Abstract
Purpose
The aim of this study was to determine the impact of different compressive forces on deproteinized bovine bone mineral (DBBM) particles covered by native bilayer collagen membrane (NBCM) during alveolar ridge preservation (ARP) in the molar area, and to identify any histomorphometric and clinical differences according to the compressive force applied.
Methods
Sockets were filled with DBBM after tooth extraction, and different compressive forces (30 N and 5 N, respectively) were applied to the graft material in the test (30 N) and control (5 N) groups. The DBBM in both groups was covered with NBCM in a double-layered fashion. A crossed horizontal mattress suture (hidden X) was then made. A core biopsy was performed using a trephine bur without flap elevation at the implant placement site for histomorphometric evaluations after 4 months. The change of the marginal bone level was measured using radiography.
Results
Twelve patients completed the study. The histomorphometric analysis demonstrated that the mean ratios of the areas of new bone, residual graft material, and soft tissue and the implant stability quotient did not differ significantly between the groups (P>0.05). However, the mean size of the residual graft material showed a significant intergroup difference (P<0.05).
Conclusions
The application of 2 compressive forces (5 N, 30 N) on particulate DBBM grafts during open-healing ARP in the posterior area led to comparable new bone formation, implant feasibility and peri-implant bone level.
Keywords: Alveolar bone grafting, Alveolar ridge augmentation, Bone substitutes, Histology, Tooth extraction
Graphical Abstract
INTRODUCTION
The authors of the present study have conducted a series of studies on open-healing alveolar ridge preservation (ARP) in the molar area for several years. First, the application of a crossed horizontal mattress suture (hidden X suture) for ARP in molar areas using deproteinized bovine bone mineral (DBBM) with 10% porcine collagen (DBBM-C) and native bilayer collagen membrane (NBCM) coverage was compared with the conventional crossed mattress suture both radiographically and histomorphometrically [1]. It was demonstrated that both techniques could successfully preserve the alveolar ridge, and that the hidden X suture better preserved the location of the mucogingival junction and maximized the keratinized gingival width. Subsequently, it was questioned whether a single-layer application of NBCM would sufficiently protect the underlying DBBM-C over the healing period, and the authors therefore compared the relevant clinical values [2]. In addition, the authors reported a case series indicating that open-healing ARP after extracting maxillary molars might reduce the necessity for sinus lift procedures [3], and similar findings have recently been also reported by other researchers [4]. In our most recent study, we found that implants placed after open-healing ARP showed a 100% implant success rate and significantly lower marginal bone loss at a 1-year follow-up after the final prosthetic placement [5]. Based on the results of these studies, it was concluded that the application of open-healing ARP in the molar region could yield predictable results in implant treatment.
Despite these studies, it has still not been fully elucidated whether the application of compressive forces on graft materials improves bone quality for placing dental implants by providing mechanical strength. To the best of the authors' knowledge, the compressive force does not exert a scientifically proven effect on bone graft material, but it is generally accepted that applying minimal pressure on graft materials is preferable because doing so yields substantial space between particles to allow the in-growth of new bone. To investigate this issue, different compressive forces using the established clinical study model of open-healing ARP in molars were compared using DBBM-C, and histomorphometric and radiographic analyses revealed that compressive force application led to a significant increase in new bone formation, with fewer changes to the ridge width and height, suggesting that applying compressive forces to DBBM-C graft materials could have positive clinical effects [6]. However, the effects of applying different forces to DBBM particles has not yet been determined in a clinical study. Therefore, the aims of the present study were to determine the impact of applying different compressive forces on DBBM particles covered by NBCM during ARP in the molar area, and to identify any histomorphometric and clinical differences according to the compressive force applied.
