Abstract
Simple mucinous cysts of the pancreas have an epithelial lining resembling pancreatic intraepithelial neoplasia but may have a clinical presentation similar to pre-malignant mucinous neoplasms such as intraductal papillary mucinous neoplasms. Whether the epithelial lining shares genomic alterations with other pancreatic preinvasive neoplasms such as PanIN and intraductal papillary mucinous neoplasm has not been determined. We performed targeted sequencing analysis using a custom designed MiSeq panel including the full coding regions of 18 pancreatic cancer genes on 13 clinically and pathologically well characterized simple mucinous cysts. We detected 59 mutations in 15 genes in the cohort, with a median of 4 mutations per cyst (range=0–16 mutations per cyst). The mutated genes and rate of detected mutations were: KMT2C (MLL3) (62%), KRAS (15%), BRAF (8%), RNF43 (8%), CDKN2a (8%), TP53 (15%), and SMAD4 (8%). No GNAS mutations were detected. Four cases (31%) had no mutations detected. These findings place the majority of simple mucinous cysts of the pancreas in the spectrum of early, low grade mucinous neoplasia, albeit with an different spectrum of genomic alterations than PanIN and intraductal papillary mucinous neoplasm.
Keywords: Pancreatic cyst, simple mucinous cyst, molecular, pancreas, retention cyst, pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm
1. Introduction
Localized cysts of the pancreas raise the pre-operative differential diagnosis of pre-malignant mucinous neoplasms such as intraductal papillary mucinous neoplasm or mucinous cystic neoplasm, but sometimes the histology of the resected cyst reveals a non-papillary mucinous cyst lining lacking ovarian stroma. These cysts have been previously labeled, “retention cyst involved by pancreatic intraepithelial neoplasia” or “mucinous non-neoplastic cyst [1].” In 2015, a consensus publication detailed criteria for the diagnosis of these cysts and proposed a unifying term, “simple mucinous cyst,” defined by size > 1 cm and a simple mucinous epithelial lining with absence of ovarian-type stroma or papillary architecture, features that would establish the alternative diagnoses for mucinous cystic neoplasm and intraductal papillary mucinous neoplasm, respectively. Although mucinous epithelium has been regarded as a neoplastic feature in pancreatic ductal lesions, simple mucinous cysts have not been established as bona fide neoplasms, and our understanding of the genetics and natural history is limited because there are few published reports with very limited follow up data and KRAS has been the only gene investigated [2], [3], [4].
The aim of this study is to describe the somatic mutations of simple mucinous cysts of the pancreas using targeted sequencing and to document the correlative clinicopathologic characteristics and clinical follow up over a long interval.
2. Materials and Methods
2.1. Sample selection and slide review
Samples from thirteen localized pancreatic cysts resected between 2000–2014 were obtained with the approval of the Memorial Sloan Kettering Institutional Review Board. The inclusion criteria for sample selection were 1) the surgical pathology diagnosis was retention cyst (all samples were obtained before consensus criteria for simple mucinous cyst were published); 2) on review, the lesion was lined by simple mucinous epithelium, size was ≥ 1 cm, and papillary architecture and ovarian-type stroma were lacking. Pertinent histologic features were recorded from the archival hematoxylin and eosin slide review. Cyst lining epithelium was assigned a grade of dysplasia using criteria described previously proposed for three tiers of dysplasia (i.e., PanIN 1A, 1B, 2, 3) [5].
2.2. DNA extraction and quantification
The cyst lining cells were micro-dissected by scraping 20 FFPE sections (5 micron thickness), with careful attention to avoid sampling peri-cystic pancreatic tissue and stroma. DNA was extracted from formalin fixed, paraffin embedded sections using pH 8.0 buffered phenol-chloroform.
2.3. Targeted Sequencing and Analysis
Library preparation and sequencing were performed on MiSeq platform (Illumina, San Diego, CA) according to the manufacturer instructions. Libraries were prepared for use against a custom designed MiSeq panel including the full coding regions of 18 pancreatic cancer genes: ARID1A, ARID2, ATM, BRAF, CDKN2A, CTNNB1, GNAS, KDM6A, KRAS, KTM2C, PCDH15, RNF43, SF3B1, SMAD3, SMAD4, TGFBR1, TGFBR2, TP53, a panel that includes genes commonly mutated in PanIN, mucinous cystic neoplasm, and intraductal papillary mucinous neoplasm.
