Abstract
Background
Hydroxychloroquine (HCQ) has been promoted as a potential treatment of coronavirus disease 2019 (COVID-19), but there are safety concerns.
Objective
The purpose of this study was to determine the effects of HCQ treatment on QT interval.
Methods
We retrospectively studied the electrocardiograms of 819 patients treated with HCQ for rheumatologic diseases from 2000 to 2020. The primary outcome was corrected QT (QTc) interval, by Bazett formula, during HCQ therapy.
Results
Mean patient age was 64.0 ± 10.9 years, and 734 patients (90%) were men. Median dosage of HCQ was 400 mg daily, and median (25th–75th percentile) duration of HCQ therapy was 1006 (471–2075) days. Mean on-treatment QTc was 430.9 ± 31.8 ms. In total, 55 patients (7%) had QTc 470–500 ms, and 12 (1.5%) had QTc >500 ms. Chronic kidney disease (CKD), history of atrial fibrillation (AF), and heart failure were independent risk factors for prolonged QTc. In a subset of 591 patients who also had a pretreatment electrocardiogram, mean QTc increased from 424.4 ± 29.7 ms to 432.0 ± 32.3 ms (P <.0001) during HCQ treatment. Of these patients, 23 (3.9%) had either prolongation of QTc >15% or on-treatment QTc >500 ms. Over median 5.97 (3.33–10.11) years of follow-up, 269 patients (33%) died. QTc >470 ms during HCQ treatment was associated with a greater mortality risk (hazard ratio 1.78; 95% confidence interval 1.16–2.71; P = .008) in univariable but not in multivariable analysis.
Conclusion
HCQ is associated with QT prolongation in a significant fraction of patients. The risk of QT prolongation is higher among patients with CKD, AF, and heart failure, who may benefit from greater scrutiny.
Keywords: Drug-induced arrhythmia, Drugs, Electrocardiogram, Long QT syndrome, Mortality
Introduction
Since December 2019, the novel single-stranded RNA virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused millions of cases of coronavirus disease 2019 (COVID-19) in a global pandemic, leading to substantial morbidity and mortality.1 Clinical trajectories of COVID-19 vary widely, with worse outcomes observed in older individuals and those with concomitant medical conditions.2 A significant proportion of hospitalized patients with COVID-19 have cardiovascular disease. Moreover, COVID-19 has been purported to cause several types of cardiac adverse events, including myocardial injury, acute myocardial infarction, acute heart failure, myocarditis, and arrhythmias.3, 4, 5 Although there is no approved treatment of COVID-19, several strategies are under investigation, such as antivirals, antiretrovirals, immune modulators, and hydroxychloroquine (HCQ).
Originally used to treat malaria, HCQ was approved by the Food and Drug Administration (FDA) for the treatment of systemic lupus erythematosus (SLE) in 1955.6 Currently it is the most commonly prescribed medication for SLE and frequently is used for a variety of other rheumatologic conditions. Cardiac toxicity is rare, but conduction abnormalities including bundle branch block, atrioventricular block, QT prolongation, torsades de pointes, and sudden cardiac death have been reported.7, 8, 9, 10 There is in vitro evidence that chloroquine and HCQ possess antiviral properties.11 These data have been extrapolated to hypothesize that HCQ may prevent SARS-CoV-2 infection and attenuate the course and severity of COVID-19.12 On March 28, 2020, the FDA issued an emergency use authorization for the use of HCQ sulfate (and chloroquine phosphate) in certain hospitalized patients being treated for COVID-19.13 However, recent studies showing no benefit of HCQ in patients with COVID-19 have led the FDA to remove that emergency use authorization.14, 15, 16, 17 Nonetheless, case reports and case series of COVID-19 patients treated with HCQ describe significant QT prolongation and torsades de pointes, which has expanded clinical concern regarding the arrhythmogenic risk of HCQ.18, 19, 20, 21 The purpose of this study was to determine the effects of HCQ on cardiac conduction abnormalities seen on 12-lead electrocardiogram (ECG).
Methods
This study was approved by the Minneapolis VA Medical Center Institutional Review Board. Informed consent requirement was waived.
