Abstract
Red blood cell distribution width (RDW) and neutrophil-to-lymphocyte ratio (NLR) have shown a prognostic value in various clinical settings. We aimed to investigate the association between RDW, NLR, and in-hospital mortality in patients with dyspnea. In this retrospective study with the Medical Information Mart for Intensive Care III database (version 1.4), adult patients who came to the emergency department with dyspnea were included. Patients' comorbidities, hematological parameters within the first 48 hours after admission to the emergency department, and in-hospital mortality were extracted. The relationships between RDW, NLR, and in-hospital mortality were analyzed with the receiver operating characteristic (ROC) curve analysis and multivariate logistic regression model. We found that hospital survivors had significantly lower NLR than those who died. However, RDW was not significantly increased in patients who died during the hospitalization. The area under the ROC curve of NLR for predicting in-hospital mortality was 0.62. On multivariate analysis, NLR was not independently associated with in-hospital mortality. On further analysis, lymphocyte percentage was independently associated with in-hospital mortality, with an odds ratio of 0.56. Therefore, we concluded that RDW and NLR are not reliable parameters to predict in-hospital mortality in critically ill patients admitted to the emergency department with dyspnea.
1. Introduction
Dyspnea is one of the most common symptoms at presentation to the emergency department (ED) [1]. Dyspnea can be the initial clinical manifestation of many disorders, such as congestive heart failure (CHF), pneumonia, acute myocardial infarction (AMI), and sepsis [2]. Patients with dyspnea are found to have higher mortality and readmission rate [3], and early risk stratification is crucial to improve patient outcomes [1].
Red blood cell distribution width (RDW) is a routinely reported hematology parameters that can be automatically measured by modern hematological analyzers [4]. It is a measure of heterogeneity in the size of circulating red blood cells (RBC). In the past, RDW has been used for differentiating thalassemia and iron deficiency anemia [5]. However, recent studies have revealed that RDW is also a useful test for estimating the prognosis or disease activity of various cancers [6, 7], thyroiditis [8], gastrointestinal disorders [9], cardiovascular diseases [10–13], critical illness [14–16], and autoimmune diseases [17, 18]. This may be due to the fact that RDW is an inflammatory parameter [17], and inflammatory response is involved in the progression of various disorders. To present, two studies have investigated the prognostic value of RDW in unselected dyspneic patients [19, 20], but with varying results. Therefore, further evidence is needed to clarify the prognostic value of RDW in dyspneic patients.
Similarly, the neutrophil-to-lymphocyte ratio (NLR) is an inflammatory parameter that can be easily calculated from routinely available hematology parameters [21]. Similar to RDW, NLR has been found to be a prognostic factor or diagnostic marker in various diseases, such as pregnancy-related disorders [22], thyroiditis [23], cancers [24, 25], and cardiovascular diseases [26–29]. However, the role of NLR in patients with dyspnea remains unknown. Because NLR is an inflammatory parameter and inflammatory markers are associated with prognosis in the dyspneic patients [30], we hypothesized that NLR could be used for risk stratification in dyspneic patients. In this study, we aimed to investigate the prognostic value of RDW and NLR in dyspneic patients.
2. Materials and Methods
2.1. Database and Patients
This is a retrospective study with MIMIC-III (Medical Information Mart for Intensive Care III) database (version 1.4). MIMIC III is a large, freely accessible critical care database comprising 38,645 adults and 7,875 neonates who were admitted to the intensive care unit (ICU) of Beth Israel Deaconess Medical Center between 2001 and 2012 [31, 32]. The Institutional Review Boards (IRB) of the Massachusetts Institute of Technology (MIT) approved the establishment of MIMIC III. One of the authors (ZD Hu, certification number: 1678079) has passed a web-based course and was approved for extracting data from MIMIC III for research purposes. Informed consent was waived because all data are from a publicly available database.
The inclusion criteria of this study were as follows: (i) patients who visit the emergency department (ED) complains of dyspnea; (ii) RDW determined within 48 hours after emergency department admission. Patients with aged <18 years were excluded.
2.2. Data Extraction
The clinical and demographical data of the patients were stored in 26 tables and can be extracted with structured query language (SQL). We extracted the following data from MIMIC III: age, gender, ethnicity, hematology parameters, comorbidities [33], and final diagnosis. Only the hematological parameters determined within 48 hours at the time of presentation after ED admission were included. For patients with more than one hematology parameters during the first 48 hours after ED admission, only the first result was studied. The SQL script used to extract comorbidities was available from the GitHub website (https://github.com/MIT-LCP/mimic-code/tree/master/concepts, date of access: January 2019) [33]. We used the keywords “dyspnea” and “shortness of breath” to search dyspneic patients in the ADMISSIONS table of MIMIC III.
