Abstract
Chloral hydrate is the oldest and most common sedative drug used in moderate sedation for pediatric dental patients. Hence, the purpose of this article is to review the safety and possible adverse events of this drug when used for pediatric dental treatment. A bibliographic search in PubMed, MEDLINE, Cochrane Library and KMbase, KISS, DBpia, KoreaMed, and RISS databases was performed. Using the keywords “dental sedation,” “chloral hydrate,” and “children or adolescent,” 512 scientific articles were found. Subsequently, 183 studies were individually assessed for their suitability for inclusion in this literature review. Altogether, 24 studies were selected. They included 12 cases of death before, during, or after chloral hydrate sedation for dental treatment, majorly due to dosing error and use of multiple sedatives. Additionally, intraoperative adverse events were mostly respiratory problems such as hypoxia and apnea, but most events were temporary. After treatment, prolonged sedation, including excessive sleep and less activity were the most common postoperative adverse events, and even death cases were reported. Despite the wide acceptance of chloral hydrate as a sedative-hypnotic agent, the risk of adverse events and adequate dose should be of great concern when using it for pediatric dental sedation.
Keywords: Adverse Drug Reactions, Child, Chloral Hydrate, Conscious Sedation, Pediatric Dentistry, Safety
INTRODUCTION
Dental fear and anxiety in children lead to problems with behavior management for treatment [1]. A child who had a negative experience during dental treatment develops even greater anxiety toward future dental visits, which makes future regular checkups and further treatment difficult [2]. Sedation is one of the behavior management methods that can be applied to pediatric patients who show the type of behavioral pattern that is likely to yield these results [3].
The drug is a critical factor in sedation. Drugs used for sedation must be able to induce a sedative state of desired depth after administration, with rapid onset of action and recovery. Additionally, sedative agents or medication must be reversible and above all, safe for the patient. Individuals who perform sedation must be able to select the right drug for the intended treatment procedure, know the drug interactions of sedatives, and have life-saving skills that can save the patient from an adverse event [4].
According to a survey conducted among members of the American Academy of Pediatric Dentistry (AAPD), oral administration was the most commonly used method of sedation besides nitrous oxide (N2O) inhalation, and the combination of chloral hydrate (CH), hydroxyzine, and N2O inhalation was commonly used by American pediatric dentists [5]. A survey of sedation practices in pediatric patients among members of the Korean Academy of Pediatric Dentistry (KAPD) and Korean Dental Society of Anesthesiology also showed that the most preferred route of sedative administration for children was oral, while the most preferred sedative combination was CH, hydroxyzine, and N2O inhalation sedation [6,7].
While CH oral sedation is frequently used and highly preferred in pediatric dentistry, there are still reports of significant adverse events during or after dental treatment under sedation, including death.
This study aims to evaluate the safety and adverse events of CH sedation for dental treatment in pediatric patients by reviewing previous studies and published literatures.
METHODS
We conducted a literature database search of PubMed, Embase, and Cochrane Library, and also domestic literature databases such as the Korean Medical Database (KMbase), KoreaMed, and Korean Studies Information Service System (KISS), which are CORE search databases, and DataBase Periodical Information Academic (DBpia) and Research Information Sharing Service (RISS), which are academic search engines/web portals. The search terms used were “dental sedation,” “chloral hydrate,” and “children or adolescent”. The period of literature search was from the release date of each database or journal publication to January 2020. There was no restriction on the year of publication.
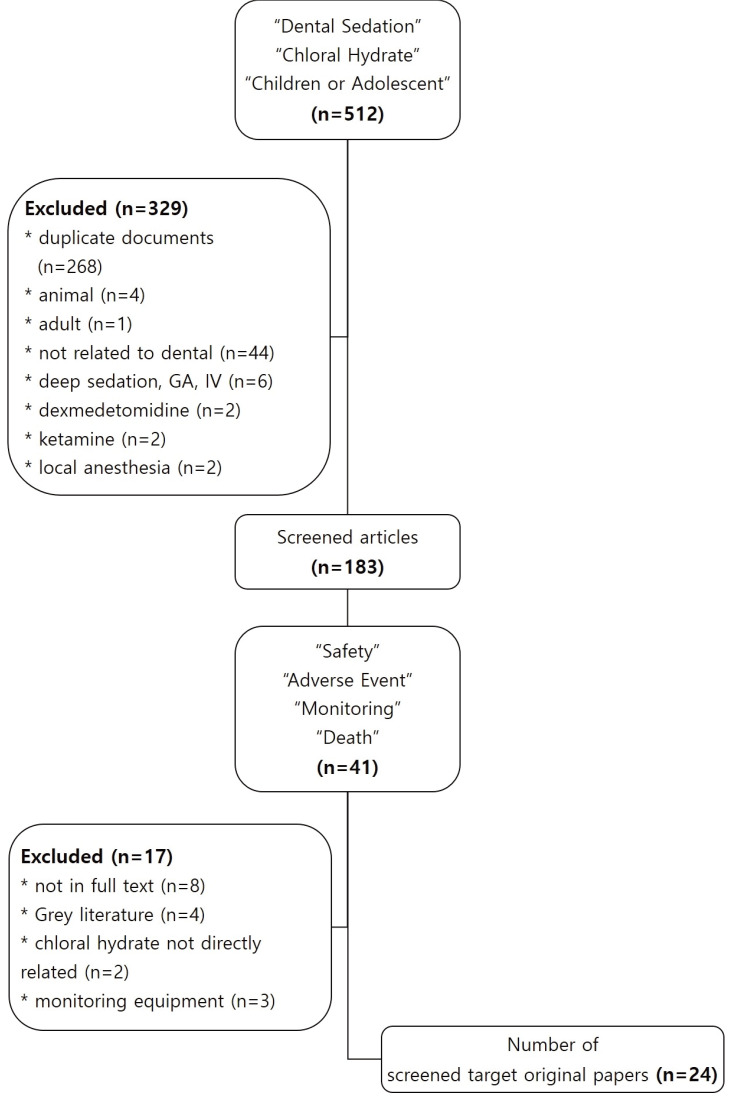
The primary search included 512 papers. Of these, 183 papers remained after checking for duplication and considering the inclusion and exclusion criteria (Table 1). In the secondary search, we manually checked the title and abstract to screen for papers related to safety, adverse event, monitoring, and death and then selected 41 papers. Of these, we excluded papers without a direct relation to CH, papers whose original text was not provided, gray literature (editor's letter, thesis paper), and papers on patient monitoring equipment (Fig. 1).
