32.14 |
| NH4OH | kg/h | 560.88 | 1682.64 | 1682.64 | - | - | - | - | - | - | - | - | - | - | - |
| Fe3O4 | kg/h | 3.33 | 10.00 | - | - | - | 10.00 | 10.00 | - | - | - | 10.00 | - | - | |
| (NH4)2SO4 | kg/h | 5.71 | 17.12 | 17.12 | - | - | - | - | - | - | - | - | - | - | - |
| Air | kg/h | - | - | - | - | - | - | - | - | 195.27 | 195.27 | 195.27 | - | - | - |
| Total | kg/h | 2110.09 | 6330.28 | 6320.28 | 10.00 | 10.00 | 4.29 | 14.29 | 14.29 | 198.40 | 198.40 | 202.38 | 10.31 | 32.14 | 32.14 |
| Total | kmol/h | 101.56 | 304.67 | 304.63 | 0.56 | 0.56 | 0.24 | 0.28 | 0.28 | 6.92 | 6.92 | 7.14 | 0.06 | 1.78 | 1.78 |
| T | K | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 298 | 368 | 318 | 308 | 523 | 523 |
| P | kPa | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 | 101.33 |
| Enthalpy | kJ/h | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20,271.67 | 20,233.54 | 38.13 | 55,152.57 | 34,880.90 |
Table 4.
Nomenclature of the processing units in Figure 5, environmental applications.
| T-01 | Storage Tank 1 | T-02 | Storage Tank 2 |
|---|---|---|---|
| M-01 | Mixer 1 | M-02 | Mixer 2 |
| R-01 | Reactor 1 | R-02 | Reactor 2 |
| R-03 | Reactor 3 | FM-01 | Magnetic Filter |
| IC-01 | Heat Exchanger 1 | A-01 | Roller Crusher |
| SA-01 | Dryer Atomizer | - | - |
Table 5.
Quantities of raw materials, products, and sub-products per hour, environmental applications.
| Materials | Quantit y (kg/h) | Quality |
|---|---|---|
| FeSO4 | 19.68 | Raw Material |
| H2O | 4620.52 | Sub-Product/Raw Material |
| NH4OH | 1691.72 | Raw Material |
| Fe3O4 | 10.00 | Raw Material |
| (NH4)2SO4 | 17.12 | Sub-Product |
Table 6.
Biotechnological applications: Streams.
| Materials | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | - |
| FeCl2·4H2O | 48.64 | 0.00 | 0.00 | 0.00 | 48.64 | 16.21 | 16.21 | 16.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - |
| CH3COONa | 0.00 | 133.26 | 0.00 | 0.00 | 133.26 | 44.42 | 44.42 | 44.42 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | 30.99 | - |
| Starch | 0.00 | 0.00 | 54.47 | 0.00 | 54.47 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 18.16 | 0.00 | 0.00 | 0.00 | - |
| H2O | 0.00 | 0.00 | 0.00 | 2174.99 | 2174.99 | 725.00 | 725.00 | 725.00 | 727.95 | 727.95 | 727.95 | 727.95 | 727.95 | 727.95 | 727.95 | 727.95 | 727.95 | 729.40 | 729.40 | 729.40 | - |
| CH3COOH | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | 9.83 | - |
| Fe(OH)2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 7.37 | 7.37 | 7.37 | 7.37 | 7.37 | 7.37 | 7.37 | 7.37 | 7.37 | 0.00 | 0.00 | 0.00 | - |
| NaCl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | 9.50 | - |
| HCl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - |
| C2H5OH | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - |
| MTBE | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - |
| Magnetite | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 6.27 | 6.27 | 6.27 | - |
| Total (kg/day) | 48.64 | 133.26 | 54.47 | 2174.99 | 2411.36 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 803.79 | 785.99 | 785.99 | 785.99 | - |
| Total (kmol/day) | 0.246 | 1.625 | 0.336 | 120.833 | 123.040 | 41.01 | 41.01 | 41.01 | 41.34 | 41.34 | 41.34 | 41.34 | 41.34 | 41.34 | 41.34 | 41.34 | 41.34 | 41.25 | 41.25 | 41.25 | - |
| Enthalpy (kJ/day) | 0.00 | 0.00 | 0.00 | 0.00 | 25,278.33 | 8426.11 | 8426.11 | 8426.11 | 17,2967.67 | 17,2967.67 | 17,2967.67 | 173,807.35 | 173,807.35 | 173,807.35 | 550,215.21 | 550,215.21 | 550,215.21 | 547,316.72 | 547,316.72 | 547,316.72 | - |
| T(K) | 298.00 | 298.00 | 298.00 | 298.00 | 300.70 | 300.70 | 300.70 | 300.70 | 353.00 | 353.00 | 353.00 | 353.35 | 353.35 | 353.35 | 473.00 | 473.00 | 473.00 | 473.00 | 473.00 | 473.00 | - |
| P(MPa) | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | - |
| - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Materials | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 |