Patient selection
We identified 2665 patients who received at least 1 prescription for HCQ dispensed at the Minneapolis Veterans Affairs (VA) Health Care System from 2000 to 2020. Each prescription represented the first dispense of HCQ until a break in prescriptions for at least 150 days.22 , 23 We excluded patients who did not have an ECG recorded while they were receiving HCQ treatment (n = 1722). After excluding 124 duplicate entries, we included 819 unique patients who received treatment in this analysis.
ECG
All ECGs were performed with the General Electric MAC 5000 (GE Healthcare, Chicago, IL) and stored in the Marquette Universal System for Electrocardiography database (GE Healthcare, Chicago IL).24 , 25 We selected for analysis the first ECG performed at least 5 days after starting HCQ treatment. If available, we also analyzed the last ECG performed before starting HCQ treatment. We recorded ventricular rate, QRS duration, QT interval, and corrected QT interval (QTc) calculated via the Bazett, Framingham, and Fridericia formulas. For patients with QRS >120 ms, we used the Bogossian formula26 to calculate a modified QT interval: QTm = QT(measured) – 48.5% ∗ QRS(measured). QTm was used to calculate QTc. We manually measured the QT interval, starting from the beginning of the Q wave to the end of the T wave on ECG, using electronic calipers in 70 randomly selected patients (8.5%) in the cohort.27 The correlation coefficient was 0.8.
Outcomes
The primary outcome variable was the QTc interval during HCQ therapy. We used the Bazett formula for QT correction because it is the most commonly used method to calculate QTc. QTc >470 ms represents the 99th percentile of QTc distribution in the general population and is a predictor of symptoms in patients with long QT syndrome.28 , 29 QTc >500 ms is associated with a higher risk of life-threatening arrhythmias.29 In a subgroup of patients in whom ECGs were recorded before and during HCQ treatment, we also assessed the change in QTc.30 Secondary outcome variable was time to death or last follow-up from the starting date of HCQ treatment as recorded in the VA vital status files.24 , 25 , 31
Predictor variables and definitions
We extracted patient demographics, comorbidities, and laboratory data from the VA corporate data warehouse (CDW) using Structured Query Language. The VA CDW contains extracts from the VA clinical and administrative system, which includes complete clinical data since October 1999.32 Prescribing reports on HCQ dosing, start and stoppage dates, and other medications also were extracted from the Veterans Health Association (VHA) CDW.22 The ECG data were obtained from the Marquette Universal System for Electrocardiography database.24 , 25 Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate (eGFR) <60 mL/min. eGFR was calculated with the Cockcroft-Gault equation: ([140 – age] × weight in kg)/(serum creatinine × 72; multiplied by 0.85 in women).
Statistical analysis
Data are presented as mean ± SD or median (interquartile range) for continuous variables, and number (percentage) for categorical variables. Comparisons of characteristics among patients with vs those without QTc prolongation were made using the χ2 test or Fisher exact test for categorical variables and the unpaired Student t test for continuous variables. The ECG characteristics before vs during HCQ therapy were compared using the paired Student t test. Independent predictors of prolonged QTc were identified by multivariable logistic regression analysis. Survival curves were constructed using the Kaplan–Meier method, and the differences of survival among patients with vs those without prolonged QTc were examined by the log-rank test. Cox proportional hazards analysis was used to assess the association between QTc prolongation and mortality, and to calculate the hazard ratio (HR) and 95% confidence interval (CI). Statistical analyses were performed using IBM SPSS Version 25 (IBM Corp, Armonk, NY).
Results
Patient characteristics
Baseline characteristics of the 819 study patients are listed in Table 1 . Mean patient age was 64.0 ± 10.9 years, and 734 of the patients (90%) were male. Cardiovascular comorbidities included hypertension (n = 532 [65%]), coronary artery disease (n = 234 [29%]), diabetes (n = 202 [25%]), and heart failure (n = 65 [8%]). A total of 99 patients (12%) had CKD (Table 1).
Table 1.