Table 1. Paper selection criteria.
| Inclusion | Exclusion |
|---|---|
| Children, adolescent group | Adult age group |
| Minimal or moderate sedation | Deep sedation, general anesthesia |
| Dental procedure, sedation | Medical procedure, sedation |
| Failure to secure the full text | |
| Gray literature (publisher's letter, thesis) |
Fig. 1. Flow diagram of the literature selection progress.
RESULTS
Altogether, 24 papers that met the criteria were included in the review. The papers are listed by year in Table 2, starting from the most recent paper based on the year of publication.
Table 2. Selected studies.
| First author | Year of publication | Type of study | Topic |
|---|---|---|---|
| Grissinger M [8] | 2019 | Review | Death |
| Abdulhamid I [9] | 2016 | Open-label study | Safety related to systemic condition |
| Huang A [10] | 2015 | Comparative study | Safety |
| Nordt SP [11] | 2014 | Case report | Death, safety |
| McCormack L [12] | 2014 | Nonrandomized cohort study | Safety |
| Kang J [13] | 2012 | Retrospective study | Safety related to systemic condition |
| Chicka MC [14] | 2012 | Review | Death, safety |
| Costa LR [15] | 2012 | Comparative study | Safety |
| Kupiec TC [16] | 2011 | Case report | Death |
| de Rezende GP [17] | 2007 | Case report | Safety |
| Martinez D [18] | 2006 | Prospective, pilot study | Safety |
| Park MK [19] | 2006 | Prospective randomized study | Safety |
| Myers GR [20] | 2004 | Randomized double-blind crossover study | Safety |
| Lee JH [21] | 2002 | Cross sectional study | Safety |
| Leelataweedwud P [22] | 2001 | Retrospective study | Safety |
| Dallman JA [23] | 2001 | Clinical trial | Safety |
| Jung JH [24] | 2001 | Cross sectional study | Safety |
| Avalos-Arenas V [25] | 1998 | Clinical trial | Safety |
| Engelhart DA [26] | 1998 | Case report | Death |
| McCann W [27] | 1996 | Clinical trial | Safety |
| Needleman HL [28] | 1995 | Retrospective study | Safety |
| Sams DR [29] | 1993 | Case controlled study | Safety |
| Wilson S [30] | 1990 | Clinical trial | Safety |
| Mueller WA [31] | 1985 | Data collection | Safety |
1. Death case report (Table 3)
Table 3. Death case report.
| First author/publication year | Case number | Age (year) | Sedative drugs (mg/kg, %) | Administered by: / at: | Detailed description | Cause |
|---|---|---|---|---|---|---|
| Grissinger M 2019 [8] | 1, 2 | Unauthorized person | Failure to recognize overdose | Dosing error | ||
| 3 | 13 | CH (6000 mg) | Dentist | Weight-based prescription | Overdose (Respiratory arrest) | |
| 4, 5 | Child | Parents | Pharmacy dispensed 500 mg/5 mL instead of 250 mg/5 mL | Dosing error | ||
| Home | ||||||
| 6 | Child | Parents | Pharmacy prescribed tenfold drug | Dosing error | ||
| Home | ||||||
| 7 | 4M | Strapped onto papoose board without proper head position | Improper patient control during treatment | |||
| 8 | Repeated “5 mL PRN” prescription | Dosing error | ||||
| Nordt SP 2014 [11] | 9 | 4F | CH (70) | Home prior to procedure | Discharge after 1 h, remained somnolent but arousable, ongoing somnolence for 6 h | Resedation after discharge (Respiratory arrest) |
| Dead after PICU | ||||||
| Chicka MC 2012 [14] | 10 | 2M | CH (unknown) | Unknown | Medical history of Russell-Silver syndrome | (Respiratory arrest) |
| Dental office | Dentist noticed respiratory rate slowed CPR, intubation, pronounced dead upon arrival at emergency department | |||||
| Kupiec TC 2011 [16] | 11 | 6M | Meth (2), Hy (1.64), CH (15), N2O-O2 | Dentist | Medical history of asthma | Cocktail (Toxicity of methadone) |
| Dental office | Patient appeared responsive but groggy after | |||||
| procedure, taken home and fell asleep | ||||||
| Dead after few hours of procedure | ||||||
| Engelhart DA 1998 [28] | 12 | 2M | CH (95), N2O-O2 | Unknown | Full arrest during surgical procedure | Combined effect of CH, lidocaine, N2O |
| Not in dental center | Transported to emergency room after 2 h, dead after 2.3 h of administration |
CH, chloral hydrate; Meth, methadone; Hy, hydroxyzine; N2O-O2, nitrous-oxygen inhalation; PRN, pro re nata, as needed; PICU, pediatric intensive care unit
Altogether, 12 deaths before, during or after dental treatment under CH sedation in pediatric patients, caused by drug administration or dosing error, overdose, or combined use of multiple sedative medications have been reported. These include patients with underlying diseases that entail high risk of respiratory obstruction, drug administration by unauthorized person, and inadequate airway management after administration.
