Abstract
目的
探究麝香通心滴丸(STDP)改善猪心肌缺血再灌注后冠脉微循环障碍(CMD)和心功能障碍的可能作用机制。
方法
根据随机数字表法,从21只小型猪随机选取14只行介入球囊阻断左前降支中部血流再灌注建立CMD模型,其余7只作为假手术组,仅行冠脉血管造影,不需要实施手术;再根据随机数字表法将14只动物随机分为CMD模型组(n=7)、CMD+STDP治疗组(n=7),3组动物均以普通饲料喂养8周。药物干预:治疗组予将麝香通心滴丸(3 mg· kg-1· d-1)拌入饲料中进行喂养8周。分别于第0周(手术前)、8周行冠脉造影、计算管腔直径和校正TIMI帧数(CTFC)、心脏彩色超声多普勒检查;8周时处死并取出左前降支冠脉远端分布区域心肌,HE染色观察心肌小血管及心肌组织的病理改变;Western blot检测前降支远端供血区心肌组织中沉默信息调节因子(Sirt1)、过氧化物酶增殖激活受体-γ共激活子-1α(PGC-1α)、过氧化物酶体增殖物激活受体α(PPARα)、细胞外信号调节激酶1/2(ERK1/2)、Toll样受体4(TLR4)、解偶联蛋白2(UCP2)的表达。
结果
实验0周和8周时,3组实验动物的冠脉左前降支血管直径差异无统计学意义(P > 0.05)。实验0周时,各组实验动物CTFC血流帧数值差异无统计学意义(P > 0.05);第8周时,与假手术组相比,模型组CTFC血流帧数显著增加;与模型组比较,STDP治疗组CTFC血流帧数减少(P < 0.05)。实验0周,3组实验动物心脏彩超各项指标差异无统计学意义(P > 0.05);实验8周时,相对于假手术组,模型组舒张期室间隔厚度、左室舒张期内径、左室舒张末容积、收缩期室间厚度、左室质量指标值增加(P < 0.05);与模型组比较,治疗组的上述指标值减小(P < 0.05)。HE染色结果显示,与假手术组相比,模型组心肌组织微血管充血、血栓形成及周边炎症细胞聚集,心肌纤维萎缩,心肌间质炎性细胞浸润;与模型组相比,治疗组未见明显充血,血管周边炎症细胞较少,心肌肌纤维萎缩程度较明显,但心肌间质炎症浸润相对较轻。相对于假手术组,其余各组的心肌组织中Sirt1、PGC-1α、PPARα表达下调,ERK1/2、TLR4、UCP2表达上调;而治疗组与模型组相比,Sirt1、PGC-1α、PPARα表达量更高,ERKI/2、TLR4、UCP2表达量更低。
结论
STDP可能通过Sirt1、PGC-1α、PPARα、ERK1/2、TLR4、UCP2发挥抗炎和调节能量代谢作用,从而改善缺血再灌注后冠脉微循环障碍和心功能障碍。
Keywords: 麝香通心滴丸, 缺血再灌注, 冠脉微循环障碍, 心功能障碍
Abstract
Objective
To investigate the mechanism by which Shexiang Tongxin dripping pills (STDP) improves coronary microcirculation disorder (CMD) and cardiac dysfunction in a porcine model of myocardial ischemia-reperfusion injury.
Methods
Fourteen minipigs were randomly selected for interventional balloon occlusion of the middle left anterior descending branch to induce CMD, and another 7 pigs received sham operation. The pig models of CMD were randomized equally into the model group and STDP-treated group. All the animals were fed with common feed for 8 weeks, and in STDP-treated group, the pigs were given STDP at the daily dose of 3 mg/kg (mixed with feed) for 8 weeks. Before and at the 8th week after the operation, the pigs underwent coronary angiography and echocardiography to determine the vessel lumen diameter and TIMI frame count (CTFC). The pathologies of the myocardium and the microvessels were examined with HE staining at the 8th week. Western blotting was used to detect the expression of silencing information regulator (Sirt1), peroxidase proliferator-activated receptor-γ coactivator-1α (PGC-1α), peroxisome proliferator-activated receptor α (PPARα), extracellular signal-regulated kinase1/2 (ERKI/2), Toll-like receptor 4 (TLR4), and uncoupling protein 2 (UCP2) in myocardial tissue.
Results
Before and at the 8th week after the operation, the diameter of the anterior descending vessel in the 3 groups did not differ significantly (P > 0.05). At the 8th week, the number of CTFC frames in the model group increased significantly compared with that in the sham-operated group, but was obviously lowered by treatment with STDP (P < 0.05). Myocardial ischemia-reperfusion injury significantly increased the interventricular septal thickness at end-diastole, left ventricular end-diastole dimension, end-diastole volume, interventricular septal thickness at end-systole and left ventricular mass at 8 weeks after the modeling (P < 0.05), but such changes were significantly alleviated by treatment with STDP (P < 0.05). STDP treatment markedly alleviated myocardial microvascular congestion, thrombosis and peripheral inflammatory cell infiltration induced by myocardial ischemia-reperfusion, but atrophy of the myocardial muscle fiber remained distinct. STDP obviously suppressed the down-regulation of Sirt1, PGC-1α, and PPARα and the up-regulation of ERK1/ 2, TLR4, and UCP2 in the myocardial tissues induced by myocardial ischemia-reperfusion injury.
Conclusion
STDP has anti-inflammatory effects and regulates energy metabolism in the myocardium through modulating Sirt1, PGC-1α, PPARα, ERKI/2, TLR4, and UCP2 to improve CMD and cardiac dysfunction after myocardial ischemia-reperfusion.