Characteristics of the 819 patients treated with hydroxychloroquine
| Age (y) | 64.0 ± 10.9 |
| Male | 734 (90) |
| Hypertension | 532 (65) |
| Coronary artery disease | 234 (29) |
| Heart failure | 65 (8) |
| Diabetes | 202 (25) |
| Atrial fibrillation | 90 (11) |
| Chronic kidney disease | 99 (12) |
| End-stage renal failure | 10 (1) |
| SLE | 786 (96) |
| Magnesium (mg/dL) | 2.0 ± 0.3 |
| Potassium (mmol/L) | 4.1 ± 0.4 |
| Creatinine (mg/dL) | 1.0 ± 0.5 |
| eGFR (mL/min/1.73 m2) | 78.6 ± 24.7 |
| eGFR (mL/min) | 100.5 ± 37.9 |
Continuous variables were given as mean ± SD. Categorical variables are given as n (%).
eGFR = estimated glomerular filtration rate; SLE = systemic lupus erythematosus.
HCQ was prescribed to treat SLE in 786 (96%) of the patients at a median starting dosage of 400 mg daily. Median (25th–75th percentile) duration of HCQ therapy was 1006 (471–2075) days.
ECG characteristics during HCQ treatment
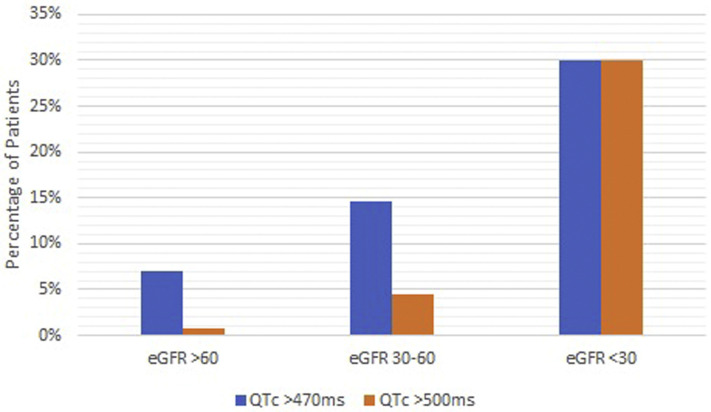
A 12-lead ECG was recorded 234 (92–586) days after starting HCQ therapy in all study patients. ECG characteristics during HCQ therapy are listed in Table 2 . Ventricular rate was 74.8 ±17.3 bpm. Mean on-treatment QTc, based on Bazett formula, was 430.9 ± 31.8 ms. A total of 55 patients (7%) had on-treatment QTc 470–500 ms, and 12 (1.5%) had QTc >500 ms (Table 2). Compared to those with QTc ≤470 ms, patients with prolonged QTc were older and more likely to have CKD, a history of atrial fibrillation, and a history of heart failure (Table 3 ). In multivariable regression, CKD (odds ratio [OR] 2.30; 95% CI 1.24–4.26; P = .008) and atrial fibrillation (OR 2.64; 95% CI 1.42–4.90; P = .002) were independent predictors of QTc >470 ms. Notably, the odds of QTc prolongation were >5 times higher (OR 5.1; 95% CI 1.23–21.1; P = .025) when eGFR was <30 mL/min. There was a negative correlation between eGFR and QTc (Figure 1 ).
Table 2.
ECG characteristics of the study patients during hydroxychloroquine treatment
| All patients (n = 819) | QTc ≤470 ms (n = 752) | QTc 471–500 ms (n = 55) | QTc >500 ms (n = 12) | P value∗ | |
|---|---|---|---|---|---|
| Ventricular rate (bpm) | 74.8 ± 17.3 | 74.1 ± 17.0 | 80.9 ± 16.5 | 85.4 ± 25.7 | .001 |
| QRS duration (ms) | 100.9 ± 22.8 | 101.0 ± 23.2 | 98.3 ± 16.3 | 104.0 ± 24.2 | .47 |
| QT interval (ms) | 392.8 ± 44.5 | 389.6 ± 42.6 | 421.3 ± 40.0 | 462.6 ± 73.6 | <.0001 |
| QTc-Bazett (ms) | 430.9 ± 31.8 | 425.5 ± 26.2 | 481.6 ± 7.9 | 535.3 ± 39.0 | <.0001 |
| QTc-Framingham (ms) | 417.8 ± 30.3 | 413.3 ± 27.3 | 457.3 ± 15.9 | 491.5 ± 45.7 | <.0001 |
| QTc-Fridericia (ms) | 418.1 ± 30.5 | 413.4 ± 27.2 | 460.2 ± 13.3 | 494.2 ± 49.4 | <.0001 |
Continuous variables are given as mean ± SD.