| FeCl2·4H2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CH3COONa | 92.98 | 0.00 | 92.98 | 0.00 | 0.00 | 0.00 | 0.00 | 92.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Starch | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| H2O | 2188.21 | 2186.22 | 142.27 | 28.21 | 0.00 | 0.00 | 28.21 | 142.27 | 73.92 | 216.19 | 0.00 | 216.19 | 216.19 | 215.76 | 0.43 | 2186.22 | 21.86 | 21.86 | 0.04 | 2164.35 | 2164.35 |
| CH3COOH | 29.48 | 29.42 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | - | 68.09 | 0.00 | 68.09 | 68.09 | 0.14 | 67.9 | 29.42 | 28.35 | 0.06 | 28.29 | 1.07 | - |
| Fe(OH)2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NaCl | 28.49 | 0.00 | 28.49 | 0.00 | 0.00 | 0.00 | 28.49 | 0.00 | 94.26 | 94.26 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| HCl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 40.82 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C2H5OH | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 886.26 | 886.26 | 0.00 | 0.00 | 0.00 |
| MTBE | 0.00 | 0.00 | 0.00 | 0.00 | 28.21 | 0.00 | 28.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Magnetite | 18.81 | 0.00 | 18.81 | 0.00 | 0.00 | 18.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Total (kg/day) | 2357.97 | 2215.64 | 282.61 | 28.21 | 28.21 | 18.81 | 56.42 | 263.90 | 114.74 | 378.54 | 94.26 | 284.28 | 284.28 | 215.90 | 68.38 | 2215.64 | 936.47 | 908.17 | 28.33 | 2165.43 | 2164.35 |
| Total (kmol/day) | 123.76 | 121.95 | 9.61 | 1.57 | 0.61 | 0.08 | 2.18 | 9.53 | 5.24 | 14.77 | 1.63 | 13.15 | 13.15 | 11.99 | 1.16 | 121.95 | 11.74 | 11.26 | 0.47 | 120.26 | 120.24 |
| Enthalpy (kJ/day) | 1,641,950 | 5,870,529 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −8416.8 | 7895.9 | 552,854 | −422,597 | 1,217,950 | 778,32.1 | 3,753,140.1 | 90,649.0 | 85,258.2 | 5398.7 | 390,831.3 | 0.00 |
| T(K) | 473.00 | 473.00 | 298.00 | 298.00 | 298.00 | 298.00 | 298.00 | 298.00 | 298.00 | 278.00 | 391.00 | 391.00 | 374.55 | 372.88 | 390.68 | 353.00 | 342.57 | 341.16 | 390.72 | 341.16 | 298.00 |
| P(MPa) | 2.00 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
Table 7.
Nomenclature of the process units in Figure 6, biotechnological applications.
| T-01 | Storage Tank 1 | T-02 | Storage Tank 2 |
|---|---|---|---|
| T-03 | Storage Tank 3 | T-04 | Storage Tank 4 |
| T-05 | Storage Tank 5 | T-06 | Storage Tank 6 |
| IC-01 | Heat Exchanger 1 | IC-02 | Heat Exchanger 2 |
| IC-03 | Heat Exchanger 3 | IC-04 | Flash Separator 4 |
| IC-05 | Heat Exchanger 5 | SF-01 | Flash Separator 1 |
| M-01 | Mixer 1 | M-02 | Mixer 2 |
| R-01 | Reactor 1 | R-02 | Reactor 2 |
| R-03 | Reactor 3 | B-01 | Pump 1 |
| B-02 | Pump 2 | B-03 | Pump 3 |
| RH-01 | Hydrothermal Reactor 1 | RH-02 | Hydrothermal Reactor 2 |
| RH-03 | Hydrothermal Reactor 3 | FM-01 | Magnetic Filter |
| E-01 | Evaporator 1 | TE-01 | Extraction Tower 1 |
| TD-01 | Distillation Tower 1 | TD-02 | Distillation Tower 2 |
| TI-01 | Ion Exchange Tower 1 | - | - |
Table 8.
Quantities of raw materials, products and sub-products per day, environmental applications.
| Materials | Quantity (kg/day) | Quality |
|---|---|---|
| FeCl2·4H2O | 48.64 | Raw Material |
| Sodium Acetate | 133.26 | Raw Material |
| Starch | 54.47 | Raw Material |
| Water | 2174.99 | Raw Material/Sub-product |
| Magnetite | 18.81 | Product |
| NaCl | 94.26 | Sub-Product |
| HCl | 40.82 | Raw Material |
| Ethanol | 886.26 | Separation Aid |
| MTBE | 28.21 | Separation Aid |
3.4.1. Raw Materials and Products
In Table 5 and Table 8 are detailed the quantities used of main raw materials, other chemicals, and products/sub-products obtained for both plants.
3.4.2. Operation and Sector Division
(a) Environmental Applications
The magnetic micro and nanoparticle production process will be carried out in three different stages. First, the FeSO4·7H2O and NH4OH solutions will be prepared in two mixers. Secondly, the reaction will be carried out in three batch reactors, and finally, the particles will be washed and dried by means of a magnetic filter and a spray dryer.
