Abstract
Aims
The present study aimed to assess the association between left atrial (LA) structure and function and the risk for cardiovascular (CV) death or heart failure (HF) hospitalization in a population with atrial fibrillation (AF).
Methods and results
In a prospective echocardiographic substudy of the Effective Anticoagulation with Factor Xa Next Generation in AF-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) study, 971 patients underwent transthoracic echocardiography. The associations between LA structure (LA volume index [LAVi]) and function (LA emptying fraction [LAEF] and LA expansion index [LAEi]) and risk for the composite endpoint of CV death or HF hospitalization, and its components, were assessed. Over a median follow-up of 2.5 years, 142 patients (14.6%) experienced CV death or HF hospitalization. Higher LAVi and lower LAEF and LAEi were each associated with a higher unadjusted risk for the composite outcome and its components. After adjustment for clinical and echocardiographic confounders, only measures of impaired LA function were predictive of the composite outcome (hazard ratio [HR] per 1 standard deviation [SD] decrease in LAEF: 1.35; 95% confidence interval [CI] 1.09–1.67 [P = 0.005]; HR per 1 SD decrease in LAEi: 1.34; 95% CI 1.06–1.69 [P = 0.012]). These findings were similar regardless of left ventricular ejection fraction, history of HF or whether patients were in AF or sinus rhythm at the time of the echocardiographic examination.
Conclusions
In patients with AF, LA dysfunction was significantly associated with an increased risk for CV death or HF hospitalization and was more predictive of these outcomes than LA size. These parameters may help to identify AF patients at greatest risk for the development of HF.
Clinical Trial Registration: ClinicalTrials.gov, NCT00781391.
Keywords: Atrial fibrillation, Left atrium, Heart failure, Cardiovascular death, Echocardiography
Introduction
Atrial fibrillation (AF) is the most common chronic arrhythmia among adults, affecting more than 33 million patients worldwide.1 It represents a significant public health burden as a result of the increased levels of risk for stroke and other thromboembolic (TE) events, heart failure (HF) and death.2 Despite current guidelines, the therapeutic management of patients affected by AF remains challenging3 and cardiovascular (CV) event rates in patients with AF have not improved substantially, especially those for CV death or hospitalization for HF.4
Left atrial (LA) enlargement is associated with greater risk for TE events in patients with AF,5 and assessment of LA structure and function has been used to predict both risk for these events and the success of restoring sinus rhythm after AF ablation.6,7 Yet although many studies have focused on the risk for stroke, AF has also been shown to be associated with higher incidences of HF and mortality, regardless of left ventricular ejection fraction (LVEF).8 However, the underlying mechanisms by which AF contributes to the increased risk for HF are less clear, despite the fact that AF and HF together increase CV mortality to a greater degree than either alone. Although LA assessment provides powerful prognostic information in the general population and in subjects with CV disease,5,9,10 its role in a selected AF cohort has not been explored. Hence, LA analysis may potentially add information that is useful for stratifying AF subjects at higher risk for CV events, beyond TE events.
To understand the impact of LA structure and function on the levels of risk for CV death or hospitalization for HF in a general AF population, patients enrolled in the prespecified echocardiographic substudy of Effective Anticoagulation with Factor Xa Next Generation in AF-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48)11 were studied.
Methods
ENGAGE AF-TIMI 48 was, as described previously,11,12 a multinational, randomized (1:1:1), double-blind, double-dummy non-inferiority design trial comparing the efficacy and safety of two dosing regimens of once-daily edoxaban vs. warfarin, respectively, titrated to an international normalized ratio of 2.0–3.0 in patients with a history of AF. The study included patients with AF at moderate to high risk for TE events defined by a CHADS2 score of ≥2. Patients with a history of LA appendage closure, intracardiac mass, moderate to severe mitral stenosis and mechanical heart valve were excluded, as were patients with a life expectancy of <12 months.11 There were no exclusions based on LVEF or HF functional class.13 The prospectively designed echocardiographic substudy of ENGAGE AF-TIMI 48 was performed at 133 sites worldwide between 2009 and 2011.14
The baseline cross-sectional analysis and protocol of the ENGAGE AF-TIMI 48 echocardiographic substudy have been reported previously.14 In the present study, the longitudinal association between baseline LA structure and function and the outcomes of CV death or HF hospitalization was examined. Patients were invited before randomization to participate and echocardiographic images were obtained within the first week after randomization. Standard two-dimensional (2D) and Doppler transthoracic echocardiography was performed, and the images were sent to the echocardiography core laboratory at Brigham and Women’s Hospital (Boston, MA, USA). Echocardiographic analyses were performed by technicians blinded to clinical information and treatment assignment, and all study measurements were confirmed by a board-certified cardiologist and echocardiographer.14 The reproducibility of echocardiographic measurements was high, with an intra-observer intra-class correlation coefficient (ICC) of 0.95 (range: 0.91–0.99) and an inter-observer ICC of 0.84 (range: 0.75–0.93).14
Echocardiography
Echocardiography was performed according to the American Society of Echocardiography (ASE) guidelines.15 Left ventricular (LV) volumes were calculated using the modified Simpson method as well as LVEF. LA diameter was assessed in the parasternal long-axis view as the 2D anterior–posterior length. LA maximal volume was measured using the modified Simpson method in the apical four- and two-chamber views at the end-systolic frame preceding mitral valve opening and was indexed to body surface area (BSA) to derive LA volume index (LAVi). Similarly, LA minimal volume was measured at the end-diastolic frame preceding mitral valve closure. LA dilatation was defined as an LAVi of ≥35 mL/m2. LA emptying fraction (LAEF) was calculated as 100 * (maximal volume–minimal volume)/maximal volume and LA expansion index (LAEi) as (maximal volume–minimal volume)/minimal volume.16 Reduced LAEF was defined as <45% and reduced LAEi as <90%.14 Early transmitral velocity (E) was measured with pulsed-wave Doppler and peak lateral and septal mitral annular early relaxation velocities (e′) were derived using tissue Doppler imaging. LV filling pressures were estimated by E wave divided by average e′ velocities (E/e′). All other measures were assessed according to ASE guidelines. Rhythm at the time of echocardiography was determined from the electrocardiographic lead recorded during the examination and by analysing the transmitral flow with pulsed-wave Doppler. Final values for all parameters, in both patients with AF and those with sinus rhythm at the time of echocardiography, were taken as the means of measurements from three cardiac cycles.14
Outcomes
The endpoints for this analysis were the composite of time to first CV death or hospitalization for HF and its components (HF hospitalization alone and CV death alone). An additional sensitivity analysis for the composite of time to first death resulting from HF or hospitalization for HF was also performed. All events were adjudicated by an independent HF clinical endpoint committee, the members of which were blinded to study assignment. The median follow-up period was 2.5 years (interquartile range [IQR] 2.3–2.8 years).