Keywords: Shexiang Tongxin dripping pills, ischemia-reperfusion, coronary microcirculation disorder, cardiac dysfunction
尽管经皮冠状动脉介入(PCI)手术的发展及时重建狭窄或闭塞的冠状动脉血流供应,极大减少了急性心梗患者的存活率。但仍有一部分患者由于缺血再灌注(I/R)引起冠脉微循环障碍(CMD),出现了急诊PCI术后慢血流或无复流现象,导致患者预后不佳甚至最后死亡[1-2]。I/R形成CMD的机制较为复杂,涉及内皮功能障碍、急性缺血缺氧导致心肌细胞及间质水肿压迫血管、斑块碎屑和白细胞-血小板的黏附、聚集堵塞微血管[3]。TLR4在心肌I/R损伤中通过激活NF-κB刺激促炎、免疫调节细胞因子基因表达,介导级联炎症反应[4];研究发现在大鼠冠脉微栓塞模型中ERK1/2激活后介导TNF-αmRNA表达和NF-kB活性以促进炎症反应[5];Sirt1是一种在心肌组织中表达较高的脱乙酰基酶,可通过去乙酰化PGC-1α及其共激活因子PPARα增加其活性,共同调节心肌I/R过程中氧化应激、炎症反应、能量代谢、心肌肥厚等病理生理过程[6]。许多研究证实I/R后心肌组织中Sirt1、PGC-1α、PPARα的表达水平降低[7-9],它们被激活后可通过抑制NF-κB的活性、上调SOD并下调MAD的表达抗氧化应激、增加ATP的含量从而减轻心肌I/R损伤[10-12]。UCP2在I/R后上调具抑制ROS、NF-κB活性以减轻氧化应激和炎症反应的作用,但也会使线粒体膜电位下降导致ATP的合成减少、诱导凋亡蛋白Bcl-2表达上升引起细胞凋亡[13]。因此Sirt1、TLR4、ERK1/2、PGC-1α、PPARα、UCP2可能参与I/R形成CMD的炎症反应过程,并通过能量代谢调节对心脏产生影响。
麝香通心滴丸(STDP)由人工麝香、丹参、人参茎叶总皂苷等七味中药组成,具有益气活血、化瘀止痛之功效。动物实验发现STDP能明显降低动脉粥样硬化家兔血清中TNF-α含量,降低血清中单核细胞趋化蛋白-1和血管细胞间黏附分子-1含量[14]。临床研究证实STDP能明显降低心血管疾病患者血清中的Hs-CRP、TNF-α、IL-18,升高IL-10水平,延缓动脉粥样硬化的进程[15],改善心力衰竭[16]。可明显使急性心梗患者PCI术后血清中eNOS的表达增强、白细胞黏附减少,加快微循环血流速度,改善心肌血流灌注和心功能[17-18]。综上所述,STDP对CMD形成的多个病理生理过程及心功能的改善具有重要作用,但具体分子作用靶点尚不清楚。本研究拟建立I/R模型,用STDP干预治疗,进一步探索STDP防治I/R后CMD形成和心功能障碍的分子作用机制。
1. 材料和方法
1.1. 实验动物
雄性五指山小型猪21只,体质量21~22 kg,由广州市饲料研究所提供,动物合格证号为SYXK(粤)2017- 125.饲料均购自于广州市饲料研究所,许可证号SYXK(粤)2018-0003。饲养温度20~26 ℃,相对湿度40%~70%,120 g· kg-1· d-1定量喂食,自由饮水。本研究已通过实验动物伦理委员会审查。
1.2. 药物及试剂
麝香通心滴丸(批号为Z20080018,购自于康恩贝药业有限公司);戊巴比妥钠溶液(北京华业寰宇化工有限公司);碘海醇注射液(通用电气药业上海有限公司);抗UCP2抗体(批号bs-20750R)、抗PGC-1α抗体(批号bs-1832R)、抗PPARα抗体(批号bs-3614R)、抗Sirt1抗体(批号bs-2257R)、抗TLR4抗体(批号bs-20595R)均购自北京博奥森生物科技公司;抗ERK1/2抗体(批号16443-1-AP,PTG公司)。蛋白裂解液、蛋白酶抑制剂苯甲基磺酰氟(批号FD100,PMSF)、BCA蛋白定量试剂盒(批号FD2001)、ECL发光液(批号FD8030)均购自弗德生物公司。
1.3. 主要仪器
Artise zee III ceiling数字减影X射线机(Siemens);桡动脉穿刺包(穿刺套管针、Runthrough细超滑钢丝和6F桡动脉鞘,日本TERUMO);经皮冠状动脉腔内成形术球囊(美敦力公司Sprinter);7108ISE型全自动生化分析仪(HITACHI);高倍显微镜(Olympus);Vivid E9心脏彩色多普勒超声仪(GE);血管内超声仪(波士顿科学公司);多功能酶标仪(上海现科仪器有限公司);灰度分析软件(Image J)。
1.4. 介入球囊堵闭冠状动脉造成缺血再灌注
小型猪缺血再灌注后CMD造模:根据随机数字表法从21只小型猪中随机选取14只对其行冠脉造影和冠脉左前降支行球囊扩张阻断血流再灌注手术;其余7只作为空白对照组(假手术组)仅行冠脉血管造影,不需要实施手术。手术前五指山小型猪禁食8 h,经耳缘静脉注射3%戊巴比妥钠溶液(30 mL/kg)进行麻醉,待动物的角膜反射消失后将其固定于仰卧位,行经胸心脏彩超、术前备皮、常规碘伏消毒、接通心电监护以及铺洞巾,打开TERUMO动脉穿刺包,使用Seldinger穿刺法把套管针经皮穿刺入右股动脉,穿刺成功后再将导引钢丝轻轻送入,接下来沿着钢丝把6 F(1 F=0.33 mm)动脉鞘管置入血管,用注射器抽取动脉血10 mL以检测血脂、肝肾功能、心激酶等生化指标,往动脉鞘的侧管注射100 U/kg肝素抗凝。在数字减影X射线光机透视下,送入6 FJL3.5冠状动脉指引导管置于左冠状动脉开口行冠脉造影。再沿鞘送入Runthrough NS指引导丝(0.014×180 cm)至左前降支远端,送入Sprinter球囊(3.5 mm×20 mm)至冠状动脉前降支中部,进行缺血预适应3次(每次球囊充盈5 atm×30 s,间隔5 min),随后以3 atm(大气压)充盈球囊完全堵闭血管10 min后撤除导丝、球囊、鞘管,无菌纱布覆盖右股动脉穿刺口,按压约30 min后使用弹力绷带进行加压包扎。大腿内侧肌肉注射青霉素钠(8×105 U/d),注射3 d,单笼喂养观察。空白对照组(假手术组)实验步骤同上,但不予球囊扩张(图 1)。
1.