The production will be carried out by means of a semicontinuous process due to the need for the reaction to be discontinuous. To ensure continuous supply to the dryer, an accumulator will be used at the outlet of the magnetic filter. The filter and reactors will be synchronized for periods of two hours (the first 40 min represent start-up).
• Preparation of Solutions
Two solutions are to be prepared in two different mixers: FeSO4 and NH4OH. The first will be prepared with solid FeSO4∙7H2O and distilled water in a mixer to obtain a concentration equal to 0.2 M. The second mixture is prepared with a NH4OH solution that has 28–39% NH3 and distilled water. The aim of this mixture is to achieve a pH of 13 and to supply the base needed for the reaction. Both solutions are obtained with constant mechanical agitation. At the end, currents flow from the mixers to the reactors.
• Reaction
Once the solutions are prepared, the reaction is carried out in batch reactors at the optimal operating conditions that have been determined experimentally [28]. The reactor consists of a tank with a perforated plate at the top that aims to distribute the FeSO4∙7H2O solution from the M-01 mixer so that this solution falls in the form of drops during 10 min (experimental reaction conditions). Then redox reaction occurs, in which iron is oxidized and oxygen is reduced. This reaction will be carried out at room temperature (25 °C) and at atmospheric pressure with constant mechanical stirring for 30 min and at a basic pH close to 13. The conversion of FeSO4 will be 100%. At the end of this time, the reaction mixture obtained will be discharged onto the magnetic filter for a processing time of 10 min. In order to have a semicontinuous process, three equal reactors (R-01, R-02 and R-03) will be used with a time difference of 20 min between their operations. The reaction mixture must be continuously stirred by a mechanical system and must have a temperature and pH control system to ensure that the reaction occurs under optimal conditions. For temperature control, the reactors have an internal coil through which water will circulate at an automatically controlled flow.
• Filtration
The reaction mixture that passes to the washing process will be composed of the obtained magnetite (Fe3O4) and the other reaction products—(NH4)2SO4—and the aqueous medium that has not reacted. To separate only the magnetite particles from this mixture, a FM-01 magnetic filter will be used, thus the reaction mixture leaving the reactors passes to a magnetic filter. This magnetic filter or high gradient magnetic filter (HGMF)-consists of a ferromagnetic wool surrounded by an electromagnetic coil that when activated generates magnetic field gradients between the fibers of the wool. Hence, as the micro and nanoparticles pass through the filter they are attracted to the wool and are retained by it while the rest of the reaction mixture passes. To eliminate the impurities that may remain on the surface of the particles, a wash is carried out in the same filter and finally the coil is deactivated and a new stream of distilled water is passed in the opposite direction to the one that has been used previously. A total humidity of 30% is obtained due to the water entering the filter. Since the filtering operation is discontinuous, an accumulator will be used at the filter outlet to provide a continuous current to the next processing unit (spray dryer). All currents involved in the above process will be at 25 °C and 1 atm.
• Drying
In the spray dryer, the current coming from the accumulator will enter and will be dried in countercurrent with a hot air stream, corresponding to atmospheric air previously heated up to 90 °C in a heat exchanger (using saturated steam at 250 °C). The dryer has a rotary atomizer and a conical drying chamber. The air taken from the atmosphere will have an average relative humidity of 80%. The humidified air will come out at 40 °C. Then, the magnetite micro and nanoparticles will come out of the drying chamber at 30 °C and 3% humidity. Finally, the obtained magnetic particles are packaged.
• Secondary Operations
In addition to the main line of the process, a series of secondary operations are carried out: the preparation of the solution for pH control in the reactor, the cleaning of the perforated plate of the reactors when the deposition of solute on the plate causes the decrease of the flow and a temperature and pH control system in the reactors.
(b) Biotechnological Applications
The process for the production of nanomagnetic particles (NMPs) can be divided into four stages: mixing, hydrothermal reaction and treatment, separation and washing of NMPs allied with the separation and purification of by-products, and finally the storage of raw materials and products. The plant will operate semicontinuously since the reactions must be carried out batchwise. To do this, we will work in three equal parallel stages, working with a seven-hour lag between them.
• Mixing, Reaction, and Hydrothermal Treatment
The raw materials are first mixed in the appropriate proportions to be subsequently fed to the mixing reactor where reaction (2) occurs. Sodium acetate and chloride are introduced with a mass ratio of 2.74, deionized water at a ratio of 44.7 L/ kg chloride, and starch with a mass ratio of 1.12 with respect to chloride. These reagents are mixed with vigorous stirring in the reactor for 20 min at 80 °C, until producing a homogeneous solution. The reaction conversion may be assumed to be 100% for the chloride.
