Abstract
Metal elements are essential components of approximately half of all cellular proteins, and approximately one-third of all known enzymes thus far are metalloenzymes. Several cellular proteins and enzymes undoubtedly impact the transduction efficiency of recombinant adeno-associated virus (AAV) vectors, but the precise role of metal ions in this process has not been studied in detail. In the present studies, we systematically evaluated the effects of all 10 essential metal ions (calcium, cobalt, copper, iron, magnesium, manganese, molybdenum, potassium, sodium, and zinc) on the transduction efficiency of AAV vectors. We report herein that five essential metal ions (iron, magnesium, manganese, molybdenum, and sodium) had little to no effect, and calcium strongly inhibited the transduction efficiency of AAV2 vectors. Whereas copper and potassium increased the transduction efficiency by ∼5-fold and ∼2-fold, respectively, at low concentrations, both essential metals were strongly inhibitory at higher concentrations. Calcium also inhibited the transduction efficiency by ∼3-fold. Two metal ions (cobalt and zinc) increased the transduction efficiency up to ∼10-fold in a dose-dependent manner. The combined use of cobalt and zinc resulted in more than an additive effect on AAV2 vector transduction efficiency (∼30-fold). The transduction efficiency of AAV serotypes 1 through 6 (AAV1–AAV6) vectors was also augmented by zinc. Similarly, the transduction of both single-stranded (ss) and self-complementary (sc) AAV3 vectors was enhanced by zinc. Zinc treatment also led to a dose-dependent increase in expression of a therapeutic protein, the human clotting factor IX (hF.IX), mediated by scAAV3 vectors in a human hepatic cell line. This simple strategy of essential metal ion-mediated enhancement may be useful to lower the dose of AAV vectors for their optimal use in human gene therapy.
Graphical Abstract
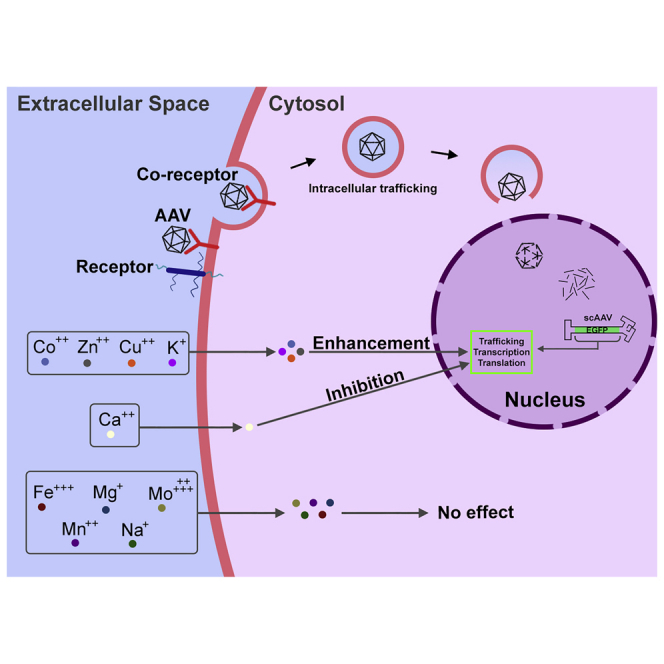
AAV vectors undoubtedly interact with host cell proteins during transduction. Since nearly half of all cellular proteins, and approximately one-third of all known enzymes, are known to be metalloproteins, Rambhai et al. systematically evaluated the effects of all 10 essential metal ions and identified zinc and cobalt as significantly enhancing the transduction efficiency of AAV vectors.
Introduction
Recombinant adeno-associated virus (rAAV) vectors have revolutionized the field of gene therapy owing to their low immunogenicity and long-term therapeutic gene expression. AAV vectors have been or are currently being used in 215 clinical trials for a wide variety of human diseases.1 During the past decade and a half, AAV vectors have also shown clinical efficacy in a number of phase I, II, and III clinical trials for several diseases such as Leber’s congenital amaurosis,2, 3, 4, 5 lipoprotein lipase deficiency,6 hemophilia B,7, 8, 9, 10 hemophilia A,11,12 aromatic l-amino acid decarboxylase deficiency,13 choroideremia,14 Leber’s hereditary optic neuropathy,15 and spinal muscular atrophy type 1.16 Thus far, three AAV therapeutics—Glybera (alipogene tiparvovec), Luxturna (voretigene neparvovec), and Zolgensma (onasemnogene abeparvovec-xioi)—have been approved (Glybera in the European Union, and Luxturna and Zolgensma in the United States).
Despite this remarkable progress, there is a dearth of knowledge on how AAV vectors navigate the host cell machinery in order to deliver therapeutic genes to the nucleus and mediate transgene expression. There is little doubt that following entry into target cells, AAV vectors must encounter cellular macromolecules, including proteins. Since metal ions are known to be essential components of nearly half of all cellular proteins, and approximately one-third of all cellular enzymes are known to be metalloenzymes,17,18 we reasoned that it is important to evaluate the role of essential metal ions in AAV vector-mediated transduction.
Herein, we describe the results of a systematic study that was carried out to evaluate the effect of all 10 essential metal ions (calcium, cobalt, copper, iron, magnesium, manganese, molybdenum, potassium, sodium, and zinc) on the transduction efficiency of both single-stranded (ss) and self-complementary (sc) AAV2 vectors expressing a reporter gene in various human cell lines. We observed that of the 10 essential metal ions, only cobalt and zinc significantly enhanced the transduction efficiency of AAV2 vectors in a dose-dependent manner. However, significantly higher concentrations of cobalt were needed to achieve the similar level of increase in the transduction efficiency that was observed with relatively low concentrations of zinc, and the combination of the two led to an additive effect. We also extended these studies to include five additional AAV serotype vectors, AAV1 through AAV6, and observed a similar increase in their transduction efficiencies. In addition, we also examined the effect of zinc on the transduction efficiency of AAV3 vectors expressing a therapeutic gene, the human clotting factor IX (hF.IX), and observed a similar dose-dependent increase in the expression of hF.IX protein as measured by quantitative western blots.
