Abstract
Background:
Rural Americans have higher prevalence of obesity and type 2 diabetes (T2D) than urban populations and more limited access to behavioral programs to promote healthy lifestyle habits. Descriptive evidence from the Rural Lifestyle Intervention Treatment Effectiveness trial (Rural LITE) delivered through local Cooperative Extension Service (CES) offices in rural areas previously identified that behavioral modification with both nutrition education and coaching resulted in a lower program delivery cost per kilogram of weight loss maintained at 2-years compared to an education-only comparator intervention.
Objective:
This analysis extended earlier Rural LITE research regarding weight loss outcomes to assess whether nutrition education with behavioral coaching delivered through CES is cost-effective relative to nutrition education only in reducing T2D cases in rural areas.
Design:
A cost-utility analysis was conducted.
Participants/setting:
Trial participants (n = 317) from June 2008 through June 2014 were adults residing in rural Florida counties with a baseline body-mass index (BMI) between 30 and 45 kg/m2, but otherwise identified as healthy.
Intervention:
Trial participants were randomly assigned to low, moderate, or high doses of behavioral coaching with nutrition education (i.e., 16, 32, or 48 sessions over 24 months) or a comparator intervention that included 16 sessions of nutrition education without coaching. Participants’ hemoglobin A1C (HbA1C) was measured at baseline and the end of the trial to assess T2D status.
Main outcome measures:
T2D categories by treatment arm were used to estimate participants’ expected annual healthcare expenditures and expected health-related utility measured as Quality Adjusted Life years (i.e., QALYs) over a 5-year time horizon. Discounted incremental costs and QALYs were used to calculate incremental cost effectiveness ratios (ICERs) for each behavioral coaching intervention dose relative to the education-only comparator.
Statistical analyses performed:
Using a third party payer perspective, Markov transition matrices were used to model participant transitions between T2D states. Replications of the individual participant behavior were conducted using Monte Carlo simulation.
Results:
All three doses of the behavioral coaching intervention had lower expected total costs and higher estimated QALYs than the education-only comparator. The moderate dose behavioral coaching intervention was associated with higher estimated QALYs but was costlier than the low dose; the moderate dose was favored over the low dose with willingness to pay thresholds over $107,895/QALY. The low dose behavioral coaching intervention was otherwise favored.
Conclusions:
Because most rural Americans live in counties with CES offices, nutrition education with behavioral coaching programs similar to those delivered through this trial may be effective and efficient in preventing or delaying T2D-associated consequences of obesity for rural adults.
Keywords: Randomized Trial, Cost-effectiveness, Behavioral Modification, Diabetes, Rural Obesity
Introduction/Background
Obesity is a major public health concern in the United States (U.S.) due to its prevalence and associated preventable health conditions such as type 2 diabetes (T2D).1–4 Rates of obesity have increased over the past several decades, with rural residents at higher risk of obesity compared to other Americans.5,6 Rural communities often lack local access to evidence-based face-to-face weight loss programs that offer strategies for behavioral health modifications and peer support associated with sustained success.7–9
The relationship between health behaviors and diabetes is well-established.4,10–16 Risk factors for prediabetes and T2D in adults includes overweight or obese body mass index (BMI), poor nutritional habits (e.g., drinking large amounts of sugar-sweetened beverages), and sedentary lifestyles.10,11,13,16,17 Consequences of poorly-controlled T2D include reduced life expectancy and medical complications such as renal disease, retinopathy, cardiovascular disease, and neuropathy.18 The economic consequences of T2D are similarly stark, with total economic costs in the U.S. estimated at $327 billion in 2017, up from $245 billion in 2012.19,20 For individuals, excess lifetime medical spending due to T2D in the U.S. was estimated at $124,600 in 2012, with decade of age at diagnosis moderating these costs.21 Thus, identifying cost-effective programs that reduce obesity-related T2D are needed, particularly in areas that may have limited access to commercial programs.8 A recent systematic review and meta-analysis that examined diabetes prevention interventions identified only eight studies that reported program cost information, but noted a wide range of costs per kilogram of net weight lost; averages ranged from approximately $34 to over $1,000 per kilogram lost.22
For rural residents, access to behavioral modification programs for local populations may be improved by providing these programs through Cooperative Extension Service (CES) offices, which are affiliated with the land-grant universities in every state and the U.S. Department of Agriculture (USDA).23,24 County CES offices are located throughout rural areas in the U.S. and have existing infrastructure and personnel to deliver educational programs. CES-based programs may result in a greater feasibility and cost efficiency for serving rural populations than storefront delivery through commercial weight loss programs. Notably, the Family and Consumer Sciences (FCS) focus of CES includes subcomponents of health and nutrition, and Extension FCS agents can deliver health promotion programs that leverage existing CES resources and infrastructure.25 A previous study, TOURS, tested the feasibility of CES-based programs for weight and lifestyle maintenance in rural areas, but results were limited to a single dose of the intervention across study arms and a descriptive analysis of programmatic costs in relation to weight loss.8,26 Further, the number of sessions used for that research, 50 over 18 months, was not considered feasible to offer as a regular CES-based program.8 The Rural Lifestyle Intervention Treatment Effectiveness (Rural LITE) trial was subsequently implemented to test alternative doses by varying the number and frequency of the intervention sessions with a CES-based behavioral program for adults with obesity in rural areas.9 Within-trial results in relation to weight loss and program delivery costs have been previously reported for Rural LITE, including a descriptive analysis of program delivery costs that increased by dose of the intervention with the lowest cost per kilogram of weight loss maintained (i.e., $22 per kg) associated with a moderate dose (i.e., 32 sessions) intervention.9