After finishing the first reaction, the obtained solution is compressed to 2 MPa, heated to the reactor operating temperature and brought to the hydrothermal reactor, where the hydrothermal treatment is carried out for 20 h at 200 °C and reactions (3) and (4) occur. Dissolved oxygen partially oxidizes iron hydroxide to form goethite until a suitable ratio of the two compounds is reached in order to form magnetite. The reaction conversion may be assumed to be 100% for the hydroxide.
• Product Separation
The products subsequently expand to atmospheric pressure in a flash, where separation of acetic acid (AcH) and water from the other components occurs. The gas stream with acetic acid and water is cooled and taken to a liquid-liquid extraction tower where they contact with methyl tert-butyl ether (MTBE), since the percentage of acetic acid in the stream is very small, which makes LL-extraction a cheaper option. The acetic acid exits with the extract, and is subsequently separated with a distillation tower, while the raffinate is taken to an ion exchange tower where pure water is obtained and returned to the storage tank. On the other hand, the liquid current that exits the flash contains the magnetite, the acetate and the salt, and is introduced in the magnetic filter in order to achieve a separation of the magnetite from the rest of the components.
To separate the nanomagnetic particles from the solution, a high gradient magnetic filter is used since it is the most efficient to separate this type of particles. This filter consists of a metallic wool matrix located inside an electromagnetic system, which when activated generates a large magnetic field that attracts the particles to the metallic wool and lets the rest of the solution pass. The solution is passed several times through the filter to ensure that all NMPs are separated. The filter is able to trap 100% of the magnetite particles.
• Flushing and Storage of NMPs
The NMPs, once trapped in the metallic wool, are subjected to a sequential washing with ethanol and water in the same filter to eliminate any impurities that they may have. The wash is performed three times, and each wash uses an amount of each component equal to half the amount of NMPs trapped in the filter. Once the washing process has been carried out, the magnetic field of the magnetic filter is deactivated and the NMPs are separated from the metallic wool by passing a stream of water, being finally stored.
• Separation, Purification and Storage of Acetic Acid
The separated solution of the NMPs containing water, acetic acid, NaCl and CH3COONa (NaAc) are taken to a mixer where hydrochloric acid is added to convert all the NaAc into acetic acid, since the separation of NaAc from NaCl is not feasible by other methods. The reaction (7) that occurs is as follows:
| CH3COONa + HCl→CH3COOH + NaCl | (7) |
where a 1:1 molar ratio will be used so that the reaction shifts completely to the right.
The final stream containing acetic acid, water and NaCl is brought to an evaporator to separate the components. On one hand, the salt will be obtained (on an aqueous form), which will be stored for possible sale, and on the other hand, the gaseous stream of acetic acid and water will be taken to a distillation tower to purify the acetic acid, which will be combined with that obtained in the LL-extraction process, to be stored.
3.5. Economic Impact and Profitability
After having determined the maximum treatment capability, the overall process engineering and characterized the different streams, we finally may compute the full economic impact and profitability of the plant.
In Table 9 and Table 10 are indicated the overall costs. In Supplementary Material details are given about the calculation of these values. It is important to notice that many of the sub-totals are calculated based on estimates and, thus, the total numbers are always estimates.
Table 9.
Overall costs of industrial implementation of NMP production for environmental applications.
| Cost | € | Invested Capital | € |
|---|---|---|---|
| 1.1 Raw Materials | 9,302,682 | 1.1 Instrumentation | 876,200 |
| 1.2 Direct Human Labor | 2,264,731 | 1.2 Initial Setup | 1,180,970 |
| 1.3 Indirect Human Labor | 503,207 | 1.3 Piping and Valves | 525,720 |
| 1.4 General Services | 1704 | 1.4 Measuring and Control | 131,430 |
| 1.5 Supplies | 41,745 | 1.5 Heat Isolation | 35,048 |
| 1.6 Conservation Expenses | 208,727 | 1.6 Electrical Installation | 131,430 |
| 1.7 Laboratory | 679,419 | 1.7 Land and Structures | 1,541,610 |
| 1.8 Board and Technical Staff | 467,565 | 1.8 Auxiliary Facilities | 350,480 |
| 1.9 Amortization | 73,880 | 1.9 Project and Design | 497,244 |
| 1.10 Packaging | 6,110,400 | 1.10 Constructor Hiring | 286,373 |
| 1.11 Taxes and Insurances | 139,151 | 1.11 Unexpected Expenses | 685,117 |
| TOTAL COST OF FABRICATION | 19,791,507 | 1.12 Preliminary Research, Study and Startup | 715,933 |
| 2.1 Comercial Expenses | 3,958,301 | TOTAL IMMOBILIZED | 6,957,556 |
| 2.2 Management | 890,618 | - | - |
| 2.3 Financial Expenses | 49,257 | CIRCULATING CAPITAL | 5,356,704 |
| 2.4 Research | 208,727 | - | - |
| 2.5 Technical Services | 33,935 | TOTAL INVESTED CAPITAL | 12,314,260 |
| TOTAL COST OF MANAGEMENT | 5,140,838 | - | - |
| TOTAL PRODUCTION COSTS | 24,932,346 | TOTAL INCOME | 30,552,000 |
Table 10.
