Abstract
Background:
Kidney tubulointerstitial fibrosis marks risk for allograft failure in kidney transplant recipients, but is poorly captured by estimated glomerular filtration rate (eGFR) or urine albumin-creatinine ratio (ACR). Whether urinary markers of tubulointerstitial fibrosis can noninvasively identify risk for allograft failure above and beyond eGFR and ACR is unknown.
Study Design:
Case-cohort study.
Setting & Participants:
The FAVORIT (Folic Acid for Vascular Outcome Reduction in Transplantation) Trial was a randomized double-blind trial testing vitamin therapy to lower homocysteine levels in stable kidney transplant recipients. We selected a subset of participants at random (n = 491) and all individuals with allograft failure during follow-up (cases; n = 257).
Predictor:
Using spot urine specimens from the baseline visit, we measured 4 urinary proteins known to correlate with tubulointerstitial fibrosis on biopsy (urine α1-microglobulin [A1M], monocyte chemoattractant protein 1 [MCP-1], and procollagen type III and type I amino-terminal amino pro-peptide).
Outcome:
Death-censored allograft failure.
Results:
In models adjusted for demographics, chronic kidney disease risk factors, eGFR, and ACR, higher concentrations of urine AIM (HR per doubling, 1.73; 95% CI, 1.43–2.08) and MCP-1 (HR per doubling, 1.60; 95% CI, 1.32–1.93) were strongly associated with allograft failure. When additionally adjusted for concentrations of other urine fibrosis and several urine injury markers, urine A1M (HR per doubling, 1.76; 95% CI, 1.27–2.44]) and MCP-1 levels (HR per doubling, 1.49; 95% CI, 1.17–1.89) remained associated with allograft failure. Urine procollagen type III and type I levels were not associated with allograft failure.
Limitations:
We lack kidney biopsy data, BK titers, and HLA antibody status.
Conclusions:
Urine measurement of tubulointerstitial fibrosis may provide a noninvasive method to identify kidney transplant recipients at higher risk for future allograft failure, above and beyond eGFR and urine ACR.
Keywords: Fibrosis, kidney transplantation, allograft failure, risk factor, inflammation, biomarker, urinary marker, tubulo-interstitial fibrosis, α1-microglobulin (A1M), monocyte chemoattractant protein 1 (MCP-1), kidney transplant recipient (KTR), end-stage renal disease (ESRD), case-cohort
In clinical practice, assessment of kidney function primarily focuses on markers of glomerular function (serum creatinine and, occasionally, cystatin C concentrations to estimate glomerular filtration rate [GFR]), as well as urine albumin-creatinine ratio (ACR) and proteinuria.1 However, tubulointerstitial fibrosis is commonly observed on kidney biopsy, even among persons with preserved estimated GFRs (eGFRs) and without elevated ACRs. Large biopsy studies of healthy kidney donors show that although tubulointerstitial fibrosis is common and increases with age, the degree of fibrosis is not associated with GFR when age is taken into account.2 The severity of tubulointerstitial fibrosis on biopsy is strongly associated with progressive loss of eGFR across kidney disease causes.3–5 Thus, tubulointerstitial fibrosis is common and of prognostic importance, yet clinical markers of kidney function do not capture its presence well.
Interstitial fibrosis and tubular atrophy (formerly chronic allograft nephropathy) are highly prevalent in kidney transplant allografts. Common causes of progressive loss of eGFR in kidney transplant recipients include recurrent acute rejection episodes, calcineurin inhibitor toxicity, and viral infections. All these are characterized by damage to tubular epithelial cells and may promote the development of interstitial fibrosis and tubular atrophy. Biopsies allow detection and quantification of tubulointerstitial fibrosis but are invasive, assess only a small portion of the kidney, and provide only a snapshot of kidney health at one point in time. Noninvasive methods to assess and monitor the degree of fibrosis may allow identification of kidney transplant recipients at high risk for allograft failure and may allow clinicians to assess the responses to changes in therapy over time.
In prior work, we have identified noninvasive markers of tubular injury and dysfunction and tubulointerstitial fibrosis in older adults and persons with human immunodeficiency virus (HIV) infection.6–12 Here, we extend that work to kidney transplant recipients for the first time. We selected 4 urinary measures that have been associated with degree of tubulointerstitial fibrosis in biopsy studies and determined their relationships with future allograft failure. We evaluated urine concentrations of α1-microglobulin (A1M), monocyte chemoattractant protein 1 (MCP-1), and procollagen amino-terminal pro-peptides of type I (PINP) and type III (PIIINP). Briefly, A1M is a low-molecular-weight protein that is freely filtered at the glomerulus but reabsorbed by proximal tubular epithelial cells under healthy conditions13; elevated urine A1M levels indicate tubulointerstitial fibrosis and decreased proximal tubular reabsorptive capacity.14,15 The potent chemokine MCP-1 is expressed by renal tubular epithelial cells, fibroblasts, and mononuclear cells, and higher concentrations in urine have been associated with degree of tubulointerstitial fibrosis in patients with immunoglobulin A nephropathy and diabetic nephropathy.16 During collagen deposition, PINP and PIIINP are cleaved from type 1 and type 3 collagen fibrils and released into urine. As with A1M and MCP-1, prior studies in a variety of kidney diseases have demonstrated that urine PIIINP concentrations are correlated with severity of tubulointerstitial fibrosis on biopsy,15,17,18 and higher baseline urine PIIINP concentrations are associated with longitudinal decline in kidney function in community-living elderly persons.10
To our knowledge, no prior study has jointly evaluated the relationship of urine concentrations of these proteins with risk for allograft failure in kidney transplant recipients. We hypothesized that higher urine concentrations of each marker would be associated with allograft failure, independent of chronic kidney disease (CKD) risk factors, baseline eGFR and ACR, and urinary concentrations of the other 3 markers.