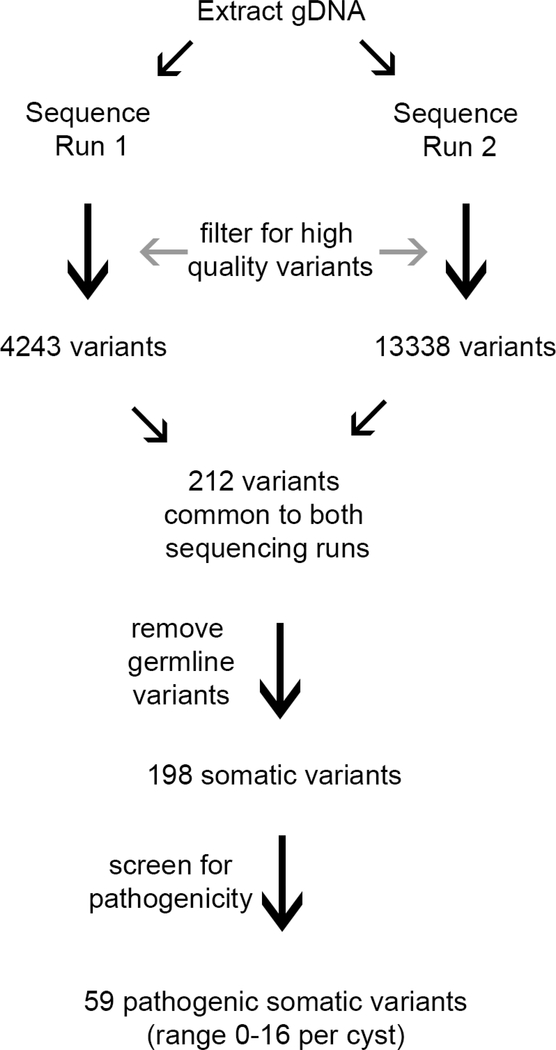
Several precautions were taken to identify high quality variants. First, each sample of DNA was used for library preparation and sequencing two independent times [6]. Second, only variants that passed MiSeq quality metrics were retained. Third, any variant that was non-coding or synonymous and that had a global minor allele frequency of >=0.05% was excluded. Fourth, only somatic variants that were present in both sequencing runs for a sample were considered. Fifth, variants were filtered against the matched normal for each patient. Samples without a matched normal were filtered against an unmatched pooled normal representing 30 patients of diverse ethnic and racial origin. Finally, this list of high quality variants was analyzed by CRAVAT (http://www.cravat.us/CRAVAT/) to identify those predicted to be functionally deleterious in nature versus passenger mutations in a driver gene.
2.4. Immunohistochemistry for driver gene expression
Sections from FFPE were stained with the following monoclonal antibodies (Manufacturer, Identifier): CDKN2A (Ventana, 725–4713), TP53 (Dako, M700101–2), SMAD4 (Santa Cruz Biotechnology, sc-7966) using previously described techniques [7]. TP53 was abnormal if expression was absent or overexpressed (>30% strong nuclear staining). CDKN2A and SMAD4 were evaluated for loss of expression, compared with intact labeling in internal normal tissues.
3. Results
3.1. Clinical data
Cysts from 13 patients met inclusion criteria. The patients were 9 women and 4 men with an age range 48–76 years (median=67 years) (Table 1). 69% of the cysts were in the pancreatic body/tail. Review of pre-operative imaging descriptions indicated that a description of a multi-septate cyst was most frequent (n=4), followed by a dilated duct with stricture (n=3). Other descriptions included multiple cysts (n=2), a unilocular cyst connected to the main pancreatic duct (n=2), and a mass/cystic mass (n=3). Three patients had serum testing for CEA and/or CA19.9 and none were outside of the reference range. Endoscopic ultrasound guided fine needle aspiration was performed in 50% of the cases with diagnoses including nondiagnostic (n=5), atypical (n=1), and mucinous neoplasm (n=1). Cyst fluid CEA ranged from 120 to 4899 ng/ml (median= 1214 ng/ml) in the four patients tested.
Table 1.