According to Cote et al. [32], drug overdose is defined as using > 1.25 times the maximum recommended dose. In the case of CH, the maximum recommended dose is 2 g, 100 mg/kg in one dose, and death from overdose of single use of CH is rare relative to its frequent usage.
While death of a patient with history of asthma has been reported [16], another patient with mild asthma was treated safely using CH with single dose of 65 mg/kg [9]; thus, asthma itself cannot be a major factor of patient death.
There has been a report of death from improper head positioning on the papoose board [8]. Immobilization devices such as the papoose board must be applied without causing airway obstruction or chest compression during sedation. In addition, the patient's head position and breathing movement must be checked frequently to secure the airway during treatment [4].
2. Adverse events before and during sedation (Table 4)
Table 4. Summary of preoperative and intraoperative adverse events.
| First author/ Publication year | Age (month) | Sample size | Sedative drugs (mg/kg, %) | Administered by: / at: | Monitoring Equipment Information Interval (minute) | Results |
|---|---|---|---|---|---|---|
| Grissinger M 2019 [8] | 48 | 1 (1M) | Strapped onto the papoose board without proper head position | |||
| Death cause: improper position to protect his airway | ||||||
| Nordt SP 2014 [11] | 36 | 1 (1M) | CH (400) | Parents Home | Somnolent after 10 min, unresponsiveness | |
| Vomiting during ambulance | ||||||
| Esmolol infusion | ||||||
| Discharge after 30 h without sequelae | ||||||
| McCormack L 2014 [12] | 55 | 40 (21M, 19F) | CH (30), Mep (2), Hy (2), N2O-O2 (30-50) | Dentist | PO, PC, Visual observation | Mdz included regimen more body movement during treatment |
| Mdz (1), Mep (2), Hy (2), N2O-O2 (30-50) | Dental office | SaO2 | ||||
| Chicka MC 2012 [14] | 96 | 1 (1M) | CH (75), Hy (4.4) | Medical history of attention deficit disorder, on medication | ||
| 50 min after administration, stopped crying, turned blue, and no pulse when placed in papoose board | ||||||
| Remained in coma for 3 days | ||||||
| Hypoxic brain damage | ||||||
| 26.4 | 1 (1M) | Hydrocodone barbiturate, CH (25), Hy (5.8), Mep (4.6) | Mother Home | 2% lidocaine (13.2 mg/kg) | ||
| Patient turned blue, no breathing during treatment | ||||||
| Naloxone administration | ||||||
| Respiratory arrest, seizure | ||||||
| CPR by parent | ||||||
| Discharged satisfactory condition | ||||||
| Costa LR 2012 [15] | 43.2 | 42 (22M, 20F) | CH (70) | PO, BPC, Visual observation | 1 case (10%) in CH 70 mg/kg group had oxygen desaturation (SaO2 90%), irritation | |
| CH (100) | Dentist | |||||
| Mdz (1.0) | Dental office | HR, SaO2, BP, RR | ||||
| Mdz (1.5) | 15 | |||||
| de Rezende GP 2007 [17] | 35 | 1 (1M) | CH (100) | Dentist | HR, SaO2, BP, RR | Became active after 15 min |
| Dental office | Vomited twice during treatment | |||||
| 15 | Abdominal pain, thirst | |||||
| Observed aggressive behavior and fell asleep | ||||||
| Park MK 2006 [19] | 44.5 | 15 (6M, 9F) | CH (60), Hy (1), N2O-O2 (50) | Dentist | PO | No hypoxia, vomit, nausea |
| 34.3 | 16 (11M, 5F) | CH (60), Hy (1), Mdz (0.1), N2O-O2 (50) | Dental office | SaO2, PR | Mean SaO2: 99.1% | |
| 2 | No hypoxia, vomit, nausea | |||||
| Mean SaO2 : 98.6% | ||||||
| SaO2, 95% once | ||||||
| Myers GR 2004 [20] | 48.9 | 40 (22M, 18F) | CH (50), N2O-O2 (50) | PO, BPC, ECG, Capno, PC | 2 oxygen desaturation (SaO2 85%, 88%) cases, resolved with head positioning and mouth suctioning | |
| CH (50), Mdz (0.2), N2O-O2 (50) | HR, SaO2, BP, RR, ETCO2 | No desaturation | ||||
| 5 | ||||||
| Lee JH 2002 [21] | 42.2 | 40 (22M, 18F) | CH (60), Hy (25 mg) | Dentist | PO, PC | Mean SaO2: 98.1% |
| Dental office | PR, SaO2, RR | No true apnea | ||||
| 5 | True desaturation (SaO2 under 95%) 0–3 times per patient | |||||
| Leelatawe edwud P 2001 [22] | 47 | 111 (57M, 54F) | CH (50), Mep (1.5), Hy (25 mg), O2 (100) | Dentist Dental office | PO, PC, Visual observation, Capno | 2 true apnea cases (no visual sign of breathing, no breath sound, Capno 0 for 25 s) |