MATERIALS AND METHODS
Study design
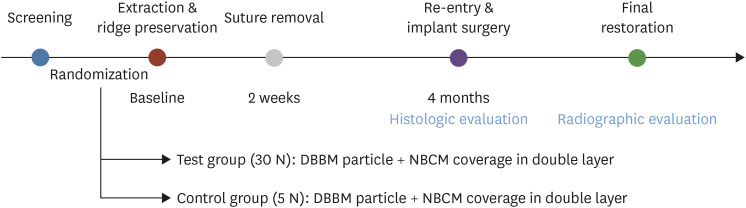
This proof-of-concept study was performed in accordance with the Declaration of Helsinki. The protocol of the study (Figure 1) was approved by the Ethics Committee of Dankook University Dental Hospital, Korea (approval No. H-1412/012/002).
Figure 1. Flow chart of the study design.
DBBM: deproteinized bovine bone mineral, NBCM: native bilayer collagen membrane.
Study population
Patients were enrolled from December 2017 to February 2019, with 29 patients screened and 12 finally included in the study. The inclusion criteria were the lack of a systemic contraindication for surgical treatment, the presence of a hopeless molar tooth [7] in the mandible or maxilla with more than 50% of bone loss in all dimensions, and the patient displaying a full understanding of the nature of the proposed surgery and agreeing to sign an informed-consent form. The exclusion criteria were a history of radiotherapy or chemotherapy within the previous 5 years, a history of autoimmune diseases or systemic diseases that may interfere with healing, a known allergy to DBBM or collagen, receiving long-term nonsteroidal anti-inflammatory drug therapy, heavy smoking (>10 cigarettes per day), the presence of untreated or uncontrolled periodontal disease, or being pregnant or lactating.
All of the details of this study were presented to the patients before they signed the informed-consent form provided by the researcher. All eligible sites were examined using a periodontal probe to assess their suitability for inclusion in the study, and dental radiographs were taken prior to performing the ARP procedure. All subjects received appropriate periodontal treatment if required.
After obtaining informed consent, the participants were allocated sequential enrollment numbers. The information on group allocation was sealed in opaque envelopes. Immediately after tooth extraction, the envelope was opened by an assistant to determine the assigned treatment group.
Experimental groups
In the test group, the sockets were filled with DBBM (Bio-Oss® small granules 0.25–1 mm, Geistlich Pharma, Wolhusen, Switzerland) with a compressive force of 30 N applied to densely compact the graft material, followed by coverage with a double layer of NBCM (Bio-Gide®, Geistlich Pharma).
In the control group, the sockets were filled with DBBM (Bio-Oss® small granules 0.25–1 mm, Geistlich Pharma) with a compressive force of 5 N applied to lightly compact the graft material, followed by coverage with a double layer of NBCM (Bio-Gide®, Geistlich Pharma).
Outcome measures
The primary outcome was a histologic comparison of the areas of new bone and residual graft material. The secondary outcome was a radiographic assessment of change of the marginal bone level and implant survival rate. Implant survival was defined as a condition capable of maintaining function with no need for removal.
Surgical procedures
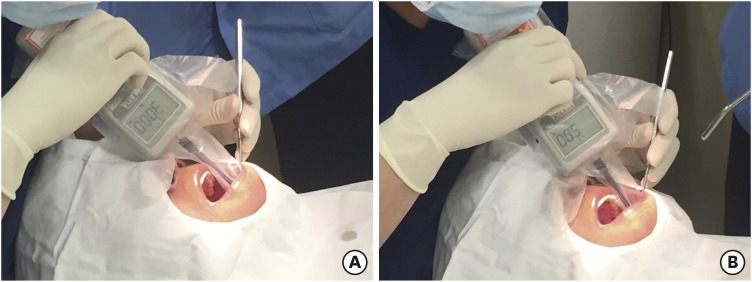
All surgical procedures were performed by periodontists (J.C.P., I.W.C., and S.J.L.). A calibration procedure was performed every 2 weeks during the experiment to equalize the pressure applied by each surgeon when inserting the graft material. To measure the applied force, forces of 5 N and 30 N were calibrated using a digital force gauge (Model DS2-50 N, Imada, Tokyo, Japan) with an A-6 extension shaft (6 mm in diameter) so that each surgeon could repeatedly reproduce and apply them [6] (Figure 2).
Figure 2. Clinical application of compressive force with the measurement with digital gauge. (A) 30 N compressive force in test group. (B) 5 N compressive force in control group.