Patient demographics
| Clinical Feature | N | % |
|---|---|---|
| Male | 4 | 31% |
| Female | 9 | 69% |
| Cyst location | ||
| Head/neck | 4 | 31% |
| Body/Tail | 9 | 69% |
| Operative procedure | ||
| Pancreatoduodenectomy | 2 | 15% |
| Distal pancreatectomy | 8 | 62% |
| Cystectomy or partial resection | 3 | 23% |
| Reason for resection | ||
| Patient choice | 2 | 15% |
| Cyst size | 4 | 31% |
| Concern for occult malignancy | 4 | 31% |
| Dilated duct | 2 | 15% |
Most patients had a pre-operative clinical diagnosis favoring IPMN except for two patients suspected to have a cyst, not otherwise specified. International guidelines for resection of IPMN did not exist for the entire period during which the samples were collected; the documented clinical reasons for resection were: large or increasing cyst size, dilated main pancreatic duct, or concern for occult malignancy based on abrupt narrowing of the pancreatic duct, as described in Table 1. Two patients chose resection after being given the option of surveillance.
3.2. Histology
By gross and histologic evaluation, most of the cystic lesions were clustered dilated ducts (10 cases, 77%), with a few (3 cases, 23%) unilocular cysts. Inclusion criteria and diagnosis of simple mucinous cyst were confirmed, including the absence of papillary architecture and ovarian-type stroma. Peri-cystic dense, pauci-cellular collagen was present in 7 cases (54%). Lobular atrophy and chronic pancreatitis were in the peri-cystic tissue in 9 cases (69%). Immunohistochemical results for ER/PR were reported as negative for two of the female patients. Connection to the main pancreatic duct was identified grossly in 2 cases. One cyst was radiologically considered a mass lesion (case 2), which was likely due to dense, inspissated mucoid material seen grossly and histologically. Columnar epithelium resembling low grade pancreatic intraepithelial neoplasia (1A=100% 1A, 1B=31%, and 2= 8%) involved the epithelial lining of all cysts, with the amount of intracytoplasmic mucin highly variable throughout the lesions (Table 2). The background pancreatic parenchyma was involved by low grade PanIN multifocally in all but 2 patients (1A=62%, 1B=38%, 2=15%). None of the patients had high grade PanIN (high nuclear-to-cytoplasmic ratio, complex architecture, and cellular disorganization). Sixty-nine percent (n=9) of patients had extensive lobular atrophy and chronic pancreatitis. 15% of patients (n=2) patients had a demonstrable cause for obstruction in the form of 1) pancreatic lithiasis (case 6) and 2) adenocarcinoma presenting 2 months later but not clinically apparent at the time of cyst resection (case 4); these are not exclusion criterion for diagnosis of simple mucinous cyst; therefore, these cases were retained in the study.
Table 2.
Clinical, radiologic, and histologic features of simple mucinous cysts
| Sample | age | Pre-operative diagnosis | Imaging findings | Gross cyst size (cm) |
PanIn grade |
Surveillance plan | Interval to last follow up (months) | Status of pancreas imaging at follow up | |
| Cyst | Background pancreas | ||||||||
| 1 | 63 | BD-IPMN | Multi-septate cyst with microcystic morphology | 1.8 | 1A, 2 | 1A,1B | YEARLY IMAGING | 84 | UNREMARKABLE |
| 2 | 51 | PDAC, IPMN | Oval mass | 2 | 1A | 0 | NONE | N/A | N/A |
| 3 | 67 | NET v PDAC | Multi-septate cyst | 1.5 | 1A | 1A, 1B | NONE | N/A | N/A |
| 4 | 70 | CYST | Dilated pancreatic duct with stricture | 1.1 | 1A | 1B | N/A | 1 | PANC HEAD MASS |
| 5 | 65 | IPMN | Multiple small cysts | 1.8 | 1A, 1B | 1A | YEARLY IMAGING | 159 | STABLE CYSTS LIKELY BD-IPMN |
| 6 | 48 | IPMN | Multi-septate cyst | 2.1 | 1A | 1A, 2 | NONE | n/a | N/A |