ECG = electrocardiogram.
Comparison of QTc ≤470 ms vs QTc >470 ms.
Table 3.
Clinical characteristics of patients based on QTc during hydroxychloroquine treatment
| QTc ≤470 ms (n = 752) | QTc 471–500 ms (n = 55) | QTc >500 ms (n = 12) | P value∗ | |
|---|---|---|---|---|
| Age (y) | 63.7 ± 10.8 | 68.3 ± 9.3 | 66.4 ± 16.9 | .002 |
| Male | 675 (90) | 48 (87) | 11 (92) | .66 |
| Hypertension | 487 (65) | 37 (67) | 8 (67) | .41 |
| Coronary artery disease | 213 (28) | 18 (33) | 3 (25) | .60 |
| Heart failure | 55 (7) | 7 (13) | 3 (25) | .03 |
| Diabetes | 179 (24) | 20 (36) | 3 (25) | .06 |
| Atrial fibrillation | 74 (10) | 13 (24) | 3 (25) | <.0001 |
| Chronic kidney disease | 83 (11) | 9 (16) | 7 (58) | .002 |
| Magnesium (mg/dL) | 2.03 ± 0.27 | 1.96 ± 0.27 | 1.97 ± 0.37 | .054 |
| Potassium (mmol/L) | 4.15 ± 0.42 | 4.04 ± 0.37 | 4.25 ± 0.49 | .21 |
| Creatinine (mg/dL) | 1.02 ± 0.30 | 1.06 ± 0.39 | 2.62 ± 2.99 | .07 |
| eGFR (mL/min/1.73 m2) | 79.2 ± 24.2 | 75.3 ± 25.9 | 53.7 ± 41.7 | .08 |
| eGFR (mL/min) | 101.2 ± 37.2 | 96.5 ± 40.4 | 72.4 ± 63.4 | .12 |
Continuous variables are given as mean ± SD. Categorical variables are given as n (%).
eGFR = estimated glomerular filtration rate.
Comparison of QTc ≤470 ms vs QTc >470 ms.
Figure 1.
Association of estimated glomerular filtration rate (eFGR) with QTc prolongation.
Paired ECG before and during HCQ treatment
A total of 591 patients (72%) had ECGs recorded before (median 345 [98–833] days) and after (median 204 [84–489] days) starting HCQ treatment. On these paired ECGs, there was modest prolongation of QTc from 424.4 ± 29.7 ms to 432.0 ± 32.3 ms (P <.0001) during HCQ treatment (Table 4 ). A total of 23 patients (3.9%) had either >15% increase in QTc or QTc >500 ms with HCQ treatment. These patients were more likely to have CKD (44% vs 14%; P = .0001) than the rest of the cohort with paired ECGs.
Table 4.
Characteristics of the 591 paired ECGs before and during hydroxychloroquine treatment
| Pretreatment | On treatment | P value | |
|---|---|---|---|
| Ventricular rate (bpm) | 72.3 ± 14.9 | 74.9 ± 17.7 | .001 |
| QRS duration (ms) | 98.1 ± 20.2 | 101.7 ± 23.3 | <.0001 |
| QT interval (ms) | 392.6 ± 42.8 | 393.9 ± 45.2 | .49 |
| QTc-Bazett (ms) | 424.4 ± 29.7 | 432.0 ± 32.3 | <.0001 |
| QTc-Framingham (ms) | 412.7 ± 28.3 | 416.8 ± 29.9 | .006 |
| QTc-Fridericia (ms) | 412.9 ± 28.3 | 417.4 ± 30.4 | .003 |
Continuous variables were given as mean ± SD.
ECG = electrocardiogram.