Statistical methods
Summary statistics for clinical and echocardiographic characteristics are presented as the mean ± standard deviation (SD) and count (percentage) for continuous and categorical data, respectively. Statistical comparisons were made according to LA dilatation (LAVi ≥35 mL/m2) and function impairment (LAEF ≤45%) using t-tests for continuous variables and chi-squared tests for categorical variables. Univariate and multivariable Cox proportional hazards models were used to assess the associations between covariates (LAVi, LAEF, LAEi) and risk for the analysed endpoints. The unadjusted association of each measure of cardiac structure and function and clinical variables with the composite outcome of CV death or hospitalization for HF was assessed (online supplementary Table S1). A multivariable Cox model was developed using the significant variables at the unadjusted analysis and with selection of the high-risk demographic and clinical confounders. The study was not powered to assess the effect modification of the allocated therapy (warfarin vs. high-dose or low-dose edoxaban regimens) by features of LA structure and function on the outcomes. However, to control for treatment assignment in analysing the association between LA measurements and outcomes, allocated therapy was included as a covariate in the multivariate models and the potential interaction with outcomes was analysed. The final model included age, sex, diabetes, heart rate, history of HF, history of myocardial revascularization, pattern of AF (paroxysmal, persistent or permanent),17 allocated therapy, LVEF, BSA indexed LV end-diastolic volume, BSA indexed LV mass and E/e′. As LAEF and LAEi were highly collinear (r = 0.98), these two variables were not included in the same multivariable model (online supplementary Figure S1). The proportional hazards assumption was tested for the final model using Schoenfeld residuals and there was no evidence of violation of the proportional hazards assumption.
Effect modification of history of HF, LVEF and rhythm at the time of echocardiography on the relationship between LA structure and function, and the composite endpoint was tested. Outcomes according to rhythm at the time of echocardiography (i.e. sinus rhythm vs. AF) and the association of LA function in both groups were examined. Time-to-event data for the composite endpoint were also evaluated with the use of Kaplan–Meier estimates. The primary analysis was performed using available data, including missing values. An additional sensitivity analysis was performed using multiple imputation for missing data. Multiple imputation by chained equations, an iterative imputation procedure, was used. Imputation was performed for each echocardiographic and clinical measure with any missing data, and was based on linear and logistic regression and derived for 20 imputations.
All analyses were performed using Stata Version 14 (StataCorp LP, College Station, TX, USA) and P-values of <0.05 were considered to indicate differences of statistical significance.
Results
Clinical and echocardiographic characteristics
Among the 971 patients enrolled in the ENGAGE AF-TIMI 48 echocardiographic substudy, the mean age was 71 ± 9.4 years (Table 1) and the majority were male (65.6%). A total of 530 (54.6%) patients had a history of HF at baseline. Paroxysmal, persistent and permanent AF were reported by investigators in 32.8%, 20.8% and 46.3% of cases, respectively. Overall, a CHADS2 score of ≥4 was observed in 21.9% and a CHA2DS2-VASc score of ≥5 in 42.0% patients. At the time of echocardiography, 321 patients (33.1%) were in sinus rhythm. Of these, the majority (74.0%) had a history of paroxysmal AF.
Table 1.
