Abstract
BACKGROUND AND PURPOSE:
Mechanical circulatory support (MCS) devices are commonly used in heart failure patients. These devices carry risk for presumably embolic and additionally hemorrhagic stroke. Alterations in blood flow play a key role in stroke pathophysiology, and we aimed to learn more about hemodynamic compromise. In this study, we used transcranial Doppler (TCD) ultrasound to define hemodynamics of commonly used nonpulsatile MCS devices, as well as pulsatile devices, with special attention to the total artificial heart (TAH).
METHODS:
From 2/2013 through 12/2016, we prospectively enrolled patients with MCS who underwent TCD imaging. We analyzed TCD parameters, including peak systolic velocity, end-diastolic velocity, pulsatility indices (PIs), and number of high-intensity transient signals. Waveform morphologies were compared between various MCS devices.
RESULTS:
We performed 132 TCD studies in 86 MCS patients. Waveforms in patients supported by venoarterial-extracorporeal membrane oxygenation demonstrated continuous flow without clear systolic peaks with an average (±SD) PI of .43 (±.2). PIs were low in patients with continuous-flow left ventricular assist devices with a mean PI of .32 (±.13). Impella patients had morphologically distinct pulsatile waveforms and a higher mean PI of .65 (±.24). In intra-arterial balloon pump patients, mean PI was 1.01 (±.16) and diastolic upstrokes were pronounced. In TAH patients, mean middle cerebral artery velocity of 79.69 (±32.33) cm/seconds and PI of .74 (±.14) approached normal values.
CONCLUSION:
TCD can detect characteristic waveforms in patients supported by various MCS devices. These device-specific TCD patterns are recognizable and reproducible.
Keywords: Transcranial Doppler ultrasound, stroke, left ventricular assist device, heart failure, high-intensity transient signal
Introduction
Bridging terminally ill cardiac failure patients to transplantation or recovery with a mechanical circulatory support (MCS) device has proven feasible and effective. Early survival after implantation is satisfactory, although at times at the expense of device-related complications.1-3 Significant improvement of the hardware and implantation procedure has greatly reduced device failure.4-6 Neurologic adverse events continue to represent a significant challenge to the long-term outcomes after MCS device placement.2,5,7-11
Multiple devices are used in current practice. Venoarterial-extracorporeal membrane oxygenation (VA-ECMO) and left ventricular assist device (LVAD) assist in blood perfusion and provide significant benefit for long-term circulatory support.1,9 Intra-aortic balloon pumps (IABPs) are inserted directly into the aorta and use counterpulsion to increase cardiac output thereby reducing afterload.12 The total artificial heart (TAH) is a biventricular pulsatile pump that replaces both native cardiac ventricles and all four cardiac valves.3,4
Cerebral thromboembolism is the most common neurologic complication reported during support from a mechanical circulatory device, ranging from 1.6% to 47% depending on the device2,5,7-9,11 The incidence of ischemic stroke in TAH-supported patients ranges from 1.6% to 8%.7,13,14 The incidence of hemorrhagic stroke in TAH-supported patients is 7%.14 TAH patients are often more critically ill preoperatively, suffer a significantly greater rate of adverse events, and have an overall higher mortality than patients supported by other types of device.15 The survival of those with thrombotic events ranges from 70-75.2%.15 Hemodynamic compromise due to device failure also presents with neurological symptoms, but less likely focally.
The use of transcranial Doppler (TCD) ultrasound in patients supported by MCS devices offers significant diagnostic value. TCD provides direct measurement of the pattern of cerebral blood flow, hemodynamic reserve, and allows for the detection of microbemboli.16,17 TCD use in MCS has been described with a greater focus on LVAD18-22 and ECMO23-26 supported patients; however, there has been limited attention to TCD in TAH patients. We sought to use TCD to better characterize the patterns of cerebral arterial waveforms associated with state-of-the-art MCS devices, with special attention to the TAH.