| 7 | 72 | CA, IPMN | Dilated pancreatic duct with stricture | 1 | 1A, 1B | 2 | YEARLY IMAGING | 12 | UNREMARKABLE |
| 8 | 76 | BD-IPMN | Multiple cysts | 4 | 1A, 1B | 0 | YEARLY IMAGING | 87 | STABLE CYSTS LIKELY BD-IPMN |
| 9 | 69 | CYST | Ill-defined mass | Multiple cysts up to 1.4 cm | 1A, 1B | 1A | N/A | 33 | NO RESIDUAL PANCREAS |
| Sample | age | Pre-operative diagnosis | Imaging findings | Gross cyst size (cm) | PanIn grade | Surveillance plan | Interval to last follow up (months) | Status of pancreas imaging at follow up | Sample |
| 10 | 55 | IPMN | Cystic mass with mild wall thickening | Multiple cysts up to 1.0 cm | 1A | 1A, 1B | YEARLY IMAGING | 61 | STABLE MD DILATATION |
| 11 | 76 | MD-IPMN, PDAC | Dilated pancreatic duct with stricture | 7 cm segment of DD up to 1.0 cm | 1A | 1A | YEARLY IMAGING | 60 | STABLE CYSTS |
| 12 | 75 | IPMN | Cyst connecting to main pancreatic duct | 2 | 1A | 1A | YEARLY IMAGING | 58 | STABLE CYSTS AND MD DILATATION |
| 13 | 66 | BD-IPMN | Multi-septate cyst connecting to main pancreatic duct | 2 | 1A | 1B | YEARLY IMAGING | 21 | UNREMARKABLE |
Abbreviations: BD= branch duct, CA=carcinoma, MD= main duct, IPMN= intraductal papillary mucinous neoplasm, PDAC=pancreatic ductal adenocarcinoma, DD=dilated pancreatic duct, NET=neuroendocrine tumor
3.3. Clinical outcome
The surgical pathology diagnosis of retention cyst with PanIN conflicted with the presumptive clinical diagnosis of IPMN in 10 patients. Subsequently, follow up recommendations were as follows: 8 patients had annual pancreatic angiography, 3 patients had no prescribed follow up pancreatic imaging due to no perceived risk of recurrence, 1 patient required no follow up due to no residual pancreatic tissue, and one patient developed a mass (previously undetected) two months following cyst resection in the residual pancreas, which was confirmed to be adenocarcinoma (case 4). For the 8 patients undergoing surveillance, follow up ranged from 12–159 months (median 60 months), during which 5 patients had residual cystic lesions and dilated pancreatic ducts that remained stable and 3 patients had no recurrence/residual abnormalities.
3.4. Molecular characterization of cyst epithelium with histologic and immunophenotypic correlation
Targeted sequencing analysis of 18 genes associated with ductal neoplasia in the pancreas detected a total of 59 mutations in this cohort, involving 15 genes, with up to 16 detected mutations per cyst (median=4 mutations per cyst), as detailed in Table 3. No mutations were detected in 4 cysts, one of which was the cyst (case 6) associated with lithiasis. The most frequently altered gene was KMT2C (MLL3), occurring in 8 cysts (62%). Mutations in genes altered early in non-invasive pancreatic neoplasia were detected at the following rates: KRAS (15%), BRAF (8%), RNF43 (8%). Several genetic alterations associated with advanced dysplasia were detected at the following rates: CDKN2a (8%), TP53 (15%), and SMAD4 (8%). No mutations were detected in GNAS, CTNNB1, and SMAD3. Six of the cysts showed multiple unique missense mutations in the same gene; genes with multiple mutations per cyst included: ARID2, ATM, KMT2C, SF3B1, TGFBR2. Two cases in this cohort had cysts with possible upstream obstruction. Case 6, with lithiasis, did not have mutations detected, but 3 other cysts had no mutations and no indication of duct obstruction. Case 6, with the subsequently detected adenocarcinoma had multiple mutations. We do not know the genotype of the adenocarcinoma to assess whether the lesions were related, but they were not close to each other in the gland.
Table 3.