Overall costs of industrial implementation of NMP production for biotechnological applications.
| Cost | € | Invested Capital | € |
|---|---|---|---|
| 1.1 Raw Materials | 15,888,945 | 1.1 Instrumentation | 1,714,802 |
| 1.2 Direct Human Labor | 2,193,938 | 1.2 Initial Setup | 1,668,069 |
| 1.3 Indirect Human Labor | 602,897 | 1.3 Piping and Valves | 1,028,881 |
| 1.4 General Services | 9,048,525 | 1.4 Measuring and Control | 514,441 |
| 1.5 Supplies | 432,988 | 1.5 Heat Isolation | 120,036 |
| 1.6 Conservation Expenses | 863,263 | 1.6 Electrical Installation | 342,960 |
| 1.7 Laboratory | 658,181 | 1.7 Land and Structures | 6,257,220 |
| 1.8 Board and Technical Staff | 507,229 | 1.8 Auxiliary Facilities | 685,921 |
| 1.9 Amortization | 128,721 | 1.9 Project and Design | 1,284,795 |
| 1.10 Taxes and Insurances | 577,318 | 1.10 Constructor Hiring | 739,940 |
| TOTAL COST OF FABRICATION | 30,902,006 | 1.11 Unexpected Expenses | 2,096,496 |
| 2.1 Comercial Expenses | 6,180,401 | 1.12 Preliminary Studies | 10,103,064 |
| 2.2 Management | 479,412 | 1.13 Preliminary Startup | 2,309,272 |
| 2.3 Financial Expenses | 5,776,170 | - | - |
| 2.4 Research | 1,154,636 | TOTAL IMMOBILIZED | 28,865,898 |
| 2.5 Technical Services | 1,904,636 | CIRCULATING CAPITAL | 9,641,903 |
| TOTAL COST OF MANAGEMENT | 14,340,620 | TOTAL INVESTED CAPITAL | 38,507,801 |
| TOTAL PRODUCTION COSTS | 45,242,625 | TOTAL INCOME | 75,000,000 |
At the end we get a maximum net benefit of 4,214,741 €/year, a net profitability of 34.2%, and a minimum recovery time of 2.92 years for the environmental applications case, and a maximum net benefit of 22,318,031 €/year, a net profitability of 58.0%, and a minimum recovery time of 1.73 years for the biotechnological applications case.
4. Discussion
Each intended application of the particles determines the characteristics of the obtained product that reflects on the final engineering process options and manufacturing process. In fact, for environmental applications the final nanomagnetic particles will possess a large polidispersity (even mixed-size: micro- and nanosized particles) and are low-cost bare particles in dry media, while for biotechnological applications the final product must present the desired monodispersity, a surface layer ready for functionalization being highly-priced particles in liquid media. The manufacturing processes are very different: particles are obtained by the reverse co-precipitation method for the environmental case while hydrothermal method is used in the biotechnological case. The production rate is about 15 times higher for the environmental applications plant. The difference in the final product also justifies that the biotechnological plant requires more process steps than the environmental applications plant. Nonetheless, both plants work in a semicontinuous way and both present the step of magnetic filtering for the recovery of the magnetic particles after the reaction stage. The biotechnological plant will be larger than the environmental plant, and the size and cost of the former will be higher than the latter (the total invested capital is almost three times higher); nonetheless profitability is expected to be higher in the biotechnological case (58% opposing to 34.2% in the environmental plant). The maximum net benefit in the biotechnological plant is also more than five times higher than in the environmental case. Anyway, it is important to notice that due to a much higher-level of competition with other companies, the biotechnological applications plant represents a riskier investment.
5. Conclusions and Future Perspectives
We have fully designed and studied the industrial implementation of two plants to produce micron and nanomagnetic particles for two different applications (environmental and biotechnological). Both have proven to be viable economically and technically. Full process engineering, including energy and mass balances, was also conducted for both plants. Since the profit is larger than regular bank benefits, investors may consider attractive the construction and startup of any of the designed industrial plants. Additionally, it is expected that this work will serve as the basis for other similar studies for different products, showing the connection between science and practical applications, and to allow scientific improvement of processes bearing in mind their possible industrial implementation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/11/2477/s1, Table S1: Costs of raw materials/income of selling subproducts (environmental applications), Table S2: Costs of raw materials/income of selling subproducts (biotechnology applications), Table S3: Labor costs for the environmental applications case (direct human labor), Table S4: Labor costs for the biotechnology applications case (direct human labor), Table S5: Labor costs for the environmental applications case (indirect human labor), Table S6: Labor costs for the biotechnology applications case (indirect human labor), Table S7: General services of the environmental applications plant, Table S8: Labor costs for the environmental applications case (chief personnel), Table S9: Chief personnel labor costs for the biotechnology applications case, Table S10: Management personnel labor costs for the biotechnology applications case, Table S11: Technical services labor costs for the environmental applications case, Table S12: Fixed immobilized capital (for EA plant), Table S13: Fixed immobilized capital (for BA plant).