Methods
This administrative data review was approved by the Cedars-Sinai Institutional Review Board, which granted waiver from informed consent. We performed TCD studies in MCS patients from January 2013 through December 2016 in patients who were 18 years of age or older. Baseline TCD studies were performed prior to implantation when able. Standard TCD protocols were used, and all studies were performed by registered vascular technologist.16 All TCDs were obtained on either Sonara (Natus Neurology, Middleton, WI) or Spencer TCD machines (Spencer Technologies, Redmond, WA).
We collected variables known to influence cerebral blood flow and stroke risk: age, gender, ethnicity, etiology of heart failure, concomitant use of antithrombotics, including aspirin, clopidogrel, enoxaparin, warfarin, and heparin, TCD parameters, including peak systolic, end diastolic velocities, mean flow velocities, and number of high-intensity transient signals (HITS), the type and dose of anticoagulation, cerebral ischemic or hemorrhagic events, and the clinical outcomes of these patients, including modified Rankin Scale;27 mean pulsatility indices (PIs) were calculated for each patient using Gosling’s PI formula: (peak systolic velocity (PSV)–end diastolic velocity (EDV))/mean flow velocity (MFV).28
Where needed, data were summarized as arithmetic mean and simple standard deviation. Demographic group differences were comparted using ANOVA for continuous and Kruskal-Wallis test for categorical variables. MFV and PI values for each device were compared to studies obtained in post heart transplant patients. Waveform parameters were compared for preimplantation baseline TCD studies with post TAH implantation TCDs using t-test, two-tailed, with significant P value less than .05. Analyses were performed using SPSS (ver25).
Results
We identified 86 MCS patients who underwent 132 TCD studies (Fig 1). Of these, 28 were VA-ECMO, 1 was VV-ECMO, 21 were HeartWare LVAD (HVAD), 5 were HeartMate IILVAD (HMII), 14 were impella, 24 were TAH, and 7 were IABP. Preimplantation baseline TCD was obtained in 20 patients. Our institution implanted 76 TAH, 301 VADs, and 257 ECMOs between 2013 and 2016 (Fig 2). Basic characteristics for included patients are presented in Table 1.
Fig 1. CONSORT diagram of participants included for analysis.
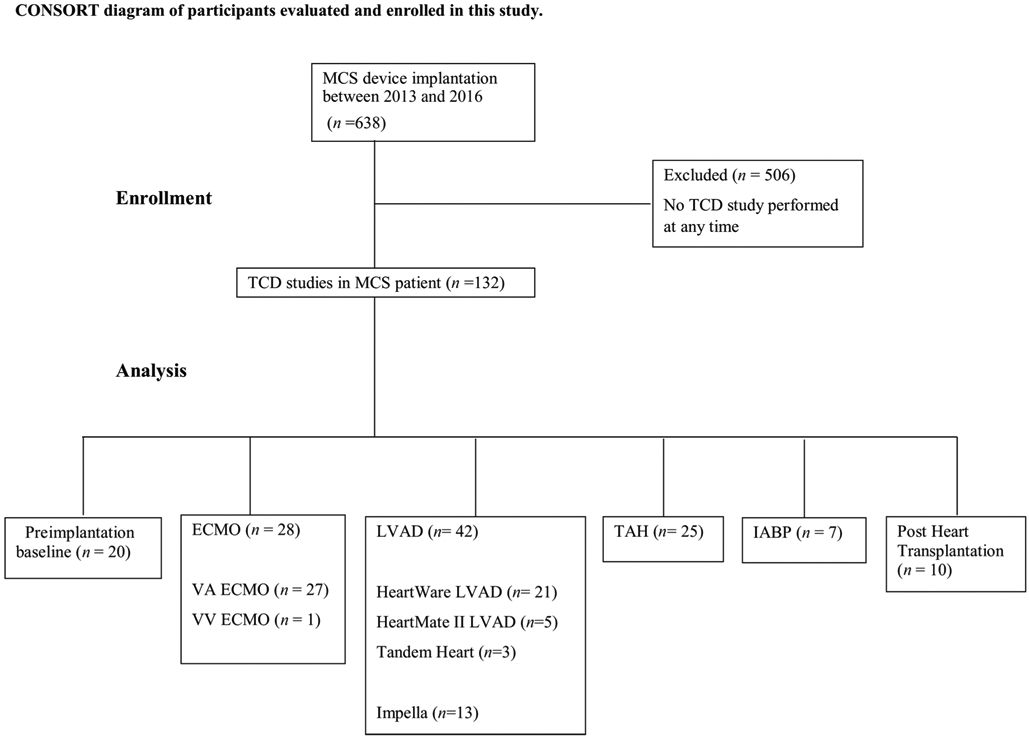
ECMO = extracorporeal membrane oxygenation; IABP = intra-arterial balloon pump; LVAD = left ventricular assist device; MCS = mechanical circulatory support; n = number of patients; TAH = total artificial heart; TCD = transcranial Doppler; VA = venoarterial; VV = venovenous.