This paper builds on the previously-published descriptive cost-outcome comparison for the Rural LITE trial 9 (ClinicalTrials.gov Identifier: NCT00912652) to estimate T2D health status effects and related participant healthcare utilization costs. A cost-effectiveness analysis was needed to inform which dose of a behavioral coaching with nutrition education intervention, if any, represents the most efficient use of resources to reduce costs and health consequences of T2D for a time horizon up to five years after the intervention is delivered.
Materials and Methods
Rural LITE trial data were used along with parameters from published population-based studies to calculate the incremental cost effectiveness of three doses of a behavioral coaching with nutrition education intervention compared to an education-only comparator intervention. The analysis used the Consolidated Health Economics Evaluation Reporting Standards (CHEERS) guidelines and recommended practices for conducting cost-effectiveness analyses alongside implementation efforts from the International Society of Pharmacoeconomics and Outcomes Research (ISPOR).27,28 The University of Florida’s Institutional Review Board approved the Rural LITE study protocol. All trial participants provided written informed consent for collection and analysis of the study data.
Study Sample and Data Sources
The Rural LITE trial was conducted using existing infrastructure of the CES in 10 rural counties of Florida between June 2008 and June 2014. Rural residents of these counties 21-75 years of age with a body-mass index (BMI kg/m2) between 30 and 45 and without cardiovascular, hepatic, renal, or cerebrovascular disease within the past year were eligible to participate.9 Trial participants were randomly assigned to receive one of three doses of a behavioral coaching with nutrition education or a comparator intervention that included nutrition education without coaching. Potential participants were excluded if they used medications to affect body weight, had a weight change of at least 4.5 kg in the six months prior to screening, or had conditions that limited walking for 30 minutes.9 Eligible participants were further screened to ensure they did not have uncontrolled hypertension or diabetes.9 The present analysis identified the subsample of Rural LITE participants with hemoglobin A1C (HbA1c) measured at both the beginning and end of the trial (n = 317 of 612 participants enrolled). The sub-sample with complete HbA1C data was further checked for similarity to the overall sample of participants at baseline: 77.3% female (vs. 78.3% overall), 54.4 years of age (vs. 52.3 overall), and body mass index (BMI) of 36.1 (vs. 36.3 overall).9
Full details regarding data collection and primary 2-year outcomes of weight change and program costs from the Rural LITE intervention are available elsewhere, but are summarized here.9 Behavioral treatment methods were grounded in social cognitive theory.29,30 The framework included a standardized 6-month weight loss program that included nutrition education based on Diabetes Prevention Program (DPP) principles (phase 1) followed by an 18-month program for targeted maintenance (phase 2) which allowed two years of active participation and data collection for evaluation.16 Participants were randomly assigned to receive 8, 16, or 24 weekly nutrition education sessions for each phase. Didactic education content for all participant groups was the same. Participants assigned to the LOW (8 sessions per phase), MOD (16 sessions per phase), or HIGH (24 sessions per phase) intervention doses received behavioral coaching during both phases, with the primary difference across doses related to the available time for discussion and problem solving (i.e., time dedicated to coaching participants). Behavioral coaching included instruction in goal setting regarding diet intake (generally 1200-1500 kcal per day) and exercise (30 minutes of walking per day) coupled with instructions for written self-monitoring of caloric intake and minutes of exercise. The self-monitoring logs were reviewed at each session and problem solving was used to assist participants who were not achieving their caloric intake or energy expenditure goals. Treatments were delivered using a mixture of telephone-based and office-based sessions to reduce travel burden and improve convenience for participants, with the number of sessions using each delivery method proportional across the doses. The phase 2 targeted maintenance program included periodic campaigns to enhance participant motivation using specific targets for weight loss and incentives for goal attainment, with these campaigns also proportionally related to the intervention dose – one campaign for the LOW dose, two for the MOD dose, and three for the HIGH dose. Participants randomized to the comparator intervention (EDUC) received the 8 didactic sessions as the LOW dose group for each phase, but without behavioral coaching. Figure 1 provides a schematic of the study design and sessions for all the intervention groups.
