Abstract
Objective:
To determine the prevalence of probiotic administration in preterm infants over time, as well as the association between probiotic administration and select adverse outcomes.
Study design:
We performed a multicenter cohort study of infants 23–29 weeks gestational age admitted to 289 neonatal intensive care units (NICUs) from 1997–2016. We evaluated the type of probiotics given and prevalence of exposure to probiotics over time and by site. We matched probiotic-exposed infants by several factors to unexposed infants receiving enteral feeds on the same postnatal day. We performed conditional logistic regression to evaluate the association between probiotics exposure and adverse outcomes, including necrotizing enterocolitis (NEC), bloodstream infections, meningitis, and death.
Results:
Of 78,076 infants, 3626 (4.6%) received probiotics. Probiotic use increased over the study period and varied among NICUs. We matched 2178 infants exposed to probiotics to 33,807 without exposure. Probiotic administration was associated with a decrease in NEC (odds ratio [OR] 0.62, 95% confidence interval [CI] 0.48–0.80) and death (OR 0.52, 95% CI 0.39–0.70), an increase in Candida infection (OR 2.23, 95% CI 1.29–3.85), but no increase in bloodstream infection (OR 0.86, 95% CI 0.70–1.05) or meningitis (OR 1.18, 95% CI 0.40–3.46).
Conclusions:
Probiotic use increased over time and was associated with decreased odds of NEC and death. Prospective, randomized-controlled studies of specific probiotic products are needed to further investigate the safety and efficacy of probiotics in preterm infants.
Infants born preterm are at high risk of gut dysbiosis, which is characterized by overgrowth of pathogenic bacteria such as Enterobacter and Pseudomonas species.1 Such overgrowth causes compromise of the intestinal wall barriers, translocation of pathogenic bacteria, and potential necrotizing enterocolitis (NEC).1 NEC remains a leading cause of mortality in very low birth weight (VLBW) infants, especially those born less than 1000 grams; survivors are at risk for neurodevelopmental impairment.1–4 Probiotics, which are live bacterial organisms intended to alter the gut microbiota, may reduce the risk of NEC.3 Potential benefits of probiotics in preterm infants include improved mucosal junctional barriers to decrease the translocation of bacteria, better modulation of the innate and humoral immune responses, promotion of protein and carbohydrate breakdown for better enteral absorption, and resistance to the overgrowth of potentially more pathogenic bacteria such as Enterococcus.1,4–7
Although several studies report that probiotic use reduces the incidence of NEC,4,8–12 there remains considerable debate over probiotic use in preterm infants.3,12–14 The concerns stem from lack of regulation for probiotic products and lack of consensus for the safest and most effective strains. United States Food and Drug Administration (FDA) approval and regulation of therapeutic agents require supporting data from well-designed clinical trials with limited bias that provide evidence the product benefits outweigh the risk, yet such trials have been difficult to perform in the VLBW population. Probiotics, as a dietary supplement, do not require strict regulation by the FDA, although the FDA recently issued guidance for early clinical trials with live biotherapeutic products.15 There are currently no FDA-approved probiotics for preterm infants.16 The use of probiotics as a non-regulated FDA product leads to potential risk of contamination, due to inconsistent quality control, as well as varying amounts and strains of bacteria within the products.4 Mucormycosis was reported in a 29-week infant who died following exposure to probiotics contaminated with mold, specifically Rhizopus species.17 Further research, including randomized-controlled trials and cohort studies among high-risk preterm infants, are necessary to assess the safety of specific probiotic preparations. The objectives of this study were to quantify probiotic use among preterm, hospitalized infants over time and to compare the prevalence of NEC, bloodstream infection, non-Candidal fungal infections, meningitis, and mortality between probiotic-exposed and unexposed infants.