METHODS
Study Population
The current report was designed as an ancillary study of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial (ClinicalTrials.gov study number: NCT00064753), a multicenter, double-blind, randomized, controlled trial designed to determine whether lowering homocysteine levels with vitamin therapy reduced the rate of cardiovascular outcomes. The FAVORIT Trial protocol was approved by institutional review boards at participating institutions and the data coordinating center (study # 98–0449), and all participants provided written informed consent. The trial design and primary results have been described in detail elsewhere.19–22 From August 2002 through January 2007, a total of 4,110 kidney transplant recipients aged 35 to 75 years who were at least 6 months post— kidney transplantation were enrolled at 30 transplantation centers in the United States, Canada, and Brazil. Participants were randomly assigned to either a standard multivitamin with high doses of folic acid, vitamin B6, and vitamin B12 or a multivitamin containing low doses of vitamin B6 and vitamin B12 with no folic acid. Entry criteria included elevated serum homocysteine level (≥11 μmol/L for women; ≥ 12 mmol/L for men) and stable kidney function, defined as estimated creatinine clearance ≥ 30 mL/min in men and ≥25 mL/min in women. Follow-up contacts occurred every 6 months through January 31, 2010, to obtain study-related outcomes through June 24, 2009. The primary outcome was pooled incident or recurrent cardiovascular disease (CVD) events. Transplant failure and all-cause mortality were secondary outcomes. As reported previously, there were no significant differences between treatment groups for primary or secondary outcomes.21
We designed this analysis as a case-cohort study to minimize specimen needs and expense while retaining statistical power. We identified a 530-member subcohort randomly selected from the entire cohort that had been included in a prior ancillary study; these participants had been selected irrespective of whether they had allograft failure during follow-up. We also selected 293 allograft failure events, regardless of whether they were sampled in the subcohort. From these, we excluded participants with missing urine samples and key covariates at baseline, resulting in a subcohort of 491 individuals and 257 allograft failure cases (Fig 1). Characteristics of the 491-member subcohort were similar compared with those of the overall FAVORIT Trial participants (Table S1, available as online supplementary material). Of the allograft failure case patients, 49 were already randomly selected within the subcohort, and 208 were not. Thus, our study resulted in a final analytic sample of 699 individuals.
Figure 1.
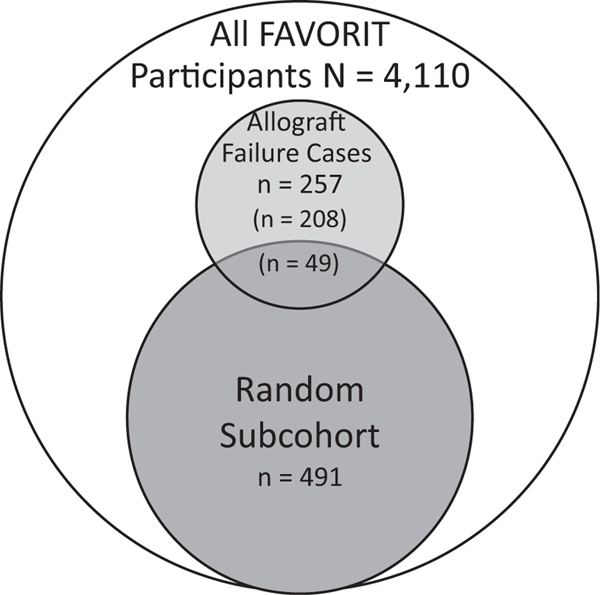
Sampling for this study within the FAVORIT (Folic Acid for Vascular Outcome Reduction in Transplantation) Trial. Among the 4,110 FAVORIT participants, we randomly selected 491 into the subcohort irrespective of allograft failure case status. We separately sampled all participants who had allograft failure during follow-up (cases). Of the 257 allograft failure cases, 49 had already been sampled into the subcohort, the remaining 208 were sampled outside of the subcohort.
Urine Biomarkers of Fibrosis
Urine A1M, MCP-1, PIIINP, and PINP were measured at the University of Vermont in spot urine samples obtained at the baseline FAVORIT Trial visit and stored at −80°C. Specimens had been thawed once previously for measurement of urine injury biomarkers, including neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), interleukin 18 (IL-18), and liver-type fatty acid—binding protein (L-FABP).23 All assays were performed in 2015. To improve precision, we measured fibrosis biomarkers twice in each urine specimen and averaged results. Urine A1M was measured on a Siemens BNII nephelometer. The lower limit of detection was 0.5 mg/dL, and estimates of interassay coefficients of variation (CVs) ranged from 1.87% to 5.03%. Urine MCP-1 was measured using an enzyme-linked immunosorbent assay (R&D Systems) after diluting urine samples 1:2. The acceptable analytic range was 2 to 4,000 pg/mL, and interassay CVs were 5.9% to 9.2% across the analytic range. Urine PIIINP was measured by a radioimmunoassay from ORION Diagnostica, as in our prior work.10 The lower limit of detection was 0.02 μg/L and inter-assay CVs ranged from 11.0% to 16.3%. Similarly, we used a radioimmunoassay (ORION Diagnostica) to measure urine PINP. The lower limit of detection was 0.1 mg/L and interassay CVs ranged from 6.8% to 9.2%. When urine samples were assayed but the biomarker concentration was below the detectable range, we imputed concentrations as the lower limit of detection. Of the 699 study participants, 145 (13.3%), 5 (0.5%), 26 (2.4%), and 124 (11.4%) had urine A1M, MCP-1, PIIINP, and PINP levels below the detectable range, respectively.
Allograft Failure
Allograft failure was defined as initiation of dialysis therapy, as ascertained by local study staff. Time to event was considered from randomization to transplant failure, last follow-up visit, or end of the study period. We censored for death; thus, death with a functioning transplant was not considered an allograft failure in these analyses.
Other Measurements
Demographic characteristics (age, sex, race, and country of origin), smoking status (current, former, or never), medical history (CVD and diabetes mellitus), transplantation characteristics (living donor kidney transplant and time since transplantation), physical examination findings (body mass index and systolic and diastolic blood pressure), and standard laboratory measurements, including serum creatinine and urine ACR, were obtained at the time of study enrollment. Race was recorded as white, black, or other. Baseline blood pressure was the average of 2 measurements. Diabetes was defined as the use of insulin or oral hypoglycemic medications or participant self-report. History of CVD was determined by self-report at baseline and included prior myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, and/or lower-extremity arterial revascularization or nontraumatic amputation above the ankle. Body mass index was calculated using the formula: weight [kg]/height [m]2. Serum creatinine was measured using an alkaline picrate kinetic method on an Olympus AU 400e (Olympus America Inc) instrument that was calibrated to an isotope-dilution mass spectrometry—traceable standard, and eGFR was computed using the 2009 CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.24 Urine albumin and creatinine were measured in spot urine samples. Urine albumin was measured using an immunoturbidimetric assay. Intra-assay CV was 2%, and interassay CV was 4%.