Clinical and echocardiographic characteristics of the ENGAGE AF-TIMI 48 echocardiographic substudy in the overall population and stratified according to left atrial structure and function
| Overall population (n = 971) | Normal LA volume (LAVi <35 mL/m2) (n = 591) | Enlarged LA volume (LAVi ≥35 mL/m2) (n = 380) | P-value | Normal LA function (LAEF >45%) (n = 252) | Reduced LA function (LAEF ≤45%) (n = 717) | P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 71 ± 9.4 | 71.2 ± 9.5 | 72.7 ± 9.3 | 0.02 | 71.8 ± 9.4 | 71.8 ± 9.4 | 0.94 |
| Women | 334 (34.4%) | 198 (33.5%) | 136 (35.8%) | 0.46 | 94 (37.3%) | 239 (33.3%) | 0.25 |
| CHA2DS2-VASc | 0.08 | 0.96 | |||||
| 2 | 70 (7.2%) | 46 (7.8%) | 24 (6.3%) | 18 (7.1%) | 52 (7.3%) | ||
| 3 | 205 (21.1%) | 138 (23.4%) | 67 (17.6%) | 51 (20.2%) | 154 (21.5%) | ||
| 4 | 288 (29.7%) | 181 (30.6%) | 107 (28.2%) | 81 (32.1 %) | 207 (28.9%) | ||
| ≥5 | 408 (42.0%) | 226 (38.2%) | 182 (47.9%) | 102 (41.0%) | 304 (43.0%) | ||
| History of heart failure | 530 (54.6%) | 282 (47.7%) | 248 (65.3%) | <0.001 | 120 (47.6%) | 410 (57.2%) | 0.009 |
| NYHA class I–II | 382 (39.4%) | 204 (34.5%) | 178 (46.8%) | <0.001 | 82 (32.5%) | 300 (41.8%) | 0.009 |
| NYHA class III–IV | 117 (12.0%) | 56 (9.5%) | 61 (16.1%) | 0.002 | 28 (11.1%) | 89 (12.4%) | 0.59 |
| Sinus rhythm at the time of echocardiography | 321 (33.1%) | 265 (44.8%) | 56 (14.7%) | <0.001 | 132 (52.4%) | 187 (26.1%) | <0.001 |
| Diabetes | 346 (35.6%) | 220 (37.2%) | 126 (33.2%) | 0.2 | 90 (35.7%) | 256 (35.7%) | >0.99 |
| Dyslipidaemia | 623 (64.2%) | 398 (67.3%) | 225 (59.2%) | 0.01 | 177 (70.2%) | 446 (62.2%) | 0.022 |
| History of hypertension | 917 (94.4%) | 555 (93.9%) | 362 (95.3%) | 0.37 | 232 (92.1%) | 683 (95.3%) | 0.06 |
| History of myocardial revascularization | 199 (20.5%) | 123 (20.8%) | 76 (20.0%) | 0.75 | 47 (18.7%) | 152 (21.2%) | 0.4 |
| Current smoking | 100 (10.3%) | 70 (11.8%) | 30 (7.9%) | 0.048 | 39 (15.5%) | 61 (8.5%) | 0.002 |
| BMI, kg/m2 | 29.9 ± 6.3 | 30.3 ± 6.6 | 29.3 ± 5.9 | 0.011 | 29.3 ± 6.4 | 30.2 ± 6.3 | 0.06 |
| Heart rate, b.p.m. | 73 ± 15 | 72.4 ± 15.2 | 75.0 ± 13.4 | 0.008 | 72.5 ± 14.5 | 73.8 ± 14.6 | 0.22 |
| Systolic blood pressure, mmHg | 130.3 ± 17.1 | 131.0 ± 17.5 | 129.2 ± 16.4 | 0.11 | 130.1 ± 17.5 | 130.3 ± 16.9 | 0.87 |
| Treatment with β-blockers | 657 (67.7%) | 393 (66.5%) | 264 (69.5%) | 0.33 | 159 (63.1%) | 497 (69.3%) | 0.07 |
| Treatment with ACEi or ARB | 565 (58.2%) | 319 (54.0%) | 246 (64.7%) | <0.001 | 137 (54.4%) | 427 (59.6%) | 0.15 |
| Treatment with MRA | 573 (59.0%) | 324 (54.8%) | 249 (65.5%) | <0.001 | 140 (55.6%) | 432 (60.3%) | 0.19 |
| Treatment with diuretics | 579 (59.6%) | 320 (54.1%) | 259 (68.2%) | <0.001 | 124 (49.2%) | 454 (63.3%) | <0.001 |
| Treatment with CCB | 287 (29.6%) | 180 (30.5%) | 107 (28.2%) | 0.44 | 87 (34.5%) | 199 (27.8%) | 0.043 |
| AF pattern at randomization | <0.001 | <0.001 | |||||
| Paroxysmal | 319 (32.9%) | 242 (40.9%) | 77 (20.3%) | 125 (49.6%) | 192 (26.8%) | ||
| Persistent | 202 (20.8%) | 130 (22.0%) | 72 (18.9%) | 42 (16.7%) | 160 (22.3%) | ||
| Permanent | 450 (46.3%) | 219 (37.1%) | 231 (60.8%) | 85 (33.7%) | 365 (50.9%) | ||
| History of AF ablation | 59 (6.1%) | 43 (7.3%) | 16 (4.2%) | 0.050 | 17 (6.7%) | 42 (5.9%) | 0.61 |
| CrCl, mL/min | 77 ± 32 | 79.9 ± 32.8 | 72.2 ± 30.9 | <0.001 | 76.4 ± 35.1 | 77.1 ± 31.2 | 0.78 |
| Treatment with anti-arrhythmic drugs | 186 (19.2%) | 136 (23.0%) | 50 (13.2%) | <0.001 | 64 (25.4%) | 121 (16.9%) | 0.003 |
| Treatment with ASA | 292 (30.1%) | 198 (33.5%) | 94 (24.7%) | 0.004 | 82 (32.5%) | 209 (29.1%) | 0.31 |
| Allocated therapy | 0.48 | 0.74 | |||||
| Warfarin | 327 (33.7%) | 207 (35.0%) | 120 (31.6%) | 84 (33.3%) | 242 (33.8%) | ||