| PR, SaO2, BP, RR, ETCO2 | 3 prolonged sedation (need more than 30 min after treatment for discharge) cases | |||||
| 5 | 1 vomiting case | |||||
| Dallman JA 2001 [23] | 41.8 | 31 (23M, 8F) | Mdz (0.2), N2O-O2 (25-50) | Dentist | HR, SaO2, BP, RR | No vomit case |
| CH (62.5), PZ (12.5 mg), N2O-O2 (25-50) | Dental office | 5 | 1 vomiting case | |||
| Jung JH 2001 [24] | 30 | 71 (40M, 31F) | CH (60), Hy (25 mg) | Dentist | PO | Temporary hypoxia (SaO2 under 95%) 42.2% |
| Dental office | SaO2 | |||||
| Avalos-Arenas V 1998 [25] | 28.58 | 40 | CH (40), Hy (2), N2O-O2 (50) | Dentist | PO | At least 10% cases of hypoxia (SaO2 under 90%) |
| CH (40), Hy (2) | Dental office | HR, SaO2, BP, RR | ||||
| 15 | ||||||
| McCann W 1996 [27] | 45 | 40 (26M, 14F) | CH (40), Hy (2), N2O-O2 (50) | Dentist | PO | No desaturation (SaO2 under 95%) episodes |
| CH (40), Hy (2) | Dental office | HR, SaO2, BP, expired | ||||
| CO2 level | ||||||
| 5 | ||||||
| Needleman HL 1995 [28] | 31.2 | 382 (216M, 166F) | CH (55), Hy (1), N2O-O2 (40-60) | PO, PC | Intraoperative vomiting 8.1% | |
| HR, SaO2, RR | 21% of patients desaturation (SaO2 under 95%) | |||||
| 5 | ||||||
| Sams DR 1993 [31] | 31 | 24 | CH (50), PZ (1), N2O-O2 (< 50) | Dentist | HR, SaO2, BP, RR, | 2 desaturation (SaO2 90-95%) |
| 35.8 | Mep (1), N2O-O2 (< 50) | Dental office | Temp | |||
| 15 | ||||||
| Wilson S 1990 [30] | 28.8 | 12 | CH (40), Hy (2) | Dentist | PO, BPC, CO2 monitor, brain monitor | 13% true desaturation (SaO2 < 95%) |
| 30.1 | 10 | CH (25, 50, 70) | Dental office | HR, SaO2, BP, RR, expired CO2 level, EMG | 10% true desaturation (SaO2 under 95%) | |
| No significant difference between dosage of CH | ||||||
| Mueller WA 1985 [31] | 20 | CH (100), N2O-O2 (50) | Dentist | PO | 35% decreased SaO2 (SaO2 under 95%) | |
| Dental office | HR, BP, RR | |||||
| 5 |
CH, chloral hydrate; Hy, hydroxyzine; Mdz, midazolam; Mep, meperidine; PZ, promethazine; PO, pulse oximetry; PC, precordial stethoscope; SaO2, oxygen saturation; BPC, blood pressure cuff/sphygmomanometer; BP, blood pressure; HR, heart rate; RR, respiratory rate; PR, pulse rate; ECG, electrocardiography; Capno, capnography; ETCO2, end tidal carbon dioxide; EMG, electromyogram
In the dental treatment under CH sedation of pediatric patients, the most common adverse event that occurred during treatment after sedative administration was hypoxia (oxygen desaturation). Gastrointestinal problems, including vomiting, nausea, abdominal pain, and paradoxical reaction (an hyperactivity reaction) were also observed during treatment. As with any sedative agent, CH sedation can result in residual sedation, disorientation, paradoxical excitement, delirium, ataxia, headache, nightmares, hallucinations, and paranoid behavior [33]. CH sedation for dental treatment in pediatric patients was often accompanied by respiratory problems like desaturation and apnea during treatment.
According to the American Dental Association (ADA) guidelines, sedation can be largely divided into four stages: minimal sedation, moderate sedation, deep sedation, and general anesthesia [34]. Although deeper levels of sedation (moderate to deep sedation) can be achieved by adjusting the dose, given that pharmacological reaction cannot be reversed and individual patient response is highly diversified, only minimal to moderate sedation can be considered in the rational use of oral CH sedation [35]. For CH administration aimed at minimal and moderate sedation, the oxygen saturation, heart rate, blood pressure, and respiratory rate must be monitored during treatment, while electrocardiogram (ECG) and capnography are recommended [4]. The name and dose of all administered drugs, route, site, and time of administration, including local anesthetic, must be recorded. Additionally, the heart rate, respiratory rate, blood pressure, oxygen saturation, and level of consciousness must be recorded at least every 10 min [4,34].