Statistical Methods
We categorized participants from the subcohort into quartile-based urine fibrosis biomarker concentrations and compared differences in demographics and risk factors across quartiles. Within the subcohort, we calculated Spearman correlation coefficients among the 4 urine fibrosis markers, eGFR, urine ACR, and the previously measured urine injury biomarkers. In the correlation matrix, we indexed all markers to urine creatinine because all urine markers were measured on the same urine sample; thus, identical urine tonicity across markers may have led to spuriously strong intercorrelations if they were not indexed to urine creatinine.
To take into account the case-cohort study design, weighted Cox proportional hazards regression was used to assess associations with allograft failure.25,26 These analyses did not index urine markers to urine creatinine concentrations, but rather adjusted for urine creatinine, such that the hazard ratios (HRs) evaluate the biomarker only, but still account for differences in urine tonicity. We evaluated models indexing to urine creatinine in sensitivity analyses. To provide an equal comparison across biomarkers, each urine marker was modeled by quartiles, setting the lowest quartile as the reference category. The proportions with allograft failure in each quartile were tabulated; event rates (per 100 person-years) were calculated among individuals in the subcohort. We also evaluated each marker as a continuous predictor variable. Given right-skewed distributions, we log transformed each biomarker on the log-base-2 scale such that coefficients can be interpreted as “per doubling” or “per 2-fold higher” of the biomarker. A series of multivariable-adjusted models were tested. Model 1 adjusted for urine creatinine level, age, sex, race, randomization arm, systolic blood pressure, prevalent CVD, diabetes, smoking status, time since transplantation, and living donor status. Model 2 additionally adjusted for eGFR and urine albumin level to allow assessment of the degree of attenuation by clinically available measures of kidney health. Model 3 additionally adjusted for urinary NGAL, IL-18, KIM-1, and L-FABP levels to determine whether each urine fibrosis biomarker was associated with allograft failure independent of previously assessed markers of kidney tubule cell injury. Model 4 additionally adjusted for all the other 3 urine fibrosis markers to determine the unique contributions of each with allograft failure. Last, we tested interactions in model 4 by eGFR (<60 vs ≥60 mL/min/1.73 m2) and ACR (>300 vs ≤300 mg/g) categories.
All analyses were conducted using R, version 3.2.1 (R Foundation for Statistical Compputing). P < 0.05 was considered statistically significant in all analyses including interaction terms.
RESULTS
For the 491 subcohort participants, mean age was 51 ± 9 (standard deviation) years, 39% were women, 24% were nonwhite, and 31% were recruited at non-US centers. Mean eGFR at baseline was 46 ± 18 mL/min/1.73 m2, median time since transplantation was 3.9 years, and 43% had received kidneys from living donors. Distributions of all 4 urine fibrosis markers were right skewed, with median values of 1.60 (interquartile range [IQR], 0.78–3.78) mg/dL for A1M, 183 (IQR, 84–351) pg/mL for MCP-1, 3.63 (IQR, 2.09–6.15) μg/L for PIIINP, and 2.36 (IQR, 1.24–3.83) μg/L for PINP.
Subcohort participant characteristics overall and across quartiles of A1M are shown in Table 1. Compared with persons in the lowest quartile, those with higher urine A1M concentrations were more frequently men and black, had shorter times since transplantation, were more likely current smokers, and had a higher prevalence of CVD, higher blood pressures, lower eGFRs, and higher urine ACRs, but a lower prevalence of diabetes. Participant characteristics by quartiles of the other 3 fibrosis markers are shown in Tables S2 to S4.
Table 1.
Baseline Characteristics by α1-Microglobulin Quartiles in Kidney Transplant Recipients in a FAVORIT Subcohort
| Overall (n = 486) | Q1: >0.79 mg/L (n = 122) | Q2: 0.79-1.60 mg/L (n = 122) | Q3: 1.61-3.78 mg/L (n = 122) | Q4: ≥3.79 mg/L (n = 120) | |
|---|---|---|---|---|---|
| Age, y | 51 ± 9 | 52 ± 9 | 51 ± 9 | 52 ± 9 | 51 ± 9 |
| Female sex | 188 (39) | 70 (57) | 42 (34) | 43 (35) | 33 (28) |
| Race | |||||
| White | 368 (76) | 104 (85) | 93 (76) | 93 (76) | 78 (65) |
| Black | 86 (18) | 10(8) | 23 (19) | 21 (17) | 32 (27) |
| Other | 32 (7) | 8 (7) | 6 (5) | 8 (7) | 10(8) |
| Treatment group | |||||
| High-dose vitamin | 240 (49) | 56 (46) | 62 (51) | 61 (50) | 61 (51) |
| Low-dose vitamin | 246 (51) | 66 (54) | 60 (49) | 61 (50) | 59 (49) |
| Country | |||||
| United States | 337 (69) | 96 (79) | 85 (70) | 89 (73) | 67 (56) |
| Canada | 57 (12) | 15(12) | 19 (16) | 12 (10) | 11 (9) |
| Brazil | 92 (19) | 11 (9) | 18 (15) | 18 (15) | 42 (35) |
| Time since transplantation, y | 3.86 [1.70–7.05] | 4.64 [2.34–8.56] | 4.30 [1.68–7.79] | 3.77 [1.80–6.59] | 2.61 [1.04–5.85] |
| Living donor kidney | 207 (43) | 44 (36) | 59 (48) | 55 (45) | 49 (41) |
| Calcineurin inhibitor use | 427 (88) | 104 (85) | 114 (93) | 105 (86) | 104 (87) |
| Sirolimus use | 49 (10) | 11 (9) | 4 (3) | 13(11) | 21 (18) |
| CVD | 93 (19) | 16 (13) | 22 (18) | 29 (24) | 26 (22) |
| Diabetes | 178 (37) | 44 (36) | 47 (39) | 50 (41) | 37 (31) |
| Smoking status | |||||
| Never | 242 (50) | 64 (53) | 68 (56) | 55 (45) | 55 (46) |
| Current | 57 (12) | 11 (9) | 12 (10) | 10(8) | 24 (20) |
| Ever | 187 (39) | 47 (39) | 42 (34) | 57 (47) | 41 (34) |
| BMI, kg/m2 | 29.0 ± 5.9 | 28.6 ± 6.0 | 29.1 ± 6.1 | 29.6 ± 5.8 | 28.6 ± 5.9 |
| SBP, mm Hg | 136 ± 20.0 | 131 ± 19 | 133 ± 17 | 136 ± 20 | 143 ± 22 |
| DBP, mm Hg | 79 ± 13 | 76 ± 11 | 78 ± 10 | 79 ± 13 | 84 ± 14 |
| eGFR, mL/min/1.73 m2 | 46 ± 18 | 49 ± 18 | 50 ± 19 | 46 ± 18 | 40 ± 15 |
| Urine ACR, mg/g | 24.5 [9.5–105.6] | 10.7 [5.5–30.0] | 16.2 [6.9–45.6] | 41.0 [12.2–174.0] | 76.1 [19.9–257.2] |
Note: Values for categorical variables are given as count (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Among the 491 person sub-cohort, 486 had urine available for alpha 1 microglobulin measurement.