Figure 1.
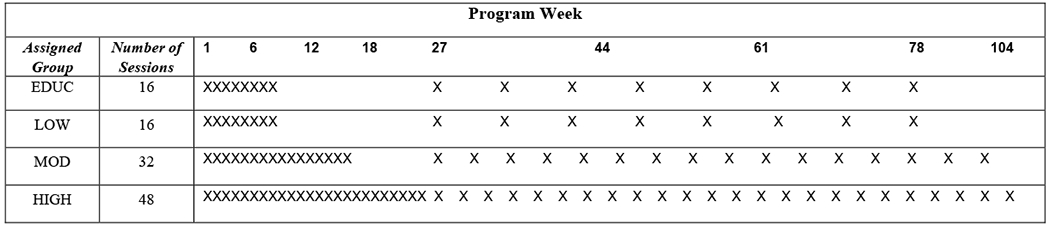
Schematic design of the Rural LITE trial, which lasted 104 weeks and was delivered through local Cooperative Extension Service (CES) offices to adults with obesity residing in rural counties. Each X represents a didactic educational session to increase knowledge regarding nutrition, weight loss, and benefits of physical activity. Phase 1, initial weight loss induction, occurred during weeks 1 to 26. Phase 2, extended care for lifestyle maintenance, occurred from weeks 27 to 104. Hemoglobin A1C (HbA1C), the marker of diabetes status captured for this study, was measured at baseline and at the end of phase 2. The number of sessions varied by intervention dose. Sessions delivered to participants in the LOW, MOD, and HIGH dose intervention groups received nutritional education and behavioral coaching to develop skills for long-term weight management. Participants in the education-only comparator intervention (EDUC) received the same educational content and number of sessions as participants assigned to LOW, but without behavioral coaching.
The interventionists for the Rural LITE trial were CES Family and Consumer Sciences Agents who teach nutrition education programs and receive ongoing training in nutrition from state Extension specialists, or individuals with bachelors or masters degrees in nutrition, exercise science, or psychology that were hired on behalf of the local CES office for the study. Study interventionists were provided with training in lifestyle treatment and nutrition education that included ten bimonthly workshops (six hours each) plus hour-long supervisory contacts by phone that occurred for every session (i.e., 16 for low, 32 for moderate, and 48 for high).
Key Measures
Weight and HbA1C of Rural LITE participants used for this analysis were measured at baseline and the end of the trial. T2D status classification was based on HbA1C levels as follows: non-diabetic (i.e., normal HbA1C) if the level was under 5.7%, pre-diabetic (i.e., elevated HbA1C) if the level was between 5.7% and 6.4%, and diabetic (i.e., high HbA1C) if the level was 6.5% or greater.31
Session delivery costs for the Rural LITE trial were previously calculated and reported.9 These costs included facility rental and telephone services for delivery of sessions, materials provided to participants and staff, staff time for training and program delivery, and other resources, such as demonstration supplies, that were needed to deliver the program. These costs reflected the varying intensity for each intervention dose, and ranged from $78 per participant for EDUC to $165 per participant for HIGH, with both LOW ($111 per participant) and MOD ($145 per participant) in between.9 Estimates for annual health care expenditures (i.e., payer costs) and health-related utility for participants were derived from published peer-reviewed studies that used population-based samples with rigorous regression-based methods to adjust for key covariates. Payer cost for non-diabetic obese adults relied on estimated incremental health services utilization and direct medical costs for a large sample of U.S. residents with normal blood glucose progressing to prediabetes (based on fasting plasma glucose between 100 and 125 or oral glucose tolerance test between 140 and 200),32 and for pre-diabetic patients progressing from prediabetes to T2D identified through ICD-9 codes, fasting plasma glucose, or impaired glucose intolerance.33
Quality Adjusted Life Years (QALYs) estimates to capture health-related utility were derived from nationally representative data across a variety of health conditions.34 The possible range for QALY values is from 0 to 1, with a QALY of 1.0 representing a fully-healthy year and lower values designating reduced quality of life due to one or more health decrements. For participants with normal HbA1c in a time period, the average utility for an otherwise healthy adult age 50-70 without T2D was estimated as 0.827. For participants with HbA1C consistent with diabetes in a time period, the corresponding utility estimate was 0.751. For participants with prediabetes, the mid-point of the two estimates, 0.789, was used. Participants who died were assigned a utility of 0 for that time period and any subsequent model years.
