Abstract
Background:
Vulvovaginal symptoms, including dryness, irritation, and pain with intercourse, are common among postmenopausal women and associated with impaired sexual functioning and quality of life. Prior assessment of treatment strategies for these symptoms has been limited by a lack of sensitive patient-centered outcome measures that assess symptom impact on functional and quality-of-life domains.
Objective(s):
We aimed to: 1) examine change in the impact of postmenopausal vulvovaginal symptoms on multiple aspects of well-being and functioning in relation to vaginal estradiol and moisturizer treatment, and 2) guide meaningful interpretation of scores on a structured-item questionnaire measure of condition-specific impact.
Study Design:
Data were drawn from postmenopausal women enrolled in the MsFLASH Vaginal Health Trial, a 12-week, double-blind, placebo-controlled randomized trial of treatment for vulvovaginal symptoms, assigned to vaginal 10-μg estradiol tablet plus placebo gel (n=98), vaginal moisturizer plus placebo tablet (n=97), or dual placebo (n=94). At baseline and 12-week follow-up, participants completed the Day-to-Day Impact of Vaginal Aging questionnaire (DIVA) to assess the impact of vaginal symptoms on four domains (activities of daily living, emotional well-being, sexual functioning, and body image), each on a 0–4 point scale. DIVA sensitivity to change was assessed by examining associations between change in DIVA domain scores and vulvovaginal symptom severity from baseline to 12 weeks with ANCOVA. Within-woman and between-group minimal clinically important improvement was assessed using an anchor-based approach relating change in DIVA domain scores with self-reported benefit from treatment.
Results:
Participants in all treatment arms (n=289) demonstrated reduced impact of vulvovaginal symptoms on all domains of well-being and functioning as assessed by DIVA at 12-week follow-up, with no significant differences in improvement between women assigned to either estradiol tablet or vaginal moisturizer compared to placebo. For all DIVA domains, mean impact scores were reduced when participants reported symptom improvement (−0.3 to −0.8 point change in DIVA scores for <2 point symptom severity change vs. −0.4 to −1.6 point change in DIVA scores for 2+ point symptom severity change, all p<0.001). Minimal clinically important change in DIVA domain scale scores, anchored to self-reported meaningful benefit from treatment at 12 weeks, ranged from −0.4 to −1.3 (within-woman) and −0.2 to −0.7 (between-group). Observed change and minimal clinically important difference were largest for the sexual functioning domain.
Conclusion(s):
The impact of vulvovaginal symptoms on day-to-day activities, sexual function, emotional well-being, and body image may be improved with low dose vaginal estradiol, moisturizer, or topical placebo. The DIVA questionnaire demonstrates sensitivity to change with treatment of vulvovaginal symptoms, particularly DIVA scales focusing on symptom impact on sexual functioning and body image. Minimal clinically important improvement in the impact of vulvovaginal symptoms as measured by the DIVA can be defined using these measures.
Keywords: Vulvovaginal symptoms, sexual functioning, atrophic vaginitis, quality of life, low dose vaginal estradiol, emotional wellbeing, body image, vaginal moisturizer, randomized controlled trial
Introduction
Bothersome vulvovaginal symptoms are common among postmenopausal women, with an estimated 40–75% reporting symptoms including vaginal dryness, irritation, and pain with intercourse1–4 frequently beginning in the late menopause transition and persisting into older age.5 These symptoms have been linked to impaired sexual functioning, reduced quality of life, and poorer emotional well-being,6,7 highlighting a need to identify effective treatment options with a meaningful impact on a range of patient-centered outcomes.
Assessment of treatment-related vulvovaginal symptom improvement has traditionally relied on limited measures that do not reflect the broad impact of these symptoms on day-to-day functioning and well-being.8,9 The Day-to-Day Impact of Vaginal Aging questionnaire (DIVA) was developed to assess the impact of vulvovaginal symptoms on multiple functional and quality-of-life domains, including activities of daily living, emotional well-being, sexual functioning, and self-concept/body image.8 While the DIVA has been used to describe symptom experience and comorbidities in observational studies,6 its ability to serve as a sensitive and reliable measure of treatment-related change has not been assessed.
The Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) clinical trials network completed a randomized, three-arm, double-blind trial of treatments commonly used for postmenopausal vulvovaginal symptoms, comparing both vaginal estradiol tablets and a vaginal moisturizer10–12 to placebo. The primary results of the trial with regard to treatment effects on vulvovaginal symptoms have been reported elsewhere.1 In the current study, we report results related to the day-to-day impact of vulvovaginal symptoms as measured by the DIVA. We examined the sensitivity of DIVA domain scales to self-reported vulvovaginal symptom change. We also used an anchor-based approach to estimate mean changes in DIVA domain scores corresponding to patient-reported meaningful benefit from treatment, in order to provide a patient-centered measure of meaningful treatment-related change.13
Materials and Methods
Study Population
The MsFLASH Vaginal Health Trial has been previously described.1 Briefly, participants were enrolled in a randomized, double-blind, placebo-controlled 12-week trial comparing the treatment efficacy of 10-μg vaginal estradiol tablets and a vaginal moisturizer to matching placebos for moderate to severe vulvovaginal symptoms. Healthy postmenopausal women were recruited between April 2016 and April 2017. The protocol was approved by the institutional review board at each clinical site and the data coordinating center; all women provided written informed consent.