2.3. Statistical Analysis
Continuous data were presented as mean and standard deviation (SD). The normal distribution of continuous variables was tested using the Kolmogorov-Smirnov test. Mann-Whitney U test or Student's t test was used for continuous data comparison between two groups, if appropriate. A Chi-square test was used for categorical variable comparison. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive accuracy of hematology parameters for in-hospital mortality. The multivariable logistic regression model was used to analyze the association between hematology parameters and in-hospital mortality. All statistical analyses were performed using R (version 3.5.0 (The R Foundation for Statistical Computing)), and p value < 0.05 was considered as statistically significant.
3. Results
3.1. Characteristics of the Patients
Figure 1 is a flowchart depicting the patient selection process. Finally, 447 patients with a RDW value were included in the present work, and 183 of them have a NLR value. The clinical characteristics of the patients are summarized in Table 1. Notably, more than half of the patients had comorbidity of CHF, and one-fourth of patients had renal failure. Compared with hospital survivors, patients who died in hospital were older and had significantly higher white blood cell (WBC) count and NLR, as well as lower lymphocyte percentage. However, we failed to find significantly higher RDW in the patients who died in the hospital as compared to survivors (p = 0.690).
Figure 1.
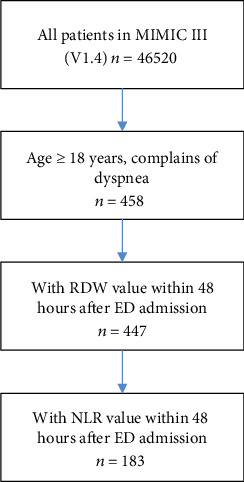
Flow chart for subject identification and inclusion.
Table 1.
Characteristics of the subjects.
| All | Survivor | Nonsurvivor | p | ||||
|---|---|---|---|---|---|---|---|
| n | Results | n | Results | n | Results | ||
| Age (years) | 447 | 68 (56–79) | 361 | 66 (56–78) | 86 | 75 (61–82) | 0.003 |
| Gender (M/F) | 447 | 213/234 | 361 | 172/189 | 86 | 41/45 | 1.000 |
| Ethnicity (white/others) | 447 | 320/127 | 361 | 246/115 | 86 | 74/12 | 0.001 |
| Hematology | |||||||
| WBC (109/L) | 447 | 8.7 (6.4–12.9) | 361 | 8.5 (6.4–12.0) | 86 | 10.6 (6.3–15.9) | 0.034 |
| Neutrophil (%) | 184 | 84 (74–90) | 143 | 84 (74–90) | 41 | 85 (76–91) | 0.398 |
| Lymphocyte (%) | 183 | 9 (5–16) | 142 | 10 (6–16) | 41 | 6 (4–10) | 0.008 |
| NLR | 183 | 9.1 (4.7–17.2) | 142 | 8.3 (4.5–15.1) | 86 | 11.4 (7.9–25.2) | 0.024 |
| Hemoglobin (g/L) | 447 | 105 (92–120) | 361 | 106 (92–120) | 86 | 101 (89–118) | 0.362 |
| RDW (%) | 447 | 15.5 (14.2–17.2) | 361 | 15.4 (14.2–17.2) | 86 | 15.7 (14.2–17.2) | 0.690 |
| MCV (fl) | 447 | 90 (86–94) | 361 | 90 (86–95) | 86 | 90 (85–94) | 0.358 |
| MCH (pg) | 447 | 29.6 (27.9–31.4) | 361 | 29.6 (28.0–31.5) | 86 | 29.6 (27.8–30.8) | 0.230 |
| MCHC (g/L) | 447 | 327 (317–338) | 361 | 328 (318–339) | 86 | 325 (317–336) | 0.336 |
| Comorbidity | |||||||
| CHF (Y/N) | 447 | 230/217 | 361 | 199/162 | 86 | 31/55 | 0.002 |
| Liver disease (Y/N) | 447 | 45/402 | 361 | 32/329 | 86 | 13/73 | 0.125 |
| Renal failure (Y/N) | 447 | 117/330 | 361 | 99/262 | 86 | 18/68 | 0.218 |
| Hypertension (Y/N) | 447 | 247/200 | 361 | 209/152 | 86 | 38/48 | 0.029 |
| Cardiac arrhythmias (Y/N) | 447 | 174/273 | 361 | 138/223 | 86 | 36/50 | 0.619 |
| Valvular disease (Y/N) | 447 | 74/373 | 361 | 62/299 | 86 | 12/74 | 0.575 |
| CPD (Y/N) | 447 | 193/254 | 361 | 158/203 | 86 | 35/51 | 0.693 |
| Hypothyroidism (Y/N) | 447 | 63/384 | 361 | 55/306 | 86 | 8/78 | 0.212 |
| Final diagnosis | |||||||
| Heart failure | 447 | 65 | 361 | 56 | 86 | 9 | 0.233 |
| Pneumonia | 447 | 35 | 361 | 28 | 86 | 7 | 0.905 |
WBC: white blood cell; NLR: neutrophil-to-lymphocyte ratio; RDW: red blood cell distribution width; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; CHF: congestive heart failure; CPD: chronic pulmonary disease.