Survival in relation to QTc
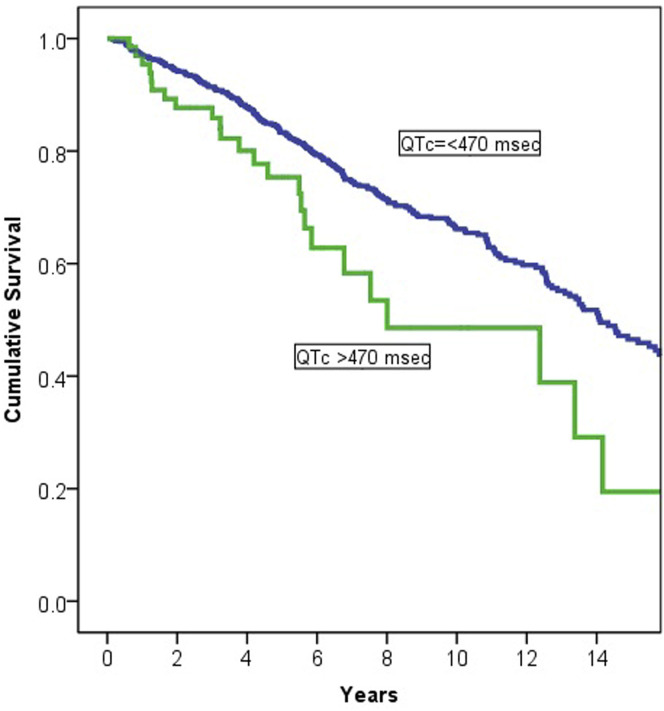
Of the 819 patients in the whole study cohort, 269 (33%) died over a median 5.97 (3.33–10.11) years of follow-up. In comparison to those who survived, the patients who died were older, more likely to be men, and more likely to have coronary disease, heart failure, and CKD. In univariable Cox regression analysis, QTc >470 ms during HCQ therapy was associated with greater mortality (HR 1.78; 95% CI 1.16–2.71; P = .008) (Figure 2 ). However, after adjustment for age, sex, and comorbidities, QTc >470 ms during HCQ treatment was no longer associated with long-term mortality. In patients with paired ECG, delta QTc was not associated with mortality (HR 1.001; 95% CI 0.998–1.005; P = .51).
Figure 2.
Survival in relation to QTc during hydroxychloroquine (HCQ) treatment. QTc >470 ms during HCQ therapy was associated with greater mortality in univariable analysis but not after adjustment for age, sex, and comorbidities.
Discussion
This investigation showed that a significant fraction of the patients (8.3%) prescribed HCQ for treatment of SLE had QTc >470 ms on ECG, including 1.5% who had QTc >500 ms. CKD, atrial fibrillation, and heart failure were associated with QTc prolongation. Among patients with ECGs recorded both before and during treatment, HCQ had a modest effect on QTc (mean increase 8 ms) except in 3.4% of the patients who had >15% increase in QTc. The QTc interval during HCQ treatment was associated with worse long-term survival in univariable analysis, whereas the change in QTc was not.
HCQ blocks the KCNH2-encoded hERG/Kv11.1 potassium channel and can contribute to prolongation of the QTc interval.12 Mutations involving these potassium channels cause the long QT syndrome, and their blockage by certain drugs can be associated with QT prolongation, torsades de pointes, and sudden death. Although QT prolongation and ventricular arrhythmias related to HCQ are uncommon, there are published case reports and postings on the FDA Adverse Events Reporting System.7 , 8 , 10 A retrospective study of 112 patients with SLE treated with HCQ reported that cardiac conduction abnormalities, including incomplete bundle branch blocks and second- and third-degree atrioventricular blocks, were present in 18% of the subjects. The presence of conduction abnormalities was not associated with mortality during the first 10 years of follow-up, but thereafter subjects with cardiac conduction abnormalities had a higher risk of death.33 In a larger series of 453 consecutive SLE patients, 84% of whom were taking HCQ, 16% had cardiac conduction abnormalities.34 Because some conduction abnormalities can be related to myocardial involvement of SLE, McGhie et al34 were not able to attribute them directly to the use of HCQ. In fact, higher cumulative doses of HCQ were associated with fewer conduction abnormalities. QTc prolongation was rare and noted in only 0.7% of patients. In 2007, a retrospective review of 85 patients taking HCQ showed that the incidences of conduction delays and QT prolongation were similar to those expected in the general public.35
There are currently more than 14 million confirmed cases of COVID-19 worldwide as of July 2020. Initially, HCQ emerged as a potential therapy for COVID-19 given in vitro evidence of antiviral properties.11 , 36 Guidelines quickly emerged cautioning use of the medication in patients with prolonged QTc. The Heart Rhythm Society published guidelines suggesting that the arrhythmic toxicity risk of HCQ likely is low given the relatively short duration of treatment for COVID-19.37 They recommend using caution in patients with known congenital long QT syndrome, renal insufficiency, or electrolyte derangements, or who are taking QT-prolonging medications (eg, certain antiarrhythmics, antipsychotics, antifungals, and macrolide antibiotics such as azithromycin).37, 38, 39 Mayo Clinic investigators recently published guidelines recommending caution when using HCQ with/without azithromycin in any patient with prolonged QTc >470 ms in males and >480 ms in females.12 Most recently, a small, randomized controlled trial and several observational studies of