Methods
We performed a multicenter retrospective cohort study of preterm infants admitted to the neonatal intensive care unit (NICU) from 1997–2016. Data were retrieved from the Pediatrix™ Clinical Data Warehouse, a multicenter clinical database including patients from 392 NICU sites in 35 states and Puerto Rico, via the BabySteps online health record (Sunrise, FL USA).18,19 Infants born at 23–29 weeks gestational age and <120 postnatal days were included. Infants who died or were discharged prior to three postnatal days were excluded. We extracted information on prenatal characteristics, demographics, exposure to medications, and interventions while in the hospital, and in-hospital clinical outcomes. The Duke University Institutional Review Board approved the study.
We defined probiotic exposure as the receipt of any probiotic during the first 120 postnatal days. The probiotic strain or product was obtained from provider notes; the brand name was sometimes, but not always, available. Outcomes of interest included medical and surgical NEC, bloodstream infection, non-Candidal fungal infection, meningitis, or death following the start day of probiotics. Using provider diagnoses, we searched for Bell stage II NEC or medically-treated NEC (medical NEC) and Bell stage III NEC or surgically-treated NEC (surgical NEC).20,21 We defined bloodstream infection as at least one positive blood culture for a bacterial or fungal pathogen at any time during postnatal days 3–120. Candida infections included a positive blood or cerebral spinal fluid (CSF) culture for Candida species. Non-Candidal fungal infections included a positive blood or CSF culture for a fungal pathogen that was not Candida. We defined meningitis as having at least one positive CSF culture for a bacterial or fungal pathogen at any time during postnatal days 3–120.22 We excluded CSF organisms considered to be contaminants. We assigned cultures positive for coagulase negative Staphylococcus based on definitions used in previous publications as: 1) definite (two positive cultures drawn on the same day); 2) probable (two positive cultures within a 4-day period, three positive cultures within a 7-day period, or four positive cultures within a 10-day period); or 3) possible (positive culture that did not meet definite or probable criteria).23
Statistical Analyses
Among the cohort that met the inclusion criteria, we evaluated probiotic type and change in prevalence of exposure to probiotics over time and by site for sites admitting >100 infants during the study period. For the remaining analyses, we excluded infants discharged before 2006 to examine use over a more recent timeframe. We divided infants discharged from 2006–2016 into two groups: infants who received probiotics prior to postnatal day 120 (exposed group); and infants who did not receive probiotics prior to postnatal day 120 (unexposed group). Exposed infants were matched to unexposed infants who received enteral feeds on the same postnatal day that probiotics were started. We also matched infants exactly on gestational age (GA), small for GA (SGA) status, inborn status, discharge year, race, history of medical or surgical NEC, and if there was or was not breast milk exposure on the start day of probiotics. When infants had multiple matching control infants, all matches were included. We summarized continuous variables by median and range and categorical variables by proportions.
In the matched cohort, we evaluated the prevalence of the outcomes of interest (any NEC, surgical NEC, bloodstream infection, Candida infection, non-Candidal fungal infection, meningitis, or death) after the start day of probiotics (exposed group) or the start day of the matching exposed infant (unexposed group). We excluded infants with a history of NEC prior to the start day of probiotics (exposed group) or the start day of the matching unexposed infant (unexposed group) from the analysis of the NEC outcome. We used conditional logistic regression (conditioned on the matched groups) to assess differences in outcomes between groups. We reported odds ratios (ORs) with 95% confidence intervals (CIs). A P value of < .05 was considered to be statistically significant. We used Stata, version 15.1 (StataCorp LLC, College Station, TX) for data analysis.
Results
Of 78,076 infants admitted to 289 NICUs across the United States who met the inclusion criteria, 3626 (4.6%) received probiotics. In 1997, at the beginning of the study period, no probiotic use was reported in the 289 NICUs included. A small number of NICU sites began probiotic use in 1998–2000, with the greatest increase in use after 2006 and again in 2014. By 2016, there were 118 infants exposed per 1000 admitted (Figure 1). The number of infants exposed to probiotics by site varied widely, from 0–841 per 1000 infants (Figure 2). The most commonly administered probiotic was Lactobacillus (71%), followed by Ultimate Flora (Bifidobacterium and Lactobacillus species), ABC Dophilus (Bifidobacterium, Lactobacillus, and Streptococcus species), and Align (Bifidobacterium); Table I (at www.jpeds.com).