| Edoxaban (low-dose regimen) | 337 (34.7%) | 204 (34.5%) | 133 (35.0%) | 92 (36.5%) | 244 (34.0%) | ||
| Edoxaban (high-dose regimen) | 307 (31.6%) | 180 (30.5%) | 127 (33.4%) | 76 (30.2%) | 231 (32.2%) | ||
| LVEF, % | 54.3 ± 10.5 | 56.4 ± 8.2 | 51.2 ± 12.7 | <0.001 | 57.1 ± 7.8 | 53.4 ± 11.1 | <0.001 |
| LVEF <50% | 217 (22.3%) | 90 (15.2%) | 127 (33.4%) | <0.001 | 37 (14.7%) | 180 (25.1%) | <0.001 |
| LVEDVi, mL/m2 | 58 ± 13 | 55.6 ± 10 | 62.6 ± 15.5 | <0.001 | 55.9 ± 10.5 | 59.2 ± 13.6 | <0.001 |
| LVMi, g/m2 | 76 ± 26 | 71.1 ± 21.4 | 83.5 ± 30.8 | <0.001 | 73.5 ± 28.1 | 76.8 ± 25.5 | 0.09 |
| LAVi, mL/m2 | 34 ± 11 | 27.4 ± 4.8 | 44.6 ± 9.7 | by design | 27.7 ± 7.4 | 36.5 ± 11.1 | <0.001 |
| LAEF, % | 37.7 ± 10 | 41.5 ± 8.8 | 31.8 ± 8.8 | <0.001 | 50.1 ± 4.0 | 33.3 ± 7.5 | by design |
| LAEi, % | 65 ± 28 | 74.9 ± 27.1 | 49.1 ± 20.4 | <0.001 | 101.7 ± 18.3 | 51.8 ± 16.5 | <0.001 |
| E/e′ average | 11.7 ± 4.6 | 11.5 ± 4.5 | 12.1 ± 4.9 | 0.042 | 11.4 ± 4.2 | 11.9 ± 4.8 | 0.19 |
| MR (moderate/severe) | 99 (10.8%) | 31 (5.6%) | 68 (18.7%) | <0.001 | 13 (5.5%) | 86 (12.6%) | 0.002 |
| RVSP, mmHga | 23.7 ± 7.7 | 22.6 ± 6.4 | 25.0 ± 8.9 | <0.001 | 22.0 ± 6.3 | 24.2 ± 8.0 | 0.004 |
Data are expressed as count (percentage) or mean ± standard deviation.
ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ASA, aspirin; BMI, body mass index; CCB, calcium channel blocker; CrCl creatinine clearance; LA, left atrial; LAEF, left atrial emptying fraction; LAEi, left atrial expansion index; LAVi, left atrial volume index; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVMi, left ventricular mass index; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; RVSP, right ventricular systolic pressure.
RVSP was measurable in 587 of 971 patients (60.5%).
On average, LVEF was preserved (mean ± SD 54.3 ± 10.5%) and 22.0% of patients had LVEF of <50%. Mean ± SD LAVi was 34.2 ± 11 mL/m2 and LA enlargement was present in 39.0% of subjects. Patients with LA enlargement were older and had higher prevalences of history of HF and permanent AF at the time of echocardiography. Higher LA volume was also related to worse LV cardiac structure and function (Table 1).
On average, LAEF was reduced (37.7 ± 10%) with 74.0% of patients showing impaired function (LAEF <45%); among patients with normal LA structure, more than half (62.0%) showed reduced LAEF. Mean ± SD LAEi was 64.8 ± 27.7% and was abnormal in 82.0% of patients and reduced in 71.0% of those with normal LAVi. Patients with reduced LAEF had higher prevalences of history of HF and permanent AF. No significant differences in LA function were observed in relation to the allocated therapy. Lower LA function was also associated with lower LVEF and higher LV volume (Table 1).
Risk for heart failure hospitalization and cardiovascular mortality
During the follow-up, 142 (14.6%) patients experienced the composite endpoint of CV death or HF hospitalization at the rate of 6.0 per 100 person-years (95% confidence interval [CI] 5.1–7.1; online supplementary Table S2). Hospitalization for HF occurred in 93 (9.6%) patients and 64 (6.6%) deaths were attributable to CV events. Hospitalization for HF was more common than TE events (9.6% vs. 4.9%) (online supplementary Table S3) and CV death was the most common cause of death (71.9%) in the total population.
Higher LAVi and lower LAEF and LAEi were significantly associated with greater levels of risk for the composite endpoints and for HF hospitalization and CV death alone in the unadjusted analysis (Table 2 and online supplementary Figure S2). The relationship between parameters of LA structure and function and the outcomes was linear and higher values of LAVi and lower values of LAEF and LAEi were associated with increased risk.
Table 2.