COVID-19 patients treated with HCQ showed no evidence of benefit and some concerning trends for harm.14, 15, 16, 17 , 40 At this time, the FDA has removed the emergency use authorization for HCQ as a therapy for COVID-19. The COVID-19 treatment guidelines put forth by the National Institutes of Health recommend against use of HCQ in combination with azithromycin, except in the context of a clinical trial.41 However, despite the negative trials the medication has received significant media attention, and it remains possible that a significant portion of the population is exposed to the medication. The association of impaired kidney function with QTc prolongation is especially concerning because reports from China indicate that patients with COVID-19 are more likely to experience renal dysfunction.42 Given the higher comorbidity burden and acute illness of patients hospitalized with COVID-19, it is possible that a higher percentage of patients would exhibit QTc prolongation with HCQ treatment.
The current report provides contemporary information on the effect of HCQ on QTc to support the recommendations discussed. Although the additional prolongation of QTc with HCQ was modest in general, substantial prolongation occurred in 3.2% of patients. Guidelines for performing screening ECGs and recommendations for monitoring patients started on HCQ therapy have been proposed.18 , 43 Therefore, it is prudent to check a baseline ECG before initiating therapy, avoid hypokalemia, and closely monitor patients with impaired kidney function and those taking other QT-prolonging medications.38 , 39 Although this study did not include genetic data, extra precautions are also recommended for patients with congenital long QT syndrome.
This study showed that 1.5% of patients taking HCQ had QTc >500 ms. The class III antiarrhythmic medications dofetilide and sotalol, used for treatment atrial fibrillation, also can cause QT prolongation. In the DIAMOND-CHF (Danish Investigators of Arrhythmia and Mortality on Dofetilide in Congestive Heart Failure) study, dofetilide was discontinued in 2% of patients due to QT prolongation.44 More recent data from general practice showed that dofetilide was discontinued in up to 17% of patients due to QT prolongation.38 Sotalol was discontinued in 4.3% of patients due to prolonged QT interval.45 The results of the current study should be evaluated within this context.
Study limitations
There may be a selection bias for patients in whom ECGs were recorded while they were receiving therapy. Patients with SLE are different from ill patients with COVID-19, who are likely to have a higher burden of cardiovascular comorbidities and impaired kidney function that make them more vulnerable to the adverse effects of HCQ. Furthermore, HCQ has a long half-life. Steady state is achieved after 3–4 months of therapy.46 At the time the ECG was performed in this study, the average time on HCQ therapy was 237 (92–575) days, suggesting that steady state had been achieved. However, patients treated for COVID-19 are likely to receive shorter courses of therapy, and this study cannot address expected ECG changes in this timeframe. Finally, this study was retrospective, lacked genetic diagnostics, and largely consisted of men.
Conclusion
HCQ therapy is associated with QT prolongation in a significant fraction of patients who were on long-term therapy with this medication for treatment of SLE. Although recent trials have not supported the expansion of HCQ use for COVID-19, it has raised significant concerns with regard to QT prolongation and arrhythmogenic risk. Avoiding hypokalemia and careful monitoring of QTc are warranted, particularly in patients with impaired kidney function and those receiving other QT-prolonging drugs.
Footnotes
Funding sources: This research did not receive specific grant from funding agencies in the public, commerical, or not-for-profit sectors. Disclosures: Dr Adabag has obtained research grants from the American Heart Association, Medtronic Inc., and Boston Scientific Inc., unrelated to this project. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2020. https://coronavirus.jhu.edu/map.html Available from.