Figure 1. Exposure to probiotics over study time period.
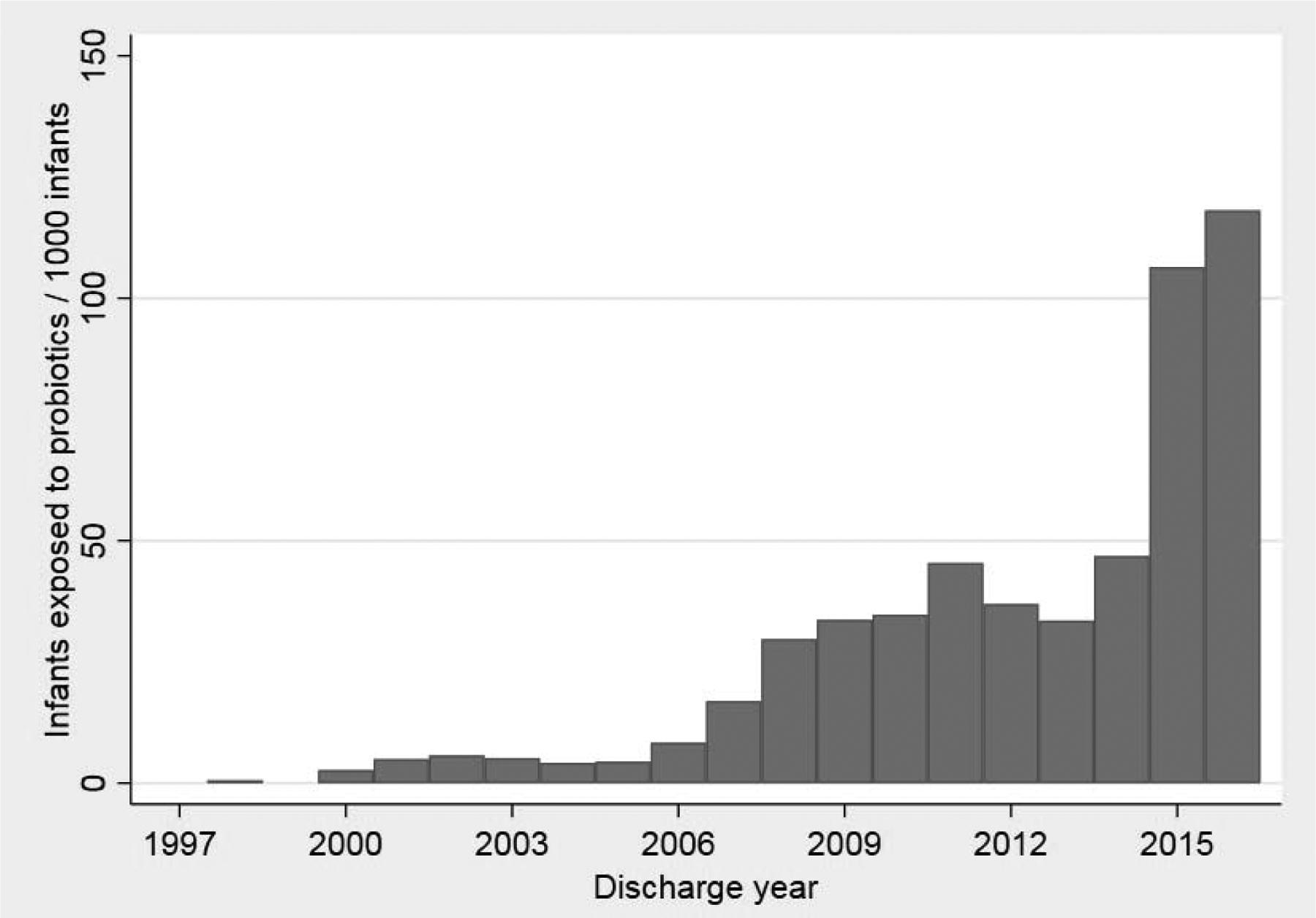
Number of infants exposed to probiotics per 1000 infants over the study time period: 1997–2016.