The literature on adverse events before and during CH sedation of pediatric dental patients included data on patient monitoring, except for three papers. In papers on patient monitoring, oxygen saturation was monitored in all patients, given that children are likely to develop respiratory problems. However, six studies used inadequate monitoring interval, did not inscribe patients' heart rate and blood pressure in the monitoring data.
Considering that CH stimulates the gastric mucosa, reports of abdominal pain or vomiting during treatment were common. If pretreatment fasting was not followed, pulmonary aspiration due to reflux of food cannot be avoided.
3. Adverse events after sedation (Table 5)
Table 5. Summary of postoperative adverse events including death case.
| First author/publication year | Age (month) | Sample size | Sedative drugs (mg/kg, %) | Monitoring period | Results |
|---|---|---|---|---|---|
| Huang A 2015 [10] | 84 | 7 | CH, Mep, Hy | 24 hours after discharge | Excessive somnolence |
| Nordt SP 2014 [11] | 48 | 1 (1F) | CH (70) | Discharge after 1 hour, remained somnolent but arousable | |
| Ongoing somnolence for 6 hours | |||||
| Post-discharge death by respiratory arrest | |||||
| McCormack L 2014 [12] | 55 | 40 (21M, 19F) | CH (30), Mep (2), Hy (2), N2O-O2 (30-50) | Post-operation before discharge, 8, 24 hours after discharge | CH combination regimen exhibited significantly more sleeping after arriving home, less talking, and greater need for postoperative pain medications up to 8 hour after discharge |
| Mdz (1), Mep (2), Hy (2), N2O-O2 (30-50) | |||||
| Costa LR 2012 [15] | 43.2 | 42 (22M, 20F) | CH (70) | 24 hours after discharge | Minor post-discharge adverse events(falling asleep, difficult to awake) were common, significantly more associated with CH than Mdz |
| CH (100) | |||||
| Mdz (1.0) | |||||
| Mdz (1.5) | |||||
| Kupiec TC 2011 [16] | 72 | 1 (1M) | Meth (2), Hy (1.64), CH (15), N2O-O2 | Medical history of asthma | |
| Patient appeared responsive but groggy after procedure, | |||||
| taken home and fell asleep | |||||
| Dead after few hours of procedure | |||||
| Post-discharge death by drug cocktail | |||||
| Martinez D 2006 [18] | 24-60 | 30 (14M, 16F) | CH (20-30), Mep (1-2), Hy (1-2), N2O-O2 (50) | Post-operation before discharge, 24 hours after discharge | Children having combination regimen containing CH were more likely to sleep on the way home and at home than those received Mdz alone |
| Mdz (0.5-0.75), those received Mdz alone N2O-O2 (50) | |||||
| Dallman JA 2001 [23] | 41.8 | 31 (23M, 8F) | Mdz (0.2), N2O-O2 (25-50) | 20 minutes after operation | Mdz group met discharge criteria more quickly than CH group |
| CH (62.5), PZ (12.5 mg), N2O-O2 (25-50) | 1 vomiting case | ||||
| Engelhart DA 1998 [28] | 24 | 1 (1M) | CH (95), N2O-O2 | Following surgical procedure, patient transported to emergency room after 2 h of administration, dead after 2.3 h of administration | |
| Post-discharge death by overdose of CH, combined effect of CH, lidocaine, N2O | |||||
| Sams DR 1990 [29] | 31 | 24 | CH (50), PZ (1), N2O-O2 (< 50) | 30 minutes, 24 hours after discharge | 2 postoperative pain |
| 6 increased anxiety/irritability | |||||
| 2 fever | |||||
| 35.8 | Mep (1), N2O-O2 (< 50) | 2 postoperative pain | |||
| 1 increased anxiety/irritability |
CH, chloral hydrate; Mep, meperidine; Hy, hydroxyzine; Mdz, midazolam; Meth, methadone; PZ, promethazine
Most adverse events occurring after CH sedation treatment in pediatric dental patients were decreased activity and excessive sleep. Cases of death after treatment termination and discharge were also reported.
The onset of CH action is usually 15–30 min after oral administration, with maximal effect occurring after more than an hour. The duration of drug action ranges from 2 to 8 h and typically lasts at least 5 h, but individual patient response varies, and the sedative effect can last up to 24 h [10,11]. The mean elimination half-life of CH is 9.7 ± 1.7 h [36]. However, it has been found that the half-life is longer in neonates and infants and that the effect of the drug outlasts the plasma half-life (related to influence of the central nerve system) [32]. Death at home after sedation treatment and discharge [11] may also be related to this.
DISCUSSION
The goals of sedation in pediatric patients are to (1) protect the safety of patients; (2) minimize physical discomfort and pain; (3) control anxiety, minimize psychological trauma, and maximize the possibility of amnesia; (4) modify behavior or movement to complete procedures safely; and (5) discharge patients in a safe state under medical/dental supervision, as determined by accepted standards [4].
The drugs used for sedation in children include N2O/oxygen, benzodiazepines such as midazolam, diazepam, and nonbarbiturate sedative-hypnotics like CH. Histamine blockers like hydroxyzine, promethazine (PZ), narcotic analgesics like meperidine (Mep) are also used. In addition, sedative like ketamine and propofol can be included as a drug for sedation in children. Each drug is used singly or in combination, and the routes of administration are oral, nasal, intramuscular, submucosal, and intravenous. Depending on the administered drug, patient characteristics, and pharmacological properties vary by route of administration [37].