These studies suggest that essential metal ion-mediated enhancement is a simple and useful strategy to further reduce the need for the high doses of AAV serotype vectors, which has implications in the optimal use of these vectors in human gene therapy.
Results
Differential Effects of Essential Metal Ions on the Transduction Efficiency of AAV2 Vectors
The effects of various concentrations of each of the 10 essential metal ions were examined using scAAV2-EGFP vectors at a multiplicity of infection (MOI) of 200 vector genomes (vg)/cell in HeLa cells. To minimize any possible influence of metal ions present in fetal bovine serum (FBS), preliminary experiments were carried out using the KnockOut (KO) serum replacement growth medium, as described under Materials and Methods. However, it was observed that the use of DMEM (Dulbecco’s modified Eagle’s medium) supplemented with FBS had a negligible effect in the transduction efficiencies of AAV2 vectors in HeLa cells treated with essential metal ions. Thus, all subsequent studies were performed with HeLa cells grown in DMEM supplemented with FBS.
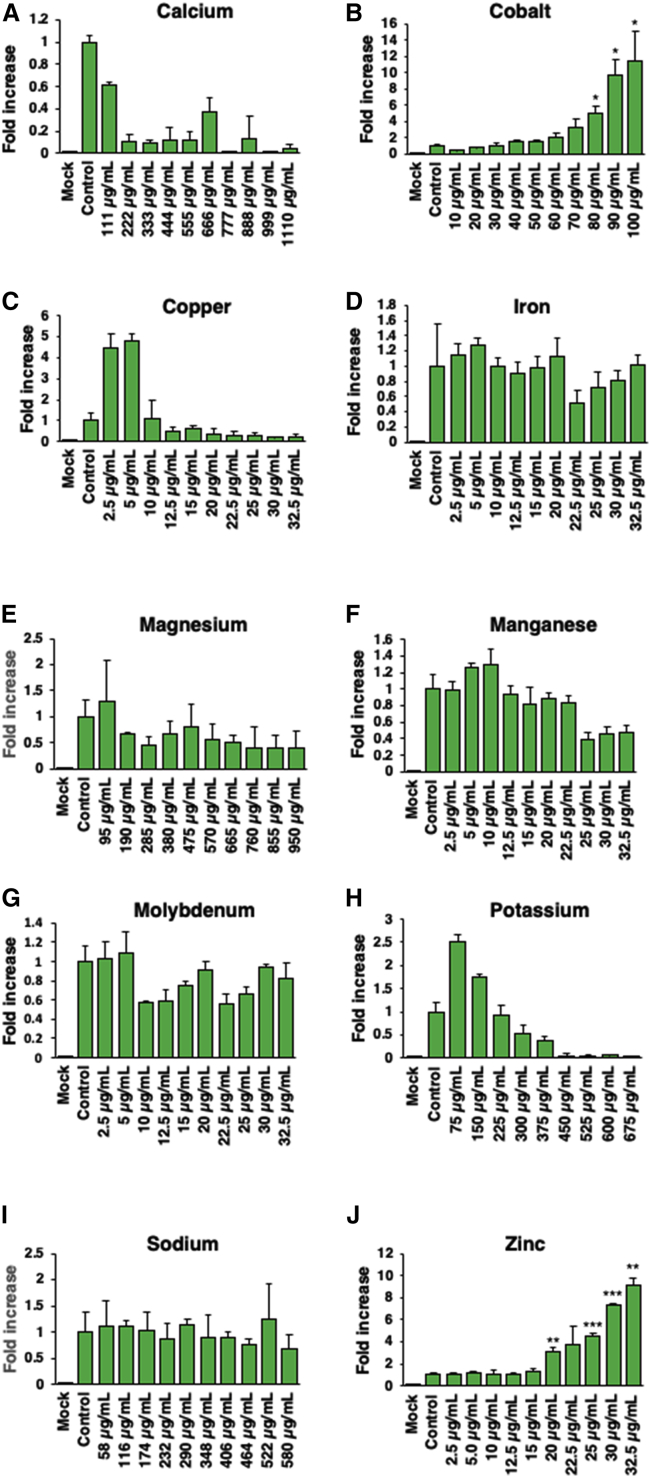
As shown in Figure 1, of the 10 essential metal ions, 4 enhanced the transduction efficiency of AAV2 vectors (cobalt, copper, potassium, and zinc), 5 had little to no effect (iron, magnesium, manganese, and sodium), and 1 was inhibitory (calcium). Although potassium showed an ∼2-fold increase at the lowest concentration of 1 mM, at higher concentrations, potassium inhibited the transduction efficiency due to cytotoxicity (Figure 1H). Magnesium and sodium affected the transduction efficiency by <1-fold across all concentrations tested (Figures 1E and 1I). Interestingly, calcium was the only essential metal ion that inhibited the AAV2 vector transduction, but without causing any cytotoxicity in HeLa cells (Figure 1A).
Figure 1.
Effect of Various Concentrations of 10 Essential Metal Ions on the Transduction Efficiency of AAV2 Vectors
HeLa cells were grown in DMEM supplemented with serum KO for at least three passages before being plated in a 96-well plate and transduced with scAAV2-CBA-EGFP at an MOI of 200 vg/cell. (A–J) Cells treated 1 h after transduction with various concentrations of each of the 10 essential metal ions separately in triplicate (A, calcium; B, cobalt; C, copper, D, iron; E, magnesium; F, manganese; G, molybdenum; H, potassium; I, sodium; J, zinc). Transgene expression was determined 48 h post-transduction as described under Materials and Methods. Values represent fold increase in mean AFU, gathered 48 h post-transduction, compared to controls. Means ± SD are plotted. ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005, based on Student’s t test of the means.
Of the six trace essential metal elements tested, iron, manganese, and molybdenum did not affect the transduction efficiency (Figures 1D, 1F, and 1G). Copper increased the transduction efficiency by up to ∼4-fold at concentrations ranging from 2.5 to 5 μg/mL before a sharp decrease due to cytotoxicity (Figure 1C).