Following the application of local anesthesia with 2% lidocaine containing 1:80,000 epinephrine, the tooth was gently extracted using luxators and extraction forceps to minimize damage to the surrounding tissues without flap elevation. The roots of multiroot teeth were separated using a high-speed handpiece and diamond bur when necessary. Granulation tissues were debrided using a surgical curette and irrigation was performed with a sterile normal saline solution.
The extraction socket was filled with DBBM in both groups, followed by the application of a compressive force of 30 N (test group) or 5 N (control group) to the graft material using a periosteal elevator. The amount of DBBM particles used in individual sockets in the test group varied with the tooth size and defect shape. After grafting, the DBBM in both groups was covered with NBCM in a double-layered fashion [2]. A crossed horizontal mattress suture (hidden X suture; 4-0 Ethilon, Ethicon, Cincinnati, OH, USA) was placed over each socket in all groups, and no attempt was made to achieve primary flap closure [1].
All patients received analgesics (talniflumate, Somalgen, Alvogen, Seoul, Korea) and antibiotics (amoxicillin sodium and sulbactam pivoxil, Sultamox, Alvogen) for 5 days, and they were instructed to rinse with mouthwash (0.05% cetylpyridinium chloride, GUM gargle, Sunstar, Osaka, Japan) twice daily. The sutures were removed 10–14 days after extraction.
Implant placement
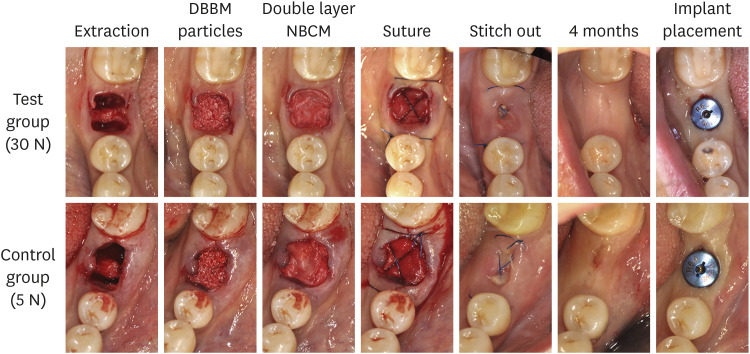
Four months later, a core biopsy was performed using a trephine bur with an internal diameter of 2.3 mm (Genoss, Seoul, Korea) without flap elevation at the implant placement site. A surgical stent was used during the biopsy procedure. Full-thickness mucoperiosteal flaps were subsequently elevated, and implants (Luna®, Shinhung, Seoul, Korea) were placed using the protocol suggested by the manufacturer (Figure 3). To achieve initial stability, a final drilling with a 1-step smaller diameter than the implant diameter was performed, and no additional guided bone regeneration (GBR) procedure was performed. All subjects were prescribed antibiotics and analgesics of the same duration and type as those used during the ARP surgery. The implant stability quotient (ISQ) was measured using resonance frequency analysis (Osstell ISQ®, Integration Diagnostics, Savedalen, Sweden) to assess the initial stability.
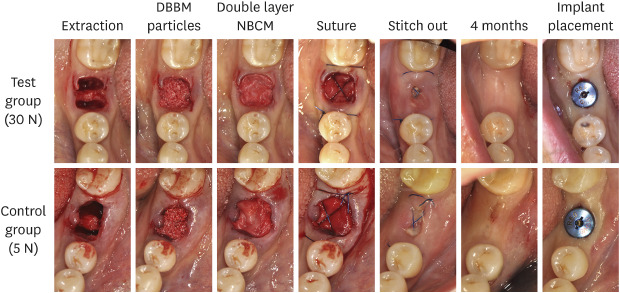
Figure 3. Clinical photographs showing the procedures from extraction to implant placement.
DBBM: deproteinized bovine bone mineral, NBCM: native bilayer collagen membrane.