Author Contributions
P.A.A.: Conceptualization; P.A.A.: Data curation; P.A.A. and T.C.-G.: Formal analysis; P.A.A., T.C.-G. and D.B.: Funding acquisition; P.A.A., T.C.-G., D.V., L.H. and A.P.: Investigation; P.A.A.: Methodology; P.A.A., T.C.-G. and D.B.: Project administration; P.A.A.: Resources; P.A.A., A.P. and D.V.: Software; P.A.A., T.C.-G, A.M.E. and D.B.: Supervision; P.A.A.: Validation; P.A.A., D.V. and A.P.: Visualization; P.A.A. and T.C.-G.: Writing—original draft; P.A.A., T.C.-G. and D.B.: Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: (a). Project MAT2016-76824-C3-2-R, MINECO, Spain; (b). Base Funding—UIDB/00511/2020 of the Laboratory for Process Engineering, Environment, Biotechnology and Energy—LEPABE—Funded by national funds through the FCT/MCTES (PIDDAC); (c). Project PTDC/QUIQFI/ 28020/2017—POCI-01-0145-FEDER-028020—Funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mehta R.V. Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology (Review) Mater. Sci. Eng. C. 2017;79:901–916. doi: 10.1016/j.msec.2017.05.135. [DOI] [PubMed] [Google Scholar]
- 2.Dabaghi M., Hilger I. Magnetic Nanoparticles Behavior in Biological Solutions; The Impact of Clustering Tendency on Sedimentation Velocity and Cell Uptake. Materials. 2020;13:1644. doi: 10.3390/ma13071644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staniland S.S., Rawlings A., Bramble J., Tolosa J., Wilson O., García-Martínez J.C., Binns C. Chapter 3—Novel Methods for the Synthesis of Magnetic Nanoparticles. In: Binns C., editor. Frontiers of Nanoscience. Volume 6. Elsevier; Amsterdam, The Netherlands: 2014. pp. 85–128. [Google Scholar]
- 4.Farag R.K., Labena A., Fakhry S.H., Safwat G., Diab A., Atta A.M. Antimicrobial Activity of Hybrids Terpolymers Based on Magnetite Hydrogel Nanocomposites. Materials. 2019;12:3604. doi: 10.3390/ma12213604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moraes S.S., Tavallaie R., Sandiford L., Tilley R.D., Gooding J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. (Camb.) 2016;52:7528–7540. doi: 10.1039/C6CC03225G. [DOI] [PubMed] [Google Scholar]
- 6.Fouriki A., Dobson J. Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts. Materials. 2013;6:255–264. doi: 10.3390/ma6010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikitin M.P., Orlov A.V., Sokolov I.L., Minakov A.A., Nikitin P.I., Ding J., Bader S.D., Rozhkova E.A., Novosad V. Ultrasensitive detection enabled by nonlinear magnetization of nanomagnetic labels. Nanoscale. 2018;10:11642–11650. doi: 10.1039/c8nr01511b. [DOI] [PubMed] [Google Scholar]
- 8.Gu T., Wang J., Xia H., Wang S., Yu X. Direct Electrochemistry and Electrocatalysis of Horseradish Peroxidase Immobilized in a DNA/Chitosan-Fe3O4 Magnetic Nanoparticle Bio-Complex Film. Materials. 2014;7:1069–1083. doi: 10.3390/ma7021069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharif H.M.A., Cheng H.-Y., Haider M.R., Khan K., Yang L., Wang A.-J. NO Removal with Efficient Recovery of N 2 O by Using Recyclable Fe 3 O 4 @EDTA@Fe(II) Complex: A Novel Approach toward Resource Recovery from Flue Gas. Environ. Sci. Technol. 2019;53:1004–1013. doi: 10.1021/acs.est.8b03934. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee R., Katsenovich Y., Lagos L., Mciintosh M., Zhang X., Li C.-Z. Nanomedicine: Magnetic nanoparticles and their biomedical applications. Curr. Med. Chem. 2010;17:3120–3141. doi: 10.2174/092986710791959765. [DOI] [PubMed] [Google Scholar]
- 11.Augusto P.A., Castelo-Grande T., Merchan L., Estevez A.M., Quintero X., Barbosa D. Landfill leachate treatment by sorption in magnetic particles: Preliminary study. Sci. Total Environ. 2019;648:636–668. doi: 10.1016/j.scitotenv.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 12.Augusto P.A., Castelo-Grande T., Estévez A.M., Barbosa D., Costa P.M. Method to evaluate and prove-the-concept of magnetic separation and/or classification of particles. J. Magn. Magn. Mater. 2017;426:405–414. doi: 10.1016/j.jmmm.2016.10.154. [DOI] [Google Scholar]