3.2. Accuracy of RDW and NLR for In-Hospital Mortality
Figure 2 shows ROC curve of RDW and NLR for in-hospital mortality. The area under curve (AUC) of NLR was 0.62 (95% CI: 0.51–0.72, p = 0.024). The AUC of RDW was not statistically significant (AUC = 0.51; 95% CI: 0.44–0.58; p = 0.690). In addition, we found that the AUC of the lymphocyte percentage was 0.64 (95% CI: 0.54–0.74; p = 0.008).
Figure 2.
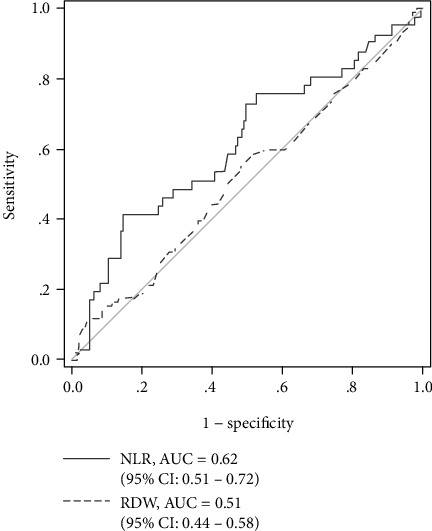
Receiver operating characteristic (ROC) curve of RDW and NLR for predicting in-hospital mortality.
3.3. Multivariate Analysis
Next, we performed a multivariable logistic regression analysis to study the association between NLR, lymphocyte percentage, and in-hospital mortality. In a multivariate model containing age, CHF, hypertension, and log-transformed NLR, we did not find an independent association between NLR and in-hospital mortality (OR = 1.42; 95% CI: 0.98–2.10; p = 0.07). Interestingly, in a model containing log-transformed lymphocyte, age, hypertension, and CHF, log-transformed lymphocyte percentage was independently associated with in-hospital mortality (OR = 0.56, 95% CI: 0.35–0.88, p = 0.01). We did not perform a multivariate model with age, CHF, and both log-transformed NLR and lymphocyte percentage because of the collinearity between NLR and lymphocyte percentage.
4. Discussion
There are some interesting findings in this study. First, RDW was not associated with in-hospital mortality in dyspneic patients. Second, the predictive accuracy of NLR for in-hospital mortality is fair, it was not independently associated with in-hospital mortality after age, and the comorbidity of heart failure was adjusted. Third, increased lymphocyte percentage was independently associated with low in-hospital mortality.
Previous two studies have indicated that RDW was independently associated with 30-day and 1-year mortality in dyspneic patients [19, 20]. However, the prognostic value of RDW was not found in this study. The inconsistency between the present and previous studies may have two possible explanations. First, all of the patients in the present study have critical illness and are admitted to the intensive care unit after ED admission. Notably, the in-hospital mortality in the present study was 19.2% (86/447), while the 30-day and 1-year mortality in previous studies was 9.5% [19] and 12.5% [20], respectively, indicating that the patients in the present study had severe disease. Inflammatory response has been proposed to be a mediator between RDW, NLR, and prognosis of dyspneic patients. In dyspneic patients with critical illness, strong inflammatory response is common, and therefore, the effects of inflammatory markers on the prognosis of patients may be attenuated. Second, the mean hemoglobin level in the present study was 107 g/L, which seems lower than that in previous studies [19, 20]. It is widely accepted that RDW is greatly affected by hemoglobin levels [34]. Indeed, the mean RDW level present was 15.9%, which seems higher than that in previous studies. Taken together, these results indicate that RDW may have a limited prognostic value in dyspneic patients with low hemoglobin and critical illness.