Radiographic analysis
Panoramic radiographs were taken at 2 time points: after implant placement and at a follow-up period of 9–13 months after loading. Image software (PACSPLUS viewer, Medical Standard Co., Ltd., Seoul, Korea) was used to measure the linear distance between the fixture shoulder and the first bone-to-implant contact in the mesial/distal aspect. The mean change of the peri-implant marginal bone level was calculated.
Histologic and histomorphometric analyses
All specimens were fixed with 10% buffered neutral formalin (Sigma Aldrich, St. Louis, MO, USA) for 2 weeks and then decalcified in Calci-Clear Rapid (National Diagnostics, Atlanta, GA, USA). The specimens were processed with a tissue processor (ASP300S, Leica, Wetzlar, Germany) and embedded in paraffin.
Each specimen was microtomed into 2 sections with a thickness of 4 μm and stained with Masson trichrome or hematoxylin and eosin. Images of sections were obtained with the aid of an optical microscope (BX51, Olympus, Tokyo, Japan) equipped with a digital camera (SPOT Insight 2Mp, Diagnostic Instruments, Sterling Heights, MI, USA). Adobe Photoshop (version CC2015, Adobe Systems, San Jose, CA, USA) was used to measure the ratios of the area of residual graft material, new bone, and soft tissue from 2 different sections at the same magnification in order to ensure reliability. The particle size of the residual graft material on the longest axis was measured using CaseViewer software (version 2.0, 3DHISTECH, Budapest, Hungary). All of the specimens were measured by a single researcher (S.J.L.).
Statistical analyses
The statistical analyses were performed using commercially available software (SPSS version 21.0, IBM Corporation, Armonk, NY, USA). Means, standard deviations, medians, and 95% confidence intervals (CIs) were calculated. The Shapiro-Wilk test was used to check conformity to a normal distribution. The unpaired t-test was used to check for significant differences in the ratios of the area of residual graft material, new bone, and soft tissue, and in the particle size. Intraclass correlation coefficient (ICC) estimates with 95% CIs were calculated. The significance of differences in the ISQ values and the change of the marginal bone level between the 2 groups was assessed using the Mann-Whitney test. The criterion for statistical significance was set at P<0.05.
RESULTS
Clinical and radiographic observations
In total, 12 patients (6 patients per group) underwent a core biopsy for histologic evaluation. One patient in each group refused to receive the follow-up radiographic evaluation. The open-healing extraction socket without primary closure was covered with soft tissue after 2–4 weeks. None of the cases required additional GBR for implant treatment. The healed soft tissue over the extraction socket was stable, with keratinized tissue of sufficient width. The ISQ value of the implants was 68.00±9.67 (mean±standard deviation) in the control group and 74.50±16.22 in the test group (P>0.05). Implant placement was delayed due to insufficient initial stability in 1 case in the control group after core biopsy (Table 1).
Table 1. Demographic information of included patients.
| Group | Patient ID | Sex | Age (yr) | Amount of material (g) | Diameter (mm) | Length (mm) | Tooth position | ISQ | Reason for extraction |
|---|---|---|---|---|---|---|---|---|---|
| Test | 1 | Male | 64 | 0.50 | 5 | 7.0 | 47 | 42 | Endodontic |
| Test | 2 | Female | 62 | 0.50 | 5 | 8.5 | 47 | 83 | Endodontic |
| Test | 3 | Female | 61 | 0.50 | 5 | 10.0 | 46 | 75 | Crack tooth |
| Test | 4 | Female | 50 | 0.50 | 5 | 8.5 | 47 | 81 | Periodontal |
| Test | 5 | Female | 66 | 0.75 | 5 | 8.5 | 46 | 83 | Root fracture |
| Test | 6 | Female | 49 | 0.50 | 5 | 8.5 | 46 | 83 | Periodontal |
| Control | 7 | Female | 75 | 0.25 | 5 | 8.5 | 47 | 61 | Periodontal |
| Control | 8 | Female | 51 | 0.25 | 5 | 8.5 | 36 | 77 | Periodontal |
| Control | 9 | Female | 26 | 0.25 | 5 | 8.5 | 16 | 56 | Root fracture |
| Control | 10 | Male | 48 | 0.25 | 5 | 8.5 | 37 | 78 | Periodontal |
| Control | 11 | Female | 56 | 0.25 | 5 | 8.5 | 16 | 68 | Periodontal |
| Control | 12a) | Female | 52 | 0.25 | N/A | N/A | 17 | N/A | Periodontal |
ISQ: implant stability quotient.
a)Patient ID 12 in control group showed insufficient initial stability for implant placement at 4 months after alveolar ridge preservation.