Analysis Methods
This cost-effectiveness study used a patient-level Markov model35 to predict transitions across T2D states for a five-year time horizon after the end of the Rural LITE trial. Costs and QALYs associated with normal, prediabetes, and diabetes states were then linked directly to the population-based estimates from the literature as described above. For each simulation year following the end of the intervention, participants’ ending T2D state and related total healthcare costs and utilities were estimated and recorded. Annual healthcare expenditures and QALYs were then aggregated to provide a discounted total cost and total utility for each individual participant. Monte Carlo simulation was used to generate 1,000 patient transitions for each trial arm and control group.35 Incremental Cost Effectiveness Ratios (ICERs) were calculated as the difference in cost divided by the difference in QALYs for each dose of the intervention relative to the comparator condition. A pairwise comparison of incremental QALYs, incremental costs, and ICERs was used to identify intervention doses that had lower costs and/or higher utility relative to the comparator. A probabilistic sensitivity analysis was conducted to assess the cumulative influence of uncertainty in model inputs on model outcomes. Additional sensitivity analysis tested similarity of Markov model results using multiple imputation methods to estimate outcomes for participants who did not complete the entire intervention, with the assumption that participants with missing data had either similar or dissimilar patterns of outcomes compared to participants with complete data.
Figure 2 illustrates the Markov model transitions across T2D categories for Rural LITE participants. The nodes (circles) represent each participant’s state while the arcs (arrows) indicate the direction of possible year-to-year transitions. For example, participants with HbA1C in the Normal range may remain in that state, transition to the Pre-diabetes state, or transition to Mortality (i.e., die) after a year. Other nodes and arcs can be interpreted similarly. Each participant’s probability of transition across health states depends on their current health state and age-related probability of mortality. Participants move across all other categories as year-to-year transitions with mortality as the only absorbing state, meaning that once a participant is in this state, they remain in that state for all future years. The observed transitions during the trial did not record any cases where a participant moved directly from normal HbA1C to having T2D, so the model assumptions reflected this with a transition probability of 0.
Figure 2.
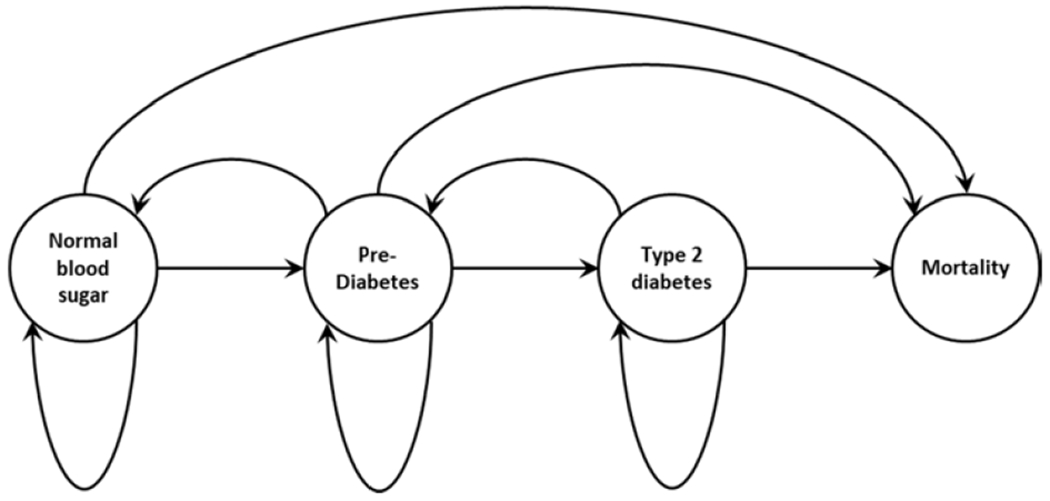
Schematic design of the Rural LITE trial, which lasted 104 weeks and was delivered through local Cooperative Extension Service (CES) offices to adults with obesity residing in rural counties. This flow diagram illustrates how participant T2D status and year-to-year transitions were modeled for the cost-effectiveness analysis. Measured hemoglobin A1C (HbA1C) values were used to assess each participant’s health state during the study, then modeled for possible transitions from one year to the next for up to 5 years after the intervention ended. Mortality using age-adjusted probabilities was included as a possible absorbing state for the Markov model. The figure indicates that participants could remain in the same state or, unless in the mortality state, transition to any of the other three states from one year to the next.