A total of 302 women enrolled. Eligible participants were aged 45–70, had their last menstrual period at least 2 years prior to study enrollment, and reported moderate-severe vulvovaginal itching, pain, irritation, or dryness at least weekly in the past 30 days, or vulvovaginal pain with penetration in the past month. Exclusion criteria included vulvar dermatoses, assessed by pelvic exam; current vaginal infection; use of hormonal medication in past 2 months; use of antibiotics, vaginal moisturizer, probiotic, prebiotic, or douche in past month; and chronic premenopausal vulvovaginal symptoms.
Study procedures
Study recruitment, randomization, and intervention procedures have been described elsewhere.1 Women were recruited through direct mailings and Facebook ads targeted to women aged 50 to 70 years within 20 miles of clinical sites. Randomization by permuted blocks of 9 and stratified by site was conducted in a secure web-based database and implemented via a computerized inventory system for dispensing identical-appearing tablets in bottles and gel in tubes. Participants, study personnel, and clinical providers were blinded to treatment assignments.
Participants were randomly assigned 1:1:1 to 10-μg vaginal estradiol tablet (Vagifem; Novo Nordisk, Plainsboro, New Jersey) + placebo vaginal gel; placebo vaginal tablet + vaginal moisturizer (Replens; Church & Dwight, Ewing, New Jersey); or placebo vaginal tablet + placebo vaginal gel.1 Participants were instructed to use the vaginal tablet (active or placebo) daily for 2 weeks, and then twice weekly for 10 weeks. Participants were instructed to use the vaginal moisturizer (or placebo gel) every 3 days throughout the trial. Participants were advised to use the vaginal tablet in the morning and vaginal gel in the evening for the first 2 weeks, and then to use the products on alternate days.
Telephone contact at 1, 3, and 11 weeks after randomization assessed protocol adherence and adverse events. Participants completed questionnaires and underwent vaginal sample collection at 4- and 12-week in-person follow-up visits. They were also asked to bring medications to these visits; remaining pills were counted and gel tubes were weighed to assess medication adherence.
Measurements
Impact of vaginal symptoms on well-being and functioning:
Participants completed the DIVA, a questionnaire that has previously demonstrated good face validity, construct validity, and internal and test-retest reliability among postmenopausal women with vaginal symptoms.8 It includes four domain-specific scales to assess the impact of symptoms on: 1) activities of daily living (5 items); 2) emotional well-being (4 items); 3) sexual functioning (5 items); and 4) self-concept and body image (5 items). All questions have 5-point ordered response scales (0–4); higher numbers indicate more severe impact. Domain scale scores are calculated by averaging the scores of individual items in each domain. In this analysis, we used scores from the short-form DIVA sexual functioning scale, consisting of five questions applicable to all women regardless of whether they are currently sexually active.
Severity of most bothersome vulvovaginal symptom:
At trial enrollment, participants identified their most bothersome symptom (MBS) as either vulvovaginal itching, pain, dryness, irritation, or pain with penetration. Severity of this symptom was rated as 0–3 (none, mild, moderate, or severe)14 at baseline and at 12 weeks. For this analysis, change in MBS severity was quantified in two ways. First, the differences between MBS severity scores at the baseline visit and week 12 were categorized as a decrease of <2 points or 2+ points. A decrease of 2+ points reflects improvement in symptom severity from moderate-severe to mild-none.1 Second, at week 12, participants retrospectively reported change in MBS severity since the baseline visit on a 7-level Likert scale. Responses were collapsed into three categories for analysis: “worse/no change” (“very much worse”, “worse”, “slightly worse”, “no change”), “some improvement” (“slightly improved”, “improved”) and “very much improved”.
Meaningful benefit from treatment:
The week 12 questionnaire asked participants whether they had experienced meaningful benefit from study medications during trial participation (yes/no). Women who responded “yes” were considered to have met the minimum threshold for clinically meaningful benefit from treatment.
Additional measurements:
Descriptive data were drawn from baseline questionnaires, including demographic factors, smoking status, alcohol intake, health status, mood, and sexual function. Mood was assessed using the Patient Health Questionnaire (PHQ-8)15 and the Generalized Anxiety Disorder (GAD-7)16 questionnaires. Sexual function was assessed using the Female Sexual Function Index (FSFI)17. Vaginal pH was assessed from vaginal samples collected at baseline and categorized as ≤ 5 vs. >5.14
Statistical Analyses
A modified intent-to-treat analysis included all randomized participants who provided baseline and 12-week DIVA assessments, regardless of treatment adherence. Characteristics of the analytic sample (total and by intervention arm) were summarized by means with standard deviations for continuous variables, and frequencies with percentages for categorical variables.