- 13.Castelo-Grande T., Augusto P.A., Carron I., Barbosa D. Ultrasound Supercritical Extraction as an innovative method to remediate soils: From Laboratorial Experiments into Industrial Implementation. JEM. 2020 submitted. [Google Scholar]
- 14.Vian-Ortuño Á. El pronóstico Económico en Química Industrial. EUDEMA, S.A.; Madrid, Spain: 1991. [Google Scholar]
- 15.Sinnot R. Diseño en Ingeniería Química. Reverté; Barcelona, Spain: 2012. [Google Scholar]
- 16.Estevez A.M., Rodriguez J.M., Alvaro A., Augusto P.A., Jiménez O., Castelo-Grande T., Barbosa D. Preparation, characterization, and testing of magnetic carriers for arsenic removal from water. IEEE Trans. Magn. 2008;44:4436–4439. doi: 10.1109/TMAG.2008.2001998. [DOI] [Google Scholar]
- 17.Chang M., Chang M.-Y., Lin W.-S., Gabayno J.L. Magnetic Control of Fe3O4 Nanomaterial for Fat Ablation in Microchannel. Materials. 2015;8:7813–7820. doi: 10.3390/ma8115429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Zhang Z., Zhao Y., Xia K., Guo Y., Qu Z., Bai R. A Mild and Facile Synthesis of Amino Functionalized CoFe2O4@SiO2 for Hg(II) Removal. Nanomaterials. 2018;8:673. doi: 10.3390/nano8090673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardino S., Estrela N., Ochoa-Mendes V., Fernandes P., Fonseca L.P. Optimization in the immobilization of penicillin G acylase by entrapment in xerogel particles with magnetic properties. J. Sol-Gel Sci. Technol. 2011;58:545–556. doi: 10.1007/s10971-011-2426-7. [DOI] [Google Scholar]
- 20.Yiu H.H.P., McBain S.C., Lethbridge Z.A.D., Lees M.R., Palona I., Olariu C.I., Dobson J. Novel Magnetite-Silica Nanocomposite (Fe3O4-SBA-15) Particles for DNA Binding and Gene Delivery Aided by a Magnet Array. J. Nanosci. Nanotechnol. 2011;11:3586–3591. doi: 10.1166/jnn.2011.3615. [DOI] [PubMed] [Google Scholar]
- 21.Nanzhaxi S., Aimin S., Lichao Y., Zhuo Z., Wei Z., Xiqian Z. Effect performance of the nanomagnetic properties of Ni–Cu–Co ferrites by Al3+ ions adulteration. Mod. Phys. Lett. B. 2020;34:2050059. [Google Scholar]
- 22.Bomatí-Miguel O., Morales M.P., Serna C., Veintemillas-Verdaguer S. Magnetic nanoparticles prepared by laser pyrolysis. IEEE Trans. Magn. 2002;38:2616–2618. doi: 10.1109/TMAG.2002.801961. [DOI] [Google Scholar]
- 23.Ghazanfari M.R., Kashefi M., Shams S.F., Jaafari M.R. Perspective of Fe3O4 Nanoparticles Role in Biomedical Applications. Biochem. Res. Int. 2016;2016:7840161. doi: 10.1155/2016/7840161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyergyek S., Makovec D., Kodre A., Arcon I., Jagodi M., Drofenik M. Influence of synthesis method on structural and magnetic properties of cobalt ferrite nanoparticles. J. Nanopart. Res. 2010;12:1263–1273. doi: 10.1007/s11051-009-9833-5. [DOI] [Google Scholar]
- 25.Gopalan E.V., Joy P.A., Al-Omari I.A., Kumar D.S., Yoshida Y., Anantharaman M.R. On the structural, magnetic and electrical properties of sol-gel derived nanosized cobalt ferrite. J. Alloy. Compd. 2009;485:711–717. doi: 10.1016/j.jallcom.2009.06.033. [DOI] [Google Scholar]
- 26.Peng J., Hojamberdiev M., Xu Y., Cao B., Wang J., Wu H. Hydrothermal synthesis and magnetic properties of gadolinium-doped CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2011;323:133–138. doi: 10.1016/j.jmmm.2010.08.048. [DOI] [Google Scholar]
- 27.Augusto P.A., Castelo-Grande T., Augusto P. Magnetic classification in health sciences and in chemical engineering. Chem. Eng. J. 2005;111:85–90. doi: 10.1016/j.cej.2005.02.013. [DOI] [Google Scholar]
- 28.Augusto P.A., Castelo-Grande T., Vargas D., Hernández L., Merchán L., Estevez A.M., Barbosa D. Water decontamination with magnetic particles by adsorption and chemical degradation. Influence of the manufacturing parameters. Materials. 2020;13:2219. doi: 10.3390/ma13102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aono H., Hirazawa H., Naohara T., Maehara T., Kikkawa H., Watanabe Y. Synthesis of fine magnetite powder using reverse coprecipitation method and its heating properties by applying AC magnetic field. Mater. Res. Bull. 2005;40:1126–1135. doi: 10.1016/j.materresbull.2005.03.014. [DOI] [Google Scholar]