Fig 2. Mechanical circulatory support devices implanted between 2013 and 2016 at our institution.
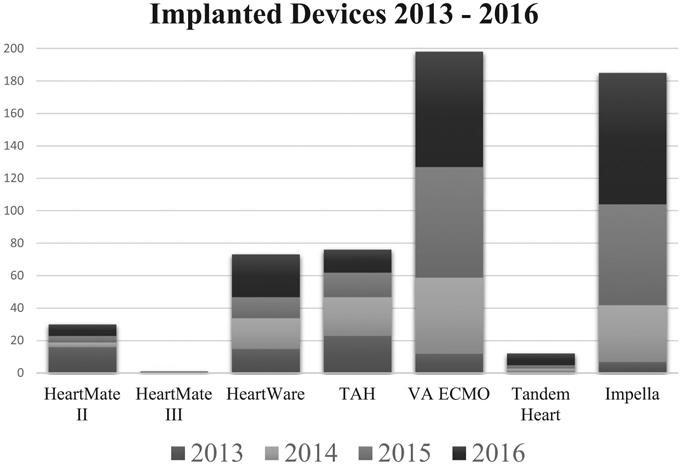
TAH = total artificial heart; VA-ECMO = venoarterial-extracorporeal membrane oxygenation.
Table 1.
Patient Characteristics by Device Type
| Variable | ECMO | HVAD | HMII | TAH | Impella | IABP | P Value |
|---|---|---|---|---|---|---|---|
| Number of patients with TCD studies | 22 | 16 | 5 | 12 | 7 | 7 | |
| Mean age (SD) (years) | 57 (11) | 55 (12) | 62 (14) | 54 (11) | 58 (12) | 60 (14) | .8 |
| Female | 5 (23) | 2 (13) | 0 (0) | 1 (8.3) | 1 (14) | 1 (14) | .3 |
| Ethnicity | |||||||
| African American | 1 (5) | 2 (13) | 1 (20) | 2 (17) | 0 (0) | 0 (0) | .7 |
| Asian | 5 (23) | 1 (6) | 0 (0) | 3 (25) | 1 (14) | 3 (43) | .2 |
| Caucasian | 12 (55) | 7 (44) | 4 (80) | 5 (42) | 5 (71) | 4 (57) | .4 |
| Hispanic | 2 (9) | 2 (31) | 0 (0) | 2 (17) | 1 (14) | 0 (0) | .5 |
| Median preimplant NYHA [IQR] (years) | 4 [4,4] | 4 [3,4] | 3.5 [3,4] | 4 [3,4] | 4 [4,4] | 3.5 [3,4] | .3 |
Note: Numbers are reported as number of patients (percent of group) unless otherwise specified.
BMI = body mass index; ECMO = extracorporeal membrane oxygenation; HMII = Heartmate II; HVAD = HeartWare ventricular assist device; IABP = intra-arterial balloon pump; IQR = interquartile range; NYHA = New York Heart Association; SD = standard deviation; TAH = total artificial heart; TCD = transcranial Doppler.