Treatment effects on change in each of the four DIVA domains (activities of daily living, emotional well-being, sexual functioning, and self-concept and body image) from baseline to week 12 were assessed using ANCOVA models. Week 12 domain scores were modeled as a function of intervention arm, baseline domain score and clinical center. To evaluate intervention effects on DIVA domain scores among adherent participants, these analyses were repeated in the subset of participants who reported 80% or better adherence to their assigned intervention.
To evaluate the sensitivity of the DIVA to self-reported change in vulvovaginal symptoms, ANCOVA models were fit to each week 12 domain score as a function of MBS severity change from baseline to 12 weeks (<2 points vs. 2+ point decrease) and self-reported improvement in MBS at week 12 (worse/no change, some improvement, or very much improved). Models were adjusted for baseline DIVA domain scores. A separate model was fit with the week 12 domain score as a function of the trend over the median self-reported MBS score within the three groups.
An anchor-based approach was used to estimate the within-woman minimal clinically important (MCI) 12-week change and the between-group minimal clinically important difference (MCID)18,19 for each DIVA domain scale. For within-woman MCI change, we estimated the mean and 95% DIVA domain score change in participants who reported “yes” when asked if they experienced a meaningful benefit from treatment at week 12. This estimate was selected as a threshold to reflect clinically meaningful, though not necessarily maximal, improvement.13 To evaluate how treatment was related to domain-specific MCI change, we estimated the percentages of participants who met the MCI change threshold both overall and within each intervention group. We then calculated the MCID as the mean difference between change in each DIVA domain score for women who reported meaningful benefit from treatment and those who did not report meaningful benefit from treatment at 12 weeks. All analyses were conducted in SAS for Windows Version 9.4 (SAS Institute Inc., Cary, NC).
Results
Sample characteristics
The analytic sample included 289 women (mean age 61, SD 4 years) with complete study data for DIVA outcomes at baseline and 12 weeks. Data from 13 randomized participants (4 assigned to estradiol tablet, 3 to vaginal moisturizer, and 6 to placebo) were excluded due to incomplete DIVA data (Figure 1). Overall, participants were largely white (89%), well-educated (67% college graduates), married (or similar; 86%), and currently sexually active (81%). The most common bothersome symptom was pain with vaginal penetration (61%), followed by vaginal dryness (21%) (Table 1). On average, baseline DIVA domain scores indicated a modest impact of vulvovaginal symptoms on activities of daily living (mean 0.5, SD 0.6) and emotional well-being (mean 1.1, SD 0.9), and moderate-severe impact on sexual functioning (mean 2.5, SD 1.0) and self-concept/body image (mean 2.5, SD 1.1; range for all scores 0–4). Treatment adherence did not vary significantly across treatment groups (estradiol tablets, 83 of 98 [85%] vs placebo tablets, 78 of 94 [83%]; p=.88; vaginal moisturizer, 72 of 97 [74%] vs placebo gel, 77 of 94 [82%]; p=.12).
Figure 1. CONSORT diagram: Randomization and follow-up of participants.

Table 1.
Baseline characteristics of the sample
| Total (n=289) | Vaginal estradiol (n=98) | Vaginal moisturizer (n=97) | Dual placebo (n=94) | |
|---|---|---|---|---|
| Age in years, mean (SD) | 61 (4) | 61 (4) | 61 (4) | 61 (4) |
| <55 | 4 (1%) | 3 (3%) | 0 (0%) | 1 (1%) |
| 55–59 | 116 (40%) | 32 (33%) | 42 (43%) | 42 (45%) |
| 60–64 | 108 (37%) | 42 (43%) | 39 (40%) | 27 (29%) |
| 65+ | 61 (21%) | 21 (21%) | 16 (16%) | 24 (26%) |
| Race/ethnicity | ||||
| White | 257 (89%) | 84 (89%) | 88 (91%) | 85 (90%) |
| Black | 12 (4%) | 7 (7%) | 3 (3%) | 2 (2%) |
| Other/unknown | 20 (7%) | 7 (7%) | 6 (6%) | 7 (7%) |
| Body mass index, kg/m2 | ||||
| <25 | 133 (46%) | 39 (46%) | 44 (45%) | 50 (53%) |
| 25–<30 | 98 (34%) | 36 (37%) | 40 (41%) | 22 (23%) |
| 30+ | 54 (19%) | 20 (20%) | 13 (13%) | 21 (22%) |
| Education | ||||