Cobalt and zinc significantly enhanced (∼10-fold) the transduction efficiency of AAV2 vectors in HeLa cells in a dose-dependent manner (Figures 1B and 1J). Cobalt increased the transduction efficiency at the peak concentration of 100 μg/mL, although signs of cytotoxicity were observed at concentrations ranging from 90 to 100 μg/mL. Similarly, at the highest concentration (32.5 μg/mL) of zinc, the highest increase in the transduction efficiency was achieved, but cytotoxicity was also observed at 30–32.5 μg/mL.
Additive Effect of Cobalt and Zinc on the Transduction Efficiency of AAV2 Vectors
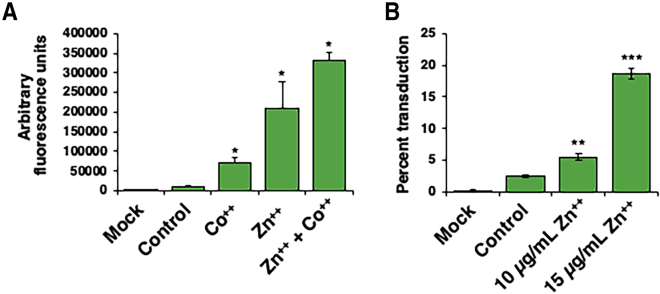
In order to determine which of the two essential metal elements (cobalt and zinc) was more effective in enhancing transduction efficiency of AAV2 vectors, a side-by-side comparison was performed under identical conditions as described above, except that cobalt and zinc concentrations used were 80 and 25 μg/mL, respectively. These concentrations were used at which no signs of cytotoxicity were observed. The results are shown in Figure 2A. Consistent with the data shown above, cobalt increased the transduction efficiency by ∼3-fold, and zinc enhanced the transduction efficiency of AAV2 vectors by ∼17-fold. Furthermore, when cobalt and zinc were used in combination, the enhancement in transduction efficiency was more than additive (∼30-fold) (Figure 2A). Because zinc was the most effective essential metal ion in increasing the transduction efficiency of AAV2 vectors and showed high reproducibility and overall consistency compared with cobalt, all subsequent studies were carried out with zinc. Since all previous data were obtained using fluorescence microscopy, we also wanted to corroborate these results using flow cytometry, as described under Materials and Methods. As shown in Figure 2B, zinc increased the transduction efficiency of AAV2 vectors in a dose-dependent manner by up to ∼10-fold.
Figure 2.
Effect of Cobalt and Zinc, and the Combination of Both, on the Transduction Efficiency of AAV2 Vectors
(A) HeLa cells transduced with scAAV2-CBA-EGFP vectors at an MOI of 200 vg/cell and either mock treated or treated with 80 μg/mL of CoCl2, 30 μg/mL ZnCl2, or a combination of both. Values represent mean AFU levels gathered 48 h post-transduction by fluorescence microscopy. (B) Flow cytometric analysis of Huh7 cells transduced with scAAV3-CBA-EGFP vectors at a MOI of 500 vg/cells treated with various concentrations of zinc. Cells were washed with PBS twice before being resuspended in a mixture of PBS and 2% FBS. Values represent the mean percentage of live cell populations expressing EGFP. Each sample was run until the live cell gate reached 10,000 events. Means ± SD are plotted. ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005, based on Student’s t test of the means.
Determination of the Safe and Effective Dose of Zinc
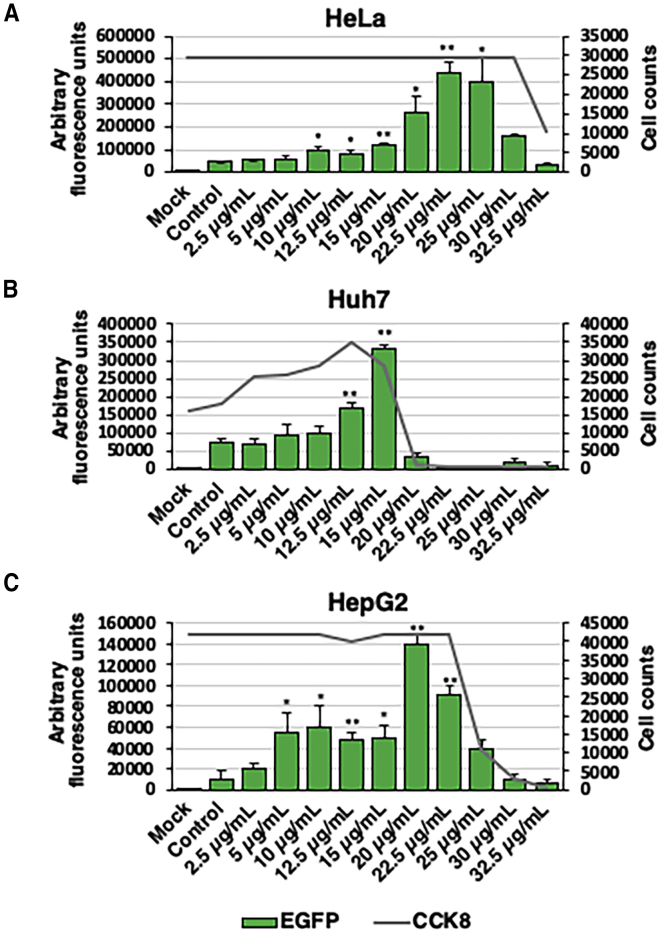
Since all of the data above were obtained with one cell line, we next wanted to evaluate the efficacy as well as cytotoxicity of zinc in additional human cell lines. Thus, in addition to HeLa cells, two human hepatocellular carcinoma cell lines, Huh7 and HepG2, were used to determine the 50% cytotoxic concentration (CC50) of zinc. HeLa cells and AAV2 vectors were used as controls. Huh7 and HepG2 cells were transduced with AAV3-EGFP vectors at an MOI of 200 vg/cell. All transductions and transduction efficiency assays were performed, followed by a cell viability assay, prior to fluorescence microscopy, as described under Materials and Methods. Preliminary studies were conducted to ensure that the Cell Counting Kit-8 (CCK-8) solution does not interfere with cell viability or fluorescence imaging. In HeLa cells, the peak transduction efficiency was observed a concentration of at 22.5 μg/mL of zinc and CC50 at 30–32.5 μg/mL of zinc (Figure 3A). The peak transduction efficiency of AAV3 vectors in Huh7 cells was at a concentration of 15 μg/mL of zinc, and a CC50 ranging from 22.5 to 25 μg/mL (Figure 3B). Out of the three cell lines tested, HepG2 cells showed the greatest increase in overall transduction efficiency (∼14-fold) at a concentration of 20 μg/mL of zinc and CC50 at ∼25 μg/mL of zinc (Figure 3C). Furthermore, a significant increase in the transduction efficiency was observed at a low concentration of zinc (5 μg/mL) compared with HeLa and Huh7 cells. Overall, HepG2 cells displayed an optimal range (5–15 μg/mL) of zinc concentrations that enhanced the transduction efficiency (∼5-fold) with no signs of cytotoxicity.