CH is one of the oldest sedative-hypnotic used for sedation since it was first synthesized by Justus von Liebig in 1832 by ethanol chlorination and was introduced to medicine by Liebreich in 1869. It was first used in children in 1894 [38] and has been widely used as an oral sedative in pediatric dentistry since the mid-1950s. In Korea, it is the only oral sedative approved for use in children by the Ministry of Food and Drug Safety. It has been sold in a 95 ml bottle at 100 mg/ml concentration by one pharmaceutical company, but since June 2018, only 5 ml and 10 ml bottles at 100 mg/m are being sold. In the USA, the manufacturing of CH solution was discontinued in 2012 under the US Food and Drug Administration (FDA) guidelines; thus, usage is limited [35]. CH is an alcohol derivative, which is rapidly absorbed through the gastrointestinal tract into the cardiovascular system after oral administration, it undergoes first-pass metabolism in the liver and kidneys, and converted to its active form, trichloroethanol (TCE). It is conjugated in the liver and then excreted in urine [35].
CH usage in pediatric dentistry for sedation has a recommended oral dose based on body weight of 25–100 mg/kg, the recommended hypnotic dose is 50 mg/kg and single maximum dose is 1 g [33]. Although there are reports of death at a dose of approximately 4 g, the toxic dose for oral administration is 10 g [39], and the maximum recommended dose is 100 mg/kg, up to 2 g [32]. However, even at the therapeutic dose, it can weaken the muscle tone of the tongue, causing tongue retraction toward the posterior oropharyngeal wall and airway problem. Therefore, proper patient monitoring is required for use in pediatric patients [35].
In pediatric dentistry, careful consideration should be taken in multiple sedative administrations. Since CH has the disadvantage of stimulating the gastrointestinal mucosa and inducing adverse effects such as nausea and vomiting, it is often used in combination with hydroxyzine, which has anti-vomiting and sedative effects [35]. According to a survey of KAPD members by Yang et al. [6], a higher proportion of participants preferred a combination of two or more drugs and routes of administration including N2O inhalation to single-drug treatment, and 67.6% of participants used a combination of sedatives, CH, hydroxyzine, and N2O. However, administering two or more sedatives can increase the risk of adverse events [4]. Cote et al. [32] demonstrated a significant correlation between the use of a combination of three or more drugs and adverse events. Unlike single-drug administration, combined use with inhalation sedation or narcotics can prolong the drug action and increase the depth of sedation; thus, it is recommended to reduce the dose of CH [3].
Generally, in sedation other than general anesthesia, the American Society of Anesthesiologists (ASA) physical status classification (levels 1 and 2) correspond to the indications. In the case of ASA levels 3 and 4 and tonsillar hypertrophy, special attention is required as there is a high risk of respiratory obstruction [4]. Furthermore, as the case of preoperative oral administration at home and post-discharge death suggest [11], drugs for sedation should not be administered without direct supervision of a skilled medical practitioner [4].
In the case of deep sedation, ECG and capnography must be monitored including oxygen saturation, heart rate, blood pressure, and respiratory rate data. Moreover, the sedation depth may be greater than the intended depth; therefore, during CH sedation, the practitioner must be capable of airway management, suction, continuous positive pressure ventilation, and bag-valve-mask ventilation at the level of deep sedation [4].
According to the preoperative fasting guideline proposed by the ASA, a minimum fasting period of 2 h is recommended for clear liquids like water or fruit juice, 4 h for breast milk, 6 h for infant formula, milk, light meal (e.g., toast and clear liquid). For fried or fatty foods, the fasting period must be longer than that for a light meal, as they take longer to digest [4,34].
In 2019, the AAPD proposed the discharge criteria in a guideline (Table 6). Patients who received moderate sedation must be postoperatively monitored in an environment equipped with a suction device and positive pressure ventilation (bag-valve-mask) that can supply ≥ 90% oxygen that is suitable for the age and body size of the pediatric patient. The patient's vital signs should be monitored every 5 min, and once the child begins to awaken, the recording intervals may be increased to 10 to 15 minutes. If the patient is not fully awake, oxygen saturation and heart rate should be continuously monitored until the discharge criteria is satisfied [4].
Table 6. Recommended discharge criteria.
| 1. Cardiovascular function and airway patency are satisfactory and stable |
| 2. The patient is easily arousable, and protective airway reflexes are intact |
| 3. The patient can talk (if age appropriate) |
| 4. The patient can sit up unaided (if age appropriate) |
| 5. For a very young child or a child with disability who is incapable of the usually expected responses, the presedation level of responsiveness or a level as close as possible to the normal level for that child should be achieved. |
| 6. The state of hydration is adequate |
Cote et al. [32] showed that adverse events following procedural sedation in pediatric patients are strongly correlated with nonhospital-based facilities and dental practitioners. In pediatric dentistry, N2O which is commonly used for anxiolysis is known to have little effect on respiration and level of consciousness if used alone [32], but when used in combination with other sedatives, the depth of sedation may be shifted to excessive levels and special attention must be taken.