- 30.Chomoucka J., Drbohlavova J., Huska D., Adam V., Kizek R., Hubalek J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010;62:144–149. doi: 10.1016/j.phrs.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Kazemzadeh H., Ataie A., Rashchi F. In situ synthesis of silica-coated magnetic nanoparticles by reverse coprecipitation method. J. Supercond. Nov. Magn. 2012;25:2803–2808. doi: 10.1007/s10948-011-1270-x. [DOI] [Google Scholar]
- 32.Mahmed N., Heczko O., Lancok A., Hannula S.-P. The magnetic and oxidation behavior of bare and silica-coated iron oxide nanoparticles synthesized by reverse co-precipitation of ferrous ion (Fe2+) in ambient atmosphere. J. Magn. Magn. Mater. 2014;353:15–22. doi: 10.1016/j.jmmm.2013.10.012. [DOI] [Google Scholar]
- 33.Mahmed N., Heczko O., Söderberg O., Hannula S.-P. Room Temperature Synthesis of Magnetite (Fe3-O4) Nanoparticles by a Simple Reverse Co-Precipitation Method. IOP Conf. Ser. Mater. Sci. Eng. 2011;18:032020. doi: 10.1088/1757-899X/18/3/032020. [DOI] [Google Scholar]
- 34.Vangijzegem T., Dimitri-Stanicki I., Laurent S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019;16:69–78. doi: 10.1080/17425247.2019.1554647. [DOI] [PubMed] [Google Scholar]
- 35.Cruz M.M., Ferreira L.P., Alves A.F., Mendo S.G., Ferreira P., Godinho M., Deus-Carvalho M. Nanostructures for Cancer Therapy. Elsevier; Amsterdam, Netherlands: 2017. Chapter 19—Nanoparticles for magnetic hyperthermia; pp. 485–511. [Google Scholar]
- 36.Mao G.Y., Yang W.J., Bu F.X., Jiang D.M., Zhao Z.J., Zhang Q.H., Fang Q.C., Jiang J.S. One-step hydrothermal synthesis of Fe3O4@C nanoparticles with great performance in biomedicine. J. Mater. Chem. B. 2014;2:4481–4488. doi: 10.1039/c4tb00394b. [DOI] [PubMed] [Google Scholar]
- 37.Sun X., Sun K., Liang Y. Hydrothermal synthesis of magnetite: Investigation of influence of aging time and mechanism. Micronano Lett. 2014;10:99–104. doi: 10.1049/mnl.2014.0344. [DOI] [Google Scholar]
- 38.Iwasaki T., Mizutani N., Watano S., Yanagida T., Kawai T. Hydrothermal synthesis of magnetite nanoparticles via sequential formation of iron hydroxide precipitates. J. Exp. Nanosci. 2012;7:355–365. doi: 10.1080/17458080.2010.515250. [DOI] [Google Scholar]
- 39.Setyawan H., Widiyastuti W. Progress in the Preparation of Magnetite Nanoparticles through the Electrochemical Method. KONA Powder Part. J. 2019;36:145–155. [Google Scholar]
- 40.Mizutani N. Effect of ferrous/ferric ions molar ratio on reaction mechanism for hydrothermal synthesis of magnetite nanoparticles. Bull. Mater. Sci. 2008;31:713–717. [Google Scholar]
- 41.ICONO Documento de Trabajo ICONO:Evolución de la Nanotecnología en España. Fundación Española para la Ciencia y la Tecnología (FECYT). Observatorio español de I+D+i. [(accessed on 5 May 2020)];2018 Available online: https://icono.fecyt.es/informes-y-publicaciones/documento-de-trabajo-evolucion-de-la-nanotecnologia-en-espana.
- 42.Global Market for Nanomaterials (Zion Market Research, 2016) [(accessed on 5 May 2020)]; Available online: https://www.zionmarketresearch.com/
- 43.Gómez-Pastora J., Bringas E., Ortiz I. Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem. Eng. J. 2014;256:187–204. [Google Scholar]
- 44.Tang S.C., Lo I.M. Magnetic Nanoparticles: Essential Factors for Sustainable Environmental Applications. Water Res. 2013;47:2613–2632. doi: 10.1016/j.watres.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 45.Skyspring Nanomaterials Inc. [(accessed on 5 May 2020)]; Available online: https://ssnano.com/
- 46.James K., Highsmith J., Evers P. Drug Development and Delivery. [(accessed on 5 May 2020)]; Available online: https://drug-dev.com/nanotechnology-market-nanotechnology-markets-in-healthcare-medicine/
- 47.The Maturing Nanotechnology Market: Products and Applications Nov 2016|NAN031G|BCC Publishing. [(accessed on 5 May 2020)]; Available online: https://www.bccresearch.com/market-research/nanotechnology/nanotechnology-market-products-applications-report.html.
- 48.Sigma-Aldrich. [(accessed on 5 May 2020)]; Available online: https://www.sigmaaldrich.com/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.