Abstract
Background:
Hypoplastic left heart syndrome (HLHS) is strongly associated with Turner syndrome (TS); outcome data when these conditions coexist is sparse. We aimed to investigate long-term survival and causes of death in this population.
Methods:
The Texas Birth Defects Registry was queried for all live born infants with HLHS during 1999–2007. We used Kaplan–Meier and Cox regression analyses to compare survival among patients with HLHS with TS (HLHS/TS+) to patients who had HLHS without genetic disorders or extracardiac birth defects (HLHS/TS−).
Results:
Of the 542 patients with HLHS, 11 had TS (2.0%), 71 had other extracardiac birth defects or genetic disorders, and 463 had neither. The median follow-up time was 4.2 y (interquartile range [IQR] 2.1–6.5). Comparing those with HLHS/TS+ to HLHS/TS−, 100% versus 35% were female (P < .001), and median birth weight was 2140 g (IQR 1809–2650) versus 3196 g (IQR 2807–3540, P < .001). Neonatal mortality was 36% in HLHS/TS+ versus 27% in HLHS/TS− (log rank = 0.431). Ten of the 11 TS+ patients died during the study period for cumulative mortality of 91% versus 50% (hazard ratio (HR) for TS+: 2.90, 95% CI 1.53–5.48). Six patients died prior to surgery, 5 underwent Stage 1 palliation (S1P), 3 died after S1P, 2 survived past S2P, and one of these died at age 19 mo. The underlying cause of death was listed as congenital heart disease on all the death certificates of HLHS/TS+ patients. In multivariable analysis controlling for low birth weight (<2500 g), TS remained associated with significantly increased cumulative mortality, although females without TS had higher mortality than males (HR for TS+ versus males: 2.42, 95% CI 1.24–4.73; HR for TS− females versus males: 1.41, 95% CI 1.08–1.83).
Conclusion:
TS with HLHS is associated with significant mortality. The increased mortality in females without documented TS calls to question if TS is undetected in a portion of females with HLHS.
Keywords: Turner syndrome, hypoplastic left heart syndrome, sex, gender, female, population
1 ∣. BACKGROUND
Turner syndrome (TS) is a common genetic disorder caused by partial or complete absence of an X chromosome.1 The prevalence of TS is estimated at 1 in 2500 live-born females.2
TS is frequently associated with congenital heart disease (CHD), which is reported in 23–50% of affected females.3 TS-associated CHD most often involves left sided cardiac structures (e.g., bicuspid aortic valve and coarctation of the aorta) or venous anomalies (e.g., partial anomalous pulmonary venous return and left superior vena cava).2,4-6)
There were isolated early reports in the 1960s and 1970s of an association between TS and hypoplastic left heart syndrome (HLHS), the most severe form of left-sided CHD,7-10 but it was not until the mid-1980s that it became widely accepted that a small but significant percentage of individuals with TS also have HLHS.11,12 Estimates of the prevalence of TS in live-born infants with HLHS range from 1 to 7%.12-15 The prevalence is likely even higher in fetal life, given high fetal loss and termination rates for fetuses with TS and left-sided obstructive lesions.16 Studies in this population have reported the prevalence and outcomes of fetal TS and HLHS,17-19 surgical outcomes,15,20,21 and long-term outcomes.22,23 However, the largest study of long-term outcomes for patients with TS to date was from Reis et al. and used data from the 1990s, was limited to only 10 patients, and did not compare the TS cohort to the larger HLHS cohort.22
Therefore, the purpose of our study was to (1) describe mortality in a more recent cohort of patients with HLHS and TS, (2) determine if TS is an independent risk factor for death in patients with HLHS, and (3) describe the causes of death in patients with HLHS and TS in a large, ethnically and socioeconomically diverse group of patients.
2 ∣. METHODS
2.1 ∣. Database and study population
This was a retrospective, population based, cohort study using the Texas Birth Defects Registry (TBDR), which has actively conducted statewide surveillance of all delivery units and pediatric hospitals in the state of Texas since 1999. The TBDR is maintained by the Birth Defects Epidemiology and Surveillance Branch of the Texas Department of State Health Services. Detailed birth, diagnostic and mortality data were available for infants with a registry-monitored birth defect diagnosed within 1 y of delivery to Texas resident mothers. Detailed surgical information was not universally available (i.e., most records had operative reports, but some had only passing references to surgery).
The TBDR classifies birth defects using 6-digit birth defect codes based on the British Pediatric Association Classification of Diseases (1979) and the International Classification of Diseases, ninth revision, clinical modification (1979). We included live births occurring between January 1, 1999 through December 31, 2007, with the codes for HLHS (746.700), aortic valve atresia (746.480), or hypoplastic left ventricle (746.881). To ensure an accurate diagnosis, a pediatric cardiologist (S.A.M.) reviewed available TBDR clinical data. A diagnosis of HLHS required ≥1 of the following:
Severe mitral and aortic obstruction, hypoplasia, or atresia and a hypoplastic left ventricle.
Hypoplastic left heart syndrome diagnosed on echocardiography, cardiac catheterization, and cardiac surgical, or pathologic report.