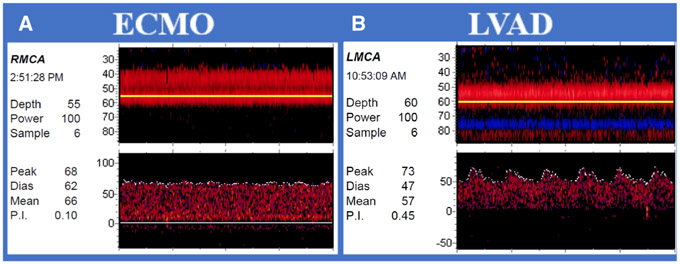
Waveforms in patients supported by VA-ECMO demonstrated continuous flow without clear systolic peaks (Fig 3A). The average (±SD) middle cerebral artery (MCA) MFV was 56.07 (±21) cm/seconds and PI was .43 (±.2) (Table 2). We observed a mean (±SD) of 2.81 (±.09) HITS per minute in HITS monitoring studies in 22 VA-ECMO patients. In a single VV-ECMO patient, the mean MCA velocity was 56.00 and the PI was 1.11 (±.03).
Fig 3. Transcranial Doppler waveforms obtained from patients with continuous flow devices.
(A) Venoarterial-extracorporeal membrane oxygenation (VA-ECMO): Waveforms in patient supported by VA-ECMO demonstrate continuous flow without clear systolic peaks. (B) Left ventricular assist devices (LVADs): Low pulsatility indices in patient with continuous-flow LVAD with native flow.
ECMO = extracorporeal membrane oxygenation; LMCA = left middle cerebral artery; LVAD = left ventricular assist device; RMCA = right middle cerebral artery; Peak = peak systolic velocity; Dias = diastolic velocity; Mean = mean flow velocity; P.I. = pulsatility index.
Table 2.
Mean Systolic Velocities, Pulsatility Indices, and HITs by Device
| Waveform Parameters | n | MFV cm/second of MCA | P Value | MCA PI | P Value | Mean HITS/minute | P Value |
|---|---|---|---|---|---|---|---|
| Preimplantation baseline | 20 | 50.86 (12.31) | .9 | 1.05 (.56) | .5 | .01 (.05) | .3 |
| VA-ECMO | 27 | 56.07 (21) | .7 | .43 (.25) | <.01** | 2.81 (.09) | .04* |
| HVAD | 21 | 48.94 (15.93) | .2 | .35 (.17) | <.01** | .00 (.01) | .3 |
| HMII | 5 | 57.30 (12.9) | .8 | .32 (.13) | <.01** | .02 (.06) | .4 |
| Tandem heart | 3 | 33.25 (9.36) | .03* | .38 (.025) | <.01* | 1.07 (1.51) | .5 |
| TAH | 25 | 79.69 (32.33) | .2 | .74 (.14) | .5 | 12.55(12.04) | .02* |
| Impella | 13 | 53.72 (11.74) | .2 | .65 (.24) | .07 | 8 (27) | .02* |
| IABP | 7 | 59.79 (11.54) | .7 | 1.01 (.16) | .7 | .01 (.03) | .4 |
| HT | 10 | 56.11 (16.39) | 1.12 (.52) | 0(0) |
Note: Numbers are reported as mean (standard deviation) unless otherwise specified. P value is obtained from comparisons to HT studies.
P < .05
P < .01.
FiO2 = fraction inspired oxygen; HITS/min = high-intensity transient signal observed per minute; HMII = Heartmate II; HT = heart transplantation; HVAD = HeartWare ventricular assist device; IABP = intra-arterial balloon pump; MCA = middle cerebral artery; MFV = mean flow velocity; n = number of transcranial Doppler studies for each device; PI = pulsatility index; TAH, total artificial heart; VA-ECMO = venoarterial-extracorporeal membrane oxygenation.