A third-party payer perspective was used for the analysis, as the analytic results may be useful in guiding payer decisions regarding the relative value (i.e., cost per QALY) of each intervention dose relative to the comparator or other interventions that might be covered through insurance or other sponsored health programs. Costs, as described above, included both the direct program delivery costs for each treatment arm previously described and reported for the Rural LITE intervention along with estimates of annual healthcare expenditures for healthy adults and added medical costs associated with prediabetes or diabetes from other published studies for up to five years after completing the intervention.9,32,33
Following standard methods for economic evaluation, all costs were adjusted to reflect constant 2015 dollars and both utilities and costs were discounted by 3% for the years following the end of the trial to reflect time preferences for present versus future values.27,36 The analysis was conducted using Microsoft® Excel®.37 The research team cross-validated model inputs and findings to assure consistency of interpretation. Full details of the model input assumptions and sensitivity analysis are available by request from the corresponding author.
Results
For the 317 Rural LITE participants with complete HbA1C data, baseline values indicated that 16.7% of the participants had T2D, 49.5% were pre-diabetic, and 33.8% had values within the normal range. At the end of the intervention, T2D percentages and net weight change from baseline were: 10.0% (−4.0 kg) for the LOW, 10.5% (−8.3 kg) for the MOD, and 11.4% (−8.4 kg) for the HIGH dose of the behavioral coaching with nutrition education intervention, versus 15.7% (−3.5 kg) for the education-only comparator intervention (EDUC). Per-participant program delivery costs in constant 2015 dollars were: $128 (LOW), $167 (MOD), and $191 (HIGH), versus $90 (EDUC). Expected annual health care costs and utility by T2D status category was estimated as: $6,600 and 0.83 QALY (normal HbA1C), with related values for prediabetes ($7,116, 0.81 QALY) and T2D ($8971, 0.79 QALY), with $12,100 estimated healthcare expenditures for the last year of life for all participants regardless of prior-year T2D status. These measures provided key parameter estimates required for cost effectiveness analysis.
Using a five-year time horizon following the end of the intervention, the analysis identified that participants in the LOW, MOD, and HIGH intervention groups had lower costs and higher QALYs relative to the EDUC comparator intervention, which indicated that the all three doses of the behavioral coaching intervention were “dominant” relative to the EDUC comparator intervention (see Table 1). The LOW dose behavioral coaching intervention was favored in relation to cost differences (i.e., approximately $888 less than EDUC), while the MOD dose was most favorable in relation to utility differences (i.e., approximately 0.02 higher than EDUC). For decision-making purposes, the moderate dose would be selected if payers are willing to pay approximately $107,895 or more per QALY gained (see the ICER comparing the LOW and MOD dose in table 2). The LOW dose would be favored otherwise.
Table 1.
Costs, Quality-Adjusted Life Years (QALYs), and Incremental Cost Effectiveness Ratios (ICERs) for the Rural LITE trial based on a 5-year post-intervention time horizon
| Intervention | Expected Total Costa | QALYsb | Incremental Costc | Incremental QALYd | ICER per QALYe |
|---|---|---|---|---|---|
| Highf | $32,733 | 3.76 | |||
| Educ | $33,398 | 3.74 | $(665) | 0.01 | Dominant |
| Low | $32,510 | 3.76 | |||
| Educ | $33,398 | 3.74 | $(888) | 0.01 | Dominant |
| Mod | $32,633 | 3.76 | |||
| Educ | $33,398 | 3.74 | $(766) | 0.02 | Dominant |
| Mod | $32,633 | 3.76 | |||
| Low | $32,510 | 3.76 | $123 | <0.01g | $107,895h |
Expected Total Cost = average total program costs and health utilization costs per participant over the 5-year time horizon.