Abbreviations: ACR, albumin-creatinine ratio; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FAVORIT, Folic Acid for Vascular Outcome Reduction in Transplantation; Q, quartile; SBP, systolic blood pressure.
Table 2 shows correlations of the 4 urine fibrosis markers indexed to urine creatinine with one another, with eGFR, with urine ACR, and with 4 kidney tubule cell injury biomarkers. We observed the strongest correlation between urine A1M and L-FABP levels (r = 0.77). Other observed correlations were weak to moderate in strength. Relationships of the 4 urine fibrosis markers with eGFRs were −0.08 to −0.25, whereas correlations with urine ACRs were 0.21 to 0.51.
Table 2.
Spearman Correlation Matrix of Urine Fibrosis Markers, eGFR, Urine ACR, and Urine Injury Biomarkers in FAVORIT
| A1M/Cr | MCP-1/Cr | PIIINP/Cr | PINP/Cr | eGFR | ACR | NGAL/Cr | IL-18/Cr | KIM-1/Cr | L-FABP/Cr | |
|---|---|---|---|---|---|---|---|---|---|---|
| A1M/Cr | 1.000 | |||||||||
| MCP-1/Cr | 0.292a | 1.000 | ||||||||
| PIIINP/Cr | 0.641a | 0.134a | 1.000 | |||||||
| PINP/Cr | 0.407a | 0.138a | 0.465a | 1.000 | ||||||
| eGFR | −0.250a` | −0.099a | −0.080 | −0.096a | 1.000 | |||||
| ACR | 0.508a | 0.475a | 0.304a | 0.214a | −0.211a | 1.000 | ||||
| NGAL/Cr | 0.395a | 0.293a | 0.332a | 0.305a | −0.234a | 0.343a | 1.000 | |||
| IL-18/Cr | 0.297a | 0.170a | 0.194a | 0.146a | −0.011 | 0.242a | 0.382a | 1.000 | ||
| KIM-1/Cr | 0.113b | 0.554a | −0.024 | −0.013 | −0.052 | 0.319a | 0.243a | 0.157a | 1.000 | |
| L-FABP/Cr | 0.768a | 0.361a | 0.495a | 0.302a | −0.257a | 0.593a | 0.413a | 0.317a | 0.212a | 1.000 |
Note:Spearman correlation matrix is limited to persons sampled within the subcohort. All urine measures are indexed to urine creatinine.
Abbreviations: A1M, α1-microglobulin; ACR, albumin-creatinine ratio; Cr, creatinine; eGFR, estimated glomerular filtration rate; FAVORIT, Folic Acid for Vascular Outcome Reduction in Transplantation; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; IL-18, interleukin 18; L-FABP, liver-type fatty acid binding protein; MCP-1, monocyte chemoattractant protein 1; PINP, procollagen type I; PIIINP, procollagen type III.
P,< 0.01.
P ,< 0.05.
As described in the Methods section, we evaluated 257 allograft failure cases in FAVORIT (of which 49 overlapped with the 491-member subcohort); these events occurred at a median 3.46 years of follow-up. Using the overall 699-individual analytic sample, we next evaluated associations of the 4 urine fibrosis markers with allograft failure (Table 3). We observed a strong and graded relationship between urine A1M levels and allograft failure. Each doubling of A1M levels was associated with a more than 2-fold risk for allograft failure, and a more than 13-fold gradient in risk was observed comparing the highest to the lowest quartile after adjustment for demographics and kidney disease risk factors (model 1). With additional adjustment for eGFR and urine ACR (model 2), doubling of A1M levels remained associated with 70% higher risk and individuals in the fourth quartile remained at a more than 7-fold risk for allograft failure when compared to the first quartile. The magnitude of the association was only minimally influenced by additional adjustment for urine injury biomarkers and the other 3 urine fibrosis markers (model 4).
Table 3.