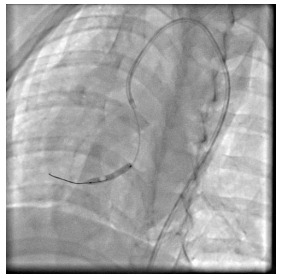
实验动物置入球囊于冠脉前降支中部的图像(头位30°)
Coronary angiography showing balloon placement in the middle of the anterior descending coronary artery
1.5. 动物术后的分组及处理
除假手术组的动物以外,手术过后的动物按随机数字表法分成2组,即微循环障碍模型组和麝香通心滴丸治疗组。手术过后,假手术组和CMD模型组以普通饲料喂养,治疗组则将药物(3 mg· kg-1· d-1)拌入饲料中喂养,药物剂量计算依据药理试验中动物与人的等效剂量换算[19],手术后连续给药8周。
1.6. 血管造影检查、血管狭窄及微循环灌注分析
血管造影机系统自备的二维定量冠状动脉造影原厂工作站定量分析各组实验动物冠脉左前降支血管两幅角度不同的造影图像,计算动脉血管直径,指引导管直径对软件分析进行校正(1 F=0.33 mm),测量心脏在舒张期末时的显影图像,放大图像进行测量以提高准确性,每一段血管分析3次以减少误差,统计分析记录后的数据均值。实际动脉直径通过计算血管校正放大因子获得,放大因子=体外测量导引导管直径÷造影片导引导管直径。测量管腔直径(LD)后,计算管腔丧失=扩张后直径(mm)-管腔直径(mm)。
造影剂完全或近乎完全充盈冠状动脉起始部的第1帧定义为首帧,造影剂进入远端分支血管并使特点解剖标志显影定义为末帧。计算首帧和末帧之间的帧数。CTFC=左前降支TIMI帧数(TFC)/1.7。
1.7. HE病理染色
使用戊巴比妥钠溶液处死动物。取动物冠状动脉左前降支远段供血心肌,生理盐水洗净,立即用4%多聚甲醛溶液固定,石蜡包埋,切片,二甲苯脱蜡,HE染色,梯度乙醇脱水,透明,封片,予光学显微镜下观察(×200)。
1.8. Western blot
同时取猪冠状动脉左前降支远段供血区心肌组织,称取100 mg放入玻璃匀浆器中,加入1 mL裂解液(蛋白裂解液与PMSF比例为100:1),在冰上充分研磨;吸入到预冷1.5 mL无菌EP管中;12 000 r/min 4 ℃,离心10 min,取上清;BCA试剂盒测定蛋白浓度,蛋白变性后,每孔上样20 μL,电泳后转膜3 h,一抗孵育1 h后,暗室中加入ECL发光液后,曝光30 min,扫描图像后Image J软件处理系统分析目标带的光密度值,目的蛋白与内参β-actin条带的光密度值比较后,再各组比较。
1.9. 经胸心脏彩超检查
各试验猪分别于实验0周及实验8周时用Vivid E9心脏彩色多普勒超声仪行经胸心脏彩超检查,测量舒张期室间隔厚度(IVSd)、收缩期室间隔厚度(IVSs)、舒张期左室内径(LVIDd)、收缩期左室内径(LVIDs)、舒张期左室后壁厚度(LVPWd)、收缩期左室后壁厚度(LVPWs)、左室舒张末容积(EDV)、左室收缩末容积(ESV)、射血分数EF(EF)、左室短轴缩短率(FS)、心搏量(SV)、左室质量指数(LV Mass)。
1.10. 统计学处理
采用SPSS 21.0的软件包对数据进行分析,计量数据用均数±标准差表示,实验数据先进行正态性检验和方差齐性检验,组间均数比较在方差齐时用单因素方差分析,方差不齐时用非参数检验。以P < 0.05为差异具有统计学意义。
2. 结果
2.1. STDP改善缺血再灌注后慢血流,减轻冠脉微循环障碍
本实验总共纳入小型猪21只,在建模的过程中,2只死亡,随机选取其中6只作为假手术组(其余12只全部建模成功,随机分为2组。第0周和第8周的各组实验动物行冠脉造影后计算管腔直径(P > 0.05)差异无统计学意义(P > 0.05,表 1);术前各组实验动物的CTFC血流帧数值差异无统计学意义(P > 0.05),排除各组实验动物血管原发性狭窄病变等因素干扰。8周时,经予球囊封堵冠状动脉前降支后,模型组和治疗组的帧数均相对于假手术组显著增多(P < 0.05)。给药组的帧数明显少于模型组(P < 0.05,表 1)。冠脉造影未见各组动物冠脉前降支血管直径有显著差异,假手术组血管充盈良好,可见较多的远端血管显影;手术组远端血管充盈不佳,显影不明显;治疗组可见远端血管充盈显影(图 2)。
1.
实验0、8周各组实验动物冠状动脉管腔直径消失比较分析结果
Comparison of coronary artery lumen loss in each group at 0th and 8th weeks (mm, mean ± SD, n=18)
| Group | 0 week | 8 week | Lumen loss |
| Sham-operated | 2.52±0.03 | 2.46±0.02 | 0.06±0.02 |
| Model | 2.42±0.08 | 2.10±2.21 | 0.31±0.28 |
| Drug-treated | 2.5±0.06 | 2.09±0.19 | 0.42±0.19 |
2.
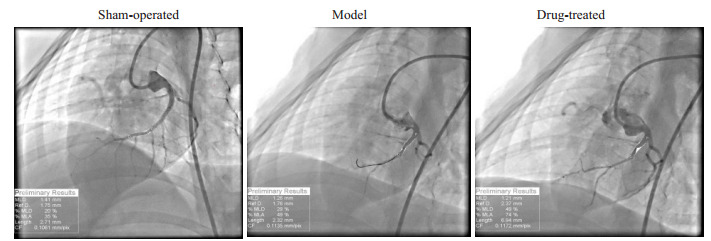
实验动物行冠脉封堵术后8周的血管充盈造影情况
Coronary angiography at 8 weeks after balloon occlusion of the coronary artery in the 3 groups
2.
实验0、8周各组动物校正TIMI帧数(CTFC)比较
Comparison of the CTFC among the 3 groups at week 0th and 8th (mean ± SD, n=18)
| Week | Sham-operated | Model | Drug-treated |
| *P < 0.05 vs Sham-operated group; #P < 0.05 vs Model group. | |||
| 0 | 27.50±1.29 | 28.17±2.79 | 28.67±2.80 |
| 8 | 28.00±1.83 | 40.50±1.87* | 36.50±1.05*# |
2.2. STDP减轻冠脉微循血管充血和炎症浸润
HE染色的结果显示,相对于空白对照组,其余各组的冠脉供血区域的心肌微小血管均出现充血、周边炎性细胞浸润。模型组可见血管充血及周围炎症浸润;治疗组则可见血管周围以脂肪变性为主,充血及炎症浸润程度较轻。各组动物心肌纤维排列呈束状,结构清楚,未见坏死病灶。模型组心肌纤维萎缩,心肌间质炎性细胞浸润;而治疗组的肌纤维萎缩程度较明显,但炎症浸润相对较轻(图 3)。
3.
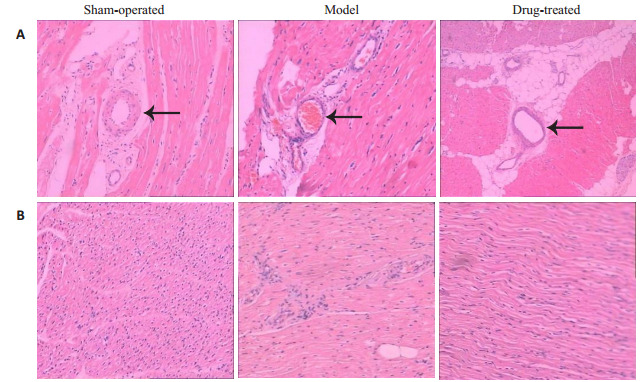
实验8周各组实验动物冠脉供血区的心肌HE染色结果
Myocardial HE staining showing the coronary blood supply area in each group at 8 weeks (Original magnification: × 200). A: Microvessels in the myocardial tissue; B: Myocardial interstitial tissues
2.3. STDP改善舒张功能及心室重构
第0周时各组心脏彩色彩色多普勒的各项指标差异无统计学意义。第8周时,相对于空白对照组,模型组和治疗组的IVSd、LVIDd、IVSs、EDV、LVd Mass显著增加(P < 0.05),但模型组比治疗组增加地更明显(P < 0.05,表 3、4)。各组动物在实验第8周的心超M型图见图 4。
3.