Patients were followed up for an average of 10.9 months (range, 9–13 months) after placement of the implant fixture. The change of the marginal bone loss was measured for 5 patients in the test group and 5 patients in the control group; the marginal bone level was 0.27±0.87 mm and 0.47±0.27 mm, respectively, showing no significant intergroup difference (P>0.05). All groups had a 100% implant survival rate.
Histologic observations
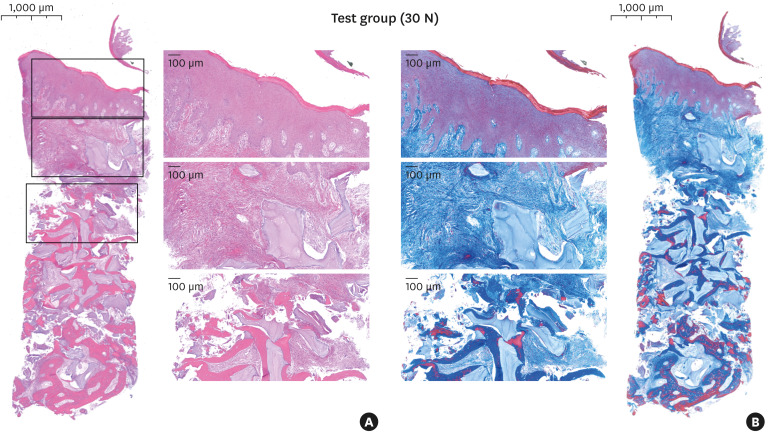
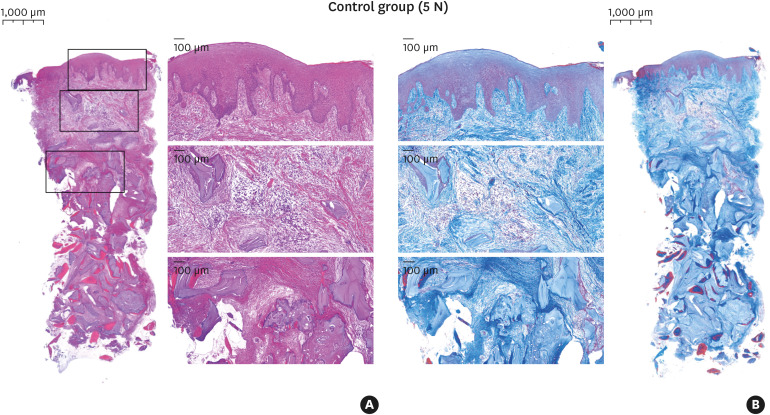
Most cases showed a firm and dense layer of keratinized mucosa and a few DBBM particles encapsulated with loose connective tissue underneath. The amount of new bone surrounding and bridging the DBBM particles appeared greater toward the apical area of the specimens. The new bone originated from the adjacent native bone around the graft-material particles, and only thin new bone had formed on the surface of the DBBM particles distant from the adjacent natural bone. Soft tissue composed of dense connective tissue was observed in the interstitial space. Large numbers of crushed particles were observed in the test group, and relatively small particles were gathered more densely than in the control group at the same magnification (Figures 4 and 5).
Figure 4. Histologic features in the test group (30 N) for hematoxylin and eosin staining (A) and Masson's trichrome staining (B).
Figure 5. Histologic features in the control group (5 N) for hematoxylin and eosin staining (A) and Masson's trichrome staining (B).