| High school diploma | 10 (3%) | 2 (2%) | 3 (3%) | 5 (5%) |
| School after high school | 84 (29%) | 30 (31%) | 26 (27%) | 28 (30%) |
| College graduate | 194 (67%) | 65 (66%) | 68 (70%) | 61 (65%) |
| Marital status | ||||
| Never married | 14 (5%) | 8 (8%) | 2 (2%) | 4 (4%) |
| Divorced or widowed | 27 (9%) | 9 (9%) | 7 (7%) | 11 (12%) |
| Married or like relationship | 248 (86%) | 81 (83%) | 88 (91%) | 79 (84%) |
| Sexually active | ||||
| Yes | 235 (81%) | 78 (80%) | 78 (80%) | 79 (84%) |
| No | 54 (19%) | 20 (20%) | 19 (20%) | 15 (16%) |
| Most bothersome symptom | ||||
| Vulvar and/or vaginal itching | 19 (7%) | 10 (10%) | 3 (3%) | 6 (6%) |
| Vulvar and/or vaginal soreness | 13 (4%) | 5 (5%) | 6 (6%) | 2 (2%) |
| Vulvar and/or vaginal irritation | 18 (6%) | 7 (7%) | 4 (4%) | 7 (7%) |
| Vaginal dryness | 60 (21%) | 20 (20%) | 17 (18%) | 23 (25%) |
| Pain with vaginal penetration | 175 (61%) | 53 (54%) | 67 (69%) | 55 (59%) |
| Self-reported health | ||||
| Excellent | 70 (24%) | 25 (26%) | 26 (27%) | 19 (20%) |
| Very good | 138 (48%) | 40 (41%) | 54 (56%) | 44 (47%) |
| Good | 75 (26%) | 32 (32%) | 14 (14%) | 29 (31%) |
| Fair/poor | 6 (2%) | 1 (1%) | 3 (3%) | 2 (2%) |
| Depressive symptoms*, mean (SD) | 3.5 (3.3) | 3.4 (3.5) | 3.2 (2.9) | 3.7 (3.4) |
| Anxiety**, mean (SD) | 3.8 (4.1) | 4.2 (4.5) | 3.0 (3.2) | 4.3 (4.3) |
| Sexual function***, mean (SD) | 15.5 (6.4) | 15.2 (6.0) | 15.1 (6.6) | 16.2 (6.6) |
| Vaginal pH | ||||
| ≤5 | 38 (13%) | 18 (18%) | 12 (12%) | 8 (9%) |
| >5 | 246 (85%) | 77 (79%) | 84 (87%) | 85 (90%) |
Effect of treatment on DIVA scores
On average, DIVA domain scores improved from baseline to follow-up in all treatment arms. No significant between-group differences were detected in the DIVA domain scores (Table 2). Results were similar in sensitivity analyses limited to participants who were adherent to treatment (data not shown).
Table 2.
Mean change from baseline to week 12 in DIVA domain scores by treatment arm
| Vaginal estradiol | Dual placebo | Difference | ||||
|---|---|---|---|---|---|---|
| Domain | n | Mean (95% CI) | n | Mean (95% CI) | Mean (95% CI) | p-value1 |
| Activities of daily living | 98 | −0.4 (−0.5, −0.3) | 94 | −0.3 (−0.4, −0.1) | −0.1 (−0.3, 0.0) | 0.45 |
| Emotional well-being | 98 | −0.8 (−1.0, −0.6) | 94 | −0.6 (−0.8, −0.4) | −0.2 (−0.5, 0.0) | 0.10 |
| Sexual functioning | 98 | −1.2 (−1.5, −1.0) | 94 | −1.1 (−1.3, −0.8) | −0.1 (−0.5, 0.2) | 0.59 |
| Self-concept and body image | 98 | −1.1 (−1.3, −0.8) | 94 | −0.8 (−1.1, −0.6) | −0.2 (−0.5, 0.1) | 0.16 |
| Vaginal moisturizer | Dual placebo | Difference | ||||
| Domain | n | Mean (95% CI) | n | Mean (95% CI) | Mean (95% CI) | p-value1 |
| Activities of daily living | 97 | −0.3 (−0.4, −0.2) | 94 | −0.3 (−0.4, −0.1) | −0.1 (−0.2, 0.1) | 0.10 |
| Emotional well-being | 97 | −0.7 (−0.8, −0.5) | 94 | −0.6 (−0.8, −0.4) | −0.1 (−0.3, 0.1) | 0.19 |
| Sexual functioning | 97 | −1.1 (−1.3, −0.8) | 94 | −1.1 (−1.3, −0.8) | 0.0 (−0.3, 0.4) | 0.37 |
| Self-concept and body image | 97 | −0.9 (−1.1, −0.6) | 94 | −0.8 (−1.1, −0.6) | −0.0 (−0.3, 0.3) | 0.95 |
p-value from an ANCOVA model of week 12 DIVA domain score as a function of intervention arm, clinical center and baseline DIVA domain score
DIVA sensitivity to change
Self-reported improvement in the severity of vulvovaginal symptoms was accompanied by improvement in all DIVA domain scores (Table 3). Specifically, significantly greater reductions in all DIVA domain scores were seen among women with ≥2-point decrease (vs. <2-point decrease) in MBS severity from baseline to 12 weeks. The average domain score improvement for women reporting a decrease of at least 2 points in MBS severity was greatest for the sexual functioning scale (1.6 points, 40% reduction from baseline), followed by self-concept and body image (1.3 points, 33% reduction), emotional well-being (0.9 points, 23% reduction) and activities of daily living (0.4 points, 10% reduction). For all DIVA domains, a statistically significant linear trend was seen in the relationship between DIVA score improvement from baseline to 12 weeks and self-reported categorical ratings of symptom improvement at week 12 (Figure 2).