Figure 2. Exposure to probiotics by site.

Number of infants exposed to probiotics per 1000 infants by site over the study time period (1997–2016)*.
*Includes sites discharging at least 100 infants during the study period and excludes 88 sites where no infants were exposed.
Table I (online only).
Distribution of probiotic exposure by type
| Probiotic Type | N=3626 n (%)* |
|---|---|
| Lactobacillus | 2592 (71) |
| Ultimate Flora® (bifidobacterium and lactobacilli species) | 944 (27) |
| ABC Dophilus® (bifidobacterium, lactobacilli, and streptococcus species) | 232 (6) |
| Align® (bifidobacterium) | 13 (0.4) |
| Other | 106 (3) |
Percentages total >100% due to exposure to multiple probiotics.
Among the exposed infants from 2006–2016, 2178 could be matched with 33,807 unexposed infants (Table II). The median (25th, 75th percentile) number of control infants matched to probiotic-exposed infants was 45 (12, 120). The overall median gestational age and birth weight of the matched cohort were 28 weeks (26, 29) and 1020 grams (826, 1218). A small proportion of infants had a history of NEC in the exposed and unexposed group prior to the start of probiotics (2% vs. 0.2% respectively, Table II). The median start day of probiotics was 4 (2, 18) and the median duration of exposure was 50 days (32, 68).
Table II.
Demographics of matched cohort (all values reported as N [%])
| Administered Probiotics N=2178 (6) | Never Administered Probiotics N=33,807 (94) | |
|---|---|---|
| Gestational age* | ||
| 22–24 weeks | 296 (14) | 2956 (9) |
| 25–28 weeks | 1352 (62) | 21,482 (64) |
| 29–30 weeks | 530 (24) | 9369 (28) |
| Small for gestational age* | 256 (12) | 1941 (6) |
| Inborn* | 1963 (90) | 31,859 (94) |
| History of NEC prior to start day* | 41 (2) | 51 (0.2) |
| Any breast milk exposure prior to start day* | 1705 (78) | 31,288 (93) |
| Birth weight | ||
| <500 g | 47 (2) | 265 (1) |
| 500–749 g | 474 (22) | 5058 (15) |
| 750–999 g | 670 (31) | 10,222 (30) |
| 1000–1499g | 922 (42) | 16,999 (50) |
| ≥1500g | 65 (3) | 1263 (4) |
| Cesarean section | 1662 (77) | 24,095 (72) |
| Antenatal steroids | 1839 (84) | 29,111 (86) |
| Antenatal antibiotics | 1279 (59) | 19,264 (57) |
| Male | 1167 (54) | 17,585 (52) |
| Maternal age | ||
| ≤19 years | 190 (9) | 3137 (9) |
| 20–29 years | 1036 (48) | 16,467 (49) |
| 30–39 years | 856 (39) | 12,682 (38) |
| ≥40 years | 92 (4) | 1405 (4) |
NEC, necrotizing enterocolitis
Indicates variable on which infants were matched exactly. Apparent imbalances in these variables are due to variation in the number of multiple matches per infant administered probiotics.