Moreover, dental treatment is performed under conditions vulnerable to airway obstruction, such as abnormal head, tongue positions and presence of foreign bodies, including rubber dam, exogenous water, saliva, and blood. Since pediatric patients have poor airway management ability and respiratory problems are likely to occur, all responsible practitioners and personnel must be capable of pediatric advanced life support to cope with emergencies.
CONCLUSIONS
CH has been used safely in pediatric patients for over 100 years. In countries such as USA, midazolam syrup is widely used, and it can be an alternative oral sedatives to CH. However, in Korea, CH syrup is the only oral sedative that can be used in pediatric patients.
Unlike sedation in adults, in pediatric patients, the younger the age and the worse the physical condition of the child, the faster and longer the drug acts and the deeper the sedation level can be, requiring close attention. Systemic condition of the patient must be assessed before sedation, and close monitoring is required, starting from drug administration. Keeping in mind that drug reaction is amplified when used in combination with other sedatives, dosage should be in great concern, as N2O/oxygen inhalation sedatives and local anesthetics are often used in combination in pediatric dentistry.
It should be remembered that the goal in oral CH sedation is minimal to moderate sedation, and patients' vital signs must be closely monitored during treatment. The practitioner should remember that sedation depth might be increased after drug administration, and should be capable of advanced life support to respond to respiratory problems. Following treatment termination, the patient should be closely monitored until discharge and full explanation of possible adverse events after discharge should be given to caregiver.
While it is a drug that has been used widely for a long time, there have been multiple cases of death; thus, full attention is required for oral CH sedation in pediatric dentistry.
Footnotes
- Sol Song: Writing — original draft, Writing — review & editing.
- Miran Han: Supervision, Data Curation.
- Jongbin Kim: Conceptualization, Supervision.
DECLARATION OF INTEREST: No potential conflict of interest was reported by the authors.
References
- 1.Klingberg G, Broberg AG. Dental fear/anxiety and dental behaviour management problems in children and adolescents: a review of prevalence and concomitant psychological factors. Int J Paediatr Dent. 2007;17:391–406. doi: 10.1111/j.1365-263X.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 2.Locker D, Shapiro D, Liddell A. Negative dental experiences and their relationship to dental anxiety. Community Dent Health. 1996;13:86–92. [PubMed] [Google Scholar]
- 3.Korean Academy of Pediatric Dentistry. Textbook of Pediatric Dentistry. 5th ed. Seoul: Yenang INC; 2014. pp. 225–259. [Google Scholar]
- 4.Coté CJ, Wilson S. Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures. Pediatr Dent. 2019;41:26E–52E. [PubMed] [Google Scholar]
- 5.Wilson S, Houpt M. Project USAP 2010: Use of sedative agents in pediatric dentistry-a 25-year follow-up survey. Pediatr Dent. 2016;38:127–133. [PubMed] [Google Scholar]
- 6.Yang YM, Shin TJ, Yoo SH, Choi SC, Kim JY, Jeong TS. Survey of sedation practices by pediatric dentists. J Korean Acad Pediatr Dent. 2014;41:257–265. [Google Scholar]
- 7.Bae CH, Kim H, Cho KA, Kim MS, Seo KS, Kim HJ. A survey of sedation practices in the korean dentistry. J Korean Dent Soc Anesthesiol. 2014;14:29–39. [Google Scholar]
- 8.Grissinger M. Chloral hydrate: is it still being used? Are there safer alternatives? P T. 2019;44:444–445. 459. [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulhamid I, Tremblay M, Stenger J, Tutag Lehr V. Chloral hydrate for sedation of children with asthma during dental treatment. Eur J Paediatr Dent. 2016;17:141–146. [PubMed] [Google Scholar]
- 10.Huang A, Tanbonliong T. Oral sedation postdischarge adverse events in pediatric dental patients. Anesth Prog. 2015;62:91–99. doi: 10.2344/0003-3006-62.3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordt SP, Rangan C, Hardmaslani M, Clark RF, Wendler C, Valente M. Pediatric chloral hydrate poisonings and death following outpatient procedural sedation. J Med Toxicol. 2014;10:219–222. doi: 10.1007/s13181-013-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormack L, Chen JW, Trapp L, Job A. A comparison of sedation-related events for two multiagent oral sedation regimens in pediatric dental patients. Pediatr Dent. 2014;36:302–308. [PubMed] [Google Scholar]
- 13.Kang J, Vann WF, Jr, Lee JY, Anderson JA. The safety of sedation for overweight/obese children in the dental setting. Pediatr Dent. 2012;34:392–396. [PubMed] [Google Scholar]
- 14.Chicka MC, Dembo JB, Mathu-Muju KR, Nash DA, Bush HM. Adverse events during pediatric dental anesthesia and sedation: a review of closed malpractice insurance claims. Pediatr Dent. 2012;34:231–238. [PubMed] [Google Scholar]