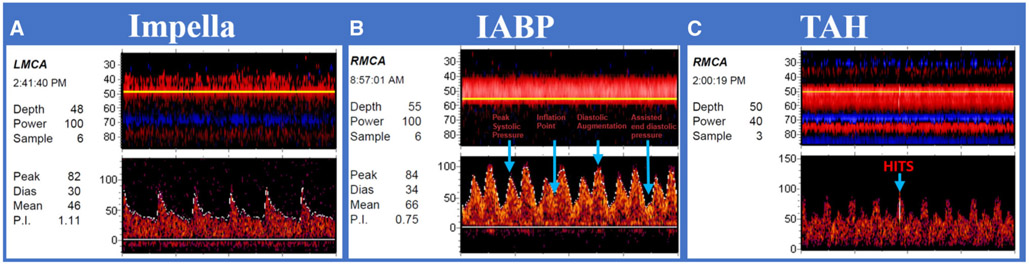
We evaluated flow patterns in individual types of LVAD devices. In general, PIs were low in patients with continuous-flow LVADs (Fig 3B). HMII average (±SD) MCA MFV was 57.3 (±12.9) cm/seconds with a PI of .32 (±.13). In HVAD patients, the average (SD) MCA MFV was 48.94 (±15.93) cm/seconds and PI of .35 (±.17). Both HMII and HVAD had very low rates of observed HITS per minute (Table 2). Impella patients had morphologically distinct pulsatile waveforms compared to other types of VADs (Fig 4A); average (±SD) MCA MFV was 53.72 (±11.74) cm/seconds and a PI of .65 (±.24). All 14 studies in impella patients included monitoring for emboli detection, with an observed mean HITS rate (±SD) of 18 (±27) per minute. Oxygen administration did not change the observed HITS burden. In the 7 IABP patients, there was an average (SD) MCA MFV of 59.79 (±11.54) cm/seconds and PI was 1.01 (±.16). These waveforms demonstrated pronounced diastolic upstrokes not present in other devices (Fig 4B).
Fig 4. Transcranial Doppler waveforms obtained from patients with pulsatile flow devices.
(A) Impella patient: Impella patients had morphologically distinct pulsatile waveforms compared to other types of ventricular assist devices. (B) Intra-arterial balloon pump (IABP) patient: Pronounced diastolic upstrokes not present in other devices, with evidence of unsupported systolic peak, inflation point, augmented diastole, and unassisted diastole. (C) Total artificial heart (TAH) patient: Waveforms resemble hemodynamics of unassisted patient.
IABP = intra-arterial balloon pump; LMCA = left middle cerebral artery; RMCA = right middle cerebral artery; TAH = total artificial heart; TCD = transcranial Doppler; Peak = peak systolic velocity; Dias = diastolic velocity; Mean = mean flow velocity; P.I. = pulsatility index.
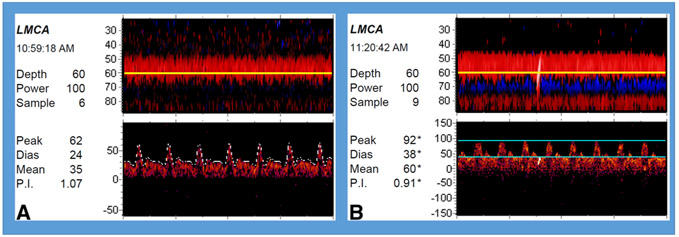
TCDs were obtained in TAH patients after a medium [interquartile range] of 18 [4-32] days following implantation. In these patients, mean (±SD) MCA MFV was 79.69 (±32.33) cm/seconds and PI of .74 (±.14) (Fig 4C), which approach our laboratory values of normal.28 Mean (±SD) PSV was 113.84 (±38.89), and mean (±SD) EDV was 55.38 (±28.87). An example of a single patient with pre- and post TAH implantation TCD studies is demonstrated in Figure 5. This patient had nonischemic cardiomyopathy due to amyloidosis. Preimplantation NYHA was 4. TCDs were obtained 1 day pre implantation and 48 hours post implantation. No significant differences were noted when comparing the waveform parameters of preimplantation TCDs and post TAH implantation TCDs (Table 3).
Fig 5. Transcranial Doppler waveforms obtained from single patient pre and post total artificial heart (TAH) implantation.
(A). Preimplantation baseline 24 hours prior to implantation. (B) 48 hours post implantation with TAH: Waveforms resemble hemodynamics of unassisted patient. High-intensity transient signal is observed during recording.
LMCA = left middle cerebral artery; Peak = peak systolic velocity; Dias = diastolic velocity; Mean = mean flow velocity; P.I. = pulsatility index.
Table 3.