实验0、8周各组实验动物心超各项指标比较分析结果
Comparison of the indexes of cardiac ultrasound among the 3 groups at weeks 0 and 8 (mean ± SD, n=18)
| Group | Ivsd (mm) | LVIDd (mm) | LVPWd (mm) | IVSs (mm) | LVIDs (mm) | LVPWs (mm) |
| *P < 0.05 vs sham-operated group; #P < 0.05 vs Model group. IVSd: Interventricular septal thickness at end-diastole; LVIDd: Left ventricular enddiastole dimension; LVPWd: Left ventricular posterior wall thickness at end- diastole; IVSs: Interventricular septal thickness at endsystole; LVIDs: Left ventricular end-systole dimension; LVPWs: Left ventricular posterior wall thickness at end-systole | ||||||
| Blank control | ||||||
| 0 week | 5.70±0.29 | 28.17±1.15 | 5.18±0.68 | 7.36±0.99 | 22.31±2.30 | 11.14±1.19 |
| 8 week | 6.0±0.57 | 28.21±0.78 | 5.23±0.62 | 7.19±0.78 | 21.80±1.79 | 12.18±1.86 |
| Model | ||||||
| 0 week | 6.09±0.69 | 29.45±1.05 | 5.86±0.84 | 9.71±1.17 | 22.25±3.18 | 11.49±1.43 |
| 8 week | 8.13±0.61* | 31.21±1.2* | 7.6±0.76* | 12.04±1.02* | 22.04±2.61 | 12.43±1.69 |
| Drug-treated | ||||||
| 0 week | 6.12±0.74 | 27.90±0.74 | 6.17±0.46 | 8.98±0.81 | 19.52±1.88 | 9.39±1.35 |
| 8 week | 7.65±0.1*# | 28.10±0.74*# | 6.36±0.81* | 8.11±0.67*# | 21.99±3.32 | 9.96±1.44 |
4.
实验0、8周各组实验动物心超各项指标比较分析结果
Comparison of the indexes of cardiac ultrasound among the 3 groups at weeks 0 and 8 (mean ± SD, n=18)
| Group | EDV (mL) | ESV (mL) | EF (%) | FS (%) | SV (mL) | LVd Mass (g) |
| *P < 0.05 vs sham-operated group; #P < 0.05 vs Model group. EDV: End-diastole volume; ESV: End-systole volume. EF: Ejection fraction; FS: Short-axis fractional shortening; SV: Stroke volume; LV Mass: Left ventricular mass | ||||||
| Sham-operated | ||||||
| 0 week | 30.50±1.29 | 17±4.55 | 74.75±8.5 | 43.25±7.59 | 51.75±11.17 | 29.97±2.12 |
| 8 week | 33.5±1.29 | 19±6.98 | 70.25±8.26 | 39.75±6.85 | 43.5±11.03 | 33.28±2.43 |
| Model | ||||||
| 0 week | 30.16±1.72 | 16.5±4.32 | 69.67±6.47 | 40.33±5.81 | 42.33±11.08 | 30.05±3.01 |
| 8 week | 52.83±5.85* | 13.67±2.07 | 76±5.02 | 45.33±3.67 | 48.33±9.27 | 54.89±10.78* |
| Drug-treated | ||||||
| 0 week | 32.33±3.08 | 12.83±5.49 | 70.5±12.37 | 36.5±6.12 | 24.67±3.67 | 30.85±2.64 |
| 8 week | 36.83±2.48*# | 15.83±1.17 | 65.67±10.41 | 38.17±9.54 | 31.83±10.03 | 41.12±6.39*# |
4.
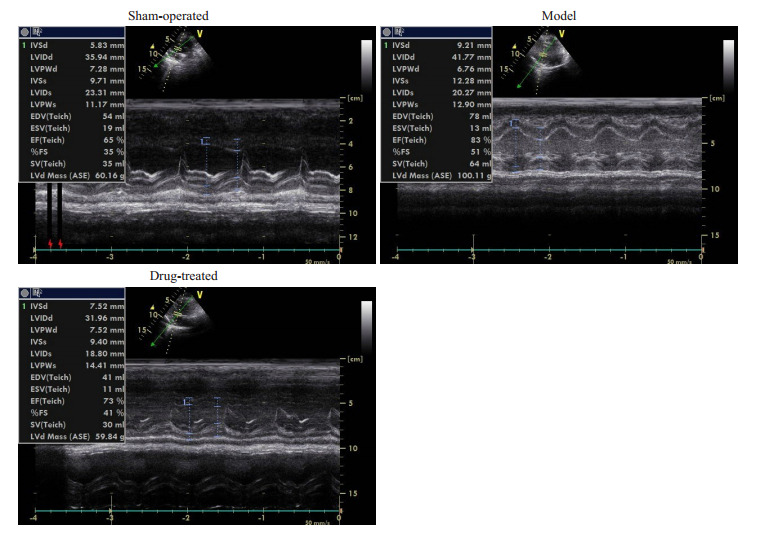
实验8周各组动物心超M型图
M-mode ultrasonogram in each group at the 8th week
2.4. STDP在冠脉微循环障碍中上调Sirt1、PGC-1α、PPARα表达,下调TLR4、ERK1/2、UCP2表达
相对于假手术组,其余各组的心肌组织中Sirt1、PGC-1α、PPARα表达下调,ERKI/2、TLR4、UCP2表达上调(P < 0.05);而治疗组与模型组相比,Sirt1、PGC-1α、PPARα表达量更高,ERKI/2、TLR4、UCP2表达量更低(P < 0.05,图 5)。
5.