Association of left atrial structure and function and the analysed endpoints
| Unadjusted |
Adjusted |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Events/person-years at risk | HR (95% CI) | P-value | n | Events/person-years at risk | HR (95% CI) | P-value | |
| HF hospitalization or CV death (n = 142) | ||||||||
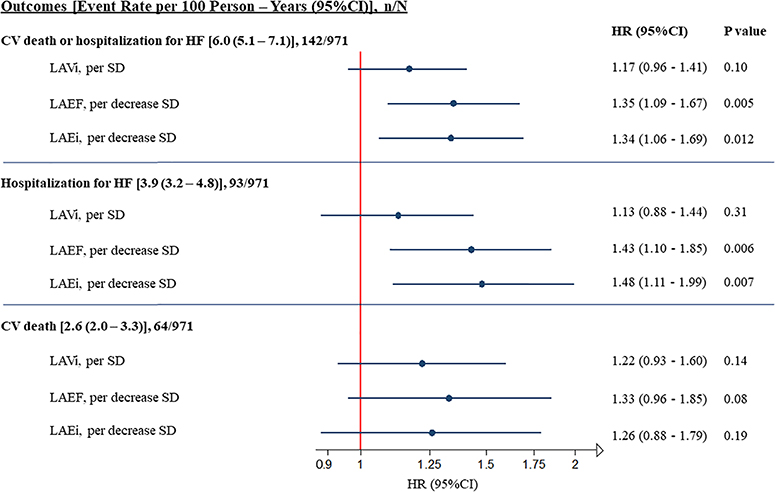
| LAVi, per SD | 971 | 142/2338 | 1.34 (1.17–1.52) | <0.001 | 776 | 103/1888 | 1.17 (0.96–1.41) | 0.10 |
| LAEF, per decrease SD | 969 | 142/2333 | 1.41 (1.19–1.66) | <0.001 | 776 | 103/1888 | 1.35 (1.09–1.67) | 0.005 |
| LAEi, per decrease SD | 969 | 142/2333 | 1.40 (1.16–1.68) | <0.001 | 776 | 103/1888 | 1.34 (1.06–1.69) | 0.012 |
| HF hospitalization (n = 93) | ||||||||
| LAVi, per SD | 965 | 93/2338 | 1.32 (1.12–1.55) | <0.001 | 776 | 69/1888 | 1.13 (0.88–1.44) | 0.31 |
| LAEF, per decrease SD | 969 | 93/2333 | 1.42 (1.15–1.75) | 0.001 | 776 | 69/1888 | 1.43 (1.10–1.85) | 0.006 |
| LAEi, per decrease SD | 969 | 93/2333 | 1.44 (1.14–1.81) | 0.002 | 776 | 69/1888 | 1.48 (1.11–1.99) | 0.007 |
| CV death (n = 64) | ||||||||
| LAVi, per SD | 971 | 64/2462 | 1.36 (1.13–1.65) | 0.001 | 776 | 44/1983 | 1.22 (0.93–1.60) | 0.14 |
| LAEF, per decrease SD | 969 | 64/2457 | 1.41 (1.10–1.81) | 0.006 | 776 | 44/1983 | 1.33 (0.96–1.85) | 0.08 |
| LAEi, per decrease SD | 969 | 64/2457 | 1.38 (1.05–1.82) | 0.018 | 776 | 44/1983 | 1.26 (0.88–1.79) | 0.19 |
Adjustment for: age, sex, diabetes, heart rate, history of heart failure (HF), history of myocardial revascularization, atrial fibrillation pattern, allocated therapy, left ventricular (LV) end-diastolic volume index, LV ejection fraction, LV mass index and E/e′. CI, confidence interval; CV, cardiovascular; HR, hazard ratio; LAEF, left atrial emptying fraction; LAEi, left atrial expansion index; LAVi, left atrial volume index by body surface area; SD, standard deviation.
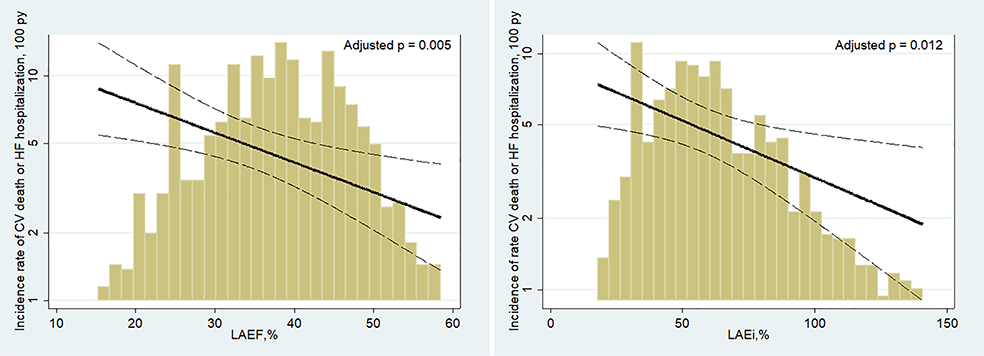
When analysed by subgroup according to the degree of functional impairment, LAEF and LAEi improved risk stratification for the composite outcome (online supplementary Figure S3), with event rates among patients with severely impaired LA function (LAEF of ≤25% and LAEi of ≤60%) rising significantly over time (P-values for trend: 0.003 and 0.004, respectively). After adjustment for demographic, clinical and echocardiographic confounders, LAVi was no longer significantly associated with all the analysed outcomes. However, each 1-SD decrease in LAEF was associated with a 35% higher risk for the composite endpoint of CV death or HF hospitalization (hazard ratio [HR] per 1-SD decrease 1.35; 95% CI 1.09–1.67; P = 0.005) and each 1-SD decrease in LAEi was associated with a 34% higher risk (HR per 1-SD decrease 1.34; 95% CI 1.06–1.69; P = 0.012) (Table 2; Figures 1 and 2). Similar results were observed for HF hospitalization alone and for the composite of death attributable to HF or HF hospitalization (online supplementary Table S4). Although point estimates were similar for CV death alone, this endpoint was underpowered (Table 2 and Figure 2).
Figure 1.
Adjusted association of left atrial emptying fraction (LAEF) and left atrial expansion index (LAEi) for the composite endpoint of cardiovascular (CV) death or hospitalization for heart failure (HF). The gold bars represent the distribution of LAEF and LAEi measures reported as percentages. The solid black lines represent an estimation by Poisson regression of the association between LAEF and LAEi and the outcome after adjusting for age, sex, diabetes, heart rate, history of HF, history of myocardial revascularization, atrial fibrillation pattern, allocated therapy, left ventricular (LV) end-diastolic volume index, LV mass index, LV ejection fraction and E/e′. Dashed lines represent 95% confidence intervals. Incidence rates are displayed on the y-axis. py, person-years.