Table 3.
Sensitivity to change: Mean change from baseline to week 12 in DIVA domain scores, stratified by self-reported change from baseline in severity of most bothersome symptom over 12 weeks
| Most bothersome symptom severity decrease over 12 weeks | |||
|---|---|---|---|
| < 2 points (n=160) | 2+ points (n=125) | ||
| Domain | Mean (95% CI) | Mean (95% CI) | p-value |
| Activities of daily living | −0.3 (−0.3, −0.2) | −0.4 (−0.5, −0.3) | <0.001 |
| Emotional well-being | −0.6 (−0.7, −0.5) | −0.9 (−1.0, −0.7) | <0.001 |
| Sexual functioning | −0.8 (−0.9, −0.6) | −1.6 (−1.8, −1.4) | <0.001 |
| Self-concept and body image | −0.6 (−0.8, −0.5) | −1.3 (−1.5, −1.1) | <0.001 |
p-value from a linear ANCOVA of week 12 DIVA domain score as a function of MBS change subgroup indicator and baseline DIVA domain score
Figure 2. Mean change from baseline to week 12 in DIVA domain scores by self-reported improvement from baseline in most bothersome symptoms at week 121.
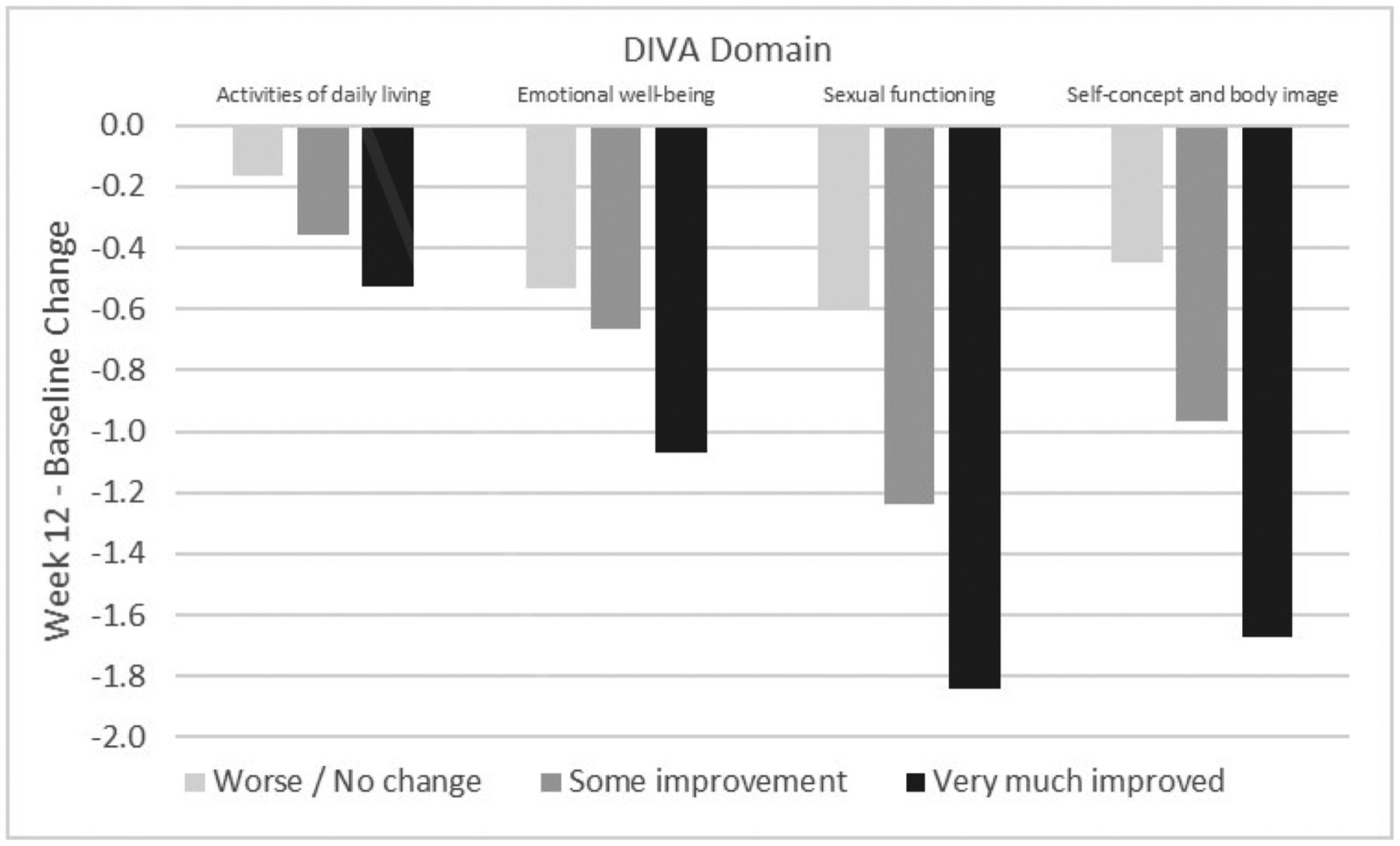
1p<.001 for all domains in linear ANCOVA models of week 12 DIVA domain score as a function of trend over median self-reported change in most bothersome symptom and baseline DIVA domain score.