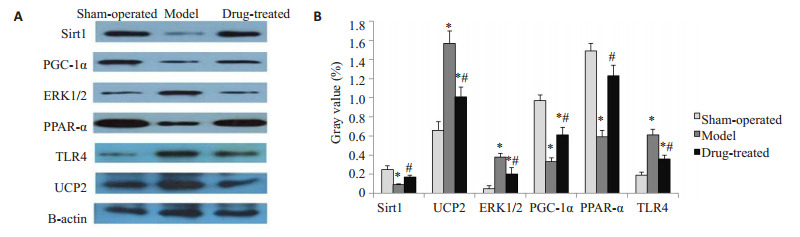
实验8周各组实验动物冠脉供血区心肌组织中SIRT1、ERK1/2、TLR4、UCP2、PGC-1α、PPARα的表达情况
Expression of SIRT1, ERK1/2, TLR4, UCP2, PGC-1α, and PPARα in myocardial tissue of the coronary blood supply area in each group at the 8th week. *P < 0.05 vs Sham-operated group; #P < 0.05 vs Model group, A: Electrophoresis image of each protein; B: Comparison of expression of various proteins between groups
3. 讨论
由于冠脉微循环的机制尚不完全清楚,模拟不同发病机制的动物模型在研究疾病发生和药物干预中具有重要作用。目前研究CMD的动物模型包括主要有冠脉微栓塞模型和心肌缺血再灌注无复流动物模型。冠状动脉血流阻断再灌法充分排除了冠脉血管无复流过程动脉粥样硬化和斑块破裂导致的冠脉栓塞等混杂干扰因素,在无复流相关的机制和药物防治研究中应用较广[20]。临床较常用的评估CMD的方法有TIMI评分及TFC等。TIMI评分无法排除不同个体的心率、血压、右心房压和造影导管直径对血流影响的差异性,也不能直接反映心脏微循环灌注状态,所以因无法精确评估微循环而限制了其在微循环障碍诊断上的应用。CTFC通过帧数计数能定量评估冠脉血流状态,弥补TIMI评分的不足,是冠脉微循环灌注情况的临床评估手段之一[21]。猪的冠状动脉分布及侧枝循环与人类相近,生理病理情况也与人类相似,因此以猪制作心血管相关疾病的动物研究模型更接近临床实际情况。本研究采用介入球囊技术对小型猪冠脉前降支远端的血流进行堵闭再灌注后,用CTFC评估微循环灌注情况。手术前后动物的血管直径、术前的CTFC血流帧数值均无统计学差异,排除了原发血管狭窄病变因素干扰,术后第8周模型组CTFC帧数值明显较假手术组增多,提示存在CMD。我们对前降支供血区心肌进行HE染色,显微镜下观察到模型组术后微小血管充血、血栓形成并见周边炎症浸润,本研究并未涉及PCI术后动脉粥样硬化斑块破裂脱落造成微血管堵塞,因此更符合I/R后微血栓和白细胞-血小板黏附、聚集堵塞微血管这一CMD发生机制。CMD使心肌持续处于灌注不足的状态,又进一步使炎症反应加重,可见炎症反应贯穿CMD形成和发展的全过程。I/R后第8周Western blot的结果显示,相对于假手术组,模型组动物的前降支远端供血区的心肌组织中Sirt1、PGC-1α、PPARα的表达下调,TLR4、ERK1/2、UCP2的表达均有所上调,依据病理结果,提示它们可能主要参与CMD炎症反应机制。上述蛋白分子表达结果与许多研究报道类似,但它们互相之间是否形成一条炎症通路尚不明确。实验研究发现在高脂血症动物模型中,Sirt1蛋白水平与空白组相比显著降低,TLR4和NF-κB显著上调,分别予Sirt1抑制剂和TLR4抑制剂干预后发现Sirt1/TLR4/NF-κB信号通路可能是炎症干预内皮损伤的新的通路[22]。大量研究表明ERK1/2在正常心肌细胞中的表达很低,当受到I/R刺激时,ERK1/2被激活并上调以发挥抗凋亡作用。实验发现Sirt1在心肌I/R中被激活后可能通过ERK1/2信号通路抑制内质网应激相关凋亡的指标的蛋白表达[23];但长时间I/R中ERK的激活则以介导细胞损伤为主[24]。使用ERK1/2特异性抑制剂处理冠脉微栓塞的动物后,其心肌TNF-αmRNA表达和NF-kB活性显著受到抑制[5]。各分子在CMD中的上下游关系如何还有待进一步研究。
术后8周行心脏彩超对心功能进行评估,结果提示心脏舒张功能较有所下降,且左室质量指数增大。在行手术时球囊扩张阻断血流的时间并未过长,是以本实验中HE染色的结果并未见到心肌坏死、纤维化等病理征象,左室质量指数的增加考虑与心肌缺血缺氧后心肌细胞和间质水肿引起的相关可能。既往有CMD使得冠脉血流阻力增加进而影响心脏舒张功能的相关报道[25]。然而,有研究证明心肌I/R后舒张功能异常的出现早于收缩功能障碍[26]。急性缺血、缺氧使线粒体氧化磷酸化功能受损导致ATP生成减少,能量不足使心肌内质网摄钙的含量下降,心肌胞浆钙浓度升高导致心肌兴奋-收缩脱偶联障碍,造成左室舒张功能障碍。冠状动脉血流供应主要在舒张期,所以舒张期异常对心肌灌注起着重要的作用,所以心脏舒张功能障碍又会进一步引起心肌灌注不足,加重CMD。心脏舒张是一个主动耗能的的过程,所需能量主要来自线粒体氧化磷酸化所产生的ATP,减轻心肌细胞线粒体损伤可改善心脏舒张功能障碍[27]。PGC-1α即可以促进线粒体的合成代谢,增加线粒体数目,提高ATP的含量,也可以介导UCP2的表达,减少ATP的产生[28]。UCP2上调可导致心肌能量供应不够[13]。PPARα在心脏内调节脂肪和能量代谢,通过调控基因编码线粒体脂肪酸β-氧化途径,为心脏以ATP的形式提供主要能量来源。PPARα缺陷小鼠的脂肪酸运输和代谢减少,引起心肌脂质堆积和ATP含量减少。结合文献报道,根据本研究中PGC-1α、PPARα下调和UCP2上调的结果,我们推测本实验中CMD也存在心肌能量代谢障碍的问题,但这仍需通过测定ATP及相关酶分子的含量来进一步证明。
本研究中,I/R后经予STDP治疗8周,动物CTFC血流帧数值显著减少,且HE染色结果显示充血及炎症浸润程度减轻,提示冠脉微循环障碍得到一定程度的改善,心脏舒张功能及心室重构得到改善,这和既往研究的结果一致。进一步研究发现经STDP治疗的动物心肌组织中Sirt1、PGC-1α、PPARα的蛋白表达有所上调,而TLR4、ERK1/2、UCP2的表达均下调了,这一结果提示这几个蛋白可能是STDP改善CMD炎症反应和调节能量代谢的作用靶点分子。Sirt1的过表达导致PPARα共激活因子PGC-1α脱乙酰化[29],被的激活PGC-1α信号通路介导的线粒体生成和氧化应激调节,防治心肌细胞氧化损伤和线粒体损伤[12];还可使NF-κB去乙酰化而失去活性,抑制血管炎症损伤[30]。研究发现PPAR-a激动剂可抑制NF-κB活性而减少炎性因子的释放,减少中性粒细胞聚集,降低游离脂肪酸水平,缩小心梗面积和改善心功能[10]。既往也有关于组成STDP药物中的个别活性成分作用于上述分子的文献报道。丹参酮ⅡA磺酸钠可能通过抑制磷酸化ERK1/2表达来抑制血管紧张素Ⅱ诱导的心肌肥大[31],还可通过调节Sirt1抑制氧化应激和炎症反应防治心肌重构[32];丹参多酚酸酯可通过作用ERK1 / 2信号通路减少小鼠心肌I/R损伤[33];隐丹参酮可通过增加PGC-1α和线粒体转录因子A的表达修复线粒体的部分功能,从而减轻阿霉素诱导的大鼠线粒体功能障碍[34];丹参酸酚B可激活Sirt1减轻肝氧化应激损伤[35];丹参酮ⅡA抑制TLR4和TNF-α的表达发挥抗动脉粥样硬化作用[36]。人参茎叶总皂苷可以上调肥胖小鼠脂肪组织中PPARα,PGC-1α的基因表达[37],其活性成分人参皂苷Rg3可以通过激活心肌组织中PGC-1α和NRF-2蛋白的表达,从而增强TFAM,调节线粒体的结构与功能,从而改善心脏功能[38];人参皂苷Rg1可通过上调PGC-1α,减少细胞外液中游离脂肪酸,有效改善由异丙肾上腺素诱导的乳鼠心肌肥大[39]。
本研究揭示STDP对I/R引起的冠脉微循环障碍中心脏Sirt1、PGC-1α、PPARα、ERKI/2、TLR4、UCP2的表达均有影响,我们首次提出STDP作用抗炎、调节能量代谢障碍相关的分子靶点发挥防治I/R后冠脉微循环障碍和心功能障碍。这一发现对进一步探索CMD的发病机制及其治疗靶点,以及STDP的药理作用分子机制均具有重要意义。
Biography
姚玉斯,硕士,E-mail: 15989275369@163.com
Funding Statement
苏州工业园区心馨心血管健康基金会微血管创新基金项目(2018-CCA-CMVD-10);广东省教育厅项目(2019KCXTD018);广东省中医药局科研课题(20191203);广东省临床教学基地教学改革研究项目课题(2018JD028)
Contributor Information
姚 玉斯 (Yusi YAO), Email: 15989275369@163.com.