- 15.Costa LR, Costa PS, Brasileiro SV, Bendo CB, Viegas CM, Paiva SM. Post-discharge adverse events following pediatric sedation with high doses of oral medication. J Pediatr. 2012;160:807–813. doi: 10.1016/j.jpeds.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Kupiec TC, Kemp P, Raj V, Kemp J. A fatality due to an accidental methadone substitution in a dental cocktail. J Anal Toxicol. 2011;35:512–515. doi: 10.1093/anatox/35.7.512. [DOI] [PubMed] [Google Scholar]
- 17.de Rezende GP, da Costa LR, da Costa PS. The use of chloral hydrate for pediatric dental sedation. Spec Care Dentist. 2007;27:85–86. doi: 10.1111/j.1754-4505.2007.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 18.Martinez D, Wilson S. Children sedated for dental care: a pilot study of the 24-hour postsedation period. Pediatr Dent. 2006;28:260–264. [PubMed] [Google Scholar]
- 19.Park M, Kim Y, Jung S, Beak K. Safety and efficacy of submucosal midazolam when combined with oral chloral hydrate, hydroxyzine and nitrous oxide sedation by using Houpt's Scale. J Korean Dent Soc Anesthesiol. 2006;6:103–112. [Google Scholar]
- 20.Myers GR, Maestrello CL, Mourino AP, Best AM. Effect of submucosal midazolam on behavior and physiologic response when combined with oral chloral hydrate and nitrous oxide sedation. Pediatr Dent. 2004;26:37–43. [PubMed] [Google Scholar]
- 21.Lee JH, Park HW. Assessment of vital signs in pediatric dental sedation using chloral hydrate and hydroxyzine. J Korean Acad Pediatr Dent. 2002;29:155–162. [Google Scholar]
- 22.Leelataweedwud P, Vann WF., Jr Adverse events and outcomes of conscious sedation for pediatric patients: study of an oral sedation regimen. J Am Dent Assoc. 2001;132:1531–1539. doi: 10.14219/jada.archive.2001.0086. [DOI] [PubMed] [Google Scholar]
- 23.Dallman JA, Ignelzi MA, Jr, Briskie DM. Comparing the safety, efficacy and recovery of intranasal midazolam vs. oral chloral hydrate and promethazine. Pediatr Dent. 2001;23:424–430. [PubMed] [Google Scholar]
- 24.Jung JH, Park KT. Evaluation of success rate and temporary hypoxia in pediatric dental sedation using chloral hydrate and hydroxyzine. J Korean Acad Pediatr Dent. 2001;28:337–344. [Google Scholar]
- 25.Avalos-Arenas V, Moyao-García D, Nava-Ocampo AA, Zayas-Carranza RE, Fragoso-Ríos R. Is chloral hydrate/hydroxyzine a good option for paediatric dental outpatient sedation? Curr Med Res Opin. 1998;14:219–226. doi: 10.1185/03007999809113362. [DOI] [PubMed] [Google Scholar]
- 26.Engelhart DA, Lavins ES, Hazenstab CB, Sutheimer CA. Unusual death attributed to the combined effects of chloral hydrate, lidocaine, and nitrous oxide. J Anal Toxicol. 1998;22:246–247. doi: 10.1093/jat/22.3.246. [DOI] [PubMed] [Google Scholar]
- 27.McCann W, Wilson S, Larsen P, Stehle B. The effects of nitrous oxide on behavior and physiological parameters during conscious sedation with a moderate dose of chloral hydrate and hydroxyzine. Pediatr Dent. 1996;18:35–41. [PubMed] [Google Scholar]
- 28.Needleman HL, Joshi A, Griffith DG. Conscious sedation of pediatric dental patients using chloral hydrate, hydroxyzine, and nitrous oxide--a retrospective study of 382 sedations. Pediatr Dent. 1995;17:424–431. [PubMed] [Google Scholar]
- 29.Sams DR, Russell CM. Physiologic response and adverse reactions in pediatric dental patients sedated with promethazine and chloral hydrate or meperidine. Pediatr Dent. 1993;15:422–424. [PubMed] [Google Scholar]
- 30.Wilson S. Conscious sedation and pulse oximetry: false alarms? Pediatr Dent. 1990;12:228–232. [PubMed] [Google Scholar]
- 31.Mueller WA, Drummond JN, Pribisco TA, Kaplan RF. Pulse oximetry monitoring of sedated pediatric dental patients. Anesth Prog. 1985;32:237–240. [PMC free article] [PubMed] [Google Scholar]
- 32.Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633–644. doi: 10.1542/peds.106.4.633. [DOI] [PubMed] [Google Scholar]
- 33.Ratnapalan S. Chloral hydrate sedation in children. Clin Pediatr (Phila) 2014;53:933–936. doi: 10.1177/0009922813508000. [DOI] [PubMed] [Google Scholar]
- 34.American Dental Association. Guidelines for the use of sedation and general anesthesia by dentists. 2016. Oct, Available from http://www.ada.org/~/media/ADA/Education%20and%20Careers/Files/ADA_Sedation_Use_Guidelines.pdf.
- 35.Wilson S. Oral sedation for dental procedures in children. Berlin Heidelberg: Springer; 2015. pp. 46–48. [Google Scholar]
- 36.Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DK, Kasian GF. Chloral hydrate disposition following single-dose administration to critically ill neonates and children. Dev Pharmacol Ther. 1991;16:71–77. [PubMed] [Google Scholar]
- 37.The Korean Dental Society of Anesthesiology. Dental Anesthesiology. 3rd ed. Seoul: Koonja; 2015. pp. 362–365. [Google Scholar]
- 38.Buck ML. Chloral hydrate use during infancy. Neonatal Pharmacol Q. 1992;1:31–37. [Google Scholar]
- 39.Malamed S. Sedation: A Guide to Patient Management. 5th ed. Amsterdam: Elsevier; 2009. p. 111. [Google Scholar]