Waveform Parameters of Transcranial Dopplers Studies Obtained Pre and Post TAH Implantation
| Waveform Parameters | Preimplantation | TAH Support | P Value |
|---|---|---|---|
| n | 20 | 25 | |
| PSV cm/second | 87.37 (34.87) | 113.33 (46.53) | .4 |
| EDV cm/second | 34.67 (7.37) | 58.83 (28.20) | .2 |
| MFV cm/second | 50.86 (12.31) | 79.69 (32.33) | .1 |
| PI | 1.05 (.56) | .74 (.14) | .2 |
Note: Numbers are reported as mean (standard deviation) unless otherwise specified.
EDV = end diastolic velocity; MFV = mean flow velocity; n = number of transcranial Doppler studies; PI = pulsatility index; PSV = peak systolic velocity; TAH = total artificial heart.
We performed 60 minutes of emboli detection monitoring in all patients with TAH, and HITS was detected in 10 (43%) studies, significantly greater than in preimplantation baseline studies (P = .02). A mean (±SD) 326.25 (±702.02) HITS was found in these 10 patients, or 11.10 (±23.88) per minute. These 10 patients were monitored for 30 additional minutes with administration of 100% oxygen, after which time only 77.67 (±136.28) HITS, or 2.59 (±4.54) HITS per minute was observed. Administration of 100% oxygen suppressed 79% of HITS in TAH patients.
Discussion
In our cohort of patients supported by various MCS devices that underwent TCD evaluation, we found unique and characteristic cerebral flow waveforms specific to each MCS device. We found low or low-normal PIs across all patients supported by continuous flow devices, consistent with previous reports in VA-ECMO patients.24,25 PIs approached normal ranges in patients supported by pulsatile flow devices, also previously described.19,22,26
We demonstrate for the first time that TAH cerebral hemodynamics approaches those patients who are not supported by MCS. The PSV, EDV, and MFV observed in patients supported by TAH did not differ from preimplantation baseline TCD studies. The PIs, which correlated with cerebral vascular resistance, trended lower in TAH patients than in preimplantation patients, although there was no significant difference.
The TAH replaces both failing ventricles with prosthetic ventricles typically attached with the native right and left atria. A diaphragm controlled from an external driver moves back and forth to create air pressure pulses, replicating native pulsatility.3 TAH as bridge to transplant is associated with a 79% survival.4 As the TAH becomes more commonly used, better understanding of the impact on cerebrovascular is imperative, both from perspective of hemodynamics and challenges associated with anticoagulation. Here, we see minimal impact on cerebral hemodynamics based on the TCD studies performed at our center.
Limitations to this study include the fact that the cohort originated from a single center. However, given the number of patients supported by cardiac devices at this center, this is one of the first studies to compare various devices from a single TCD laboratory, thus minimizing interobserver variability. As performance of TCDs can be susceptible to user variability, demonstrating variations in patients from a single center by similarly well-trained and proficient technicians allows for adequate comparisons to be made.
We found that HITS was not universally present and intermittently suppressible by oxygen, suggesting that some may be gaseous in nature. Such suppressible HITS may represent nitrogenous cavitations that are suppressible with increasing partial pressure of oxygen, as reported previously in prosthetic valve recipients during continuous TCD emboli monitoring.21 Characterization of the ultrasound findings via TCD evaluation with embolus detection can elucidate insight into the exact cerebrovascular hemodynamics of these patients, leading to improved anticoagulation options.17 For example, future studies will be needed to determine if long-term monitoring for microemboli can be used–perhaps in conjunction with thromboelastography (TEG)–to guide antithrombotic therapy. Conventional coagulation assays determine the adequacy of coagulation factor levels or degree of their inhibition in plasma, whereas TEG is a functional assay that reflects the interaction of platelets and other cellular contributors with factors in whole blood and is a closer representation of in-vivo processes.