Figure 3.
Effect of Zinc on Cell Viability
Transduction efficiency of scAAV2-EGFP vectors was evaluated as a function of viable cell numbers in various cell lines (HeLa, Huh7, HepG2) treated with increasing concentrations of zinc. (A–C) HeLa cells were transduced with scAAV2-CMV-EGFP vectors (A), and Huh7 (B) and HepG2 (C) cells were transduced with scAAV3-CBA-EGFP vectors at an MOI of 200 vg/cells, and transgene expression was evaluated 48 h post-transduction. CCK-8 solution was added 48-h post transduction before fluorescence imaging and incubated for an additional 2.5 h. Absorbance readings were recorded using CLARIOstar Plus plate reading at a wavelength of 450 nm. Total viable cell numbers were determined using standard curves previously established for each cell line. Cell count averages are plotted. AFU means ± SD are plotted. ∗p < 0.05, ∗∗p < 0.005.
Effect of Zinc on the Transduction Efficiency of AAV Serotypes Vectors 1 through 6
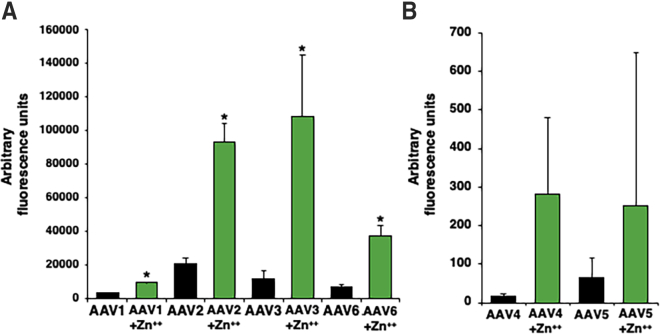
In addition to AAV2 and AAV3 serotype vectors, we also evaluated whether zinc could enhance the transduction efficiency of AAV1, AAV4, AAV5, and AAV6 serotype vectors. HeLa cells were transduced with AAV serotype vectors 1 through 6 expressing EGFP at an MOI of 500 and a final concentration of 30 μg/mL of zinc. Zinc-mediated enhancement of AAV transduction efficiency was observed across all serotypes. AAV2 vectors had a similar level of relative increase (∼5-fold) as AAV6 vectors, but differed only in terms of control signals due to differences in transducibility of these vectors (Figure 4A). Zinc enhanced the transduction efficiency of AAV1 and AAV3 serotype vectors in HeLa cells by ∼3- and 9-fold, respectively. AAV4 and AAV5 vectors transduce HeLa cells poorly, especially at low MOIs. Thus, few transduced cells were observed in control HeLa cells. However, HeLa cells treated with zinc displayed EGFP-positive cells transduced with from AAV4 and AAV5 serotype vectors (Figure 4B). Thus, the underlying mechanism of zinc-mediated enhancement of the transduction efficiency of AAV serotype vectors appears to be similar.
Figure 4.
Effect of Zinc on the Transduction Efficiency of AAV Serotype Vectors 1 through 6
(A and B) HeLa cells were transduced with (A) AAV1, AAV2, AAV3, and AAV6 vectors, and (B) with AAV4 and AAV5 vectors driving a CMV promoter, with and without treatment with 30 μg/mL of ZnCl2. Transgene expression was evaluated 48 h post-transduction. Values represent fold increase in mean AFU. Means ± SD are plotted. ∗p < 0.05, based on Student’s t test of the means.
Effect of Zinc on the Transduction Efficiency of ssAAV Vectors
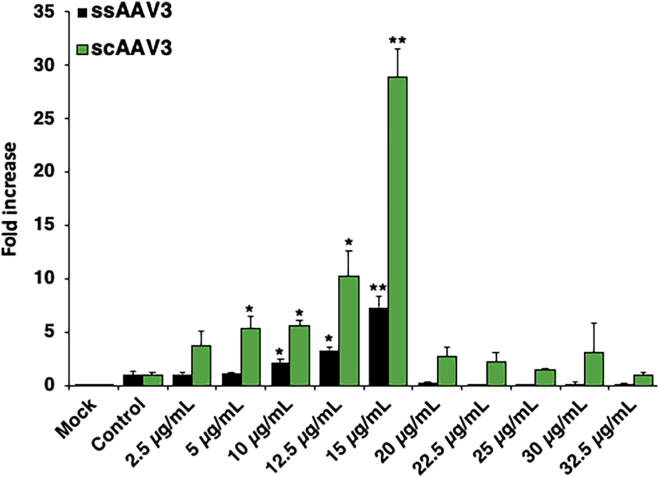
Since all of the data described above were obtained using scAAV vectors, we next examined whether a zinc-mediated increase in the transduction efficiency could also be achieved with ssAAV vectors. In a side-by-side transduction assay, Huh7 cells were transduced with ssAAV3-EGFP and scAAV3-EGFP vectors at MOIs of 200 vg/cell followed by treatment with various increasing concentrations of zinc under identical conditions. As shown in Figure 5, a zinc-mediated maximal increase in the transduction efficiency was observed with both ssAAV3 (∼7-fold) and scAAV3 (∼29-fold) vectors at a zinc concentration of 15 μg/mL. Since scAAV vectors obviate the requirement for the rate-limiting step of the viral second-strand DNA synthesis,19, 20, 21, 22 these results suggest that the zinc-mediated increase in the transduction efficiency by ssAAV vectors is independent of viral second-strand DNA synthesis.