Left ventricular hypoplasia with evidence of Stage 1 palliation (S1P) and no more appropriate diagnosis.
We excluded infants with alternative cardiac diagnoses, including atrioventricular septal defect and double outlet or double inlet ventricle. We also excluded infants with gestational age <23 wk or very low birthweight (<400 g).
Of infants born with HLHS, we compared patients with HLHS and diagnosed with TS (HLHS/TS+) to a subset of patients with HLHS who did not have any other genetic disorders, major extracardiac birth defects, or more than three minor extracardiac birth defects (HLHS/TS). Known genetic disorders and extracardiac birth defects are carefully catalogued by the TBDR.
2.2 ∣. Covariates
Maternal socioeconomic status was estimated from the percent of population living in poverty in the maternal census tract, according to the 2000 US Census.24,25 When a post office box or a nonmappable rural route was the residential address listed, the zip code was used. To define poverty status, a cutoff of ≥20% was used in accordance with the US Census Bureau.26
Preterm birth was defined as <37 wk, low birthweight as <2500 g, and small for gestational age as <10% of expected weight using published criteria.27 Birth era was divided into 1999 through 2002 and 2003 through 2007. Presence of partial anomalous pulmonary venous return was also noted. Because restrictive atrial septum information was not available in ≈25% of infants, it was not included in the analyses. Institutional surgical volume was not included in the analysis given many patients with TS did not undergo surgery, and the 11 patients with TS were cared for at 10 different institutions.
2.3 ∣. Ascertainment of mortality
Children were classified as deceased based on the TBDR and Texas vital records. Neonatal death was defined as death prior to 28 d, and infant death was defined as death <1 y of age.
2.4 ∣. Analysis
For demographic and birth variables, comparisons between HLHS/TS+ and HLHS/TS− were performed using chi-square, Fisher’s exact, or Wilcoxon rank-sum tests when appropriate. The probability of freedom from death was estimated using the Kaplan–Meier Product-Limit method with birth as time 0; the Wilcoxon log-rank test was used for comparison of curves. Patients who died were censored at the time of the event, and survivors were censored at the end of the follow-up period (December 31,2007). We used the Wilcoxon log-rank test to determine whether the distributions of time to the earliest occurrence of death differed by presence or absence of TS (all patients and limited to surgical patients), or differed by sex and presence of TS. Univariate and multivariate Cox proportional hazards regression hazard ratios (HR) were used to estimate effect sizes. For multivariate models, backward elimination was used to minimize model size, using variable entry criteria of P < .10, and retaining variables with P < .05. P values <.05 were considered statistically significant. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
The study was approved by the Institutional Review Boards of Baylor College of Medicine and the Texas Department of State Health Services with a waiver of consent.
3 ∣. RESULTS
From 1999 to 2007, 3 401 057 infants were born to Texas-resident mothers. Five hundred forty-two patients had HLHS. Seventy-nine patients had genetic anomalies or extracardiac birth defects: eleven patients had HLHS/TS+, representing 2.0% of newborns with HLHS; 68 had other extracardiac birth defects or genetic disorders and were excluded from further analysis (Table 1). Four hundred sixty-three patients had HLHS/TS– with no other defects.
TABLE 1.
Genetic defects and/or extracardiac birth defects in patients with hypoplastic left heart syndrome, Texas, 1999–2007
| Diagnosis/anomaly | n |
|---|---|
| With genetic diagnosis | 34 |
| Turner syndrome | 11 |
| Chromosome 8p abnormalities | 4 |
| 8p duplication | 2 |
| 8p inverted duplication and deletion | 1 |
| Unbalanced translocation of 8p and 13q | 1 |
| Trisomy 13 | 3 |
| Trisomy 18 | 3 |
| Trisomy 18 only | 2 |
| Trisomy 18 and 1q deletion | 1 |
| VATER syndrome | 2 |
| Kabuki syndrome | 2 |
| Rubinstein-Taybi syndrome | 2 |
| Jacobsen syndrome | 1 |
| Goldenhar syndrome | 1 |
| Hirschprung diseases-mental retardation syndrome (suspected) | 1 |
| Unbalanced translocation of chromosomes 11 and 13 | 1 |
| Chromosome 2 deletion (not otherwise specified) | 1 |
| Chromosome 15q deletion | 1 |
| Mosaic Trisomy 21 | 1 |
| Without known/documented genetic diagnosis | 45 |
| Multiple congenital anomalies and/or significant dysmorphisms | 31 |
| Congenital diaphragmatic hernia (CDH) | 6 |
| CDH with genitourinary anomalies | 4 |
| Isolated CDH | 2 |
| Isolated genitourinary anomalies | 3 |
| Isolated cleft lip and/or palate | 2 |
| Isolated tracheoesophageal fistula | 1 |
| Pyloric stenosis | 1 |
| Isolated multicystic dysplastic kidney | 1 |
Patient characteristics are delineated in Table 2. Of the 11 HLHS+/TS patients, 6 had karyotype of 45, X and 5 had a mosaic karyotype. The median birthweight of the HLHS/TS+ group (2140 g) was significantly lower than the HLHS/TS− group (3196 g) (P < .001). The TS patients also were more premature (median = 35 wk gestation, interquartile range [IQR] 34–37.5) than their counterparts (median = 39 wk gestation, IQR 38–39) (P < .001).