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. Published online March 27, 2020. JAMA Cardiol https://doi.org/10.1001/jamacardio.2020.1286 [DOI] [PubMed]
- 4.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardeny O., Madjid M., Solomon S.D. Applying the lessons of influenza to COVID-19 during a time of uncertainty. Circulation. 2020;141:1667–1669. doi: 10.1161/CIRCULATIONAHA.120.046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration PLAQUENIL® Hydroxychloroquine Sulfate, USP. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/009768s041lbl.pdf Available from. Accessed April 10, 2020.
- 7.Morgan N.D., Patel S.V., Dvorkina O. Suspected hydroxychloroquine-associated QT-interval prolongation in a patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:286–288. doi: 10.1097/RHU.0b013e31829d5e50. [DOI] [PubMed] [Google Scholar]
- 8.O’Laughlin J.P., Mehta P.H., Wong B.C. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Rep Cardiol. 2016;2016:4626279. doi: 10.1155/2016/4626279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.-Y., Wang F.-L., Lin C.-C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol. 2006;44:173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 10.FDA Adverse Events Reporting System (FAERS) Public Dashboard. https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/59a37af8-d2bb-4dee-90bf-6620b1d5542f/state/analysis Available from.
- 11.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Published online March 9, 2020. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed]
- 12.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinton D. United States Food and Drug Administration; Washington, DC: March 28, 2020. Re: Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease [response letter to R. Bright] [Google Scholar]
- 14.Tang W., Cao Z., Han M. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahévas M., Tran V.-T., Roumier M. Clinical efficacy of hydroxychloroquine in patients with Covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magagnoli J., Narendran S., Pereira F. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Preprint. Posted online April 21, 2020. medRxiv. 2020 doi: 10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra R.L., Greenstein S.A., Epstein L.M. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: possible benefits of intravenous lidocaine. HeartRhythm Case Rep. 2020;6:244–248. doi: 10.1016/j.hrcr.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szekely Y., Lichter Y., Shrkihe B.A., Bruck H., Oster H.S., Viskin S. Chloroquine-induced torsades de pointes in a patient with coronavirus disease 2019. Published online May 5, 2020. Heart Rhythm. https://doi.org/10.1016/j.hrthm.2020.04.046 [DOI] [PMC free article] [PubMed]
- 20.Jankelson L., Karam G., Becker M.L., Chinitz L.A., Tsai M.-C. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Published online May 11, 2020. Heart Rhythm. https://doi.org/10.1016/j.hrthm.2020.05.008 [DOI] [PMC free article] [PubMed]
- 21.Chorin E., Wadhwani L., Magnani S. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Published online May 12, 2020. Heart Rhythm. https://doi.org/10.1016/j.hrthm.2020.05.014 [DOI] [PMC free article] [PubMed]
- 22.Rector T.S., Adabag S., Cunningham F., Nelson D., Dieperink E. Outcomes of citalopram dosage risk mitigation in a veteran population. Am J Psychiatry. 2016;173:896–902. doi: 10.1176/appi.ajp.2016.15111444. [DOI] [PubMed] [Google Scholar]
- 23.Westermeyer J., Adabag S., Anand V., Thuras P., Yoon G., Batres-y-Carr T. Methadone maintenance dose/weight ratio, long QTc, and EKG screening. Am J Addict. 2016;25:499–507. doi: 10.1111/ajad.12423. [DOI] [PubMed] [Google Scholar]
- 24.Moulki N., Kealhofer J.V., Benditt D.G. Association of cardiac implantable electronic devices with survival in bifascicular block and prolonged PR interval on electrocardiogram. J Interv Card Electrophysiol. 2018;52:335–341. doi: 10.1007/s10840-018-0389-0. [DOI] [PubMed] [Google Scholar]
- 25.Coumbe A.G., Naksuk N., Newell M.C., Somasundaram P.E., Benditt D.G., Adabag S. Long-term follow-up of older patients with Mobitz type I second degree atrioventricular block. Heart. 2013;99:334–338. doi: 10.1136/heartjnl-2012-302770. [DOI] [PubMed] [Google Scholar]