Without accounting for the number of matches per exposed infant, the prevalence of outcomes appeared similar in the two groups (Table III). Non-Candidal fungal infection occurred in one infant, who was not exposed to probiotics. In the conditional logistic regression analysis, infants exposed to probiotics had significantly lower odds of any NEC (OR 0.62; 95% CI 0.48–0.80, p<0.001) compared with unexposed infants. The odds of death were lower in infants exposed to probiotics (OR 0.52, 95% CI 0.39–0.70, p<0.001). The odds of Candida infection was higher in infants exposed to probiotics (OR 2.23, 95% CI 1.29–3.85, p=0.004). There was no significant association between probiotics exposure and surgical NEC (OR 0.81, 95% CI 0.54–1.22; p=0.32), bloodstream infection (OR 0.86, 95% CI 0.70–1.05, p=0.14), or meningitis (OR 1.18, 95% CI 0.40–3.46, p=0.76).
Table III.
Outcomes of infants in the matched cohort (all values reported as N [%])
| Outcome | Administered Probiotics N=2178 (6) | Never Administered Probiotics N=33,807 (94) |
|---|---|---|
| NEC* | 87/2137 (4%) | 2087/33,756 (6%) |
| Surgical NEC* | 37/2137 (1.7%) | 578/33,756 (1.7%) |
| Bloodstream infection | 172 (8%) | 2622 (8%) |
| Candida infection | 23 (1%) | 142 (0.4%) |
| Non-candidal fungal infection | 1 (0%) | 0 (0%) |
| Meningitis | 6 (0.3%) | 66 (0.2%) |
| Death | 82 (4%) | 1396 (4%) |
NEC, necrotizing enterocolitis
Denominators differ from overall totals due to the fact that we excluded infants with a history of NEC prior to the start day of probiotics (exposed group) or the start day of the matching unexposed infant (unexposed group) from the analysis of the NEC outcome.
Discussion
With almost 20 years of data among centers across the United States, we showed a trend of increased use of probiotics among NICU infants. A telephone survey of hospitals participating in the Vermont Oxford Network (VON) database reported an increase in the use of probiotics in VLBW infants in 2013–2014 from 5.2% to 6.7% and increasing to 14% of NICU sites in 2015.16 The increasing trends of probiotic use in our cohort from 1998–2000 and again in 2006 and 2014 parallel the growing number of randomized control trials, cohort studies and meta-analyses reviewing the efficacy and safety of probiotics.
Although evidence is growing to support the use of probiotics, there is a lack of consensus on which strains or products to use. In our cohort, the prevalence of any probiotic treatment varied widely by site, with 88 NICUs using no probiotics at all and several NICUs using probiotics in the majority of infants meeting inclusion criteria. By not requiring matching of infants within site, we used this variation to compare otherwise similar infants from different sites. In the VON survey and in a recent meta-analysis of 25 randomized control trials, single strains of Lactobacillus species were most commonly used, followed by a combination of Bifidobacterium, Lactobacillus, and Streptococcus species.16,24 Similar to these prior reports, we found that probiotics containing Lactobacillus species were the most commonly prescribed, followed by various multi-species probiotics. Timing and duration of exposure also varied among VON sites, and indications for exposure ranged from feeding intolerance to antibiotic exposure and physician preference.16 Our study indicates there is a persistent lack of consistency among providers and sites for the use of probiotics, likely due to conflicting reports on efficacy and safety, as well as the absence of FDA approval for the therapeutic use of this product.
Previous studies evaluating the efficacy of probiotics revealed mixed data to support probiotic supplementation to reduce NEC in infants. The two early studies in 1986 and 1993 suggested that probiotics (single and combined strains) could colonize the gut; however, the studies’ results did not support the reduction of pathogenic bacteria associated with NEC.25,26 The Probiotics in Preterm Infants (PiPs) Study Collaborative assessed the safety and efficacy of a single strain (Bifidiobacterium breve) in 1315 infants, but found difference in the primary outcome and did not recommend the routine use of probiotics in this population.27 Our study included a small number of infants treated with bifidobacteria, which limited our ability to make conclusions on efficacy of this organism alone.