Histomorphometric measurements
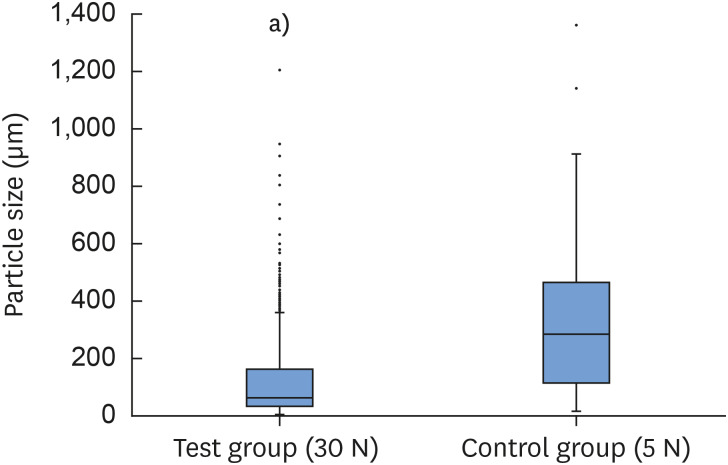
The ratios of the area of new bone, residual graft material, and soft tissue in the control group were 13.32%±8.93%, 16.19%±5.18%, and 70.49%±6.96%, respectively; the corresponding values in the test group were 14.03%±12.63%, 20.27%±3.91%, and 65.70%±13.25%, respectively. These 3 variables did not differ significantly between the 2 groups (P>0.05). The size of the residual graft material particles was 0.28±0.21 mm in the control group and 0.13±0.13 mm in the test group (P<0.05) (Table 2) (Figure 6). The ICC for evaluating the reliability of the area measurements was 0.98 (95% CI, 0.96–0.99).
Table 2. Histomorphometric measurements of the specimens.
| Group | Patient ID | New-bone area (%) | Area of residual graft material (%) | Soft-tissue area (%) | Particle size (mm) |
|---|---|---|---|---|---|
| Test | 1 | 32.75 | 19.92 | 47.33 | 0.12±0.14 |
| Test | 2 | 13.57 | 20.14 | 66.29 | 0.15±0.16 |
| Test | 3 | 3.37 | 27.09 | 69.54 | 0.14±0.14 |
| Test | 4 | 27.45 | 20.42 | 52.12 | 0.12±0.13 |
| Test | 5 | 5.98 | 19.25 | 74.77 | 0.07±0.09 |
| Test | 6 | 1.07 | 14.81 | 84.12 | 0.19±0.21 |
| Average | 14.03±12.63 | 20.27±3.91 | 65.70±13.25 | 0.13±0.13 | |
| Control | 7 | 6.39 | 24.50 | 69.12 | 0.37±0.25 |
| Control | 8 | 26.35 | 9.80 | 63.85 | 0.20±0.19 |
| Control | 9 | 10.13 | 17.66 | 72.21 | 0.21±0.14 |
| Control | 10 | 20.42 | 17.23 | 62.36 | 0.32±0.20 |
| Control | 11 | 15.45 | 11.05 | 73.50 | 0.29±0.17 |
| Control | 12 | 1.17 | 16.93 | 81.90 | 0.28±0.21 |
| Average | 13.32±8.93 | 16.19±5.18 | 70.49±6.96 | 0.28±0.21 | |
| P value | 0.916 | 0.161 | 0.468 | 0.001a) | |
Data from all specimen percentage of new bone, residual graft material, soft tissue area and particle size. Average data are mean±standard deviation values.
a)Statistically significant difference between groups (P<0.05).
Figure 6. Box plots with whiskers of the particle sizes in the test and control groups. Each box plot shows the median, first and third quartiles, and range, with outliers also indicated.
a)Statistically significant difference (P<0.05).
DISCUSSION
This prospective proof-of-concept study utilized a histomorphometric analysis to evaluate the impact of different compressive forces on DBBM particles covered by NBCM during ARP in the molar area. Previous studies have analyzed quantitative changes such as horizontal and vertical alterations of the alveolar ridge with a cast model or cone-beam computed tomography [8,9,10]. The present study instead focused on performing qualitative research through a histologic analysis.