Future studies are needed in order to explore the potential correlation of hemodynamic findings by TCD with patients’ clinical outcomes. These studies should address the pathophysiological mechanism linking flow pattern (pulsatile vs. nonpulsatile) dynamics to stroke risk. Exploration of an increased stroke risk in patients with spontaneous HITS is in order, and if so the mechanism of the stroke itself. Future prospective studies may aim to understand how TCDs can help risk stratify patients prior to device implantation. Finally, TCD studies may help guide therapeutic approaches for optimal anticoagulation strategies to decrease stroke risk in this patient population.
Patients supported by MCS devices produce unique and characteristic waveforms on TCD. Normative values in this special population have not yet been determined. This study will help generate further hypotheses related to the use, management, and care of stroke patients with mechanical circulatory devices. TCD may be a pragmatic tool to understand how coagulation and hemodynamics are altered with MCS devices.
Acknowledgments and Disclosure:
The authors wish to acknowledge the Cedars-Sinai Medical Center Cerebrovascular Ultrasound Laboratory.
Sources of Funding: Supported in part by NINDS R01 NS075930, U24 NS113452 and the Carmen and Louis Warschaw Family Foundation
Contributor Information
Kara R. Melmed, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA; Department of Neurology, New York University Langone Health, New York, NY.
Konrad H. Schlick, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA
Brenda Rinsky, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA.
Oana M. Dumitrascu, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA
Oksana Volod, Department of Pathology, Cedars-Sinai Medical Health, Los Angeles, CA.
Mani Nezhad, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA; Department of Neurology, Dignity Health Medical Foundation, San Francisco, CA.
Matthew M. Padrick, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA
Carmelita Runyan, Cedars-Sinai Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA.
Francisco A. Arabia, Cedars-Sinai Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA; Department of Surgery & Medicine, Banner-University of Arizona, Phoenix, AZ
Jaime D. Moriguchi, Cedars-Sinai Heart Institute, Los Angeles, CA
Patrick D. Lyden, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA
Shlee S Song, Department of Neurology and Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA.
References
- 1.Omar HR, Mirsaeidi M, Shumac J, et al. Incidence and predictors of ischemic cerebrovascular stroke among patients on extracorporeal membrane oxygenation support. J Crit Care 2016;32:48–51. [DOI] [PubMed] [Google Scholar]
- 2.Morgan JA, Brewer RJ, Nemeh HW, et al. Stroke while on long-term left ventricular assist device support: incidence, outcome, and predictors. ASAIO J 2014;60:284–9. [DOI] [PubMed] [Google Scholar]
- 3.Copeland JG, Smith RG, Arabia FA, et al. The CardioWest total artificial heart as a bridge to transplantation. Semin Thorac Cardiovasc Surg 2000;12:238–42. [DOI] [PubMed] [Google Scholar]
- 4.Arabia FA, Moriguchi JD. Machines versus medication for biventricular heart failure: focus on the total artificial heart. Future Cardiol 2014;10:593–609. [DOI] [PubMed] [Google Scholar]
- 5.Willey JZ, Demmer RT, Takayama H, et al. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: risk factors, diagnosis, and treatment. J Heart Lung Transplant 2014;33:878–87. [DOI] [PubMed] [Google Scholar]
- 6.Colombo PC, Mehra MR, Goldstein DJ, et al. Comprehensive analysis of stroke in the long-term cohort of the MOMENTUM 3 study. Circulation 2019;139:155–68. [DOI] [PubMed] [Google Scholar]
- 7.Copeland J, Copeland H, Nolan P, et al. Results with an anticoagulation protocol in 99 SynCardia total artificial heart recipients. ASAIO J 2013;59:216–20. [DOI] [PubMed] [Google Scholar]
- 8.Boyle AJ, Jorde UP, Sun B,et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 2014;63:880–8. [DOI] [PubMed] [Google Scholar]