曾 智桓 (Zhihuan ZENG), Email: gzzh@163.com.
References
- 1.Gibson CM, Schömig A. Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion. Circulation. 2004;109(25):3096–105. doi: 10.1161/01.CIR.0000134278.50359.CB. [Gibson CM, Schömig A. Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion[J]. Circulation, 2004, 109(25): 3096-105.] [DOI] [PubMed] [Google Scholar]
- 2.赵 映, 陈 韵岱, 田 峰, et al. 急性心肌梗死患者急诊PCI术后无复流的危险因素分析. http://www.j-smu.com/oa/pdfdow.aspx?Sid=201202261. 南方医科大学学报. 2012;32(2):261–4. [赵映, 陈韵岱, 田峰, 等.急性心肌梗死患者急诊PCI术后无复流的危险因素分析[J].南方医科大学学报, 2012, 32(2): 261-4.] [PubMed] [Google Scholar]
- 3.Stiermaier T, Thiele H, Eitel I. Coronary microvascular obstruction. http://d.old.wanfangdata.com.cn/Periodical/zgjjyx200303011. Cardio Imaging. 2017;10(6):e006568. doi: 10.1161/CIRCIMAGING.117.006568. [Stiermaier T, Thiele H, Eitel I. Coronary microvascular obstruction [J]. Cardio Imaging, 2017, 10(6): e006568.] [DOI] [PubMed] [Google Scholar]
- 4.
- 5.李 浪, 黎 东华, 曲 楠, et al. ERK1/2信号转导通路在大鼠冠状动脉微栓塞致心肌炎症及心功能损伤的作用机制. 中华医学杂志. 2010;90(8):558–63. doi: 10.3760/cma.j.issn.0376-2491.2010.08.014. [李浪, 黎东华, 曲楠, 等. ERK1/2信号转导通路在大鼠冠状动脉微栓塞致心肌炎症及心功能损伤的作用机制[J].中华医学杂志, 2010, 90(8): 558-63.] [DOI] [PubMed] [Google Scholar]
- 6.Yu LM, Sun Y, Cheng L, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res. 2014;57(2):228–38. doi: 10.1111/jpi.12161. [Yu LM, Sun Y, Cheng L, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1[J]. J Pineal Res, 2014, 57(2): 228-38.] [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Lu L, Greyson C, et al. The PPAR-alpha activator fenofibrate fails to provide myocardial protection in ischemia and reperfusion in pigs. Am J Physiol Heart Circ Physiol. 2006;290(5):H1798–807. doi: 10.1152/ajpheart.00631.2005. [Xu Y, Lu L, Greyson C, et al. The PPAR-alpha activator fenofibrate fails to provide myocardial protection in ischemia and reperfusion in pigs[J]. Am J Physiol Heart Circ Physiol, 2006, 290(5): H1798-807.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu CP, Zhai P, Yamamoto T, et al. Sirt1 Protects the Heart from Ischemia/Reperfusion. http://cn.bing.com/academic/profile?id=29b6fb41918b8cf7350e83d0ad34ce16&encoded=0&v=paper_preview&mkt=zh-cn. Circulation. 2011;122(21):2170–82. doi: 10.1161/CIRCULATIONAHA.110.958033. [Hsu CP, Zhai P, Yamamoto T, et al. Sirt1 Protects the Heart from Ischemia/Reperfusion[J]. Circulation, 2011, 122(21): 2170-82.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang JY, Lu LH, Liu Y, et al. Quercetin improve ischemia/ reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J Cellular Biochem. 2019;120(06):9747–57. doi: 10.1002/jcb.28255. [Tang JY, Lu LH, Liu Y, et al. Quercetin improve ischemia/ reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling[J]. J Cellular Biochem, 2019, 120(06): 9747-57.] [DOI] [PubMed] [Google Scholar]
- 10.Yue TL, Bao W, Jucker BM, et al. Activation of Peroxisome Proliferator-Activated Receptor- Protects the Heart From Ischemia/ Reperfusion Injury. Circulation. 2003;108(19):2393–9. doi: 10.1161/01.CIR.0000093187.42015.6C. [Yue TL, Bao W, Jucker BM, et al. Activation of Peroxisome Proliferator-Activated Receptor- Protects the Heart From Ischemia/ Reperfusion Injury[J]. Circulation, 2003, 108(19): 2393-9.] [DOI] [PubMed] [Google Scholar]
- 11.Hu MZ, Zhou B, Mao HY, et al. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts from ischemia/ reperfusion injury through sirt1/PGC-1α signaling pathway. Int Heart J. 2016;57(4):477–82. doi: 10.1536/ihj.15-506. [Hu MZ, Zhou B, Mao HY, et al. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts from ischemia/ reperfusion injury through sirt1/PGC-1α signaling pathway[J]. Int Heart J, 2016, 57(4): 477-82.] [DOI] [PubMed] [Google Scholar]
- 12.Ye QY, Ye LF, Xu XJ, et al. Epigallocatechin-3-gallate suppresses 1- methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. https://link.springer.com/article/10.1186/1472-6882-12-82. BMC Complement Altern Med. 2012;12(1):1–7. doi: 10.1186/1472-6882-12-82. [Ye QY, Ye LF, Xu XJ, et al. Epigallocatechin-3-gallate suppresses 1- methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway[J]. BMC Complement Altern Med, 2012, 12(1): 1-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshovs'ka IuV, Lisovyĭ OO, Shymans'ka TV, et al. UCP2 and UCP3 gene expression, heart function and oxygen cost of myocardial work changes during aging and ischemia-reperfusion. http://cn.bing.com/academic/profile?id=89438b3aa508788e7d452981a8d1b8e0&encoded=0&v=paper_preview&mkt=zh-cn. Fiziol Zh. 2009;55(3):26–36. [Hoshovs'ka IuV, Lisovyĭ OO, Shymans'ka TV, et al. UCP2 and UCP3 gene expression, heart function and oxygen cost of myocardial work changes during aging and ischemia-reperfusion [J]. Fiziol Zh, 2009, 55(3): 26-36.] [PubMed] [Google Scholar]
- 14.王 怡, 牛 子长, 何 斌, et al. 麝香通心滴丸对稳定动脉粥样硬化斑块的机制研究. 中西医结合心脑血管病杂志. 2011;9(9):1083–6. doi: 10.3969/j.issn.1672-1349.2011.09.033. [王怡, 牛子长, 何斌, 等.麝香通心滴丸对稳定动脉粥样硬化斑块的机制研究[J].中西医结合心脑血管病杂志, 2011, 9(9): 1083-6.] [DOI] [Google Scholar]
- 15.Xiong MQ, Jia CL, Cui JG, et al. Shexiang Tongxin dropping pill attenuates atherosclerotic lesions in ApoE deficient mouse model. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1e5c7ec6b627dfed3b4988dbe84b94d3. J Ethnopharmacol. 2015;159(7):84–92. doi: 10.1016/j.jep.2014.11.013. [Xiong MQ, Jia CL, Cui JG, et al. Shexiang Tongxin dropping pill attenuates atherosclerotic lesions in ApoE deficient mouse model [J]. J Ethnopharmacol, 2015, 159(7): 84-92.] [DOI] [PubMed] [Google Scholar]