Figure 5.
Effect of Zinc on the Transduction Efficiency of ssAAV3 and scAAV3 Vectors
Huh7 cells were transduced with either ssAAV3-EGFP or scAAV3-EGFP in 96-well plates followed by zinc treatment 1 h post-transduction. Values represent fold increase in mean AFU, gathered 48 h post-transduction. Means ± SD are plotted. ∗p < 0.05, ∗∗p < 0.005, based on Student’s t test of means versus control treatment.
Effect of Zinc on the Expression of a Human Therapeutic Gene
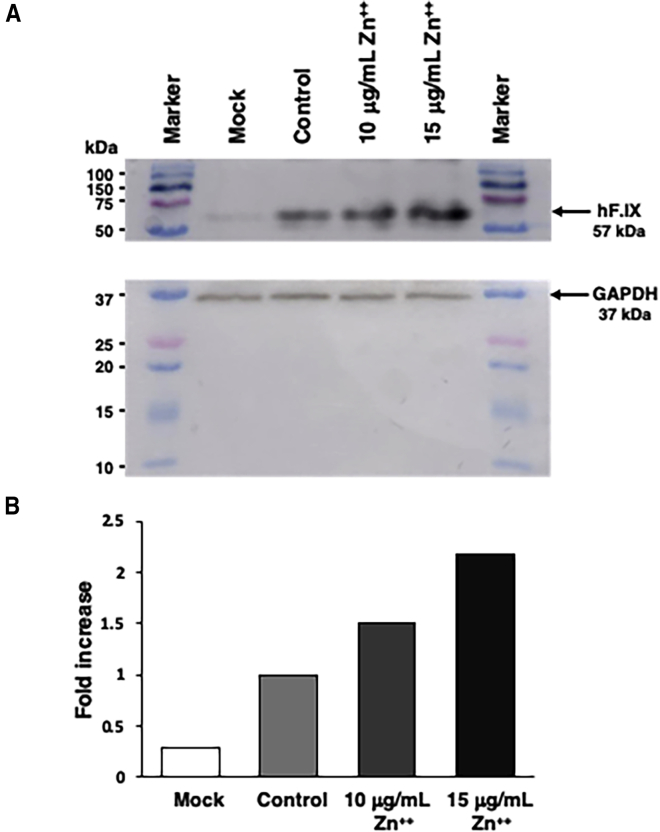
Finally, studies were designed to determine whether zinc-mediated enhancement in the transduction efficiency could also be achieved with an AAV vector expressing a human therapeutic gene. HepG2 cells, known for their ability to produce and secrete hF.IX,23,24 were either mock transduced or transduced with scAAV3 vectors expressing the hF.IX gene under the control of a synthetic liver-specific promoter25 at an MOI of 500 vg/cells, with and without treatment with increasing concentrations of zinc. Total cellular proteins were harvested and equivalent amounts were analyzed on western blots using anti-hF.IX antibodies as described under Materials and Methods. These results are shown in Figure 6. As can be seen, in HepG2 cells transduced with scAAV3-hF.IX vectors in the absence of zinc, there was an ∼4-fold increase in hF.IX protein expression compared with the endogenous levels in mock-transduced HepG2 cells. HepG2 cells treated with 10 μg/mL of zinc showed a an ∼1.5-fold increase in hF.IX expression compared with control levels, and an ∼6-fold increase compared with endogenous levels. Treatment of cells with 15 μg/mL of zinc led to an ∼2-fold increase in hF.IX protein expression compared with control levels, and an ∼8-fold increase compared with endogenous levels. The zinc concentrations used were based on previous cell viability data documenting little to no cytotoxicity. These data suggest that the observed increase in the transgene expression is independent of the promoter used, and that it is indeed possible to achieve a zinc-mediated increase in expression of therapeutic genes.
Figure 6.
Effect of Zinc on Levels of hF.IX Protein Expression
HepG2 cells plated in a six-well plate were either mock transduced or transduced with scAAV3-TTR-hF.IX.Padua vector. Cells were treated with 0, 10, or 15 μg/mL of ZnCl2 1 h post-transduction. Cells were harvested 48 h post-transduction using RIPA lysis buffer. Samples were centrifuged and supernatant was saved. Protein concentrations were determined using a BCA protein assay kit and absorbance reading. (A) Equal concentrations of each protein were loaded and analyzed on western blots using polyclonal anti- hF.IX antibody as described under Materials and Methods. (B) Quantitation of the data from (A).
Discussion
One of the well-documented approaches to augment the transduction efficiency of AAV vectors is by modifying the cellular environment to induce cellular stress.26, 27, 28 For example, DNA synthesis and topoisomerase inhibitors,29 heat shock,30 hydroxyurea,31,32 UV light,33,34 and X-rays26 have all been reported to enhance AAV transduction through activation of cellular stress pathways. Major components of all cellular environments that still remain to be explored in augmenting AAV transduction efficiency are essential metal ions. To date, there are only two studies that have begun to address the role of metal ions in AAV vector biology. In the first, arsenic trioxide, a heavy metal chemotherapeutic agent, was shown to enhance the transduction efficiency of multiple AAV serotype vectors by inducing the release of reactive oxidative species (ROS) that stabilize perinuclear accumulations of AAV capsids.35 In the second, sodium chloride was shown to improve production of various AAV vectors in a serum-free suspension manufacturing platform.36 Given that essential metal ions play a major role involving half of all known proteins and one-third of all enzymes, we undertook the present studies and carried out a systematic evaluation of the effect of each of the 10 essential metal ions on AAV vector-mediated transduction. We observed that peak transduction levels for AAV vectors were achieved as essential metal ion concentrations reached cytotoxic levels. Since all of the experiments were performed using relatively low MOIs of AAV vectors, it remains possible that cobalt and zinc increased the vector entry, which led to increased transgene expression. Although the precise underlying mechanism of the transduction enhancements of AAV remains unknown, a cellular stress pathway may be a contributing factor, but it is also clear that the observed increase is independent of promoter function as well as the viral second-strand DNA synthesis. It also remains a possibility that specific metal ions, such as zinc and cobalt, also influence signaling pathways that lead to augmented transgene expression.