Association of Urine Fibrosis Markers With Risk for Kidney Allograft Failure in FAVORIT
| No. of Events | Event Rate, per 100 PY | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|---|
| Urine AIM | ||||||
| Per log2 increase | 2.13 (1.79–2.53); | 1.73 (1.43–2.08); | 1.51 (1.12–2.04); | 1.76 (1.27–2.44); | ||
| P < 0.001 | P < 0.001 | P = 0.008 | P = 0.001 | |||
| Q1: <0.79 mg/L | 30 | 0.83 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 0.79–1.60 mg/L | 45 | 1.74 | 2.51 (1.17–5.35) | 2.81 (1.24–6.35) | 2.71 (1.41–6.44) | 4.23 (1.56. 11.42) |
| Q3: 1.61–3.78 mg/L | 76 | 2.19 | 6.79 (3.29–14.01) | 5.64 (2.53–12.61) | 4.07 (1.66–9.95) | 7.62 (2.57–22.57) |
| Q4: ≥3.79 mg/L | 104 | 2.96 | 13.49 (6.27–29.0) | 7.36 (3.21–16.89) | 3.15 (1.07–9.27) | 6.14 (1.75–21.55) |
| Urine MCP-1 | ||||||
| Per log2 increase | 2.03 (1.68–2.44); | 1.60 (1.32–1.93); | 1.47 (1.16–1.85); | 1.49 (1.17–1.89); | ||
| P < 0.001 | P < 0.001 | P = 0.001 | P = 0.001 | |||
| Q1: <84.2 pg/mL | 42 | 1.70 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 84.2–183.3 pg/mL | 58 | 1.39 | 1.99 (1.02–3.86) | 1.60 (0.78–3.30) | 1.12 (0.51–2.44) | 1.33 (0.59–3.01) |
| Q3: 183.4–351.4 pg/mL | 65 | 1.78 | 4.22 (2.09–8.52) | 2.80 (1.35–5.79) | 1.55 (0.66–3.63) | 1.89 (0.79–4.53) |
| Q4: ≥351.4 pg/mL | 88 | 3.00 | 7.57 (3.55–16.13) | 4.59 (2.10–10.05) | 2.00 (0.81–4.95) | 2.13 (0.83–5.44) |
| Urine PIIINP | ||||||
| Per log2 increase | 1.28 (1.10–1.49); | 1.12 (0.97–1.30); | 0.91 (0.78–1.06); | 0.76 (0.64–0.90); | ||
| P = 0.001 | P = 0.1 | P = 0.2 | P = 0.001 | |||
| Q1: <2.09 mg/L | 57 | 1.48 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 2.09–3.63 mg/L | 51 | 1.50 | 1.04 (0.58–1.85) | 1.03 (0.54–1.97) | 0.58 (0.30–1.12) | 0.34 (0.17–0.68) |
| Q3: 3.64–6.15 mg/L | 74 | 2.56 | 1.91 (1.09–3.35) | 1.41 (0.79–2.53) | 0.53 (0.27–1.04) | 0.19 (0.09–0.41) |
| Q4: ≥6.16 mg/L | 75 | 2.41 | 1.99 (1.08–3.68) | 0.94 (0.47–1.87) | 0.17 (0.07–0.38) | 0.04 (0.02–0.11) |
| Urine PINP | ||||||
| Per log2 increase | 1.27 (1.13–1.44); | 1.12 (0.99–1.27); | 1.00 (0.88–1.14); | 0.99 (0.87–1.14); | ||
| P < 0.001 | P = 0.07 | P = 0.9 | P = 0.9 | |||
| Q1: <1.25 mg/L | 57 | 1.13 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 1.25–2.36 mg/L | 51 | 1.85 | 1.21 (0.69–2.12) | 1.38 (0.77–2.47) | 1.23 (0.68–2.23) | 1.22 (0.67–2.21) |
| Q3 2.37–3.83 mg/L | 57 | 1.37 | 1.27 (0.70–2.31) | 0.99 (0.51–1.91) | 0.67 (0.34–1.33) | 0.61 (0.30–1.22) |
| Q4: ≥3.84 mg/L | 92 | 3.74 | 3.77 (2.12–6.71) | 1.76 (0.92–3.36) | 0.79 (0.39–1.62) | 0.60 (0.28–1.29) |
Unless otherwise indicated, values are given as hazard ratio (95% confidence interval). Events refer to kidney allograft failure events. Model 1: adjusted for urine creatinine, age, sex, race, country, randomized treatment, diabetes, systolic blood pressure, cardiovascular disease, smoking, time since transplantation, and living donor. Model 2: model 1 plus estimated glomerular filtration rate and urine albumin. Model 3: model 2 plus NGAL, IL-18, KIM-1, and L-FABP. Model 4: model 3 plus urine PIIINP, PINP, AIM, and MCP-1.
Abbreviations: A1M, α1-microglobulin; FAVORIT, Folic Acid for Vascular Outcome Reduction in Transplantation; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver-type fatty acid binding protein; MCP-1, monocyte chemoattractant protein 1; NGAL, neutrophil gelatinase-associated lipocalin; PINP, procollagen type I; PIIINP, procollagen type III; PY, patient-year; Q, quartile.
Although the association between urine MCP-1 levels and allograft failure was also graded and strong, it was weaker in magnitude compared to urine A1M. In model 1, adjusting for demographics and traditional kidney disease risk factors, doubling of MCP-1 levels was associated with approximately 2-fold higher risk for allograft failure. Additional adjustment for eGFR and urine ACR (model 2) attenuated the association moderately, but each doubling of MCP-1 level remained associated with 60% higher risk for allograft failure. Additional adjustment for levels of the urine injury biomarkers and the other 3 urine fibrosis biomarkers moderately attenuated the association further, such that MCP level doubling was associated with nearly 50% higher risk for allograft failure, an association that remained statistically significant. Associations by quartiles followed similar patterns, but the highest quartile was not statistically significant in models that included all injury and fibrosis markers.
Urine PIIINP and PINP concentrations were also associated with allograft failure when adjusted for demographics and kidney disease risk factors (model 1). However, additional adjustment for eGFR and urine ACR attenuated these associations (model 2). Surprisingly, when adjusted for the other fibrosis and tubular injury markers (model 4), higher PIIINP level was significantly associated with lower risk for allograft failure.
We evaluated whether results were modified by baseline eGFR or ACR. Results were similar in all cases except one. The association of PINP level with allograft failure was modestly stronger in persons with ACRs > 300mg/g (HR, 1.40; 95% confidence interval [CI], 1.03–1.92) than in those with lower ACRs (HR, 0.91; 95% CI, 0.77–1.08; P for interaction = 0.05). We also examined associations of each biomarker indexed to urine creatinine, rather than adjusted for it. Results were slightly stronger when indexed (Table S5). However, in each case, the interpretation was similar to that in the main models.