- 16.Huang XP, Li ZX, Wu RX. Effect of shexiang tongxin dropping pill on cardiac function in patients with chronic heart failure. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zxyjhxnxgbzz201102008. Chin J Integrat Med Cardio Disease. 2011;25(9):143–45. [Huang XP, Li ZX, Wu RX. Effect of shexiang tongxin dropping pill on cardiac function in patients with chronic heart failure[J]. Chin J Integrat Med Cardio Disease, 2011, 25(9): 143-45.] [Google Scholar]
- 17.Xiao SS, Tang B. Shexiang Tongxin Dropping Pill combined with thrombus aspiration improves myocardial perfusion after PCI in patients with STEMI: the clinical research. http://en.cnki.com.cn/Article_en/CJFDTOTAL-HNYY201704008.htm. J Hainan Medical University. 2017;23(4):31–35. [Xiao SS, Tang B. Shexiang Tongxin Dropping Pill combined with thrombus aspiration improves myocardial perfusion after PCI in patients with STEMI: the clinical research[J]. J Hainan Medical University, 2017, 23(4): 31-35.] [Google Scholar]
- 18.张 艳达, 施 珊岚, 厉 娜, et al. 麝香通心滴丸改善心肌梗死小鼠微循环障碍的作用机制. http://d.old.wanfangdata.com.cn/Periodical/zxyjhxnxgbzz201723008. 中西医结合心脑血管病杂志. 2017;15(23):2969–72. [张艳达, 施珊岚, 厉娜, 等.麝香通心滴丸改善心肌梗死小鼠微循环障碍的作用机制[J].中西医结合心脑血管病杂志, 2017, 15(23): 2969-72.] [Google Scholar]
- 19.黄 继汉, 黄 晓晖, 陈 志扬, et al. 药理试验中动物间和动物与人体间的等效剂量换算. 中国临床药理学与治疗学. 2004;9(9):1069–72. doi: 10.3969/j.issn.1009-2501.2004.09.026. [黄继汉, 黄晓晖, 陈志扬, 等.药理试验中动物间和动物与人体间的等效剂量换算[J].中国临床药理学与治疗学, 2004, 9(9): 1069-72.] [DOI] [Google Scholar]
- 20.Uitterdijk A, Sneep S, van Duin RW, et al. Serial measurement of hFABP and high-sensitivity troponin I post-PCI in STEMI: how fast and accurate can myocardial infarct size and no-reflow be predicted? Am J Physiol Heart Circ Physiol, 2013;305(7):H1104–10. doi: 10.1152/ajpheart.00447.2013. [Uitterdijk A, Sneep S, van Duin RW, et al. Serial measurement of hFABP and high-sensitivity troponin I post-PCI in STEMI: how fast and accurate can myocardial infarct size and no-reflow be predicted[J]? Am J Physiol Heart Circ Physiol, 2013, 305(7): H1104-10.] [DOI] [PubMed] [Google Scholar]
- 21.Umman B, Nisanci Y, Sezer M, et al. The relationship between corrected TIMI frame count and myocardial fractional flow reserve. http://cn.bing.com/academic/profile?id=041dc42b794c21089793e57e5b467c7a&encoded=0&v=paper_preview&mkt=zh-cn. J Invasive Cardiol. 2002;14(3):125–8. [Umman B, Nisanci Y, Sezer M, et al. The relationship between corrected TIMI frame count and myocardial fractional flow reserve [J]. J Invasive Cardiol, 2002, 14(3): 125-8.] [PubMed] [Google Scholar]
- 22.Jie H, Hua-Qin T, Song-Yi C, et al. Effect of kangshuanyihao formula on the inflammatory reaction and SIRT1/TLR4/NF-r, κr, B signaling pathway in endothelial injury. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429935/ Evid Based Complement Alternat Med. 2017;2017:1–11. doi: 10.1155/2017/9019765. [Jie H, Hua-Qin T, Song-Yi C, et al. Effect of kangshuanyihao formula on the inflammatory reaction and SIRT1/TLR4/NF-r, κr, B signaling pathway in endothelial injury[J]. Evid Based Complement Alternat Med, 2017, 2017:1-11.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.吴倩. SIRT1对大鼠心肌缺血再灌注时内质网应激相关凋亡的影响及ERK1/2通路的作用[D].重庆: 重庆医科大学, 2015.http://cdmd.cnki.com.cn/Article/CDMD-10631-1016008287.htm
- 24.Jiang M, Wang L, Jiang HH. Role of spinal MAPK-ERK signal pathway in myocardial ischemia-reperfusion injury. http://d.old.wanfangdata.com.cn/Periodical/zgddekzz201305017. Chin J Contemp Pediatr. 2013;15(5):387–91. [Jiang M, Wang L, Jiang HH. Role of spinal MAPK-ERK signal pathway in myocardial ischemia-reperfusion injury[J]. Chin J Contemp Pediatr, 2013, 15(5): 387-91.] [PubMed] [Google Scholar]
- 25.Remmelink M, Sjauw KD, Yong ZY, et al. Coronary microcirculatory dysfunction is associated with left ventricular dysfunction during follow-up after STEMI. Neth Heart J. 2013;21(5):238–44. doi: 10.1007/s12471-013-0382-2. [Remmelink M, Sjauw KD, Yong ZY, et al. Coronary microcirculatory dysfunction is associated with left ventricular dysfunction during follow-up after STEMI[J]. Neth Heart J, 2013, 21(5): 238-44.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leather HA, De Wolff MH, Wouters PF. Effects of propofol on the systolic and diastolic performance of the postischaemic, reperfused myocardium in rabbits. Eur J Anaesthesiol. 2003;20(3):191–8. doi: 10.1097/00003643-200303000-00003. [Leather HA, De Wolff MH, Wouters PF. Effects of propofol on the systolic and diastolic performance of the postischaemic, reperfused myocardium in rabbits[J]. Eur J Anaesthesiol, 2003, 20(3): 191-8.] [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Zhang Z, Hu F, et al. 17β-estradiol prevents cardiac diastolic dysfunction by stimulating mitochondrial function: a preclinical study in a mouse model of a human hypertrophic cardiomyopathy mutation. https://www.scholars.northwestern.edu/en/publications/17%CE%B2-estradiol-prevents-cardiac-diastolic-dysfunction-by-stimulati. J Steroid Biochem Mol Biol. 2015;147(5):92–102. doi: 10.1016/j.jsbmb.2014.12.011. [Chen Y, Zhang Z, Hu F, et al. 17β-estradiol prevents cardiac diastolic dysfunction by stimulating mitochondrial function: a preclinical study in a mouse model of a human hypertrophic cardiomyopathy mutation[J]. J Steroid Biochem Mol Biol, 2015, 147 (5): 92-102.] [DOI] [PubMed] [Google Scholar]