One limitation of our current studies is that we did not evaluate the efficacy of essential metal ions in an animal model in vivo. Further studies are warranted regarding the delivery of essential metal ions to target tissues and may need to be investigated prior to administration of AAV vectors. Various parameters such as route of essential metal ion administration, their retention, and the optimal time for administration are all questions that we hope to address in our future studies. This limitation notwithstanding, the simple strategy of essential metal ion-mediated enhancement of AAV vector transduction efficiency may further reduce the doses needed for therapeutic purposes. This is particularly relevant in the context of all current clinical trials being performed for the potential gene therapy of hemophilia, where relatively large vector doses are required to achieve clinical efficacy.7, 8, 9,11,12
There is overwhelming evidence that the immune response to AAV vectors correlated directly with the vector dose.37, 38, 39, 40, 41, 42, 43 Current AAV clinical trial practices consist of treating patients prophylactically with immunosuppressant steroids, such as prednisolone, prior to AAV vector administration.7, 8, 9, 10, 11, 12, However, while this practice has improved the outcomes of some clinical trials, it could be argued that decreasing the vector dose would also reduce the likelihood of the host immune response. The use of essential metal ions, either via systemic administration, or through dietary supplementation, could be a possible approach to augment AAV transduction efficiency, especially since our data corroborate that low levels of zinc can augment the expression of hF.IX from an AAV3 vector. Thus, the combined use of zinc and the more efficient capsid-modified AAV3 vectors44, 45, 46, 47 have implications in the potential gene therapy of hemophilia B.
Materials and Methods
Cells and Cell Cultures
HepG2, HeLa, and Huh7 cell lines were purchased from ATCC (Manassas, VA, USA). All cells were maintained in DMEM (Gibco, Waltham, MA, USA) supplemented with 10% FBS (Gibco, Waltham, MA, USA) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA), as described previously.48 Cultures were grown in incubators at 37°C and 5% CO2. HeLa cells were passaged three times and maintained in Dulbecco’s modified Eagle’s minimal essential medium (MEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with KO serum replacement (Thermo Fisher Scientific) prior to treatment with essential metal ions.
Recombinant AAV Vectors
Highly purified and concentrated stocks of recombinant scAAV2-EGFP, scAAV3-EGFP, and ssAAV3-EGFP vectors expressing the EGFP reporter gene under the control of a chicken β-actin (CBA) promoter and scAAV3-FIX vectors expressing the hF.IX gene driven by transthyretin (TTR) promoter were custom generated by SAB Technology (Philadelphia, PA, USA). Recombinant scAAV1-EGFP, scAAV2-EGFP, scAAV3-EGFP, scAAV4-EGFP, scAAV5-EGFP, and scAAV6-EGFP vectors expressing a cytomegalovirus (CMV) promoter-driven EGFP gene were generated by triple transfection of HEK293T cells as previously described with modifications.49, 50, 51 Quantification of genome titers were carried out by treating 10 μL of rAAV stocks with Benzonase supplemented with MgCl2 for 1 h at 37°C, and NaOH for an additional 30 min at 65°C. Plasmid standard concentrations were quantified by a Thermo Scientific NanoDrop light spectrophotometer, and extracted rAAV genomes were then purified using Zymo-Spin columns (Zymo Research, Irvine, CA, USA). Genome copy numbers were quantified with qPCR primer ITR2 forward (5′-GGAACCCCTAGTGATGGAGTT-3′) and ITR2 reverse (5′-CGGCCTCAGTGAGCGA-3′). Titers were determined by running standards and unknowns in triplicate in a Bio-Rad CFX96 qPCR system.
Reagent Preparation
Metal ion stock solutions were made by weighing reagents by difference and dissolving in double-distilled H2O (ddH2O) under sterile conditions. Stock solutions for manganese chloride, iron chloride, cobalt chloride, copper chloride, zinc chloride, and molybdenum chloride were prepared at a concentration of 2 mg/mL, and stock solutions for sodium chloride, potassium chloride, magnesium chloride, and calcium chloride were prepared at a concentration of 1 M.
AAV Vector Transduction Assay
96-well plates were seeded with 1.2 × 104 cells per well and incubated at 37°C overnight. Growth medium was removed and replaced with 37°C serum-free medium containing AAV vectors or a mock treatment at a specified MOI followed by incubation for 1 h at 37°C. Treatments of various metal ion concentrations and KO serum replacement or FBS-enriched DMEM was added in triplicate and incubated for 48 h. Wells were washed with phosphate-buffered saline (PBS) and imaged with a Leica fluorescence microscope. Wells were imaged in triplicate using a ×5 visual field, and total fluorescence was calculated as integral density of EGFP fluorescence or arbitrary fluorescence units (AFU) using NIH ImageJ software.
Flow Cytometric Analysis
12-well plates seeded with 1.2 × 105 Huh7 cells per well were transduced with scAAV3-CBA-EGFP vectors at an MOI of 500 vg/cell or mock transduced. Cells transduced with AAV3-EGFP vectors were exposed to three concentrations of ZnCl2 (0, 10, and 15 μg/mL) in triplicate after 1 h. Cells were then trypsinized 48 h later and washed with PBS before being resuspended in PBS enriched with 2% FBS. Samples were run on a BD Accuri C6 flow cytometer for flow analysis. Gates were set for live cells and EGFP (488 nm) to evaluate the percentage of EGFP-positive cells versus live cell counts. Samples were run until the live cell gate reached 10,000 events, and analysis was conducted using FCS Express 6 software.
Cell Viability Assay
Standard curves were generated by performing serial dilutions in triplicate of HeLa, HepG2, and Huh7 cell lines in a clear 96-well plate. Cells were incubated for less than 24 h (until adherence) in growth medium before adding 20 μL of CCK-8 (Sigma, St. Louis, MO, USA) solution into each well. Plates were left to incubate for 2.5 h and cooled for 15 min before inserting into a CLARIOstar Plus plate reader recording absorbance at a wavelength of 450 nm. Absorbances were recorded as averages minus the backgrounds recorded by blanks. A standard curve equation was generated by plotting absorbance readings versus number of cells.