To provide a frame of reference of the relative strengths of association, we compared associations of the highest versus lowest quartile of each urine fibrosis marker with allograft failure to that of extreme quartiles of eGFR and urine ACR (Fig 2). The point estimate for the association of the 4th quartile of urine A1M with allograft failure was stronger than that of eGFR and was approximately half as strong as the highest quartile of urine ACR. Urine MCP-1 was also strong, with the high quartile having a similar magnitude of association with allograft failure as the lowest eGFR quartile (the low quartile had eGFRs < 33 mL/min/1.73 m2 vs the high quartile with eGFRs $ 57 mL/min/1.73 m2). It should be noted that associations of levels of the fibrosis markers were adjusted for eGFR and ACR in Fig 2, so the depicted risk shows information garnered above and beyond these standard clinical markers of kidney function.
Figure 2.
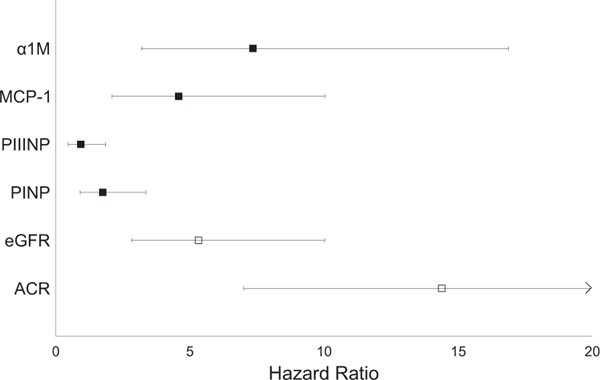
Association with allograft failure of quartile 4 versus quartile 1 of each marker. Point estimates reflect the highest versus lowest quartile of the marker, with the exception of estimated glomerular filtration rate (eGFR), for which the lowest (worst kidney function) is compared to the highest quartile. All models are adjusted for urine creatinine, age, sex, race, country, FAVORIT (Folic Acid for Vascular Outcome Reduction in Transplantation) randomization group, diabetes, systolic blood pressure, cardiovascular disease, smoking, time since transplantation, living donor status, eGFR, and urine albumin-creatinine ratio (ACR), corresponding to model 2 from Table 3. For eGFR, individuals in quartile 4 had eGFRs < 33 mL/min/ 1.73 m2, and those in quartile 1 (reference category) had eGFRs ≥ 57 mL/min/1.73 m2. For urine ACR, individuals in quartile 4 had urine ACRs ≥ 105 mg/g, and those in quartile 1 (reference category) had urine ACRs < 10 mg/g. Error bars reflect 95% confidence interval limits. For ACR, the upper bound of the 95% confidence interval is truncated for improved visual appearance of the other markers; the upper limit of the 95% confidence interval is at 29.5. Abbreviations: α1M, α1-microglobulin; MCP-1, monocyte chemoattractant protein 1; PINP, procollagen type I; PIIINP, procollagen type III.
DISCUSSION
Among stable kidney transplant recipients, we found that higher concentrations of 2 urinary markers of tubulointerstitial fibrosis, urine A1M and MCP-1, are strongly associated with future allograft failure. These associations are independent of eGFR, urine ACR, other kidney disease risk factors, and urine concentrations of kidney tubule cell injury biomarkers. The associations are strong in comparison to eGFR and urine ACR, and if confirmed, these findings may have important implications for monitoring kidney transplant recipients.
We have been interested in identifying novel noninvasive markers of kidney tubule fibrosis in nontransplantation settings6–12 and extend that work to kidney transplant recipients for the first time here. Tubular damage and tubulointerstitial fibrosis are common features of several causes of decreased kidney allograft function, including acute rejection, BK nephropathy, and long-term calcineurin inhibitor exposure. Because eGFR and urine ACR primarily mark glomerular health, these commonly used clinical measures are insensitive to detection of tubulointerstitial damage or fibrosis.2 For this reason, despite the invasive nature of kidney biopsies, some transplantation centers have adopted protocols for surveillance biopsies to detect acute rejection and other pathologic processes that may be missed by laboratory monitoring alone.27–30 Noninvasive markers that provide insight into tubulointerstitial fibrosis may therefore have a clinical role if they add information about risk for allograft failure above and beyond eGFR and ACR.
To our knowledge, this is the first study to demonstrate the relationship of urine A1M and MCP-1 levels with future risk for kidney allograft failure after accounting for eGFR and urine ACR. Because of this and the strength of associations observed here, our findings suggest that urine A1M and MCP-1 may ultimately provide a useful tool to identify kidney transplant recipients who are at higher risk for allograft failure. Moreover, because A1M and MCP-1 levels are associated with allograft failure independent of one another and are only moderately correlated with one another, they may provide complementary information if measured together. Future studies are required to determine whether acute changes in levels of these markers can identify individuals who may benefit from kidney biopsy or whether changes in levels of these markers may allow evaluation of response to changes in drug therapy.
As far as we are aware, this is also the first study to compare strengths of associations with those of eGFR and ACR. The associations of these 2 markers with allograft failure were strong in comparison, even when adjusted for eGFR and ACR. For an additional point of comparison, a recent study from FAVORIT23 evaluated associations of 4 kidney injury markers (NGAL, KIM-1, IL-18, and L-FABP) with subsequent allograft failure. In models adjusted for similar covariates, the highest urine NGAL quartile was associated with 2.6-fold increased risk for allograft failure; this effect was much weaker in comparison to that of A1M (HR, 7.36) and MCP-1 (HR, 4.59) in the current report. The markers KIM-1, IL-18, and L-FABP were not independently associated with allograft failure in that previous study.
Some prior studies have measured urine A1M in kidney transplant recipients. Our findings are consistent with these studies. Evaluating 136 kidney transplant recipients at a single center, Teppo et al31 demonstrated that urine A1M concentrations 8 days after transplantation correlated with pretransplantation cold ischemia time; in addition, urine A1M concentrations increased 1 to 4 days before acute rejection episodes and subsequently declined during treatment for acute rejection. Two prior studies have demonstrated that urine A1M concentrations are correlated with degree of tubulointerstitial fibrosis on protocol biopsies.15,32 The relationship between urine A1M levels and longitudinal changes in kidney function has not been extensively studied. In a single-center study, higher urine A1M level was significantly associated with allograft failure; however, only 12 allograft failure events occurred during follow-up and the study did not adjust for eGFR or other kidney disease risk factors.32 We previously reported that higher urine A1M concentrations are associated with longitudinal decline in eGFR among HIV-infected and -uninfected individuals and that urine A1M level had a stronger association with this outcome compared with levels of several kidney tubule injury biomarkers (KIM-1, IL-18, and NGAL).11 The current study extends the work by evaluating a large multicenter sample of kidney transplant recipients with considerably greater numbers of allograft failure events during follow-up, by demonstrating that the association of urine A1M level with allograft failure is independent of eGFR and urine ACR, and by putting the strength of association into context relative to clinically available markers of kidney health and other urine injury biomarkers.