TABLE 2.
Characteristics of patients with hypoplastic left heart syndrome with and without Turner syndrome, Texas, 1999–2007
| Characteristic | HLHS/TS+, n = 11 | HLHS/TS−, n = 463 | P value |
|---|---|---|---|
| Female, n (%) | 11 (100) | 160 (35) | <.001 |
| Birth weight, median (IQR) | 2140 g (1809–2650) | 3196 g (2807–3538) | <.001 |
| Low birth weight, n (%) | 6 (55) | 50 (10) | .001 |
| Gestational age, median (IQR) | 35 wk (34–37.5) | 39 wk (38–39) | <.001 |
| Preterm, n (%) | 6 (55) | 59 (13) | .001 |
| Small for gestational age, n (%) | 3 (27) | 38 (8) | .061 |
| Karyotype, n (%) | |||
| 45, X | 6 (55) | na | |
| Mosaic | 5 (46) | na | |
| Maternal race/ethnicity, n (%) | |||
| Non-Hispanic white | 7 (64) | 208 (45) | 1.000 |
| Non-Hispanic black | 1 (9) | 46 (10) | |
| Hispanic | 3 (27) | 199 (43) | |
| Others | 0(0) | 10 (2) | |
| Poverty by census tract ≤20%, n (%) | 4 (36) | 144 (31) | .746 |
| Prenatal diagnosis, n (%) | 7 (64) | 180 (36) | .122 |
| PAPVR, n (%) | 0 (0) | 13 (2.8) | 1.000 |
| Birth year, n (%) | |||
| 1999–2002 | 3 (27) | 200 (43) | .366 |
| 2003–2007 | 8 (73) | 263 (57) | |
HLHS/TS+:, patients with hypoplastic left heart syndrome with Turner syndrome; HLHS/TS−, patients with hypoplastic left heart syndrome without Turner syndrome; na, not applicable; PAPVR, partial anomalous pulmonary venous return. Low birthweight defined as <2.5 kg; preterm defined as birth before 37 wk gestation.
3.1 ∣. Survival
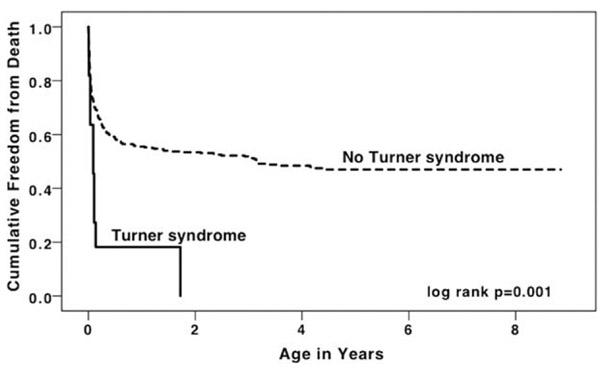
Median follow-up time was 4.2 y (IQR 2.1–6.5 y). Neonatal mortality was 36% in HLHS/TS+, and 27% in HLHS/TS− (HR for TS+: 1.48, 95% CI 0.55–4.02, log-rank = 0.431). When comparing infant mortality, there was a stark difference between the two groups, with 82% mortality in the HLHS/TS+ patients compared to 51% of the HLHS/TS− patients (log-rank = 0.006; HR for TS+: 2.45, 95% CI 1.26–4.79, P = .008). The cumulative survival curves for both groups are depicted in Figure 1 and demonstrate significantly increased mortality in the TS group (log-rank = 0.001). The hazard ratio for cumulative survival in the HLHS/TS+ group compared to the HLHS/TS− group was 2.90 (95% CI 1.53–5.48, P = .005, Table 3). In multivariable analysis, the presence of TS remained associated with higher mortality (Table 3, Model 1; HR for TS+: 2.12, 95% CI 1.10–4.09, P = .025).
FIGURE 1.
Kaplan–Meier survival curve for infants with hypoplastic left heart syndrome, with and without Turner syndrome, Texas, 1999–2007
TABLE 3.