To the best of our knowledge, this is the first study investigating the prognostic value of NLR in dyspneic patients. We found that the predictive accuracy of NLR and lymphocyte percentage for in-hospital mortality is mild, with AUCs of 0.62 and 0.64, indicating that NLR and lymphocyte percentage may not be useful prognostic factors in dyspneic patients. Indeed, NLR was not independently associated with in-hospital mortality in a multivariable logistic regression model. However, considering that the patients in the present study are severely diseased, further studies with common dyspneic patients are needed to evaluate the prognostic value of NLR and lymphocyte percentage.
The present study has some limitations. First, this is a retrospective study with a small sample size. Second, to define dyspneic patients, we used the keywords “dyspnea” and “shortness of breath” to search the variable of DIAGNOSIS in the ADMISSIONS table. The variable DIAGNOSIS provides a free text and preliminary diagnosis for the patient on hospital admission. It is usually assigned by the admitting caregivers and does not use a systematic ontology. Therefore, some dyspneic patients may have been missed by the search keywords. In addition, because some clinical data are missing in the MIMIC III database, the confounding factors adjusted in multivariable regression analysis are limited.
In conclusion, we found that both NLR and RDW have a limited value for predicting in-hospital mortality in severely diseased patients with dyspnea. Giving the retrospective design and small sample size of the present study, further studies with a large sample size, full adjustment, and prospective design are needed to validate the findings of the present study.
Acknowledgments
We thank Dr. Hemant Goyal MD FACP from the Wright Center for Graduate Medical Education for manuscript editing.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Z.D Hu designed the study, extracted data, performed data analyses, and revised the manuscript. L Yan performed the statistical analysis and drafted the manuscript. All authors approved for submission.
References
- 1.Parshall M. B., Schwartzstein R. M., Adams L., et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. American Journal of Respiratory and Critical Care Medicine. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Januzzi J. L., Jr., Camargo C. A., Anwaruddin S., et al. The N-terminal pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. American Journal of Cardiology. 2005;95(8):948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Safwenberg U., Terent A., Lind L. The emergency department presenting complaint as predictor of in-hospital fatality. European Journal of Emergency Medicine. 2007;14(6):324–331. doi: 10.1097/MEJ.0b013e32827b14dd. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G., Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clinical Chemistry and Laboratory Medicine. 2014;52(9):1247–1249. doi: 10.1515/cclm-2014-0585. [DOI] [PubMed] [Google Scholar]
- 5.Urrechaga E., Hoffmann J. Critical appraisal of discriminant formulas for distinguishing thalassemia from iron deficiency in patients with microcytic anemia. Clinical Chemistry and Laboratory Medicine. 2017;55(10):1582–1591. doi: 10.1515/cclm-2016-0856. [DOI] [PubMed] [Google Scholar]
- 6.Montagnana M., Danese E. Red cell distribution width and cancer. Annals of Translational Medicine. 2016;4(20):p. 399. doi: 10.21037/atm.2016.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao T., Cui L., Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomarkers. 2016;16(4):507–512. doi: 10.3233/CBM-160591. [DOI] [PubMed] [Google Scholar]
- 8.Aktas G., Sit M., Dikbas O., et al. Could red cell distribution width be a marker in Hashimoto's thyroiditis? Experimental and Clinical Endocrinology and Diabetes. 2014;122(10):572–574. doi: 10.1055/s-0034-1383564. [DOI] [PubMed] [Google Scholar]
- 9.Aktas G., Alcelik A., Tekce B. K., Tekelioglu V., Sit M., Savli H. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Prz Gastroenterol. 2014;9(3):160–163. doi: 10.5114/pg.2014.43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fava C., Cattazzo F., Hu Z. D., Lippi G., Montagnana M. The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: useful or hype? Annals of Translational Medicine. 2019;7(20):p. 581. doi: 10.21037/atm.2019.09.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese E., Lippi G., Montagnana M. Red blood cell distribution width and cardiovascular diseases. Journal of Thoracic Disease. 2015;7(10):E402–E411. doi: 10.3978/j.issn.2072-1439.2015.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montagnana M., Cervellin G., Meschi T., Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clinical Chemistry and Laboratory Medicine. 2012;50(4) doi: 10.1515/cclm.2011.831. [DOI] [PubMed] [Google Scholar]