Minimal clinically important change
In analyses estimating within-woman MCI, mean change in DIVA domain scores from baseline to 12 weeks among women who reported meaningful benefit from treatment at 12 weeks ranged from a 0.4 point (10%) reduction for the activities of daily living scale to a 1.3 point (33%) reduction for the sexual functioning scale (Table 4). The percentage of women whose baseline-to-12-week change in scores met these thresholds varied by domain and differed across treatment arms. These ranged from a low of 49% in the dual placebo arm meeting MCI change thresholds for the activities of daily living and emotional well-being domains, to as many as 67% in the vaginal estradiol meeting the threshold in the self-concept/body image domain. Overall, the percentage of women reaching the MCI change threshold was highest for all domains in the vaginal estradiol arm (61–67%), and generally lowest in the dual placebo arm (49–61%) (Table 5). In analyses estimating the between-group MCID, the mean difference between change in DIVA domain scores for women who did and did not report meaningful benefit from treatment ranged from −0.2 for the activities of daily living scale to −0.7 for the sexual functioning scale (Table 4).
Table 4.
Minimal clinically important change: Mean change from baseline to week 12 in DIVA domain scores by meaningful benefit from treatment at week 12
| Within-woman MCI1 | Between-group MICD2 | |
|---|---|---|
| Domain | Mean (95% CI) | Mean (95% CI) |
| Activities of daily living | −0.4 (−0.5, −0.3) | −0.2 (−0.3, −0.1) |
| Emotional well-being | −0.8 (−0.9, −0.7) | −0.3 (−0.5, −0.1) |
| Sexual functioning | −1.3 (−1.5, −1.2) | −0.7 (−0.9, −0.4) |
| Self-concept and body image | −1.1 (−1.3, −1.0) | −0.6 (−0.8, −0.4) |
Mean change from baseline to week 12 in DIVA domain scores among women who reported meaningful benefit at week 12 (n=196)
Mean difference between change from baseline to week 12 in DIVA domain scores between women who reported meaningful benefit at week 12 (n=196) and those who did not (n=93)
Table 5.
Frequency and percentage of participants meeting the within-woman minimal clinically important change threshold for each DIVA domain, by intervention arm.
| Total (n=289) | Vaginal Estradiol (n=98) | Vaginal Moisturizer (n=97) | Dual placebo (n=94) | |
|---|---|---|---|---|
| DIVA domain | n (%) | n (%) | n (%) | n (%) |
| Activities of daily living | 162 (56%) | 63 (64%) | 53 (55%) | 46 (49%) |
| Emotional well-being | 166 (57%) | 60 (61%) | 60 (62%) | 46 (49%) |
| Sexual functioning | 176 (61%) | 64 (65%) | 55 (57%) | 57 (61%) |
| Self-concept and body image | 180 (62%) | 66 (67%) | 58 (60%) | 56 (60%) |
Comment
Principal Findings
Using data from a multicenter, double-blind, randomized clinical trial,1 we compared the effects of vaginal estradiol, moisturizer, and placebo on the functional and quality-of-life impact of postmenopausal vulvovaginal symptoms as measured by the DIVA questionnaire, examined the sensitivity of DIVA domain scales to change in symptom impact, and identified mean changes in DIVA domain scores corresponding with a pre-defined minimal clinically important change in perceived treatment benefit. Vaginal estradiol, vaginal moisturizer, and matched placebos were similarly effective in improving DIVA scores, particularly sexual functioning and self-concept/body image. Self-reported meaningful benefit from treatment was reflected by quantifiable changes in DIVA domain scores that may provide meaningful targets for research and clinical efforts.13
Results
The impact of vulvovaginal symptoms on multiple aspects of well-being and functioning improved in all treatment arms, with no benefit of estradiol tablet or vaginal moisturizer over placebo. This is consistent with the main finding of the MsFLASH Vaginal Health Trial, with no difference across all treatment arms in self-reported 12-week change in severity of women’s most bothersome vulvovaginal symptom.1 The MsFLASH network also reported no differences in the effect of treatment assignment on anxiety, depressive symptoms, and psychosocial functioning,20 mirroring the equivalent effect of estradiol tablet, moisturizer, and placebo on the impact of vulvovaginal symptoms on DIVA-assessed emotional well-being. Similarly, no differential treatment effects on sexual function as measured by the FSFI and selected items from the FSDS-R have been identified.1 However, women assigned to estradiol tablet did report significantly greater improvement in menopause-related quality of life and sexual functioning as assessed by the MENQOL compared to placebo.20
Overall, improvement in the unidimensional severity of vulvovaginal symptoms was reflected by substantial changes in DIVA scores. On average, women who reported a 2-point or greater improvement in symptom severity also demonstrated pronounced improvement in the impact of symptoms on sexual functioning and self-concept/body image. To a lesser degree, reduced impact of symptoms on emotional well-being and activities of daily living were also seen among women who reported symptom improvement.