Univariable and multivariable hazard ratios (HR) and 95% confidence intervals (CI) for mortality of patients with hypoplastic left heart syndrome with and without Turner syndrome, Texas, 1999–2007
| Characteristic | Univariable HR | P value | Multivariable HR | P value |
|---|---|---|---|---|
| Model 1 | ||||
| Turner syndrome | 2.90 (1.53–5.48) | .005 | 2.12 (1.10–4.09) | .025 |
| Low birth weight | 2.20 (1.58–3.08) | <.001 | 2.05 (1.45–2.89) | <.001 |
| Preterm | 1.89 (1.36–2.63) | <.001 | – | – |
| Model 2 | ||||
| Sex/Turner syndrome (TS) | ||||
| Male | 1.00 (ref) | 1.00 (ref) | ||
| Female non-TS | 1.43 (1.10–1.86) | .008 | 1.41 (1.08–1.83) | .011 |
| Female TS | 3.31 (1.73–6.31) | <.001 | 2.42 (1.24–4.73) | .009 |
| Low birth weight | 2.20 (1.58–3.08) | .001 | 2.01 (1.42–2.84) | <.001 |
| Preterm | 1.89 (1.36–2.63) | <.001 | – | |
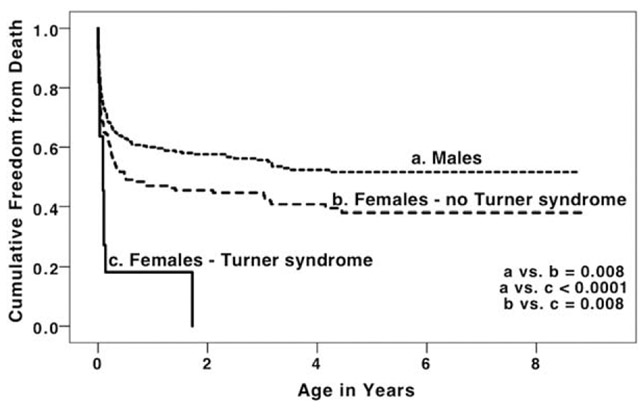
Further analysis by sex and TS status demonstrated significantly increased mortality in females without TS compared to males, and increased mortality in females with TS compared to females without TS (Figure 2 and Table 3, Model 2). This difference in mortality remained clinically and statistically significant in multivariable analysis (Table 3, Model 2).
FIGURE 2.
Kaplan–Meier survival curve for hypoplastic left heart syndrome in males, females without Turner syndrome, and female with Turner syndrome, Texas, 1999–2007
3.2 ∣. Timing of death by stage of palliation
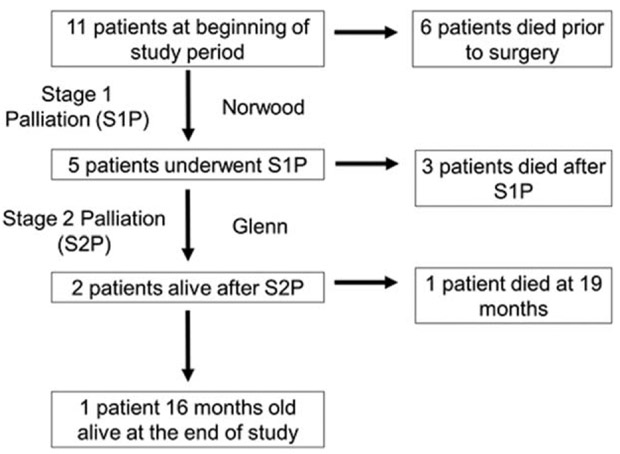
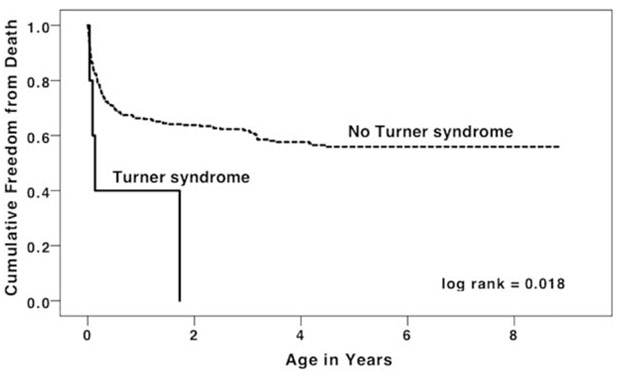
As displayed in Figure 3, there were 11 patients with HLHS/TS+ born during the study period. Three infants died without undergoing any cardiac surgical or catheter interventions and died at ages 4, 12, and 33 d old. One infant underwent an unspecified surgery at age 2 d and died. For two infants, the chart did not specify if any interventions were performed; both died at age 39 d. Information about whether these 6 infants received comfort care or died despite a plan for surgery is not available. Five patients underwent S1P. Three of these patients died in the interstage period between S1P and stage 2 palliation (S2P), at 12, 33, and 50 d of life. Two patients survived through S2P. One of these patients died at 19 mo of age; the other patient was still alive at the end of the study period and was 16 mo old. When only including infants with HLHS who were documented in the TBDR to have undergone S1P (n = 390), there was still a significantly higher mortality in infants with HLHS/TS+ (log-rank = 0.018, Figure 4).
FIGURE 3.
Timing of death in patients with hypoplastic left heart syndrome and Turner syndrome
FIGURE 4.
Kaplan–Meier survival curve for infants with hypoplastic left heart syndrome with and without Turner syndrome who have undergone Stage I palliation, Texas 1999–2007
3.3 ∣. Cause of death
Causes of death from the death records of all patients with HLHS/TS+ who died were reviewed. CHD was listed as the underlying cause of death on death records. One patient had respiratory arrest after a procedural complication and one patient died of a dysrhythmia. Typical findings in patients with severe TS like edema or frank anasarca were not reported.