Few studies have evaluated urine MCP-1 concentrations in kidney transplant recipients. MCP-1 is principally expressed by monocytes, fibroblasts, and endothelial cells. Its main function is as a potent chemoattractant molecule expressed and released in response to tissue injury. In biopsy studies, urine MCP-1 concentrations are correlated with the degree of tubulointerstitial fibrosis, tubule atrophy, and inflammation in kidney transplant recipients.33 In a single-center study of 231 kidney transplant recipients who had 20 allograft failure events, urine MCP-1 level was found to be associated with allograft failure.33 This association remained statistically significant after adjustment for recipient age, pretransplantation donor-specific antibody titer, and occurrence of delayed graft function; however, no adjustment for eGFR, urine ACR, or other kidney disease risk factors was undertaken. Thus, the present study confirms the association of MCP-1 level with future allograft failure in a larger sample and demonstrates for the first time that the association is independent of traditional kidney disease risk factors, baseline eGFR, urine ACR, A1M level, and levels of several kidney injury biomarkers.
In contrast to urine A1M and MCP-1, urine PIIINP and PINP concentrations were not found to be associated with future allograft failure when models were adjusted for eGFR and urine ACR. In final models, higher urine PIIINP level was significantly associated with lower risk for allograft failure. Types I and III collagen are synthesized as procollagen molecules, and the amino-terminal pro-peptides (PINP and PIIINP, respectively) are cleaved during collagen deposition in the extracellular matrix and released into urine. Several studies have reported that urine PIIINP concentrations are associated with tubulointerstitial fibrosis on biopsy.15,17,18 In an elderly community-living population, we recently reported that higher urine PIIINP concentrations are associated with longitudinal decline in kidney function independent of baseline eGFR, ACR, and other kidney disease risk factors.10 Less is known about urine PINP. However, types I and III collagen are the main constituents of renal fibrosis, and type 1 fragments were recently identified as predictors of CKD progression by an untargeted proteomic approach.34 The reasons why urine PIIINP and PINP concentrations were not associated with allograft failure in this study are uncertain, but the different setting and/or use of immunosuppression are possible contributors. We were surprised by higher urine PIIINP levels being associated with lower risk for allograft failure in final models. Given the change in direction of associations across subsequent models, it is possible that this finding reflects overadjustment and/or that the other urine injury and fibrosis markers are marking the biological processes more precisely than urine PIIINP.
We selected the 4 urine markers based on their relationships with tubulointerstitial fibrosis in prior biopsy studies. However, only 2 were found to be associated with allograft failure, and A1M and MCP-1 both provide insights into other factors beyond fibrosis. A1M is freely filtered and avidly reabsorbed by proximal tubule cells in healthy kidneys, and higher urine concentrations may therefore mark defects in proximal tubule reabsorptive capacity in addition to relationships with fibrosis. Similarly, MCP-1 is a potent chemoattractant molecule and may therefore mark intrarenal inflammatory stress above and beyond its relationships with fibrosis. As collagen markers, PIIINP and PINP may be more specific to fibrosis only. Whether non—fibrosis-related biological pathways may be the predominant factors driving associations with allograft failure rather than fibrosis per se requires further study.
Strengths of the present study include the large sample of kidney transplant recipients recruited from many centers in 3 countries. The long-term follow-up and large number of allograft failure events provided substantial statistical power. Availability not only of kidney disease risk factors, eGFR, and urine ACR, but also of multiple urine tubule injury biomarkers are an additional strength. These data allowed us to assess the correlation of urine fibrosis markers with a reasonably large panel of other kidney disease measures and determine the degree to which the fibrosis markers identify risk for allograft failure independent of the others.
The study also has important limitations. We lack data for kidney biopsies, BK viremia status, and HLA antibody status. Whether these factors may influence levels of urine fibrosis markers is uncertain and requires future study. However, a noninvasive method to assess fibrosis may prove useful regardless of these measures to identify kidney transplant recipients at risk for progression and potentially to noninvasively track response to changes in therapy. We measured urine fibrosis markers at one point in time. Thus, the stability of urine concentrations of fibrosis markers over time and the relationship of trajectory of change with allograft function are uncertain. Given emerging data from other studies suggesting that urine A1M may change dynamically in the setting of acute rejection,31 these questions should be a high priority for future studies. As with any observational study, the possibility of residual confounding cannot be excluded; however, the strong associations reported here and the relatively modest attenuation observed with statistical adjustment argue against this possibility.
In conclusion, among stable kidney transplant recipients, urine A1M and MCP-1 concentrations are strongly associated with risk for kidney allograft failure. These associations are independent of baseline eGFR and urine ACR and of urine concentrations of several markers of kidney tubule cell injury. If the findings are confirmed, A1M and MCP-1 measurement may provide an opportunity to identify kidney transplant recipients at higher risk for allograft failure, for whom closer surveillance may be warranted.
Supplementary Material
Table S1: Comparison of characteristics in subcohort vs all of FAVORIT.
Table S2: Baseline characteristics in subcohort, by MCP-1 quartile.
Table S3: Baseline characteristics in subcohort, by PIIINP quartile.
Table S4: Baseline characteristics in subcohort, by PINP quartile.
Table S5: Association of urine fibrosis markers indexed to creatinine with risk of kidney allograft failure in FAVORIT.