QALY = Quality Adjusted Life Year.
Incremental Cost = difference in Expected Total Cost between successive intervention pairs.
Incremental QALY = difference in QALY between successive intervention pairs.
ICER = Incremental Cost Effectiveness Ratio = Incremental Cost divided by Incremental QALY. High, Mod, and Low represent the three intervention doses and Educ is the comparator. An ICER per QALY listed as “Dominant” indicates that the intervention has both a lower incremental expected total costs and higher incremental QALYs relative to the comparator, Educ. The ICER per QALY of $107,900 indicates that the Mod dose would be selected over the Low dose if payers are willing to pay $107,900 or more per QALY gained.
Treatment groups included a LOW, MOD, and HIGH dose intervention groups that received nutritional education along with behavioral coaching to develop skills for long-term weight management. Participants in the comparator condition (EDUC) received the same educational content and number of sessions as participants in the LOW intervention dose, but without behavioral coaching.
The calculated incremental QALY gain for participants in the MOD versus LOW intervention group was approximately 0.00114.
The ICER is calculated as $123 divided by 0.00114
Table 2.
Probabilistic sensitivity analysis summary statistics of the Rural LITE trial. Summary statistics from the probabilistic sensitivity analysis for a five-year horizon and n = 1,000 simulated participants
| Statistic | Treatment Arma | |||||||
|---|---|---|---|---|---|---|---|---|
| Educ | Low | Mod | High | |||||
| QALYsb | Costsc | QALYs | Costs | QALYs | Costs | QALYs | Costs | |
| Average | 3.7439 | $33,341 | 3.7587 | $32,446 | 3.7598 | $32,573 | 3.7565 | $32,666 |
| Std. Errd | 0.0021 | $984 | 0.0021 | $985 | 0.0021 | $984 | 0.0021 | $984 |
| 95%e | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 |
| Lowerf | 3.7398 | $31,413 | 3.7546 | $30,516 | 3.7557 | $30,644 | 3.7524 | $30,737 |
| Upperg | 3.7479 | $35,269 | 3.7627 | $34,376 | 3.7639 | $34,502 | 3.7605 | $34,596 |
Treatment Arms included a LOW, MOD, and HIGH dose intervention groups that received nutritional education along with behavioral coaching to develop skills for long-term weight management. Participants in the comparator condition (EDUC) received the same educational content and number of sessions as participants in the LOW intervention dose, but without behavioral coaching
QALYs = Quality Adjusted Life Years.
Costs = Total program costs plus healthcare utilization costs per participant over the five-year horizon.
Std. Err. = Estimated standard error = Estimated standard deviation / Square root (1,000).
95% = 95% confidence interval estimate from a standard normal distribution.
Lower = Lower confidence interval estimate, Average − 1.96 × Std. Err.
Upper = Upper confidence interval estimate, Average + 1.96 × Std. Err.
Results from a probabilistic sensitivity analysis, which incorporated uncertainty around utility (i.e., QALYs), costs, and the discount rate, produced consistent findings. All three doses of the behavioral coaching with nutrition education intervention were estimated to result in higher QALYs and lower costs relative to the comparator intervention (see Table 1). The LOW dose intervention was the most favorable in relation to cost savings whereas the MOD dose had the highest incremental gain in QALYs.
The cost-effectiveness of the behavioral coaching with nutrition education intervention is reinforced in Figure 3, which illustrates the cost effectiveness frontier by comparing the average cost and average QALY for each intervention dose. The HIGH, MOD, and LOW interventions had lower average costs and higher average QALYs than EDUC. The LOW and MOD doses resulted in both more QALYs and lower cost than the HIGH dose. This suggests that there may be some efficiency associated with lower intensity doses. The MOD dose resulted in more QALYs than the LOW dose; however, it was also more costly. Results from a sensitivity analysis (see Table 2, online only) that used estimated values for participants with incomplete data to assess T2D status were consistent with the findings reported in Table 1 and Figure 3. Specifically, the sensitivity analysis consistently identified that the LOW dose intervention had the greatest expected total cost differences relative to EDUC, while the MOD dose intervention had the highest incremental difference in QALYs compared to EDUC.