Distribution of distinct gene mutations for each simple mucinous cyst
| Number distinct mutations per gene | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | ARID1A | ARID2 | ATM | BAGE2 | BRAF | CDKN2A | CTNNB1 | GNAS | KMT2C | KRAS | PCDH15 | RNF43 | SF3B1 | SMAD3 | SMAD4 | TGFBR1 | TGFBR2 | TP53 | Total mutations detected per cyst |
| 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 8 |
| 4 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 10 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| 11 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 16 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 7 |
| 13 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Total | 2 | 6 | 7 | 1 | 2 | 1 | 0 | 0 | 21 | 1 | 4 | 1 | 7 | 0 | 1 | 1 | 2 | 2 | 59 |
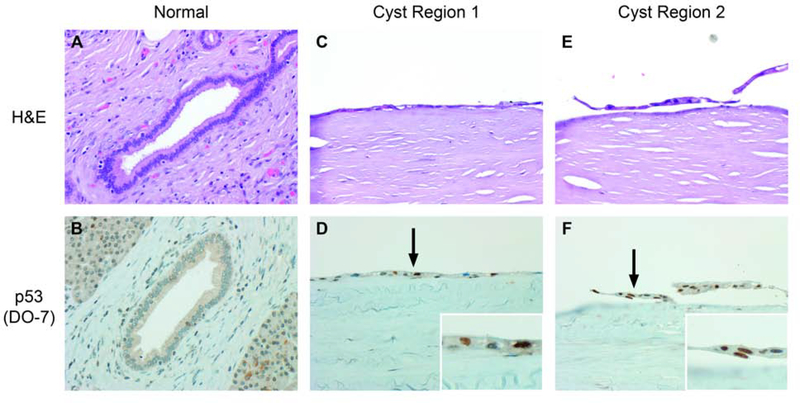
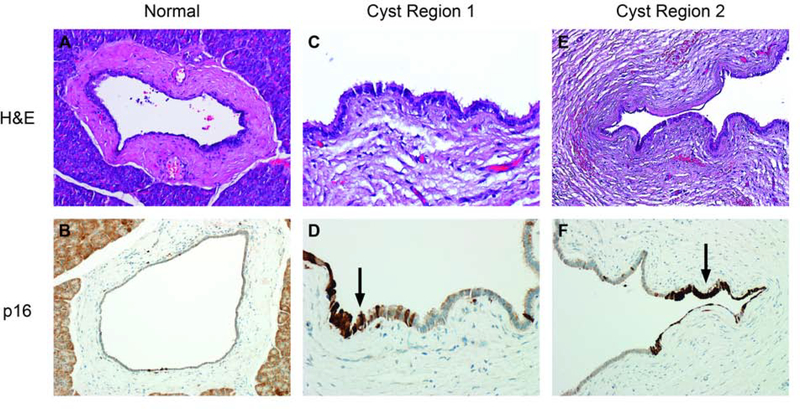
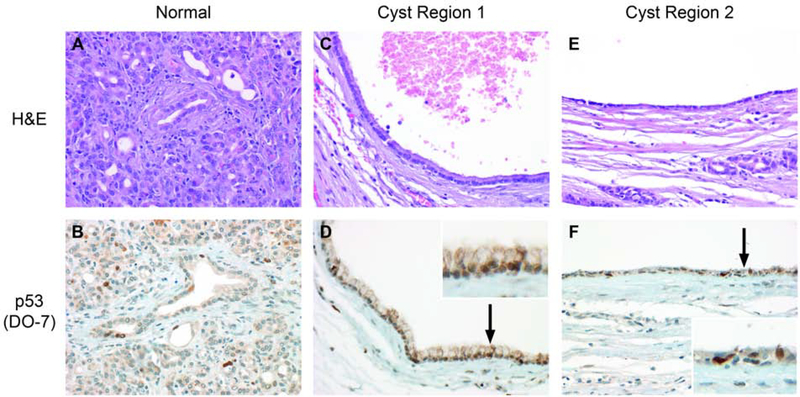
Immunohistochemical staining for p16, p53, and SMAD4 was tested on cysts showing abnormalities of the related genes CDKN2A, TP53, and SMAD4, respectively. Patchy overexpression of p16 was seen in case 1 (Figure 1). SMAD4 labeling was retained in cases 9 and 10 but rare cells in case 1 had absent expression of uncertain significance. Staining for p53 did not meet criteria for abnormal expression in cases 4 and 11, but p53 was overexpressed in case 3 (Figures 2,3).
Figure 1.
Schematic of the high quality variant detection method.
Figure 2.
Case 1 had mutations of CDKN2A and patchy, intense labeling for p16.
Figure 3.
Focal strong immunohistochemical labeling for p53 did not reach the overall threshold for abnormal staining in this case (4) with a TP53 mutation.
4. Discussion
Using a targeted genetic sequencing panel, we tested the epithelium of 13 simple mucinous cysts, originally diagnosed as retention cysts with PanIN, and detected multiple, heterogeneous driver gene mutations associated pancreatic mucinous neoplasia, which provides insight into the biology of these lesions.