MIC/MIDs varied by DIVA domain, following patterns of sensitivity to change led by more pronounced improvements in the impact of symptoms on sexual functioning and self-concept/body image in women who report vulvovaginal symptom improvement. Though they varied by domain and across intervention arms, these thresholds were met by over half of women overall, suggesting meaningful improvement for at least half of women in the study.
Clinical Implications
Our findings suggest that women using low-dose vaginal estradiol or non-hormonal moisturizer are likely to experience similar improvements in symptom-related functioning and well-being. Regardless of specific treatment approach, clinicians may anticipate that most women will perceive meaningful reduction in symptom impact. Additionally, these findings suggest that women’s perception of symptom improvement and benefit from treatment may be driven by change in the impact of vaginal symptoms on sexual functioning and body image.
Research Implications
While the DIVA questionnaire has been previously used in an observational sample to describe patient-centered experiences of vulvovaginal symptoms,21 this study provides evidence that it is a sensitive and useful instrument to assess change in symptom impact in clinical trials. This study identified target values for within-woman minimal clinically important change (MIC) and between-women minimal clinically important difference (MID) in DIVA domain scores, using an anchor-based approach13,18 correlating change in DIVA scores to self-reported meaningful benefit from treatment. By tying numerical scores to independent, patient-reported assessment of improvement, this approach identifies a measure of treatment-related change that should be meaningful to patients.13 Though replication is needed to verify and refine these MIC/MID estimates, these results will allow appropriate power calculations and interpretation of symptom improvement measurements in clinical trials, observational studies, and clinical practice.13
Strengths and Limitations
Strengths of this study include assessment within the MsFLASH Vaginal Health Trial, a well-defined, multicenter, randomized clinical trial.1 By design, all participants entered the trial with moderate-severe vulvovaginal symptoms, as would be expected of treatment-seeking women in clinical settings.10 The study used reliable and valid measures of symptom improvement and had excellent participant retention and medication adherence. Additionally, an anchor-based approach was used to examine sensitivity to change and MIC/MID from a patient-centered perspective.13 However, these findings should be interpreted in light of limitations. Data were drawn from a relatively homogenous population of women who were willing and able to participate in a clinical trial and provide long-term follow-up information, which limits generalizability. Minimal baseline impairment in DIVA Activities of Daily Living was seen in this sample, which may have limited our ability to detect change in this domain. While we identified a robust overall anchor, not identifying a different anchor for each DIVA domain scale may have limited our ability to evaluate the MCID of each DIVA scale in isolation from the others. The estimated MIC/MID needs to be confirmed and refined in other samples, and more work may be needed to evaluate the sensitivity of the DIVA questionnaire to treatment improvement in these domains relative to other assessment tools.
Conclusions
Meaningful assessment of vulvovaginal symptom treatment efficacy should involve patient-centered outcome measures, including measures that capture the impact of symptoms on multiple aspects of daily well-being and functioning. On average, participants in the MsFLASH Vaginal Health Trial reported a decreased impact of vulvovaginal symptoms on activities of daily living, emotional well-being, sexual functioning, and self-concept/body image over 12 weeks as measured by the DIVA questionnaire, regardless of treatment assignment. In addition to providing more evidence to inform treatment decisions, this study demonstrates the sensitivity of the DIVA instrument, and provides MICs and MIDs for each domain of this instrument relative to patient-reported benefit from treatment. These findings can guide patient-centered research to understand the impact of symptoms and care practices to improve symptom impact on domains important to patients, including activities of daily living, emotional well-being, sexual functioning, and self-concept and body image.
AJOG at a Glance:
A. Why was this study conducted?
To compare the effects of a vaginal 10-μg estradiol tablet and a vaginal moisturizer, each to placebo, on the day-to-day impact of postmenopausal vulvovaginal symptoms on well-being and functioning.
To examine sensitivity to change and estimate minimal clinically important change in scores on the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire, as a measure of the functional and quality of life impact of postmenopausal vulvovaginal symptoms.
B. What are the key findings?
Vaginal estradiol tablet, vaginal moisturizer, and placebo treatments resulted in similar improvements in the impact of postmenopausal vulvovaginal symptoms on well-being and functioning over 12 weeks, as measured by DIVA domain scores.
Self-reported improvement in vaginal symptoms and perceived benefit from treatment were accompanied by decreases in all DIVA domain scale scores, particularly DIVA scales assessing impact on sexual functioning and body image.
Minimum clinically important differences ranging from −0.4 to −1.3 for DIVA domain scores, associated with self-reported meaningful benefit from treatment, may be used in future studies to evaluate treatment responses.