Repeated measurements using a digital force gauge demonstrated that a minimum force of 5 N for graft packing during ARP was enough to secure the bone particles in the extraction socket. Meanwhile, 30 N was the highest compressive force that did not cause discomfort or pain to patients. Based on the present histologic observations, the greater compressive force appeared to produce relatively small DBBM particles (<0.25 mm) in the test group. It has been reported that the maximal compressive strength of DBBM particles is approximately 35 MPa [11], and the 30 N compressive force applied in the test group seemed to only affect the weaker interface between particles and the sharp edges of the graft. Then it is suspected that the compressive force induced closer aggregation of the small particles in the test group. However, the total ratio of the area of residual graft particles did not show a significant difference between the test and control groups. Vail et al. [12] reported that smaller graft particles may induce a greater inflammatory response, but no inflammatory cell infiltration or clinical inflammation was observed in the present study. A previous investigation of open-healing ARP using DBBM-C did not find fractured or crushed particles under the compressive force; instead, only the inter-space between particles was reduced, resulting in increased bone density [6]. The authors speculate that the additional 10% collagen in DBBM-C might have acted as a spatial buffer to absorb the pressure between graft particles and to maintain a certain degree of distance. However, further studies are warranted to fully elucidate this phenomenon.
The overall histologic pattern varied between specimens, which may have been due to differences in individual healing abilities. However, Lee et al. [13] reported that a wide defect or socket entrance in the molar region produced various contraction mechanisms during the healing process, such as dimensional shrinkage and dehiscence defect formation, even after a ridge preservation procedure. These diverse morphological changes could have affected various aspects of the histologic changes observed in the present study.
Although the ratio of residual graft materials did not show a statistically significant difference between groups, the test group showed a somewhat greater amount of graft particles than the control group (21.36%±3.15% vs. 16.05%±5.60%). It is speculated that the greater compressive force mechanically introduced more graft particles into the extraction sockets. Interestingly, the rate of new bone formation did not differ between the control group (15.75%±7.63%) and the test group (16.63%±12.25%), and the new bone ratio for the test group is comparable to that found in the previous study using DBBM-C.
In patient No. 12 from the control group, the initial implant stability was insufficient, and the surgeon failed to place the implant. Additionally, 3 patients showed ISQ values lower than 65, and therefore required a longer healing period than normal [14]. A potential reason for these lower ISQ values may be the degree of mechanical engagement of pristine bone with the implant apex. The authors are currently investigating this issue (manuscript in preparation) to clarify the impact of compressive force on implant stability after ARP. Interestingly, marginal bone loss was not significantly different between the 2 groups, and the extent of marginal bone loss fulfilled the criteria of implant success proposed by Albrektsson et al. [15].
This study had limitations associated with its proof-of-concept design. Further randomized controlled clinical studies with sufficient numbers of subjects are needed to determine long-term outcomes with adequate power. Within the limitations of this study, it was concluded that the application of 2 different compressive forces (5 N and 30 N) on particulate DBBM grafts during open healing ARP in the posterior area led to comparable new bone formation, implant feasibility and peri-implant bone level.
Footnotes
Funding: The present study was supported by the research fund of Dankook University (R-2017-01474).
- Conceptualization: Sung-Jo Lee, In-Woo Cho, Hyun-Seung Shin, Seung-Il Shin, Jung-Chul Park.
- Data curation: Sung-Jo Lee, Dae-Young Kang.
- Formal analysis: Sung-Jo Lee.
- Funding acquisition: Jung-Chul Park.
- Investigation: Sung-Jo Lee, In-Woo Cho, Jung-Chul Park.
- Methodology: In-Woo Cho, Hyun-Seung Shin, Seung-Il Shin, Jung-Chul Park.
- Project administration: Jung-Chul Park.
- Resources: Hyun-Seung Shin.
- Software: Sung-Jo Lee, Dae-Young Kang.
- Supervision: In-Woo Cho, Hyun-Seung Shin, Seung-Il Shin, Jung-Chul Park.
- Validation: Sung-Jo Lee, In-Woo Cho, Jung-Chul Park.