Figure 2.
Adjusted association of measures of left atrial (LA) structure and function and the composite endpoint of cardiovascular (CV) death or hospitalization for heart failure (HF) and its components. Each 1-standard deviation (SD) decrease in LA emptying fraction (LAEF) and LA expansion index (LAEi), but not LA volume index (LAVi), was associated with an increased risk for the composite endpoint of CV death or HF hospitalization and for HF hospitalization alone. Point estimates were similar for CV death alone. The reported adjusted hazard ratio (HR) and 95% confidence interval (CI) are adjusted for age, sex, diabetes, heart rate, history of HF, history of myocardial revascularization, atrial fibrillation pattern, allocated therapy, left ventricular (LV) end-diastolic volume index, LV mass index, LV ejection fraction and E/e′.
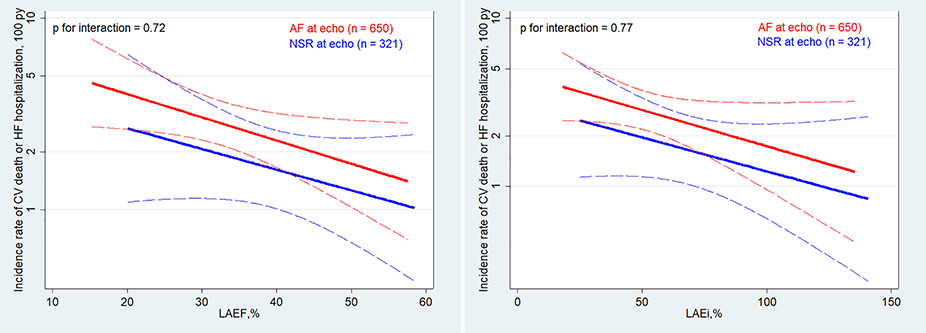
No differences were observed in the relationship between LA dysfunction and outcomes based on baseline LVEF, history of HF and allocated therapy (warfarin vs. high-dose or low-dose edoxaban regimens) (all P-values for interaction >0.05). Moreover, the prognostic value of both LAEF and LAEi was similar regardless of whether the patients were in sinus rhythm or AF at the time of echocardiography (Figure 3 and online supplementary Table S5), although patients in sinus rhythm had lower event rates (4.9 vs. 6.6 per 100 patient-years). Similar results were obtained, including for mitral regurgitation, LA dilatation, New York Heart Association class, history of AF ablation, systolic blood pressure and medical treatment, in the multivariable model (online supplementary Table S5). The overall findings with respect to LAEF and LAEi were not substantially altered in a sensitivity analysis using multiple imputation for missing data (online supplementary Table S6).
Figure 3.
Adjusted association between left atrial emptying fraction (LAEF) and left atrial expansion index (LAEi) and the composite endpoint stratified by rhythm at the time of echocardiography (atrial fibrillation [AF] vs. normal sinus rhythm [NSR]). The red and blue lines represent an estimation by Poisson regression of the association between LAEF and LAEi and the outcome stratified by cardiac rhythm at the time of echocardiography after adjusting for age, sex, diabetes, heart rate, history of heart failure (HF), history of myocardial revascularization, AF pattern, allocated therapy, left ventricular (LV) end-diastolic volume index, LV mass index, LV ejection fraction and E/e′. Dashed lines represent 95% confidence intervals. Incidence rates are displayed on the y-axis. P-values refer to interactions between the two groups. CV, cardiovascular; py, person-years.
Discussion
In a contemporary cohort of AF patients enrolled in the ENGAGE AF-TIMI 48 echocardiographic substudy, abnormalities of LA function were found to be associated with increased risk for CV death and HF hospitalization. In particular, both LAEF, a measure of global LA function, and LAEi, a measure of LA compliance affecting reservoir function, were related to outcomes, especially HF hospitalization, after multivariable adjustment for multiple confounders, including LA dilatation. LA size was related to outcomes only in unadjusted analyses. LA functional impairment was identified in approximately half of patients with normal LA structure, which suggests that LA dysfunction may precede LA enlargement, and that LA dysfunction may increase the risk for CV death and HF hospitalization despite normal LA size.
The present results corroborate those of previous studies that showed the incremental value of LA function over LA dimension for risk assessment in patients with and without heart disease.5 In particular, LAEF has been shown to be more strongly associated with mortality than LA volume in the general population.9
Left atrial dysfunction is also a strong predictor of CV outcomes in patients with HF and reduced or preserved ejection fraction, and in patients with stable coronary heart disease.9,10,18–20 However, little is known about its prognostic value in predicting CV outcomes beyond TE events in patients with AF.
In the current study, HF hospitalization occurred at a rate more than 1.5-fold higher than that of TE events. This is of particular interest, given that LA analysis has often been used for TE risk stratification even if the rate of strokes in AF is decreasing over time, and event rates related to progressive symptomatic HF and CV death have not improved substantially.1,21 AF is associated with the onset of HF with both reduced and preserved ejection fraction, and both conditions together portend a poorer prognosis in terms of CV mortality than either condition alone.8
Although it is not clear how AF contributes directly to the risk for HF, abnormalities of LA function may play a key role in this process.22 From this perspective the present findings may add significant information for use in stratifying AF subjects at high risk of events beyond thromboembolism. The left atrium is commonly considered a buffer chamber between the pulmonary circulation and the left ventricle and its changes are thought to be indirectly related to LV function.5 Chronic exposure to high LV filling pressure initiates an adaptative process leading to LA enlargement and dysfunction. However, as the current data showed that LA functional impairment was predictive of HF outcomes regardless of LA size and was independent of the common measures of LV structure and function (i.e. LV mass, LVEF and E/e′), it is possible that underlying LA functional impairment may indeed contribute to the risk for symptomatic HF and that it does not represent an innocent bystander.