C. What does this study add to what is already known?
This research adds to our understanding of patient-centered outcomes in treatment of bothersome postmenopausal vulvovaginal symptoms and provides meaningful metrics that may be used to guide future research and clinical efforts.
Funding sources/roles of funding sources:
The parent study design, methods, subject recruitment, and data collection for the MsFLASH Vaginal Health Trial was funded by the National Institutes of Health, National Institute on Aging: R01 AG048209. The sponsor was not involved in the analysis or preparation of this manuscript. This manuscript is the result of work supported with resources and the use of facilities at the San Francisco VA Health Care System and the University of California, San Francisco, and supported in part by Career Development Award Number IK2 HX002402 from the United States (U.S.) Department of Veterans Affairs Health Services R&D (HSRD) Service (CJG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Dr. Huang has received research grants from Pfizer Inc. and Astellas Pharma through the University of California San Francisco to conduct research unrelated to this manuscript. Dr. Mitchell is a consultant for Scynexis Inc., and receives research funding from Merck. Dr. Reed receives grant support from Bayer Pharmaceuticals. Dr. LaCroix has served on a scientific advisory board for Sermonix, Inc. All other authors have no conflicts of interest to declare.
Clinical trial registration: Clinicaltrials.gov: NCT02516202. Registered 8/5/2015, study enrollment began 4/2016.
Related presentations: Some findings from this manuscript will be presented at the North American Menopause Society Annual Meeting (September 25–27, 2019).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the National Institute of Aging.
Condensation of the paper: Vulvovaginal symptom treatment was accompanied by reduced impact of symptoms on well-being and functioning as measured by the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire.
References
- 1.Mitchell CM, Reed SD, Diem S, et al. Efficacy of Vaginal Estradiol or Vaginal Moisturizer vs Placebo for Treating Postmenopausal Vulvovaginal Symptoms: A Randomized Clinical Trial. JAMA Intern Med. 2018;178(5):681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minkin MJ, Reiter S, Maamari R. Prevalence of postmenopausal symptoms in North America and Europe. Menopause. 2015;22(11):1231–1238. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142. [DOI] [PubMed] [Google Scholar]
- 4.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799. [DOI] [PubMed] [Google Scholar]
- 5.Huang AJ, Moore EE, Boyko EJ, et al. Vaginal symptoms in postmenopausal women: self-reported severity, natural history, and risk factors. Menopause. 2010;17(1):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter MM, Nakagawa S, Van Den Eeden SK, Kuppermann M, Huang AJ. Predictors of impact of vaginal symptoms in postmenopausal women. Menopause. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nappi RE, Palacios S, Bruyniks N, Particco M, Panay N, investigators ES. The burden of vulvovaginal atrophy on women’s daily living: implications on quality of life from a face-to-face real-life survey. Menopause. 2018. [DOI] [PubMed] [Google Scholar]
- 8.Huang AJ, Gregorich SE, Kuppermann M, et al. Day-to-Day Impact of Vaginal Aging questionnaire: a multidimensional measure of the impact of vaginal symptoms on functioning and well-being in postmenopausal women. Menopause. 2015;22(2):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;215(6):704–711. [DOI] [PubMed] [Google Scholar]
- 10.Faubion SS, Sood R, Kapoor E. Genitourinary Syndrome of Menopause: Management Strategies for the Clinician. Mayo Clin Proc. 2017;92(12):1842–1849. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: evaluation, sequelae, and management. Am J Obstet Gynecol. 2016. [DOI] [PubMed] [Google Scholar]
- 12.Palacios S, Castelo-Branco C, Currie H, et al. Update on management of genitourinary syndrome of menopause: A practical guide. Maturitas. 2015;82(3):308–313. [DOI] [PubMed] [Google Scholar]
- 13.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–1343. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration Guidance for Industry: Estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms--Recommendations for clinical evaluation. In: Administration USFaD, ed. Rockville, MD2003. [Google Scholar]
- 15.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 17.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. [DOI] [PubMed] [Google Scholar]
- 18.Smelt AF, Assendelft WJ, Terwee CB, Ferrari MD, Blom JW. What is a clinically relevant change on the HIT-6 questionnaire? An estimation in a primary-care population of migraine patients. Cephalalgia. 2014;34(1):29–36. [DOI] [PubMed] [Google Scholar]
- 19.McElhone K, Abbott J, Sutton C, et al. Sensitivity to Change and Minimal Important Differences of the LupusQoL in Patients With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2016;68(10):1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diem SJ, Guthrie KA, Mitchell CM, et al. Effects of vaginal estradiol tablets and moisturizer on menopause-specific quality of life and mood in healthy postmenopausal women with vaginal symptoms: a randomized clinical trial. Menopause. 2018;25(10):1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter MM, Nakagawa S, Van Den Eeden SK, Kuppermann M, Huang AJ. Predictors of impact of vaginal symptoms in postmenopausal women. Menopause. 2016;23(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