4 ∣. DISCUSSION
Our intent was to use population-based data to describe mortality in patients with HLHS and TS, to determine if TS is an independent risk factor for death in patients with HLHS in the current era. We found that TS confers a significant increased risk of death in patients with HLHS. Our study is similar to the Michigan-based study by Reis et al. in size and follow up time; however the time frame in the Reis study was 1990–1997, in an era in which overall HLHS outcomes were more pessimistic (Table 4).22 It is interesting to note that this study almost exactly mirrors the outcomes in that cohort: 10 patients total, and only two surviving to S2P. In contrast, a study examining 5-y mortality in patients with HLHS treated in the late 1990s compared to the 2000s reported an 80% 5-y mortality rate in the 1990s that improved to 50% in the 2000s.28 A more contemporary study was published in 2014 by Cramer and colleagues from Wisconsin with four patients with HLHS/TS+ followed from 1999 to 2011.23 Three made it to S2P; however, one died from sepsis; one died after Glenn takedown and only one remained alive at the end of the study period.
TABLE 4.
Review of literature describing surgical and long term outcomes in patients with hypoplastic left heart syndrome with Turner syndrome
| Author | Center/database | Dates | Date of publication |
Number of patients |
Stage 1 survival (number of undergoing S1P)a |
Interstage survival (number of surviving S1P) |
Stage 2 survival (number of undergoing surgery)a |
1-y survival (number of surviving) |
Ages of survivors at time of study |
|---|---|---|---|---|---|---|---|---|---|
| Reis | University of Michigan | 1990–1997 | 1999 | 10 | 20% (n = 10) | 100% (n = 2) | 100% (n = 2) | 20% (n = 2) | 1 and 3 y |
| Madriago | Pediatric Cardiac Care Consortium Database | 1982–2006 | 2012 | 21 | 10% (n = 21)b | – | – | – | – |
| Cramer | Children’s Hospital of Wisconsin | 1999–2011 | 2014 | 4 | 100% (n = 4) | 75% (n = 4) | 67% (n = 3) | 25% (n = 1) | Not given, but one patient in palliative care |
| Lara | State of Texas | 1999–2007 | 2016 | 11 | 80% (n = 5) | 50% (n = 4) | 100% (n = 2) | 18% (n = 2) | 16 mo |
Survival for Stage 1 and Stage 2 palliation are counted if survived to 30 d postsurgery without takedown of surgery.
The paper only specified “HLH surgery,” so other surgeries may be included.
S1P, Stage 1 palliation.
Two recent studies from Madriago et al. and Patel et al. analyze surgical outcomes in this population.15,21 Patel et al. utilized data from the Society of Thoracic Surgeons Database from 2002 to 2006 and the Congenital Heart Surgeons’ Society Database from 1994 to 2001 and found a decreased 10-y survival of 25% in patients with chromosomal defects (including but not limited to TS) and HLHS compared to 54% in patients with HLHS and normal chromosomes.21 In that cohort, there were 14 patients with chromosomal defects, and 11 of those patients had TS, 9 of whom died, which is very similar to our results. Madriago et al. searched the Pediatric Critical Care Consortium Database for surgical and catheter intervention outcomes in patients with TS from 1982 to 2006.15 They found an operative mortality of 90.4% in patients with HLHS and TS compared to 70.5% in patients with HLHS without TS.
TS is a well-known cause of fetal demise. Boue et al. estimated that 1.5% of all conceptions have 45, X karyotype.29 Kajii et al. estimated the survival rate of non-mosaic fetuses with 45, X to be 1 in 300.30 Hook and Warburton estimated that 10% of all fetal deaths are due to TS and 75% of all fetuses with TS abort spontaneously.31 Iyer et al. studied all the cases of TS reported to the Congenital Anomaly Register and Information Service for Wales and found 124 cases. Pregnancy was terminated in 49% of the cases; there was spontaneous loss of the fetus in 24%. There were two cases of HLHS noted in the study; one case in a spontaneous fetal loss and one undergoing termination of pregnancy, versus none in live-born patients. When including coarctation and arch hypoplasia, 30% of those undergoing loss or termination had disease compared to 12% of live born fetuses.16 Fetal demise in TS has been traditionally attributed to hydrops and cystic hygromas, but Barr and Oman-Ganes suggested that overall cardiac hypoplasia (demonstrated by low heart weight for gestational age) is the primary lesion in TS, and that this is a major contributor to fetal death.18
Our finding of increased mortality in females with HLHS but without TS compared to males with HLHS is intriguing. Several studies have evaluated differences in outcomes based on patient characteristics including sex. Dean et al. examined this using data from the University Health System Consortium Clinical Database.32 They followed 1949 patients with HLHS throughout the three stages of palliation. They found an increased odds ratio (OR) of 1.21 (95% CI 0.96–1.52) for mortality after S1P for females with HLHS but the increase did not meet statistical significance. The OR for S2P was similar at 1.16, but was also not statistically significant. Fixler et al. found a similar pattern in a study of severe CHD also using the TBDR.33 The study found an increased hazard ratio for death of 1.21 (95% CI 0.99–1.48) for female sex. Marelli et al. examined the effect of sex on mortality in children with CHD in a population-based 2010 paper.34 When limiting the population to infants undergoing surgery categorized as RACHS 4–6 (which would include S1P for HLHS), the female OR was 1.39 (95% CI 1.16–1.67). These findings were mirrored in a study by Kochilas et al. examining mortality in the Pediatric Cardiac Care Consortium, who found increased mortality risk in females who underwent high-risk surgery at age less than 6 mo.35 However, the increased female risk was not shown in non-HLHS specific heart lesions including atrioventricular septal defect defects,36 tetralogy of Fallot with pulmonary atresia,37 or pulmonary atresia with intact ventricular septum.38