Figure 3.
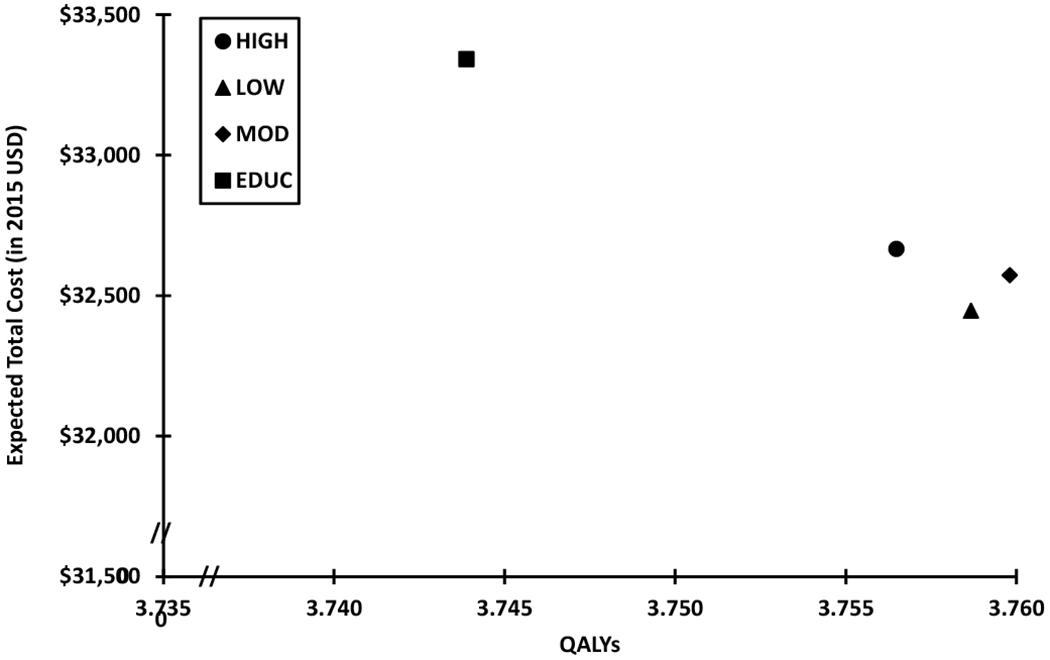
Each symbol displays the expected total healthcare costs (in 2015 USD) and Quality Adjusted Life Years (QALYs) for three doses of the Rural LITE intervention that included behavioral coaching with nutrition education (LOW, MOD, HIGH) and an education-only comparator intervention (EDUC). The expected total healthcare costs and QALYs are the averages obtained from the Monte Carlo simulation for 1,000 participants in each group. Expected total costs include both the program costs and healthcare utilization costs over the 5-year time horizon. The education-only comparator intervention, EDUC, had higher expected total costs and lower expected QALYs compared to LOW, MOD, and HIGH.
Discussion
Using a combination of trial-based data for assessing diabetes status, published population-based estimates of direct medical expenditures and utility related to diabetes status, and recommended practices for economic evaluation alongside clinical trials, results of this analysis identified that three doses of a behavioral coaching with nutrition education intervention (LOW, MOD, HIGH) was cost-effective relative to a comparator intervention (EDUC) that offered participants educational materials without behavioral coaching. The delivery costs of the programs (e.g., less than $200 per participant for the program with 48 sessions) were modest in relation to the health expenditure savings that likely result from lower risk of pre-diabetes or diabetes associated with successful integration of lifestyle modification for intervention participants. Specifically, participants in the comparator intervention (EDUC) had higher expected costs of medical care after the intervention due to higher estimated probabilities of prediabetes or diabetes observed at the end of the Rural LITE trial. After a short time horizon, relatively small differences in the program delivery costs were offset by savings in healthcare utilization, and thus all doses of the intervention with behavioral coaching demonstrated higher value to the payer relative to the comparator (EDUC). The LOW dose intervention may be favored since it requires the fewest number of sessions for participants and could potentially have higher fidelity of implementation in local areas since it requires fewer staffing resources. Over the 5-year time horizon used for the analysis, incremental QALY differences reflect 4.6 to 5.8 additional healthy days per year for participants who received behavioral coaching (LOW, MOD, and HIGH) relative to those who did not (EDUC), with the moderate dose yielding the greatest gain in QALYs.