- 9.Lazar RM, Shapiro PA, Jaski BE, et al. Neurological events during long-term mechanical circulatory support for heart failure: the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Experience. Circulation 2004;109:2423–7. [DOI] [PubMed] [Google Scholar]
- 10.Cho SM, Moazami N, Frontera JA. Stroke and intracranial hemorrhage in HeartMate II and HeartWare left ventricular assist devices: a systematic review. Neurocrit Care 2017;27:17–25. [DOI] [PubMed] [Google Scholar]
- 11.Frontera JA, Starling R, Cho SM, et al. Risk factors, mortality, and timing of ischemic and hemorrhagic stroke with left ventricular assist devices. J Heart Lung Transplant 2017;36:673–83. [DOI] [PubMed] [Google Scholar]
- 12.Pfluecke C, Christoph M, Kolschmann S, et al. Intra-aortic balloon pump (IABP) counterpulsation improves cerebral perfusion in patients with decreased left ventricular function. Perfusion 2014;29:511–6. [DOI] [PubMed] [Google Scholar]
- 13.Copeland JG, Smith RG, Arabia FA, et al. Total artificial heart bridge to transplantation: a 9-year experience with 62 patients. J Heart Lung Transplant 2004;23:823–31. [DOI] [PubMed] [Google Scholar]
- 14.Kirsch ME, Nguyen A, Mastroianni C, et al. SynCardia temporary total artificial heart as bridge to transplantation: current results at la pitié hospital. Ann Thorac Surg 2013;95:1640–6. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland JC, Naftel DC, Reece TB, et al. Survival after biventricular assist device implantation: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support database. J Heart Lung Transplant 2011;30:862–9. [DOI] [PubMed] [Google Scholar]
- 16.Alexandrov AV, Sloan MA, Wong LK, et al. Practice standards for transcranial Doppler ultrasound: part I–test performance. J Neuroimaging 2007;17:11–8. [DOI] [PubMed] [Google Scholar]
- 17.Kargiotis O, Psychogios K, Safouris A, et al. The role of transcranial Doppler monitoring in patients with multi-territory acute embolic strokes: a review. J Neuroimaging 2019;29:309–22. [DOI] [PubMed] [Google Scholar]
- 18.Marinoni M, Migliaccio ML, Trapani S, et al. Cerebral microemboli detected by transcranial Doppler in patients treated with extracorporeal membrane oxygenation. Acta Anaesthesiol Scand 2016;60:934–44. [DOI] [PubMed] [Google Scholar]
- 19.Karahan M, Kocabeyoglu SS, Kervan U, et al. Effects of continuous flow left ventricular assist devices on cerebral hemodynamics. Artif Organs 2019. 10.1111/aor.13616. [DOI] [PubMed] [Google Scholar]
- 20.Fukuma K, Seguchi O, Saito K, et al. The clinical implication of transcranial Doppler detection of microembolic signals in patients with Heartmate II. ASAIO J 2018;64:694–6. [DOI] [PubMed] [Google Scholar]
- 21.Thoennissen NH, Allroggen A, Dittrich R, et al. Can Doppler time domain analysis of microembolic signals discriminate between gaseous and solid microemboli in patients with left ventricular assist device? Neurol Res 2005;27:780–4. [DOI] [PubMed] [Google Scholar]
- 22.Potapov EV, Nasseri BA, Loebe M, et al. Transcranial detection of microembolic signals in patients with a novel nonpulsatile implantable LVAD. ASAIO J 2001;47:249–53. [DOI] [PubMed] [Google Scholar]
- 23.Ono M, Joshi B, Brady K, et al. Cerebral blood flow autoregulation is preserved after continuous-flow left ventricular assist device implantation. J Cardiothorac Vasc Anesth 2012;26:1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavi T, Esch M, Rinsky B, et al. Transcranial Doppler changes in patients treated with extracorporeal membrane oxygenation. J Stroke Cerebrovasc Dis 2016;25:2882–5. [DOI] [PubMed] [Google Scholar]
- 25.Salna M, Ikegami H, Willey JZ, et al. Transcranial Doppler is an effective method in assessing cerebral blood flow patterns during peripheral venoarterial extracorporeal membrane oxygenation. J Card Surg 2019;34:447–52. [DOI] [PubMed] [Google Scholar]
- 26.Cho SM, Ziai W, Mayasi Y, et al. Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J 2019. [DOI] [PubMed] [Google Scholar]
- 27.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. [DOI] [PubMed] [Google Scholar]
- 28.Gosling RG, King DH. Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med 1974;67:447–49. [PMC free article] [PubMed] [Google Scholar]