Other studies support a role for probiotics in the reduction of NEC. A meta-analysis including 37 trials with >10,000 infants reviewed prophylactic probiotic supplementation in preterm newborns and showed significantly reduced incidence of NEC compared with a placebo.8 Although preparations containing lactobacilli alone or in combination with bifidobacteria seemed to be most protective, the population in this meta-analysis was highly variable in the gestational age, birth weights, and strains and combinations of probiotics.8 Another retrospective cohort study of 652 infants <29 weeks treated with prophylactic probiotics (Florbaby with Bifidobacterium species and Lactobacillus rhamnosus, and Biogaia with Lactobacillus reuteri) found decreased risk for NEC (adjusted OR 0.64, 95% CI 0.410–0.996) compared with those who were not treated, but no difference in NEC in infants less than 26 weeks (p=0.95).29 Our cohort included a larger sample size over similar gestational ages, and included the use of more probiotic products compared with this study. Another large observational study of 5300 infants within 46 NICUs over a 2-year period found prophylactic use of Infloran (Lactobacillus acidophilus/Bifidobacterium infantis) reduced risk of surgical NEC in VLBW infants (4.2 vs. 2.6%, p=0.028).30 This study included infants up to 32 weeks gestation, whereas our study included more premature infants (23–29 weeks).30 Another study with a sub-analysis of 4683 ELBW infants showed that a dual-strain probiotic of Lactobacillus acidophilus and Bifidobacterium spp. (Infloran) was associated with reduced risk of NEC (adjusted hazard ratio [HR] 0.48, 95% CI 0.36–0.63, p<0.001) and overall mortality (HR 0.59, 95% CI 0.41–0.84, p=0.003).31 We showed a decrease in the odds of any NEC diagnosis, but there was not an associated reduction in surgical NEC for those exposed.
Many questions remain concerning the safety of probiotics in preterm infants. Several studies using single and multiple species reported no association between the use of probiotics and increased rates of sepsis, bacteremia, meningitis, or death.9,12,24,32 However, concerns were raised when the ABC Dophilus® probiotic was associated with a mucormycosis infection and death of an infant following exposure to product contaminated with Rhizopus oryzae.17 This report prompted the removal of the product from the market. Our study included the use of ABC Dophilus®, but did not find an association between fungal or mold infections following exposure to probiotics. This may be due to the small number of infants exposed to this probiotic, but there were no cases of fungal or mold infection when including all probiotics.
An unexpected finding in our study was the increased risk for Candida infections among infants exposed to probiotics, which is contradictory to previous studies. Earlier studies reported probiotics use reduced colonization of Candida species in preterm infants.33,34 In 2017, a systematic review of seven randomized-controlled trials, including 1371 preterm infants, showed probiotic exposure reduced the incidence of Candida colonization. The infants were exposed to both single- and multi-strain products of Bifidobacterium and Lactobacillus.34 The authors chose to exclude one study with a high baseline incidence of fungal sepsis. The absolute difference we report is less than 1% and will require confirmatory reports. Such confirmation will be challenging, given the low incidence of Candida infection, which has been decreasing over time in the NICU.35 Although the results in our study were statistically significant, the question remains as to whether the findings are clinically significant. Our study did not consider other aspects of care that contribute to the risk of Candida infection, such as unit hand hygiene practices, length of exposure to antibiotics, time to full feedings, growth velocities, or length of stay.
Strengths of our study include our report of probiotic use over time and a matched cohort design that allowed us to minimize selection bias. Our study also had certain limitations. First, the retrospective design did not allow us to account for unmeasured confounders, including variability in other clinical management from site to site. Second, similar to many previous studies, there was great variation in probiotic products and organisms, as well as a lack of dosing information, which made it unclear which product, organism, or dose might be most effective. We do not know if the providers tested any probiotic products for contamination after events of sepsis or NEC. We relied on clinician diagnosis to distinguish surgical NEC from spontaneous intestinal perforation; therefore, it is possible that some cases of surgical NEC were actually misdiagnosed cases of spontaneous intestinal perforation. Other factors that may influence the risk of NEC were not reviewed in this cohort, including percentage of mother’s milk intake in the first few weeks of life and the timing and duration of antibiotic exposure prior to a diagnosis of NEC.36,37 We did not have access to transfusion timing or the probiotic administration criteria, which may vary by site. Finally, our study population included a small number of infants less than 24 weeks or 750 g exposed to probiotics, which may indicate caution by providers in younger age groups, due to conflicting evidence.