The panel of 18 genes was designed to cover the most common mutations occurring in PanIN and IPMN, and we detected a 59 total mutations with a median of 4 mutations per simple mucinous cyst (range= 0–16 per cyst). It is difficult to place this prevalence of mutations in context with the mutational burdens of other mucinous neoplasms, since methodology varies among studies. For example, whole exome sequencing of high grade PanIN has a median of 33 mutations per lesion [8]. Targeted next generation sequencing (51 cancer-associated genes) of low grade IPMN found a median of 3 mutations per lesion (range= 0–5) [9]. Targeted sequencing (275 cancer-associated genes) on high grade IPMN has a median of 4.5 (range=0–40) [8, 9, 10]. Like IPMN and PanIN, the mutations detected in simple mucinous cysts included multiple driver genes, which provides further support that these lesions contain neoplastic epithelium rather than occurring as a consequence of obstruction, as reflected by the initial terminology of “mucinous non-neoplastic cyst.” The concept that the mucinous epithelium represents non-neoplastic metaplasia was initially propagated after a published report of polyclonality in these lesions [11]. Recently, both PanIN and IPMN have been shown to contain multiple neoplastic clones, challenging the idea that these neoplasms are monoclonal. [12, 13].
PanIN and IPMN are defined as neoplasms because they have clonal mutations of cancer associated genes and show grade progression with increasing prevalence of mutations [8, 12]. Consequently, the prevalence of activating KRAS mutations in simple mucinous cysts was the initial evidence used to support their neoplastic nature [2]. The KRAS mutation rate in our cohort was 15% (2/13). Prior reports of KRAS mutation rates for simple mucinous cysts has a wide range (13–55%), depending on whether mutations were detected in tissue or cyst fluid [2, 3]. In comparison to other mucinous neoplasms, KRAS mutants are much more consistently detected in PanIN (up to 94% of low grade PanINs), with the major caveat that in the earliest PanIN lesions, the mutations are present in a small fraction of the cells comprising the lesion [8, 14]. The prevalence of KRAS mutations in genomic studies of IPMNs covers a wide range (mean 30–40%) and depends on the prevalence of the various histologic subtypes of IPMNs tested. For example, Wu et al. reported on a cohort with a high concentration of gastric type IPMNs and found a 79% prevalence of KRAS mutations [15].
The cysts we studied consistently demonstrated low grade dysplasia, yet in addition to expected mutations for KRAS, 69% of cysts had multiple other established driver mutations, such as KMT2C (MLL3), the most prevalent which comprised 62% (21/59 mutations detected in the cohort). BRAF and CDKN2A, for example, are known drivers in KRAS negative pancreatic mucinous neoplasms. Notably, we have shown that simple mucinous cysts can contain alterations driver genes that are more often seen in advanced pancreatic neoplasia, such as SMAD4, TP53, and CDKN2A, which is not unlike prior reports that early, low grade PanINs contain KRAS, CDKN2A, GNAS, or BRAF mutations [14]. Another recent study demonstrated that driver gene heterogeneity is more prevalent in low grade than high grade IPMNs, and while we cannot draw a clear parallel based on our limited data, there is appeal to the hypothesis that we observed a similar biological phenomenon [12, 13]. The scope of our methods did not include quantification, clonality, or epigenetic studies that could provide more insight into the sequence and combination of events that lead to disease progression, which is an area of long-standing debate.
Since the epithelial lining of simple mucinous cysts with low grade dysplasia resembles the neoplastic epithelium of low grade gastric type IPMNs and PanINs, two entities with well-characterized genetics, there is an existing framework for comparison with our data from simple mucinous cysts. IPMNs, PanINs, and simple mucinous cysts seem to have overlap in genotype; all but 3 of the genes on our panel (GNAS, SMAD3, and CTNNB1) that characterize these other mucinous neoplasms were altered in at least one simple mucinous cyst [8, 13]. However, the specific mutations in simple mucinous cysts differ from those in PanINs and IPMNs, both in type and in frequency. The absence of GNAS mutation somewhat reduces the likelihood that this lesion represents an incipient IPMN, since GNAS is thought to be one of the earliest driver genes in IPMN. Even small (<1 cm) cystic lesions, so-called “incipient” IPMNs, have been shown to have a 33% rate of GNAS mutation [16]. In 50% of IPMNs, both GNAS and KRAS mutations are present (Wu Jiao 2011, Wu Matthaei 2011 [10]. The overall frequency of KRAS mutations in simple mucinous cysts was also lower than in PanINs or IPMNs, and some of the most frequently altered genes (e.g. MLL3) have not been implicated commonly in the pathogenesis of these more common pancreatic mucinous lesions.