The diagnosis of TS is often missed in the neonatal period.39,40 In fact, less than 30% of TS is diagnosed in the first year of life.41 Given the conferred additional mortality of HLHS with TS, and the increased non-TS female mortality that may be specific to HLHS, we raise the question whether the increased mortality of females without TS in our study is due to underascertainment of TS or other aberrations of the X chromosome. The prevalence of diagnosed TS in our population was 2%; however, universal karyotyping or other genetic studies were not performed, resulting in a likely underestimate of TS prevalence. Unfortunately, the true prevalence of X chromosome abnormalities in HLHS is unknown, as prior published studies have had one or more of the following limitations: performed in a small cohort, analyzed CHD lesions all together, analyzed chromosomal abnormalities all together, have not performed universal genetic evaluation, or have excluded known TS from detailed genetic descriptions.11,15,21,42,43 With these limitations, estimates of TS in live born individuals with HLHS range from 1 to 7%. The prevalence is likely even higher in fetal life, given high fetal loss and termination rates for fetuses with TS and left-sided obstructive lesions.16 In the current study, if the true prevalence of TS was 7% (assuming 28 of the 160 HLHS/TS− female patients actually have TS), and the true hazard ratio of HLHS/TS− females for mortality is the same as males (1.0) compared to 3.3 in the HLHS/TS+ patients, the calculated HR of the female group currently labeled TS− would be ~1.31. This is quite close to the calculated HR in this study of 1.43.
Our findings, in combination with the operative outcomes and the other long term follow up studies paint a grim picture for patients with HLHS/TS+. The reasons for this increased mortality are uncertain; it is possible that it may be due to downstream results of the cardiac hypoplasia noted by Barr and Oman-Ganes,18 problems related to impaired lymphatic drainage potentially affecting pulmonary function,44 or another group of currently unknown etiologies. The high risk of mortality among these patients should be shared with parents during prenatal and preoperative counseling. Finally, these results raise the question as to whether primary heart transplant would be a better option for these patients than a single ventricle palliation.
5 ∣. LIMITATIONS
A limitation of our study and all of the similar studies conducted to date is sample size. There are very few subjects in all of these studies, and it is entirely possible that with an increased study population the pattern of mortality could be different. Furthermore, while our study analyzes data from one of the most populous states with a large number of cardiac surgical centers, it is certainly possible that regional differences may affect the generalizability of these findings. It is also well known that surgical center volume has a large impact on survival after S1P,42,45,46 and this could have impacted the results. However, 7 of the 11 infants with TS were cared for at very high volume cardiac surgical centers with excellent historical S1P outcomes, with only a single survivor. A future direction of this research should be a multiinstitutional prospective study to increase the number of patients studied and broaden the number of surgical centers involved.
We could not perform mixed models to assess outcomes by center given the extremely small number of patients with TS. This would have affected our results if there was confounding by surgical center (caused by an association between surgical center and TS). However, the distribution of TS by centers showed no large predilection for center; patients were born at 10 different birth centers and were cared for at 6 different surgical centers. Thus, confounding by surgical center is an unlikely explanation for our findings.
Given the limitations of the dataset, it was not possible to determine precisely when in the time course of surgical repair the patients passed away. There is not a difference in the first month of life, when a patient with HLHS would be expected to have the Norwood palliation. The large difference comes at 12 mo. By this point, all of the patients would have been expected to have received the Glenn operation. This is the salient point—that even if a patient can make it through the most complicated operation (S1P), her ability to survive after is considerably diminished.
6 ∣. CONCLUSIONS
Patients with HLHS and TS have a significantly higher mortality than patients with HLHS without a known genetic or extracardiac abnormality. This trend continues to be significant when accounting for the lower birth weight in these infants. The increased mortality in females without documented TS calls to question if TS is undetected in a portion of the females with HLHS who do not have obvious dysmorphisms.
Footnotes
CONFLICT OF INTEREST
None.
DISCLOSURE
None.