- Visualization: Sung-Jo Lee.
- Writing - original draft: Sung-Jo Lee.
- Writing - review & editing: Dae-Young Kang, In-Woo Cho, Seung-Il Shin, Hyun-Seung Shin, Kai R. Fischer, Jung-Chul Park.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Park JC, Koo KT, Lim HC. The hidden X suture: a technical note on a novel suture technique for alveolar ridge preservation. J Periodontal Implant Sci. 2016;46:415–425. doi: 10.5051/jpis.2016.46.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi HK, Cho HY, Lee SJ, Cho IW, Shin HS, Koo KT, et al. Alveolar ridge preservation with an open-healing approach using single-layer or double-layer coverage with collagen membranes. J Periodontal Implant Sci. 2017;47:372–380. doi: 10.5051/jpis.2017.47.6.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho HY, Suh CW, Duong HP, Lee SJ, Cho IW, Shin HS, et al. Alveolar ridge preservation of maxillary molars for implant placement without sinus lift surgery: case series. J Korean Acad Oral Maxillofac Implantol. 2018;22:220–235. [Google Scholar]
- 4.Cha JK, Song YW, Park SH, Jung RE, Jung UW, Thoma DS. Alveolar ridge preservation in the posterior maxilla reduces vertical dimensional change: a randomized controlled clinical trial. Clin Oral Implants Res. 2019;30:515–523. doi: 10.1111/clr.13436. [DOI] [PubMed] [Google Scholar]
- 5.Lim HC, Shin HS, Cho IW, Koo KT, Park JC. Ridge preservation in molar extraction sites with an open-healing approach: a randomized controlled clinical trial. J Clin Periodontol. 2019;46:1144–1154. doi: 10.1111/jcpe.13184. [DOI] [PubMed] [Google Scholar]
- 6.Cho IW, Park JC, Shin HS. A comparison of different compressive forces on graft materials during alveolar ridge preservation. J Periodontal Implant Sci. 2017;47:51–63. doi: 10.5051/jpis.2017.47.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire MK, Nunn ME. Prognosis versus actual outcome. III. The effectiveness of clinical parameters in accurately predicting tooth survival. J Periodontol. 1996;67:666–674. doi: 10.1902/jop.1996.67.7.666. [DOI] [PubMed] [Google Scholar]
- 8.Cardaropoli D, Cardaropoli G. Preservation of the postextraction alveolar ridge: a clinical and histologic study. Int J Periodontics Restorative Dent. 2008;28:469–477. [PubMed] [Google Scholar]
- 9.Cardaropoli D, Tamagnone L, Roffredo A, Gaveglio L, Cardaropoli G. Socket preservation using bovine bone mineral and collagen membrane: a randomized controlled clinical trial with histologic analysis. Int J Periodontics Restorative Dent. 2012;32:421–430. [PubMed] [Google Scholar]
- 10.Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74:990–999. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- 11.Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin Oral Implants Res. 2000;11:217–229. doi: 10.1034/j.1600-0501.2000.011003217.x. [DOI] [PubMed] [Google Scholar]
- 12.Vail TB, Trotter GW, Powers BE. Equine demineralized bone matrix: relationship between particle size and osteoinduction. Vet Surg. 1994;23:386–395. doi: 10.1111/j.1532-950x.1994.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Cha JK, Kim CS. Alveolar ridge regeneration of damaged extraction sockets using deproteinized porcine versus bovine bone minerals: a randomized clinical trial. Clin Implant Dent Relat Res. 2018;20:729–737. doi: 10.1111/cid.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bornstein MM, Hart CN, Halbritter SA, Morton D, Buser D. Early loading of nonsubmerged titanium implants with a chemically modified sand-blasted and acid-etched surface: 6-month results of a prospective case series study in the posterior mandible focusing on peri-implant crestal bone changes and implant stability quotient (ISQ) values. Clin Implant Dent Relat Res. 2009;11:338–347. doi: 10.1111/j.1708-8208.2009.00148.x. [DOI] [PubMed] [Google Scholar]
- 15.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]