Our robust filtering process selected for deleterious mutations, yet immunohistochemical protein expression inconsistently correlated with the genetic abnormalities in the cases we tested. Given the evidence for the low proportion of KRAS mutated cells in low grade PanIN lesions, if these mutations in simple mucinous cysts also involve scattered lesional cells, it may be challenging to detect these alterations by immunolabeling [17].
Krasinskas et al. published the largest case series of simple mucinous cysts to date, and our smaller, representative cohort has similar clinicopathologic characteristics [3]. For example, our cohort also presented with a mean age in the mid-seventh decade, a predominance of post-menopausal females, cysts located in the pancreas body/tail, and evidence of elevated cyst fluid CEA. In contrast, this cohort had a larger proportion of multiloculated cysts and an absence of high grade dysplasia [3].
The histologic diagnosis of simple mucinous cyst is not without controversy, given the resemblance of these cysts to PanIN involving a retention cyst, mucinous cystic neoplasm, and branch duct gastric type IPMN. PanIN was less compelling as a diagnosis because most of the cysts do not have an obvious obstructing lesion and PanIN is by definition a microscopic, incidentally detected lesion (<0.5 cm). The apparent predilection for females and distal gland involvement is in common with MCN, but ovarian type stroma was consistently absent in simple mucinous cysts. It is more difficult to make an argument against the possibility these are branch duct IPMNs, particularly since occasional pancreatic duct connection has been observed (two cysts in this study) and multi-loculation/grape-like arrangements commonly described for BD-IPMN are typical [3]. Yet, IPMN is an awkward diagnosis for a cyst lacking the eponymous papillations, hence the consensus designation of these cysts as, “simple mucinous cyst.” The lack of an intestinal immunophenotype (MUC2-, MUC5AC+) in simple mucinous cysts has been repeatedly demonstrated [3, 11]. We did not perform mucin immunophenotyping but none of the cysts exhibited the hallmark distinctly elongated ovoid nuclei and goblet cells of the intestinal histologic phenotype. Furthermore, the lack of GNAS mutations, which are particularly characteristic of IPMNs, argues against the possibility simple mucinous cysts are simply IPMNs that lack papillae.
In 69% of the cysts in this series, the patients presented with a radiologic picture of IPMN, leading to a vexing clinical dilemma of what surveillance should be recommended. IPMNs are often multifocal, and patients have up to a 20% risk of recurrence or new disease in their remnant pancreas following resection [18]. Recurrent IPMN can be due to incomplete resection of the original primary, intraductal spread of the previous primary, or a new lesion, independent of the first [19]. For these reasons, IPMN surveillance entails examinations at 2 and 5 years following resection, but more frequent monitoring is often undertaken [20]. With a median follow up of 5 years, none of the 8 patients in our study with follow up (one patient had no residual pancreas) had progression or recurrent disease, with one notable exception. One patient developed a clinically evident adenocarcinoma within 2 months following resection of the cyst. The lack of progression in this small sample size is reassuring, but this event highlights how critical it is to clinically exclude an upstream obstructive lesion in the residual pancreas of patients with simple mucinous cysts, since they are possibly etiologically related to retention cysts secondary to malignant obstruction.
In conclusion, using clinically and histopathologically well characterized simple mucinous cysts we have documented the genetic composition of these neoplasms which places them in an extended spectrum of early, low grade mucinous neoplasia, thus filling a knowledge gap since previous reports were limited to the testing for KRAS [2].
Figure 4.
A TP53 mutation and diffusely abnormal immunohistochemical expression of p53 were detected in case 3.
Highlights.
Discussion
We detected 59 total mutations with a median of 4 mutations per simple mucinous cyst (range= 0–16 per cyst).
KRAS mutations were present in 15% of the cysts (2/13).
The cysts we studied were consistently low grade in histology, yet 69% had multiple established driver mutations, such as KMT2C (MLL3), the most prevalent mutation (21/59, 36%).
Eight patients had clinical follow up (median 5 years) with either no progression of residual cystic disease or recurrent cyst disease.
Acknowledgments
We gratefully acknowledge the administrative support of Rebecca Andrade and Tanisha Daniel.