In conclusion, our study builds upon prior studies supporting the safety and effectiveness of probiotics. Future studies in infants must evaluate dosing for particular strains and mechanism of action to determine which treatment yields the highest safety and efficacy. These data could inform larger, comparison trials of single probiotic strains in preterm infants to identify which strains and doses are most associated with lower rates of NEC and infection.
Acknowledgments
We thank Divine Pinson, Vivian Chu, and Amanda McMillan for their work with the 2017 DUKE STAR summer program.
Funded by the National Institute of Child Health and Human Development (NICHD) (HHSN275201000003I [to D.B.]) for the Pediatric Trials Network. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Also supported by Duke Clinical Research Institute’s R25 Summer Training in Academic Research (STAR) Program (#5R25HD076475-07). R.G. received support from industry for research services (https://dcri.org/about-us/conflict-of-interest/). The other authors have declare no conflicts of interest.
Abbreviations
- CSF
cerebral spinal fluid
- CI
confidence interval
- FDA
Food and Drug Administration
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- OR
odds ratio
- SGA
small for gestational age
- VLBW
very low birth weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millar M, Wilks M, Costeloe K. Probiotics for preterm infants? Arch Dis Child Fetal Neonatal Ed 2003;88:F354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talavera MM, Bixler G, Cozzi C, Dail J, Miller RR, McClead R Jr, et al. Quality improvement initiative to reduce the necrotizing enterocolitis rate in premature infants. Pediatrics 2016;137. [DOI] [PubMed] [Google Scholar]
- 3.Johnson-Henry KC, Abrahamsson TR, Wu RY, Sherman PM. Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Adv Nutr 2016;7:928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underwood MA. Impact of probiotics on necrotizing enterocolitis. Semin Perinatol 2017;41:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics 2016;137:e20153684. [DOI] [PubMed] [Google Scholar]
- 6.Zhang GQ, Hu HJ, Liu CY, Shakya S, Li ZY. Probiotics for preventing late-onset sepsis in preterm neonates: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Semin Pediatr Surg 2018;27:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawh SC, Deshpande S, Jansen S, Reynaert CJ, Jones PM. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ 2016;4:e2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014:CD005496. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury T, Ali MM, Hossain MM, Singh J, Yousuf AN, Yasmin F, et al. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. J Coll Physicians Surg Pak 2016;26:770–4. [PubMed] [Google Scholar]
- 11.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010;125:921–30. [DOI] [PubMed] [Google Scholar]
- 12.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis 1999;3:197–202. [DOI] [PubMed] [Google Scholar]
- 13.Samuels N, van de Graaf R, Been JV, de Jonge RC, Hanff LM, Wijnen RM, et al. Necrotising enterocolitis and mortality in preterm infants after introduction of probiotics: a quasi-experimental study. Sci Rep 2016;6:31643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costeloe K, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, et al. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess 2016;20:1–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Food and Drug Administration (FDA). Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information Guidance for Industry. FDA web site. https://www.fda.gov/media/82945/download. Published February 2012 Updated June 2016 Accessed December 2, 2019.
- 16.Viswanathan S, Lau C, Akbari H, Hoyen C, Walsh MC. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. J Perinatol. 2017;37:104. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC). Fatal gastrointestinal mucormycosis in an infant following use of contaminated ABC Dophilus Powder from Solgar Inc. 2014 (archived document). CDC web site. https://www.cdc.gov/fungal/outbreaks/rhizopus-investigation.html. Updated February 20, 2016 Accessed June 26, 2019.