The present data suggest that LA function is an important determinant of outcome regardless of rhythm, as it was predictive of outcome even in patients in sinus rhythm at the time of echocardiography. The loss of global atrial function may directly affect LV output, especially during exercise.5 In addition, reduced LA wall compliance, which influences atrial reservoir function, results in elevated LA pressure which consequently can lead to increased pulmonary venous and arterial pressure and thus HF symptoms. From this perspective, LA dysfunction may contribute to the increased incidence of outcomes in high-risk patients.
The pathological changes related to these mechanisms may reflect a spectrum of atrial tissue structural alterations leading to atrial impairment, including myocyte hypertrophy, necrosis, apoptosis and changes in the composition of the extracellular matrix.5,20,23 Atrial fibrosis and the related electrical alterations (such as ion channel dysfunction and alterations in cellular calcium handling) may represent the main trigger for episodes of AF.24 Once AF develops, it may lead to increased severity of fibrosis which, in turn, results in increased substrate for atrial arrhythmias. Because AF begets HF, this vicious circle is likely to lead to worse outcomes than those in patients without AF.25 Interestingly, the changes affecting LA function can occur before LA enlargement and may represent early processes affecting the LA wall even if the chamber is not dilated. Importantly, LA dilatation has been shown to be reversible after medical therapy,26,27 and LA reverse remodelling is associated with improved CV outcomes.28 Ablation therapy for AF has been shown to improve outcomes in patients with HF and reduced ejection fraction.29 Whether ablation therapy or the initiation of medical therapies that have been beneficial in HF at a point when LA dysfunction is detected will result in improved outcomes is unknown, and further studies will need to assess whether therapeutic interventions that improve LA function would improve HF outcomes in a broad range of patients.
By design, the ENGAGE AF-TIMI 48 enrolled a moderate- to high-risk AF population (CHADS2 score ≥2) and hence the current analysis does not include the entire spectrum of patients with AF. Although the irregular rhythm of AF leads to beat-to-beat variability in echocardiographic assessment, the present study averaged measures of cardiac structure and function over three cardiac cycles. In addition, some echocardiographic views or measures, particularly Doppler measures, were missing. However, a sensitivity analysis using multiple imputations to account for missing data showed similar findings to the primary analysis (online supplementary Table S5). The current analysis did not include novel measures of cardiac mechanics, such as strain from speckle-tracking echocardiography, which may provide measures of LA function that are less dependent on LV function and loading conditions and may add additional prognostic information.30 Data on natriuretic peptides were available in fewer than half of the study population. For this reason, biomarkers were not incorporated in the present analysis. The analysis was based on baseline LA cardiac structure and function and does not account for changes over time that may contribute to CV events. Finally, the study was not powered to assess the relationship between LA function and outcome within the individual subgroups (i.e. by history of HF or LVEF).
In conclusion, in a contemporary population of patients with AF enrolled in the echocardiographic substudy of the ENGAGE AF-TIMI 48 trial, the outcome of CV death or HF hospitalization was found to occur at a higher rate than TE events and worse LA function was associated with greater risk for events, especially HF hospitalization, even in patients with normal LA size. These parameters may be useful in clinical practice to stratify patients with AF at increased risk for death caused by CV events or hospitalization for HF, and may provide a therapeutic target for medical treatment to improve LA function and consequently survival.
Supplementary Material
Acknowledgments
Funding
The ENGAGE AF-TIMI 48 trial was funded by Daiichi Sankyo.
Conflict of interest: R.M.I. reports consulting fees from Daiichi Sankyo. R.P.G. reports the receipt of grants by his institution from Daiichi Sankyo during the conduct of the study, and the receipt of grants by his institution from Abbott Laboratories, AstraZeneca, Critical Diagnostics, Eisai, GlaxoSmithKline, Intarcia, Roche Diagnostics, Takeda, Gilead, Poxel, Novartis, MedImmune and Genzyme, and grants and personal fees from Amgen, Daiichi Sankyo, Merck and Janssen Research Development, and personal fees from Boehringer-Ingelheim, Bristol-Myers-Squibb, Lexicon, Pfizer and Portola, outside the submitted work. C.T.R. discloses awards of institutional research grants to the TIMI Study Group at Brigham and Women’s Hospital from Abbott Laboratories, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eisai, Gilead, GlaxoSmithKline, Intarcia, Janssen Research and Development, Medicines Company, MedImmune, Merck, the National Institutes of Health, Novartis, Poxel, Pfizer, Roche Diagnostics and Takeda. C.T.R. also discloses the receipt of honoraria for scientific advisory board and consulting services from Bayer, Bristol-Myers-Squibb, Boehringer Ingelheim, Daiichi Sankyo, Janssen, MedImmune, Pfizer and Portola. E.M.A. reports the receipt of grant support through his institution from Daiichi Sankyo. M.F.M. was previously employed by Daiichi Sankyo. M.A.G. is employed by Daiichi Sankyo. E.B. reports the receipt of grant support through his institution from Daiichi Sankyo, Astra Zeneca, Glaxo SmithKline, Merck, Novartis, consulting fees from Cardurion, MyoKardia, Sanofi and Verve, lecture fees from Medscape, and has delivered uncompensated consultancies and lectures for, the Medicines Company and Novartis. S.D.S. has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, the National Institutes of Health National Heart, Blood and Lung Institute, Novartis, Sanofi Pasteur and Theracos, and has consulted for Akros, Alnylam, Amgen, AstraZeneca, Bayer, BMS, Cardior, Corvia, Cytokinetics, Daiichi Sankyo, Gilead, GSK, Ironwood, Merck, MyoKardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions and Tenaya. The other authors report no conflicts of interest.