Funding disclosure and Conflict of Interest statement
This work was funded in part by R35 CA220508 awarded to CID, and in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute [P30CA008748]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Iacobuzio-Donahue discloses research support funding from Bristol Myers Squibb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brunner A, Ladurner R, Kosmahl M, Mikuz G, Tzankov A, Mucinous non-neoplastic cyst of the pancreas accompanied by non-parasitic asymptomatic liver cysts. Virchows Arch 2004;444:482–4. 10.1007/s00428-004-0999-z [DOI] [PubMed] [Google Scholar]
- [2].Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, et al. , A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 2015;39:1730–41. 10.1097/PAS.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Krasinskas AM, Oakley GJ, Bagci P, Jang KT, Kuan SF, Reid MD, et al. , “Simple mucinous cyst” of the pancreas: A clinicopathologic analysis of 39 examples of a diagnostically challenging entity distinct from intraductal papillary mucinous neoplasms and mucinous cystic neoplasms. Am J Surg Pathol 2017;41:121–7. 10.1097/PAS.0000000000000750 [DOI] [PubMed] [Google Scholar]
- [4].Schechter S, Shi J, Simple mucinous cyst of the pancreas: Review and update. Arch Pathol Lab Med 2017;141:1330–5. 10.5858/arpa.2017-0232-RA [DOI] [PubMed] [Google Scholar]
- [5].Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, et al. , An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2004;28:977–87. [DOI] [PubMed] [Google Scholar]
- [6].Cancer Genome Atlas Research Network. Electronic address aadhe, Cancer Genome Atlas Research N, Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 2017;32:185–203 e13. 10.1016/j.ccell.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Makohon-Moore AP, Zhang M, Reiter JG, Bozic I, Allen B, Kundu D, et al. , Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet 2017;49:358–66. 10.1038/ng.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hosoda W, Chianchiano P, Griffin JF, Pittman ME, Brosens LA, Noe M, et al. , Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (hg-panin) reveal paucity of alterations in tp53 and smad4. J Pathol 2017;242:16–23. 10.1002/path.4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, et al. , Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 2014;233:217–27. 10.1002/path.4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tan MC, Basturk O, Brannon AR, Bhanot U, Scott SN, Bouvier N, et al. , Gnas and kras mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J Am Coll Surg 2015;220:845–54 e1. 10.1016/j.jamcollsurg.2014.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cao W, Adley BP, Liao J, Lin X, Talamonti M, Bentrem DJ, et al. , Mucinous nonneoplastic cyst of the pancreas: Apomucin phenotype distinguishes this entity from intraductal papillary mucinous neoplasm. Hum Pathol 2010;41:513–21. 10.1016/j.humpath.2009.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fischer CG, Beleva Guthrie V, Braxton AM, Zheng L, Wang P, Song Q, et al. , Intraductal papillary mucinous neoplasms arise from multiple independent clones, each with distinct mutations. Gastroenterology 2019;157:1123–37 e22. 10.1053/j.gastro.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, et al. , Recurrent gnas mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66 10.1126/scitranslmed.3002543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, et al. , Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730–3 e9. 10.1053/j.gastro.2011.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mariot V, Wu JY, Aydin C, Mantovani G, Mahon MJ, Linglart A, et al. , Potent constitutive cyclic amp-generating activity of xlalphas implicates this imprinted gnas product in the pathogenesis of mccune-albright syndrome and fibrous dysplasia of bone. Bone 2011;48:312–20. 10.1016/j.bone.2010.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matthaei H, Wu J, Dal Molin M, Shi C, Perner S, Kristiansen G, et al. , Gnas sequencing identifies ipmn-specific mutations in a subgroup of diminutive pancreatic cysts referred to as “incipient ipmns”. Am J Surg Pathol 2014;38:360–3. 10.1097/PAS.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hong SM, Vincent A, Kanda M, Leclerc J, Omura N, Borges M, et al. , Genome-wide somatic copy number alterations in low-grade panins and ipmns from individuals with a family history of pancreatic cancer. Clin Cancer Res 2012;18:4303–12. 10.1158/1078-0432.CCR-12-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He J, Cameron JL, Ahuja N, Makary MA, Hirose K, Choti MA, et al. , Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg 2013;216:657–65; discussion 65–7. 10.1016/j.jamcollsurg.2012.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pea A, Yu J, Rezaee N, Luchini C, He J, Dal Molin M, et al. , Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (ipmn) of the pancreas. Ann Surg 2017;266:133–41. 10.1097/SLA.0000000000001817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. , International consensus guidelines 2012 for the management of ipmn and mcn of the pancreas. Pancreatology 2012;12:183–97. 10.1016/j.pan.2012.04.004 [DOI] [PubMed] [Google Scholar]