- 18.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for “meaningful use” in continuous quality improvement. Clin Perinatol 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 19.Ellsbury DL, Clark RH, Ursprung R, Handler DL, Dodd ED, Spitzer AR. A Multifaceted approach to improving outcomes in the NICU: the Pediatrix 100 000 Babies Campaign. Pediatrics 2016;137. [DOI] [PubMed] [Google Scholar]
- 20.Gordon PV, Clark R, Swanson JR, Spitzer A. Can a national dataset generate a nomogram for necrotizing enterocolitis onset? J Perinatol 2014;34:732–5. [DOI] [PubMed] [Google Scholar]
- 21.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in the infant. Clin Perinatol 2015;42:29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornik CP, Benjamin DK, Becker KC, Benjamin DK Jr, Li J, Clark RH, et al. Use of the complete blood cell count in late-onset neonatal sepsis. Pediatr Infect Dis J 2012;31:803–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang HY, Chen JH, Chang JH, Lin HC, Lin CY, Peng CC. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: an updated meta-analysis. PLoS One 2017;12:e0171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuman PD, Duckworth DH, Smith KL, Kagan R, Bucciarelli RL, Ayoub EM. Lack of effect of Lactobacillus on gastrointestinal bacterial colonization in premature infants. Pediatr Infect Dis 1986;5:663–8. [DOI] [PubMed] [Google Scholar]
- 26.Millar MR, Bacon C, Smith SL, Walker V, Hall MA. Enteral feeding of premature infants with Lactobacillus GG. Arch Dis Child 1993;69:483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016;387:649–60. [DOI] [PubMed] [Google Scholar]
- 28.Abrahamsson TR. Not all probiotic strains prevent necrotising enterocolitis in premature infants. Lancet 2016;387:624–5. [DOI] [PubMed] [Google Scholar]
- 29.Singh B, Shah PS, Afifi J, Simpson CD, Mitra S, Dow K, et al. Probiotics for preterm infants: A National Retrospective Cohort Study. J Perinatol 2019;39:533–9. [DOI] [PubMed] [Google Scholar]
- 30.Härtel C, Pagel J, Rupp J, Bendiks M, Guthmann F, Rieger-Fackeldey E, et al. Prophylactic use of Lactobacillus acidophilus/Bifidobacterium infantis probiotics and outcome in very low birth weight infants. J Pediatr 2014;165:285–9.e1. [DOI] [PubMed] [Google Scholar]
- 31.Denkel LA, Schwab F, Garten L, Geffers C, Gastmeier P, Piening B. Protective effect of dual-strain probiotics in preterm infants: a multi-center time series analysis. PloS One 2016;11:e0158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;132:1055–62. [DOI] [PubMed] [Google Scholar]
- 33.Romeo MG, Romeo DM, Trovato L, Oliveri S, Palermo F, Cota F, et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol. 2011;31:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu HJ, Zhang GQ, Zhang Q, Shakya S, Li ZY. Probiotics prevent Candida colonization and invasive fungal sepsis in preterm neonates: a systematic review and meta-analysis of randomized controlled trials. Pediatr Neonatol 2017;58:103–10. [DOI] [PubMed] [Google Scholar]
- 35.Aliaga S, Clark RH, Laughon M, Walsh TJ, Hope WW, Benjamin DK, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics 2014;133:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neu J. Preterm infant nutrition, gut bacteria, and necrotizing enterocolitis. Curr Opin Clin Nutr Metab Care 2015;18:285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colaizy TT, Bartick MC, Jegier BJ, Green BD, Reinhold AG, Schaefer AJ, et al. Impact of optimized breastfeeding on the costs of necrotizing enterocolitis in extremely low birthweight infants. J Pediatr 2016;175:100–5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]