Footnotes
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murrayet CJL. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SH, Killian JM, Weston SA, Roger VL. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med 2015;128:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 6.Gupta DK, Giugliano RP, Ruff CT, Claggett B, Murphy S, Antman E, Mercuri MF, Braunwald E, Solomon SD. The prognostic significance of cardiac structure and function in atrial fibrillation: the ENGAGE AF-TIMI 48 echocardiographic substudy. J Am Soc Echocardiogr 2016;29:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motoki H, Negishi K, Kusunose K, Popovic ZB, Bhargava M, Wazni OM, Saliba WI, Chung MK, Marwick TH, Klein AL. Global left atrial strain in the prediction of sinus rhythm maintenance after catheter ablation for atrial fibrillation. J Am Soc Echocardiogr 2014;27:1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santhanakrishnan R, Wang N, Larson M, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM, Drazner MH. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J 2013;34:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol 2012;59:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 12.Ruff CT, Giugliano RP, Antman EM, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D, McCabe CH, Braunwald E. Evaluation of the novel factor Xa inhibitor edoxabancompared with warfarin in patients with atrialfibrillation: design and rationale for the Effective a Nticoa Gulation with factor xA next GEneration inAtrial Fibrillation-Thrombolysis In MyocardialInfarction study 48 (ENGAGE AF-TIMI 48). Am Heart J. 2010;160(4):635–641. [DOI] [PubMed] [Google Scholar]
- 13.Magnani G, Giugliano RP, Ruff CT, Murphy SA, Nordio F, Metra M, Moccetti T, Mitrovic V, Shi M, Mercuri M, Antman EM, Braunwald E. Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF-TIMI 48. Eur J Heart Fail 2016;18:1153–1161. [DOI] [PubMed] [Google Scholar]
- 14.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman H, Patel I, Shi M, Mercuri M, Mitrovic M, Braunwald E, Solomon SD. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J 2014;35:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 16.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 17.Link MS, Giugliano RP, Ruff C, Scirica BM, Huikuri H, Oto A, Crompton AE, Murphy SA, Lanz H, Mercuri MF, Antman EM, Braunwald E; ENGAGE AF-TIMI 48 Investigators. Stroke and mortality risk in patients with various patterns of atrial fibrillation results from the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol 2017;10:e004267. [DOI] [PubMed] [Google Scholar]
- 18.Sargento L, Vicente Simoes A, Longo S, Lousada N, Palma dos Reis R. Left atrial function index predicts long-term survival in stable outpatients with systolic heart failure. Eur Heart J Cardiovasc Imaging 2017;18:119–127. [DOI] [PubMed] [Google Scholar]
- 19.Malagoli A, Rossi L, Bursi F, Zanni A, Sticozzi C, Piepoli MF, Quinto Villani G. Left atrial function predicts cardiovascular events in patients with chronic heart failure with reduced ejection fraction. J Am Soc Echocardiogr 2019;32:248–256. [DOI] [PubMed] [Google Scholar]
- 20.Rossi A, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction structure, function, and significance. Circ Heart Fail 2014;7:1042–1049. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano RP, Ruff CT, Wiviott SD, Nordio F, Murphy SA, Kappelhof JA, Shi M, Mercuri MF, Antman EM, Braunwald E. Mortality in patients with atrial fibrillation randomized to edoxaban or warfarin: insights from the ENGAGE AF-TIMI 48 Trial. Am J Med 2016;129:850–857. [DOI] [PubMed] [Google Scholar]
- 22.Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J. Global left atrial failure in heart failure. Eur J Heart Fail 2016;18:1307–1320. [DOI] [PubMed] [Google Scholar]
- 23.Kojima T, Kawasaki M, Tanaka R, Ono K, Hirose T, Iwama M, Watanabe T, Noda T, Watanabe S, Takemura G, Minatoguchi S. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging 2012;13:227–234. [DOI] [PubMed] [Google Scholar]
- 24.Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol 2008;51:1–11. [DOI] [PubMed] [Google Scholar]
- 25.Allessie MA. Atrial electrophysiologic remodeling: another vicious circle. J Cardiovasc Electrophysiol 1998;9:1378–1393. [DOI] [PubMed] [Google Scholar]
- 26.Thomas L, Abhayaratna WP. Left atrial reverse remodeling mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 2017;10:65–77. [DOI] [PubMed] [Google Scholar]
- 27.Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, Hall WJ, Pfeffer MA, Moss AJ; MADIT-CRT Investigators. Effect of cardiac resynchronization therapy of reverse remodeling and outcome. Circulation 2010;122:985–992. [DOI] [PubMed] [Google Scholar]
- 28.Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Køber L, Bourgoun M, McMurray JJ, Velazquez EJ, Maggioni AP, Ghali J, Arnold JM, Zelenkofske S, Pfeffer MA, Solomon SD. Left atrial remodeling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo study. Eur Heart J 2009;30:56–65. [DOI] [PubMed] [Google Scholar]
- 29.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D; CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 30.Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, Mondillo S. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol 2012;110:264–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.