- 13.Al-Najjar Y., Goode K. M., Zhang J., Cleland J. G. F., Clark A. L. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. European Journal of Heart Failure. 2009;11(12):1155–1162. doi: 10.1093/eurjhf/hfp147. [DOI] [PubMed] [Google Scholar]
- 14.Han Y. Q., Yan L., Zhang L., et al. Red blood cell distribution width provides additional prognostic value beyond severity scores in adult critical illness. Clinica Chimica Acta. 2019;498:62–67. doi: 10.1016/j.cca.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Tonietto T. A., Boniatti M. M., Lisboa T. C., et al. Elevated red blood cell distribution width at ICU discharge is associated with readmission to the intensive care unit. Clinical Biochemistry. 2018;55:15–20. doi: 10.1016/j.clinbiochem.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Badrick T. Red blood cell distribution width—a marker of fundamental importance? Journal of Laboratory and Precision Medicine. 2018;3:p. 50. doi: 10.21037/jlpm.2018.05.08. [DOI] [Google Scholar]
- 17.Hu Z. D. Red blood cell distribution width: a promising index for estimating activity of autoimmune disease. Journal of Laboratory and Precision Medicine. 2016;1:p. 4. doi: 10.21037/jlpm.2016.10.02. [DOI] [Google Scholar]
- 18.Peng Y. F., Pan G. G., Luo B., Qin Y. H., Xu H. D. Increased red blood cell distribution width in patients with polymyositis is correlated with disease activity. International Journal of Laboratory Hematology. 2016;38(2):e35–e37. doi: 10.1111/ijlh.12460. [DOI] [PubMed] [Google Scholar]
- 19.Hong N., Oh J., Kang S. M., et al. Red blood cell distribution width predicts early mortality in patients with acute dyspnea. Clinica Chimica Acta. 2012;413(11-12):992–997. doi: 10.1016/j.cca.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Turcato G., Cervellin G., Salvagno G. L., et al. The role of red blood cell distribution width for predicting 1-year mortality in patients admitted to the emergency department with severe dyspnoea. Journal of Medical Biochemistry. 2017;36(1):32–38. doi: 10.1515/jomb-2016-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y. L., Han Z. J., Hu Z. D. Red blood cell distribution width and neutrophil to lymphocyte ratio are associated with outcomes of adult subarachnoid haemorrhage patients admitted to intensive care unit. Annals of Clinical Biochemistry. 2017;54(6):696–701. doi: 10.1177/0004563216686623. [DOI] [PubMed] [Google Scholar]
- 22.Hai L., Hu Z. D. The clinical utility of neutrophil to lymphocyte ratio in pregnancy related complications: a mini-review. Journal of Laboratory and Precision Medicine. 2020;5:p. 1. doi: 10.21037/jlpm.2019.10.03. [DOI] [Google Scholar]
- 23.Aktas G., Sit M., Dikbas O., et al. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras (1992) 2017;63(12):1065–1068. doi: 10.1590/1806-9282.63.12.1065. [DOI] [PubMed] [Google Scholar]
- 24.Hu Z. D., Huang Y. L., Qin B. D., et al. Prognostic value of neutrophil to lymphocyte ratio for gastric cancer. Annals of Translational Medicine. 2015;3(4):p. 50. doi: 10.3978/j.issn.2305-5839.2015.03.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao W. K., Chen D., Li S. Q., Fu S. J., Peng B. G., Liang L. J. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14(1) doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benites-Zapata V. A., Hernandez A. V., Nagarajan V., Cauthen C. A., Starling R. C., Wilson Tang W. H. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. American Journal of Cardiology. 2015;115(1):57–61. doi: 10.1016/j.amjcard.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamhane U. U., Aneja S., Montgomery D., Rogers E. K., Eagle K. A., Gurm H. S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. American Journal of Cardiology. 2008;102(6):653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Bhat T., Teli S., Rijal J., et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Review of Cardiovascular Therapy. 2014;11(1):55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 29.Lattanzi S., Brigo F., Trinka E., Cagnetti C., di Napoli M., Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Translational Stroke Research. 2019;10(2):137–145. doi: 10.1007/s12975-018-0649-4. [DOI] [PubMed] [Google Scholar]
- 30.Wiklund K., Gränsbo K., Lund N., et al. Inflammatory biomarkers predicting prognosis in patients with acute dyspnea. American Journal of Emergency Medicine. 2016;34(3):370–374. doi: 10.1016/j.ajem.2015.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y. L., Badrick T., Hu Z. D. Using freely accessible databases for laboratory medicine research: experience with MIMIC database. Journal of Laboratory and Precision Medicine. 2017;2:p. 31. doi: 10.21037/jlpm.2017.06.06. [DOI] [Google Scholar]
- 32.Johnson A. E. W., Pollard T. J., Shen L., et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3(1) doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 34.Salvagno G. L., Sanchis-Gomar F., Picanza A., Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Critical Reviews in Clinical Laboratory Sciences. 2014;52(2):86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