For cell viability as a function of transduction efficiency experiments, a transduction assay was performed until cells were removed from incubators after 48 h. Cell numbers were determined by adding CCK-8 solution directly into the growth medium and running a cell viability assay. After recording absorption levels, cells were washed with PBS and imaged with a fluorescence microscope.
Western Blotting Assay
HepG2 cells were transduced with rAAV3-TTR-hFIX.padua vectors at an MOI of 500 vg/cell in a six-well plate. Treatments of mock, control, low zinc (10 μg of ZnCl2/mL), and high zinc (15 μg of ZnCl2/mL) were tested. Cells were harvested directly from plates chilled on ice using radioimmunoprecipitation assay (RIPA) lysis buffer (G-Biosciences, St. Louis, MO, USA). Harvested samples were then vortexed twice at 13,000 × g for 15 min at 4°C. The supernatant was then transferred to a clean tube, and protein concentrations were quantified using a Pierce bicinchoninic acid (BCA; Thermo Scientific, Waltham, MA, USA) protein assay kit. Samples were stored at −20°C until use. Polyacrylamide gels were cast in-house using 1-mm glass spacers and 12% resolving gel, and 4% stacking gel mixtures. Loaded gels were run at 80–120 V for 1.5 h before being removed and transferred onto a nitrocellulose membrane at 0.35 A for 45 min. Once transferred, the membrane was blocked for 2 h at room temperature using a 5% Omniblok (AmericanBio, Canton, MA, USA) solution. Diluted rabbit polyclonal anti-hF.IX (diluted 1:2,000; Thermo Fisher Scientific) antibody was added to the membrane and shook overnight at 4°C. The membrane was then washed with Tris-buffered saline with Tween 20 (TBST) three times before adding goat anti-rabbit secondary antibody (American Research Products, Waltham, MA, USA). After the second antibody, the membrane was washed three more times with TBST and then treated with a Thermo Fisher chemiluminescent assay kit. Relative protein concentrations were analyzed using the Amersham Imager 680 to image and evaluate volumes of each signal.
Statistical Analysis
Results are presented as a mean ± SD. Differences between treatments were evaluated using a two-tailed Student’s t test, with significance being established at p ≤ 0.05.
Author Contributions
H.K.R. and K.Q. designed and performed the experiments. F.A. assisted with some of the experiments, H.K.R, F.J.A., K.Q., and A.S. analyzed the data. A.S. conceived of the idea. H.K.R. and A.S. wrote the manuscript, and all authors read and approved the final version.
Conflicts of Interest
A.S. is a co-founder of, and holds equity in, Lacerta Therapeutics and Nirvana Therapeutics, and is an inventor on several issued patents on recombinant AAV vectors that have been licensed to various gene therapy companies. The remaining authors declare no competing interests.
Acknowledgments
We thank our laboratory colleagues, both past and current, for scientific discussions and helpful suggestions. This research was supported in part by Public Health Service grants R01 HL-097088, R41 AI-122735, and R21 EB-015684 from the National Institutes of Health; a grant from the Children’s Miracle Network; and by support from the Kitzman Foundation (to A.S.).
References
- 1.Li C., Samulski R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 2.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 3.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauswirth W.W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., Conlon T.J., Boye S.L., Flotte T.R., Byrne B.J., Jacobson S.G. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cideciyan A.V., Aleman T.S., Boye S.L., Schwartz S.B., Kaushal S., Roman A.J., Pang J.J., Sumaroka A., Windsor E.A., Wilson J.M. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudet D., Méthot J., Déry S., Brisson D., Essiembre C., Tremblay G., Tremblay K., de Wal J., Twisk J., van den Bulk N. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 12.Pasi K.J., Rangarajan S., Mitchell N., Lester W., Symington E., Madan B., Laffan M., Russell C.B., Li M., Pierce G.F., Wong W.Y. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N. Engl. J. Med. 2020;382:29–40. doi: 10.1056/NEJMoa1908490. [DOI] [PubMed] [Google Scholar]
- 13.Hwu W.L., Muramatsu S., Tseng S.H., Tzen K.Y., Lee N.C., Chien Y.H., Snyder R.O., Byrne B.J., Tai C.H., Wu R.M. Gene therapy for aromatic l-amino acid decarboxylase deficiency. Sci. Transl. Med. 2012;4:134ra61. doi: 10.1126/scitranslmed.3003640. [DOI] [PubMed] [Google Scholar]
- 14.MacLaren R.E., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Seymour L., Clark K.R., During M.J., Cremers F.P., Black G.C. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guy J., Feuer W.J., Davis J.L., Porciatti V., Gonzalez P.J., Koilkonda R.D., Yuan H., Hauswirth W.W., Lam B.L. Gene therapy for Leber hereditary optic neuropathy: low- and medium-dose visual results. Ophthalmology. 2017;124:1621–1634. doi: 10.1016/j.ophtha.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 17.Holm R.H., Kennepohl P., Solomon E.I. Structural and functional aspects of metal sites in biology. Chem. Rev. 1996;96:2239–2314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 18.Thomson A.J., Gray H.B. Bio-inorganic chemistry. Curr. Opin. Chem. Biol. 1998;2:155–158. doi: 10.1016/s1367-5931(98)80056-2. [DOI] [PubMed] [Google Scholar]
- 19.Fisher K.J., Gao G.P., Weitzman M.D., DeMatteo R., Burda J.F., Wilson J.M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari F.K., Samulski T., Shenk T., Samulski R.J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarty D.M., Monahan P.E., Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., Ma H.I., Li J., Sun L., Zhang J., Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 23.Gordon E.M., Tang H., Salazar R.L., Kohn D.B. Expression of coagulation factor IX (Christmas factor) in human hepatoma (HepG2) cell cultures after retroviral vector-mediated transfer. Am. J. Pediatr. Hematol. Oncol. 1993;15:196–203. doi: 10.1097/00043426-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 24.de la Salle H., Altenburger W., Elkaim R., Dott K., Dieterlé A., Drillien R., Cazenave J.P., Tolstoshev P., Lecocq J.P. Active gamma-carboxylated human factor IX expressed using recombinant DNA techniques. Nature. 1985;316:268–270. doi: 10.1038/316268a0. [DOI] [PubMed] [Google Scholar]