REFERENCES
- [1].Bondy CA, Turner Syndrome Study Group. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92(1):10–25. [DOI] [PubMed] [Google Scholar]
- [2].Chacko E, Graber E, Regelmann MO, Wallach E, Costin G, Rapaport R. Update on Turner and Noonan syndromes. Endocrinol Metab Clin North Am. 2012;41(4):713–734. [DOI] [PubMed] [Google Scholar]
- [3].Bondy CA. Heart disease in Turner syndrome. Minerva Endocrinol. 2007;32(4):245–261. [PubMed] [Google Scholar]
- [4].Gutmark-Little I, Hor KN, Cnota J, Gottliebson WM, Backeljauw PF. Partial anomalous pulmonary venous return is common in Turner syndrome. J Pediatr Endocrinol Metab. 2012;25(5–6):435–440. [DOI] [PubMed] [Google Scholar]
- [5].Ho VB, Bakalov VK, Cooley M, et al. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004;110(12):1694–1700. [DOI] [PubMed] [Google Scholar]
- [6].Mazzanti L, Cacciari E. Congenital heart disease in patients with Turner’s syndrome. Italian Study Group for Turner Syndrome (ISGTS). J Pediatr. 1998;133(5):688–692. [DOI] [PubMed] [Google Scholar]
- [7].Conen PE, Glass IH. 45/XO Turner’s syndrome in the newborn: report of two cases. J Clin Endocrinol Metab. 1963;23:1–10. [DOI] [PubMed] [Google Scholar]
- [8].Lintermans JP. Gonadal dysgenesis and hypoplastic left heart syndrome. J Pediatr. 1970;76(6):979. [DOI] [PubMed] [Google Scholar]
- [9].Bidot-Lopez P, Matisoff D, Talner NS, Hsia YE. Hypoplastic left heart in a patient with 45,X/46,XX/47,XXX mosaicism. Am J Med Genet. 1978;2(4):341–343. [DOI] [PubMed] [Google Scholar]
- [10].Shah A, Fay JE, Ford S, Holden JJ. 46,X,i(Xq) karyotype in a patient with hypoplastic left heart. Clin Genet. 1985;28(2):178–179. [DOI] [PubMed] [Google Scholar]
- [11].Natowicz M, Kelley RI. Association of Turner syndrome with hypoplastic left-heart syndrome. Am J Dis Child. 1987;141(2):218–220. [DOI] [PubMed] [Google Scholar]
- [12].van Egmond H, Orye E, Praet M, Coppens M, Devloo-Blancquaert A. Hypoplastic left heart syndrome and 45X karyotype. Br Heart J. 1988;60(1):69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sybert VP. Cardiovascular malformations and complications in Turner syndrome. Pediatrics 1998;101(1):E11. [DOI] [PubMed] [Google Scholar]
- [14].Tan KB, Yeo GS. Pattern of Turner syndrome in Singapore (1999–2004). Singapore Med J. 2009;50(6):587–590. [PubMed] [Google Scholar]
- [15].Madriago E, Nguyen T, McFerson M, et al. Frequency and outcomes of cardiac operations and catheter interventions in Turner syndrome. Am J Cardiol. 2012;110(4):580–585. [DOI] [PubMed] [Google Scholar]
- [16].Iyer NP, Tucker DF, Roberts SH, Moselhi M, Morgan M, Matthes JW. Outcome of fetuses with Turner syndrome: a 10-year congenital anomaly register based study. J Matern Fetal Neonatal Med. 2012;25(1):68–73. [DOI] [PubMed] [Google Scholar]
- [17].Haak MC, Bartelings MM, Gittenberger-De Groot AC, Van Vugt JM. Cardiac malformations in first-trimester fetuses with increased nuchal translucency: ultrasound diagnosis and postmortem morphology. Ultrasound Obstet Gynecol. 2002;20(1):14–21. [DOI] [PubMed] [Google Scholar]
- [18].Barr M Jr, , Oman-Ganes L. Turner syndrome morphology and morphometrics: cardiac hypoplasia as a cause of midgestation death. Teratology 2002;66(2):65–72. [DOI] [PubMed] [Google Scholar]
- [19].Surerus E, Huggon IC, Allan LD. Turner’s syndrome in fetal life. Ultrasound Obstet Gynecol. 2003;22(3):264–267. [DOI] [PubMed] [Google Scholar]
- [20].Jacobs JP, O’brien SM, Chai PJ, Morell VO, Lindberg HL, Quintessenza JA. Management of 239 patients with hypoplastic left heart syndrome and related malformations from 1993 to 2007. Ann Thorac Surg. 2008;85(5):1691–1696, discussion 7. [DOI] [PubMed] [Google Scholar]
- [21].Patel A, Hickey E, Mavroudis C, et al. Impact of noncardiac congenital and genetic abnormalities on outcomes in hypoplastic left heart syndrome. Ann Thorac Surg. 2010;89(6):1805–1813. discussion 13-4. [DOI] [PubMed] [Google Scholar]
- [22].Reis PM, Punch MR, Bove EL, van de Ven CJ. Outcome of infants with hypoplastic left heart and Turner syndromes. Obstet Gynecol. 1999;93(4):532–535. [DOI] [PubMed] [Google Scholar]
- [23].Cramer JW, Bartz PJ, Simpson PM, Zangwill SD. The spectrum of congenital heart disease and outcomes after surgical repair among children with Turner syndrome: a single-center review. Pediatr Cardiol. 2014;35(2):253–260. [DOI] [PubMed] [Google Scholar]
- [24].Pappas G, Queen S, Hadden W, Fisher G. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med. 1993;329(2):103–109. [DOI] [PubMed] [Google Scholar]
- [25].Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Poverty Definitions: U.S. Census Bureau. Available from: http://www.census.gov/hhes/www/poverty/methods/definitions.html. Accessed March 25, 2015.