- 26.Bogossian H., Frommeyer G., Ninios I. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm. 2014;11:2273–2277. doi: 10.1016/j.hrthm.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Al-Khatib S.M., LaPointe N.M.A., Kramer J.M., Califf R.M. What clinicians should know about the QT interval. JAMA. 2003;289:2120–2127. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 28.Mason J.W., Ramseth D.J., Chanter D.O., Moon T.E., Goodman D.B., Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:228–234. doi: 10.1016/j.jelectrocard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Issa Z., Miller J. Elsevier; Philadelphia: 2018. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. Third Edition. [Google Scholar]
- 30.Bart G., Wyman Z., Wang Q., Hodges J.S., Karim R., Bart B.A. Methadone and the QTc interval. J Addict Med. 2017;11:489–493. doi: 10.1097/ADM.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain R., Duval S., Adabag S. How accurate is the eyeball test?: A comparison of physician’s subjective assessment versus statistical methods in estimating mortality risk after cardiac surgery. Circ Cardiovasc Qual Outcomes. 2014;7:151–156. doi: 10.1161/CIRCOUTCOMES.113.000329. [DOI] [PubMed] [Google Scholar]
- 32.Westanmo A., Marshall P., Jones E., Burns K., Krebs E.E. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16:1019–1026. doi: 10.1111/pme.12699. [DOI] [PubMed] [Google Scholar]
- 33.Godeau P., Guillevin L., Fechner J., Bletry O., Herreman G. Disorders of conduction in lupus erythematosus: frequency and incidence in a group of 112 patients. Ann Med Interne (Paris) 1981;312:234–240. [PubMed] [Google Scholar]
- 34.McGhie T.K., Harvey P., Su J., Anderson N., Tomlinson G., Touma Z. Electrocardiogram abnormalities related to anti-malarials in systemic lupus erythematosus. Clin Exp Rheumatol. 2018;36:545–551. [PubMed] [Google Scholar]
- 35.Costedoat-Chalumeau N., Hulot J.-S., Amoura Z. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology. 2007;46:808–810. doi: 10.1093/rheumatology/kel402. [DOI] [PubMed] [Google Scholar]
- 36.Liu J., Cao R., Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakkireddy D.R., Chung M.K., Gopinathannair R. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on. Circulation. 2020;141:e823–e831. doi: 10.1161/CIRCULATIONAHA.120.047063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anand V., Vakil K., Tholakanahalli V., Li J.-M., McFalls E., Adabag S. Discontinuation of dofetilide from QT prolongation and ventricular tachycardia in the real world. JACC Clin Electrophysiol. 2016;2:777–781. doi: 10.1016/j.jacep.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Ko B., Garcia S., Mithani S., Tholakanahalli V., Adabag S. Risk of acute kidney injury in patients who undergo coronary angiography and cardiac surgery in close succession. Eur Heart J. 2012;33:2065–2070. doi: 10.1093/eurheartj/ehr493. [DOI] [PubMed] [Google Scholar]
- 40.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institutes of Health Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://covid19treatmentguidelines.nih.gov Available from. [PubMed]
- 42.Li Z, Wu M, Yao J, et al. Caution on kidney dysfunctions of COVID-19 patients. Preprint. Posted online April 1, 2020. SSRN Electron J 10.2139/ssrn.3559601 [DOI]
- 43.Jain S., Workman V., Ganeshan R. Enhanced electrocardiographic monitoring of patients with coronavirus disease 2019. Published online May 6, 2020. Heart Rhythm. https://doi.org/10.1016/j.hrthm.2020.04.047 [DOI] [PMC free article] [PubMed]
- 44.Torp-Pedersen C., Møller M., Bloch-Thomsen P.E. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. N Engl J Med. 1999;341:857–865. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 45.Weeke P., Delaney J., Mosley J.D. QT variability during initial exposure to sotalol: experience based on a large electronic medical record. Europace. 2013;15:1791–1797. doi: 10.1093/europace/eut153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan P., Brookes J.G., Nikolic G., Le Couteur D.G., Le Couteur D. Hydroxychloroquine overdose: toxicokinetics and management. J Toxicol Clin Toxicol. 1999;37:861–864. doi: 10.1081/clt-100102466. [DOI] [PubMed] [Google Scholar]