- 25.Chuah M.K., Petrus I., De Bleser P., Le Guiner C., Gernoux G., Adjali O., Nair N., Willems J., Evens H., Rincon M.Y. Liver-specific transcriptional modules identified by genome-wide in silico analysis enable efficient gene therapy in mice and non-human primates. Mol. Ther. 2014;22:1605–1613. doi: 10.1038/mt.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yalkinoglu A.O., Heilbronn R., Bürkle A., Schlehofer J.R., zur Hausen H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988;48:3123–3129. [PubMed] [Google Scholar]
- 27.Duan D., Yue Y., Yan Z., Yang J., Engelhardt J.F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson J.S., Gentzsch M., Zhang L., Ribeiro C.M., Kantor B., Kafri T., Pickles R.J., Samulski R.J. AAV exploits subcellular stress associated with inflammation, endoplasmic reticulum expansion, and misfolded proteins in models of cystic fibrosis. PLoS Pathog. 2011;7:e1002053. doi: 10.1371/journal.ppat.1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell D.W., Alexander I.E., Miller A.D. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc. Natl. Acad. Sci. USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong L., Qing K., Si Y., Chen L., Tan M., Srivastava A. Heat-shock treatment-mediated increase in transduction by recombinant adeno-associated virus 2 vectors is independent of the cellular heat-shock protein 90. J. Biol. Chem. 2004;279:12714–12723. doi: 10.1074/jbc.M310548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ju X.D., Lou S.Q., Wang W.G., Peng J.Q., Tian H. Effect of hydroxyurea and etoposide on transduction of human bone marrow mesenchymal stem and progenitor cell by adeno-associated virus vectors. Acta Pharmacol. Sin. 2004;25:196–202. [PubMed] [Google Scholar]
- 32.Johnson J.S., Samulski R.J. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J. Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teramoto S., Bartlett J.S., McCarty D., Xiao X., Samulski R.J., Boucher R.C. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J. Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zentilin L., Marcello A., Giacca M. Involvement of cellular double-stranded DNA break binding proteins in processing of the recombinant adeno-associated virus genome. J. Virol. 2001;75:12279–12287. doi: 10.1128/JVI.75.24.12279-12287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell A.M., Li C., Samulski R.J. Arsenic trioxide stabilizes accumulations of adeno-associated virus virions at the perinuclear region, increasing transduction in vitro and in vivo. J. Virol. 2013;87:4571–4583. doi: 10.1128/JVI.03443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamson-Small L., Potter M., Byrne B.J., Clément N. Sodium chloride enhances recombinant adeno-associated virus production in a serum-free suspension manufacturing platform using the herpes simplex virus system. Hum. Gene Ther. Methods. 2017;28:1–14. doi: 10.1089/hgtb.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayak S., Herzog R.W. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basner-Tschakarjan E., Mingozzi F. Cell-mediated immunity to AAV vectors, evolving concepts and potential solutions. Front. Immunol. 2014;5:350. doi: 10.3389/fimmu.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers G.L., Martino A.T., Aslanidi G.V., Jayandharan G.R., Srivastava A., Herzog R.W. Innate immune responses to AAV vectors. Front. Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colella P., Ronzitti G., Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2017;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabinowitz J., Chan Y.K., Samulski R.J. Adeno-associated virus (AAV) versus immune response. Viruses. 2019;11:102. doi: 10.3390/v11020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berns K.I., Srivastava A. Next generation of adeno-associated virus vectors for gene therapy for human liver diseases. Gastroenterol. Clin. North Am. 2019;48:319–330. doi: 10.1016/j.gtc.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng B., Ling C., Dai Y., Lu Y., Glushakova L.G., Gee S.W., McGoogan K.E., Aslanidi G.V., Park M., Stacpoole P.W. Development of optimized AAV3 serotype vectors: mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther. 2012;19:375–384. doi: 10.1038/gt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling C., Wang Y., Zhang Y., Ejjigani A., Yin Z., Lu Y., Wang L., Wang M., Li J., Hu Z. Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum. Gene Ther. 2014;25:1023–1034. doi: 10.1089/hum.2014.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S., Ling C., Zhong L., Li M., Su Q., He R., Tang Q., Greiner D.L., Shultz L.D., Brehm M.A. Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3B vectors. Mol. Ther. 2015;23:1867–1876. doi: 10.1038/mt.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vercauteren K., Hoffman B.E., Zolotukhin I., Keeler G.D., Xiao J.W., Basner-Tschakarjan E., High K.A., Ertl H.C., Rice C.M., Srivastava A. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol. Ther. 2016;24:1042–1049. doi: 10.1038/mt.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin L., Keeler G.D., Zhang Y., Hoffman B.E., Ling C., Qing K., Srivastava A. AAV3-miRNA vectors for growth suppression of human hepatocellular carcinoma cells in vitro and human liver tumors in a murine xenograft model in vivo. Gene Ther. 2020 doi: 10.1038/s41434-020-0140-1. Published online March 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ling C., Wang Y., Lu Y., Wang L., Jayandharan G.R., Aslanidi G.V., Li B., Cheng B., Ma W., Lentz T. Enhanced transgene expression from recombinant single-stranded D-sequence-substituted adeno-associated virus vectors in human cell lines in vitro and in murine hepatocytes in vivo. J. Virol. 2015;89:952–961. doi: 10.1128/JVI.02581-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ran G., Chen X., Xie Y., Zheng Q., Xie J., Yu C., Pittman N., Qi S., Yu F.X., Agbandje-McKenna M. Site-directed mutagenesis improves the transduction efficiency of capsid library-derived recombinant AAV vectors. Mol. Ther. Methods Clin. Dev. 2020;17:545–555. doi: 10.1016/j.omtm.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang H., Qing K., Keeler G.D., Yin L., Mietzsch M., Ling C., Hoffman B.E., Agbandje-McKenna M., Tan M., Wang W., Srivastava A. Enhanced transduction of human hematopoietic stem cells by AAV6 vectors: Implications in gene therapy and genome editing. Mol. Ther. Nucleic Acids. 2020;20:451–458. doi: 10.1016/j.omtn.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]