- [27].Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol.1996;87 (2):163–168. [DOI] [PubMed] [Google Scholar]
- [28].Menon SC, Keenan HT, Weng HY, et al. Outcome and resource utilization of infants born with hypoplastic left heart syndrome in the Intermountain West. Am J Cardiol. 2012;110(5):720–727. [DOI] [PubMed] [Google Scholar]
- [29].Boue J, Bou A, Lazar P. Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology. 1975;12(1):11–26. [DOI] [PubMed] [Google Scholar]
- [30].Kajii T, Ferrier A, Niikawa N, Takahara H, Ohama K, Avirachan S. Anatomic and chromosomal anomalies in 639 spontaneous abortuses. Hum Genet. 1980;55(1):87–98. [DOI] [PubMed] [Google Scholar]
- [31].Hook EB, Warburton D. The distribution of chromosomal genotypes associated with Turner’s syndrome: livebirth prevalence rates and evidence for diminished fetal mortality and severity in genotypes associated with structural X abnormalities or mosaicism. Hum Genet. 1983;64(1):24–27. [DOI] [PubMed] [Google Scholar]
- [32].Dean PN, McHugh KE, Conaway MR, Hillman DG, Gutgesell HP. Effects of race, ethnicity, and gender on surgical mortality in hypoplastic left heart syndrome. Pediatr Cardiol. 2013;34(8):1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fixler DE, Nembhard WN, Xu P, Ethen MK, Canfield MA. Effect of acculturation and distance from cardiac center on congenital heart disease mortality. Pediatrics. 2012;129(6):1118–1124. [DOI] [PubMed] [Google Scholar]
- [34].Marelli A, Gauvreau K, Landzberg M, Jenkins K. Sex differences in mortality in children undergoing congenital heart disease surgery: a United States population-based study. Circulation. 2010;122(11 Suppl):S234–S240. [DOI] [PubMed] [Google Scholar]
- [35].Kochilas LK, Vinocur JM, Menk JS. Age-dependent sex effects on outcomes after pediatric cardiac surgery. J Am Heart Assoc. 2014;3 (1):e000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morris CD, Magilke D, Reller M. Down’s syndrome affects results of surgical correction of complete atrioventricular canal. Pediatr Cardiol. 1992;13(2):80–84. [DOI] [PubMed] [Google Scholar]
- [37].Cho JM, Puga FJ, Danielson GK, et al. Early and long-term results of the surgical treatment of tetralogy of Fallot with pulmonary atresia, with or without major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg. 2002;124(1):70–81. [DOI] [PubMed] [Google Scholar]
- [38].Hanley FL, Sade RM, Blackstone EH, Kirklin JW, Freedom RM, Nanda NC. Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multiinstitutional study. J Thorac Cardiovasc Surg. 1993;105(3):406–423. 24–7; discussion 23-4. [PubMed] [Google Scholar]
- [39].Sari E, Bereket A, Yesilkaya E, Bas F, Bundak R, Aydin BK, etet al. Anthropometric findings from birth to adulthood and their relation with karyotpye distribution in Turkish girls with Turner syndrome. Am J Med Genet A. 2016;170A(4):942–948. [DOI] [PubMed] [Google Scholar]
- [40].Miguel-Neto J, Carvalho AB, Marques-de-Faria AP, Guerra-Junior G, Maciel-Guerra AT. New approach to phenotypic variability and karyotype-phenotype correlation in Turner syndrome. J Pediatr Endocrinol Metab. 2016;90(3):267–268. [DOI] [PubMed] [Google Scholar]
- [41].Massa G, Verlinde F, De Schepper J, et al. Trends in age at diagnosis of Turner syndrome. Arch Dis Child. 2005;90(3):267–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tabbutt S, Ghanayem N, Ravishankar C, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tennstedt C, Chaoui R, Korner H, Dietel M. Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: results of a seven year necropsy study. Heart.1999;82(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Loscalzo ML, Van PL, Ho VB, et al. Association between fetal lymphedema and congenital cardiovascular defects in Turner syndrome. Pediatrics. 2005;115(3):732–735. [DOI] [PubMed] [Google Scholar]
- [45].Checchia PA, McCollegan J, Daher N, Kolovos N, Levy F, Markovitz B. The effect of surgical case volume on outcome after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;129(4):754–759. [DOI] [PubMed] [Google Scholar]
- [46].Morris SA, Ethen MK, Penny DJ, et al. Prenatal diagnosis, birth location, surgical center, and neonatal mortality in infants with hypoplastic left heart syndrome. Circulation. 2014;129(3):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]