ACKNOWLEDGEMENTS
Support: This study was supported by an American Heart Association Established Investigator Award (EIA 18560026 to Dr Ix) and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01 DK098234 to Drs Ix and Shlipak). The FAVORIT Trial was supported by cooperative agreement U01 DK61700 from the NIDDK (to Dr Bostom) and by the Office of Dietary Supplements at the National Institutes of Health. The funders of this study had no role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Peer Review: Evaluated by 3 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
Because 3 authors of this article were editors for AJKD at the time of manuscript submission, the peer-review and decision-making processes were handled entirely by an Editorial Board Member (Mark M. Mitsnefes, MD) who served as Acting Editor-in-Chief Details of the journal’s procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
Published by Elsevier Inc. on behalf of the National Kidney Foundation, Inc. This is a US Government Work. There are no restrictions on its use.
SUPPLEMENTARY MATERIAL
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2016.10.019) is available at www.ajkd.org
REFERENCES
- 1.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 2.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152(9):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20(1): 1–17. [DOI] [PubMed] [Google Scholar]
- 4.Howie AJ, Ferreira MA, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant. 2001;16(6):1163–1169. [DOI] [PubMed] [Google Scholar]
- 5.Takebayashi S, Kiyoshi Y, Hisano S, et al. Benign nephrosclerosis: incidence, morphology and prognosis. Clin Nephrol. 2001;55(5):349–356. [PubMed] [Google Scholar]
- 6.Sarnak MJ, Katz R, Newman A, et al. Association of urinary injury biomarkers with mortality and cardiovascular events. J Am Soc Nephrol. 2014;25(7):1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driver TH, Katz R, Ix JH, et al. Urinary kidney injury molecule 1 (KIM-1) and interleukin 18 (IL-18) as risk markers for heart failure in older adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis. 2014;64(1): 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenstein L, Driver TH, Fried L, et al. Serum bicarbonate concentrations and kidney disease progression in community-living elders: the Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis. 2014;64(4):542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garimella PS, Biggs ML, Katz R, et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int. 2015;88(5):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ix JH, Biggs ML, Mukamal K, et al. Urine collagen fragments and CKD progression-the Cardiovascular Health Study. J Am Soc Nephrol. 2015;26(10):2494–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jotwani V, Scherzer R, Abraham A, et al. Association of urine alpha1-microglobulin with kidney function decline and mortality in HIV-infected women. Clin J Am Soc Nephrol. 2015;10(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez JR, Shlipak MG, Whooley MA, Ix JH. Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J Am Soc Nephrol. 2013;24(4):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akerstrom B, Logdberg L, Berggard T, Osmark P, Lindqvist A. alpha(1)-Microglobulin: a yellow-brown lipocalin. Biochim Biophys Acta. 2000;1482(1–2):172–184. [DOI] [PubMed] [Google Scholar]
- 14.Weber MH, Verwiebe R. Alpha 1-microglobulin (protein HC): features of a promising indicator of proximal tubular dysfunction. Eur J Clin Chem Clin Biochem. 1992;30(10): 683–691. [PubMed] [Google Scholar]
- 15.Teppo AM, Tornroth T, Honkanen E, Gronhagen-Riska C. Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003;75(12):2113–2119. [DOI] [PubMed] [Google Scholar]
- 16.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294(4):F697–F701. [DOI] [PubMed] [Google Scholar]
- 17.Ghoul BE, Squalli T, Servais A, et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol. 2010;5(2):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soylemezoglu O, Wild G, Dalley AJ, et al. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transplant. 1997;12(9):1883–1889. [DOI] [PubMed] [Google Scholar]
- 19.Bostom AG, Carpenter MA, Hunsicker L, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis. 2009;53(1):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostom AG, Carpenter MA, Kusek JW, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152(3):448. e441–448.e447. [DOI] [PubMed] [Google Scholar]
- 21.Bostom AG, Carpenter MA, Kusek JW, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011;123(16):1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiner DE, Carpenter MA, Levey AS, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012;12(9):2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal N, Carpenter MA, Weiner DE, et al. Urine injury biomarkers and risk of adverse outcomes in recipients of prevalent kidney transplants: the Folic Acid for Vascular Outcome Reduction in Transplantation Trial. J Am Soc Nephrol. 2016;27(7):2109–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50(4):1064–1072. [PubMed] [Google Scholar]
- 26.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. [DOI] [PubMed] [Google Scholar]
- 27.Kee TY, Chapman JR, O’Connell PJ, et al. Treatment of subclinical rejection diagnosed by protocol biopsy of kidney transplants. Transplantation. 2006;82(1):36–42. [DOI] [PubMed] [Google Scholar]
- 28.Nankivell BJ, Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant. 2006;6(9):2006–2012. [DOI] [PubMed] [Google Scholar]
- 29.Seron D, Moreso F. Protocol biopsies in renal transplantation: prognostic value of structural monitoring. Kidney Int. 2007;72(6):690–697. [DOI] [PubMed] [Google Scholar]
- 30.Thaunat O, Legendre C, Morelon E, Kreis H, Mamzer-Bruneel MF. To biopsy or not to biopsy? Should we screen the histology of stable renal grafts? Transplantation. 2007;84(6): 671–676. [DOI] [PubMed] [Google Scholar]
- 31.Teppo AM, Honkanen E, Ahonen J, Gronhagen-Riska C. Changes of urinary alpha1-microglobulin in the assessment of prognosis in renal transplant recipients. Transplantation. 2000;70(8):1154–1159. [DOI] [PubMed] [Google Scholar]
- 32.Amer H, Lieske JC, Rule AD, et al. Urine high and low molecular weight proteins one-year post-kidney transplant: relationship to histology and graft survival. Am J Transplant. 2013;13(3):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho J, Wiebe C, Gibson IW, et al. Elevated urinary CCL2: Cr at 6 months is associated with renal allograft interstitial fibrosis and inflammation at 24 months. Transplantation. 2014;98(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 34.Schanstra JP, Zurbig P, Alkhalaf A, et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015;26(8): 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of characteristics in subcohort vs all of FAVORIT.
Table S2: Baseline characteristics in subcohort, by MCP-1 quartile.
Table S3: Baseline characteristics in subcohort, by PIIINP quartile.
Table S4: Baseline characteristics in subcohort, by PINP quartile.
Table S5: Association of urine fibrosis markers indexed to creatinine with risk of kidney allograft failure in FAVORIT.