- 28.Oberkofler H, Klein K, Felder TK, et al. Role of peroxisome proliferator-activated receptor-Gamma coactivator-1alpha in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells. Endocrinology. 2006;147(2):966–76. doi: 10.1210/en.2005-0817. [Oberkofler H, Klein K, Felder TK, et al. Role of peroxisome proliferator-activated receptor-Gamma coactivator-1alpha in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells[J]. Endocrinology, 2006, 147(2): 966-76.] [DOI] [PubMed] [Google Scholar]
- 29.Planavila A, Iglesias R, Giralt M, et al. Sirt1 Acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_PM21115502. Cardiovasc Res. 2011;90(2):276–84. doi: 10.1093/cvr/cvq376. [Planavila A, Iglesias R, Giralt M, et al. Sirt1 Acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation[J]. Cardiovasc Res, 2011, 90(2): 276-84.] [DOI] [PubMed] [Google Scholar]
- 30.Yang HY, Zhang W, Pan H, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One. 2012;7(9):e46364. doi: 10.1371/journal.pone.0046364. [Yang HY, Zhang W, Pan H, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity[J]. PLoS One, 2012, 7(9): e46364.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li SS, Feng J, Zheng Z, et al. Effect of sodium tanshinone Ⅱ A sulfonate on phosphorylation of extracellular signal-regulated kinase 1/ 2 in angiotensin Ⅱ-induced hypertrophy of myocardial cells. Chin J Integr Med. 2008;14(2):123–7. doi: 10.1007/s11655-008-0123-3. [Li SS, Feng J, Zheng Z, et al. Effect of sodium tanshinone Ⅱ A sulfonate on phosphorylation of extracellular signal-regulated kinase 1/ 2 in angiotensin Ⅱ-induced hypertrophy of myocardial cells[J]. Chin J Integr Med, 2008, 14(2): 123-7.] [DOI] [PubMed] [Google Scholar]
- 32.Feng J, Li SS, Chen HW. Tanshinone ⅡA inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: Possible role of silent information regulator 1. https://www.sciencedirect.com/science/article/pii/S0014299916306409. Eur J Pharmacol. 2016;791(11):632–9. doi: 10.1016/j.ejphar.2016.09.041. [Feng J, Li SS, Chen HW. Tanshinone ⅡA inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: Possible role of silent information regulator 1[J]. Eur J Pharmacol, 2016, 791(11): 632-9.] [DOI] [PubMed] [Google Scholar]
- 33.Qi JY, Yu J, Huang DH, et al. Salvianolate reduces murine myocardial ischemia and reperfusion injury via ERK1/2 signaling pathways in vivo. Chin J Integr Med. 2017;23(1):40–47. doi: 10.1007/s11655-016-2621-z. [Qi JY, Yu J, Huang DH, et al. Salvianolate reduces murine myocardial ischemia and reperfusion injury via ERK1/2 signaling pathways in vivo[J]. Chin J Integr Med, 2017, 23(1): 40-47.] [DOI] [PubMed] [Google Scholar]
- 34.Zhang YS, Liang C, Fan L, et al. Cyptotanshinone protects against adriamycin-induced mitochondrial dysfunction in cardiomyocytes. https://www.tandfonline.com/doi/pdf/10.3109/13880209.2015.1029052. Pharm Biol. 2015;54(2):1–6. doi: 10.3109/13880209.2015.1029052. [Zhang YS, Liang C, Fan L, et al. Cyptotanshinone protects against adriamycin-induced mitochondrial dysfunction in cardiomyocytes [J]. Pharm Biol, 2015, 54(2): 1-6.] [DOI] [PubMed] [Google Scholar]
- 35.Zeng WJ, Shan W, Gao LL, et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. http://cn.bing.com/academic/profile?id=ee1b3d048fa00e2d3ea78322b42dcea3&encoded=0&v=paper_preview&mkt=zh-cn. Sci Rep. 2015;24(5):16013. doi: 10.1038/srep16013. [Zeng WJ, Shan W, Gao LL, et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease[J]. Sci Rep, 2015, 24(5): 16013.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.贾 连群, 冯 峻屹, 杨 关林, et al. 丹参酮ⅡA对EA.hy926细胞TLR4/NF- κB炎症信号通路的影响. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_zgshywzz201105001. 中国生化药物杂志. 2011;31(5):337–40. [贾连群, 冯峻屹, 杨关林, 等.丹参酮ⅡA对EA.hy926细胞TLR4/NF- κB炎症信号通路的影响[J].中国生化药物杂志, 2011, 31(5): 337-40.] [Google Scholar]
- 37.Chen GL, Li HJ, Zhao Y, et al. Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high-fat diet-induced obese C57BL/6 mice. Food Chem Toxicol. 2017;106:393–403. doi: 10.1016/j.fct.2017.06.012. [Chen GL, Li HJ, Zhao Y, et al. Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high-fat diet-induced obese C57BL/6 mice[J]. Food Chem Toxicol, 2017, 106: 393-403.] [DOI] [PubMed] [Google Scholar]
- 38.Sun MW, Huang CL, Wang C, et al. Ginsenoside Rg3 improves cardiac mitochondrial population quality: mimetic exercise training. http://cn.bing.com/academic/profile?id=4131abaef8461a4178446ff6b81e7abe&encoded=0&v=paper_preview&mkt=zh-cn. Biochem Biophys Res Commun. 2013;441(1):169–74. doi: 10.1016/j.bbrc.2013.10.039. [Sun MW, Huang CL, Wang C, et al. Ginsenoside Rg3 improves cardiac mitochondrial population quality: mimetic exercise training [J]. Biochem Biophys Res Commun, 2013, 441(1): 169-74.] [DOI] [PubMed] [Google Scholar]
- 39.Hui W, Hang G. Ginsenoside Rg1 protect cardiac hypertrophy induced by ISO via PGC-1α signaling pathway. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zyylylc201503017. Pharm Clin Chin Mater Med. 2015;14(3):55–9. [Hui W, Hang G. Ginsenoside Rg1 protect cardiac hypertrophy induced by ISO via PGC-1α signaling pathway[J]. Pharm Clin Chin Mater Med, 2015, 14(3): 55-9.] [Google Scholar]