Effective interventions that can reduce body weight and obesity-related diabetes risk are needed in rural areas since commercial programs with in-person meeting options, such as WW® (formerly Weight Watchers), may not be feasible at the scale of operations demanded by small communities.38 While this research provides helpful insights into the economics of a proven community-based lifestyle modification program that included HbA1C data to assess diabetes status, some limitations are noted. First, study participants volunteered for the trial and may not be representative of all adults with obesity in rural areas. Further, participants with complete HbA1C data may also differ from other trial participants in ways not captured in the imputations used for the sensitivity analysis. While identifying that the demographics and baseline BMI characteristics for participants with complete HbA1C data was similar to the characteristics of the sample without complete data is reassuring, the study did not measure all potential differences that could impact subsequent transitions across T2D categories. Second, the Markov assumptions and the model itself extend information from the end of the trial to a time horizon that is five years later. Accuracy of the model predictions regarding T2D status are heavily dependent on the data measured at the end of the intervention. Thus, the five-year extrapolation should be regarded as an expectation of what might happen based on sophisticated modeling methods that simulate outcomes rather than as a definitive outcome. Third, assumptions regarding health-related utility (i.e., QALYs) and expenditures are based on existing literature and rely on the accuracy of the data and models used. The research team confirmed that the previously published studies were appropriate and rigorous. Finally, the analysis used a single criterion for assessing diabetes status. Future research may be able to identify and collect additional indicators to identify and potentially enhance the accuracy of classifying participants.
Conclusions
The Rural LITE trial identified an innovative option to deliver rural populations a program for weight reduction with behavioral coaching to sustain weight loss. The sessions included a mix of telephone and face-to-face meetings, and was especially important to test in relation to T2D status and health-related costs since CES already serves as a non-commercial educational partner for remote populations. Results of this study suggest that CES provides a feasible program delivery option for payers or other stakeholders concerned about reducing the costs associated with diabetes in rural adult populations. Further, the analysis identified that a low to moderate dose intervention with nutrition education plus coaching following a standard, evidence-based weight reduction program was cost-effective for reducing future health consequences and costs of T2D relative to educational programming without behavioral coaching.
Research Snapshot.
Research Question:
Is an intervention that involves both nutrition education and behavioral coaching to reduce obesity also cost-effective in reducing type 2 diabetes risk and prevalence?
Key Findings:
This cost-effectiveness analysis examined participant transitions across normal blood glucose, prediabetes, and diabetes states for the intervention using HbA1C levels measured at baseline and end of a clinical trial. All three doses of the intervention (i.e., Low = 16 sessions, Moderate = 32 sessions, High = 48 sessions) were identified as cost-saving and had higher Quality Adjusted Life Years (QALYs) gained versus a comparator intervention that included education without behavioral coaching.
Acknowledgements:
The authors thank the faculty and staff of the University of Florida Weight Management Program and the Cooperative Extension Offices in Baker, Bradford, Clay, Dixie, Flagler, Lafayette, Levy, Putnam, Suwannee, and Union Counties, Florida.
Funding Acknowledgement: This study was supported by NIH/NHLBI, R18HL87800.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: There are no conflicts to report.
Contributor Information
Tiffany A. Radcliff, Department of Health Policy & Management, Texas A&M School of Public Health, 212 Adriance Lab Road, College Station, TX 77843-1266.
Murray J. Côté, Department of Health Policy & Management, Texas A&M School of Public Health, 1266 TAMU, College Station, TX 77843-1266.
Melanie D. Whittington, Department of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, 12850 E. Montview Blvd V20-1206, Aurora, CO, 80045.
Michael Daniels, Department of Statistics, University of Florida, Gainesville, FL 32611.
Linda Bobroff, Department of Family, Youth, and Community Sciences; Extension Nutrition Specialist, Institute of Food and Agricultural Sciences, University of Florida, 3026 McCarty Hall, Gainesville, FL 32611-0310.
David Janicke, Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, PO Box 100185, Gainesville, FL, 32610.
Michael G. Perri, College of Public Health and Health Professions, University of Florida, PO Box 100185, Gainesville, FL, 32610.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Befort CA, Nazir N, Perri MG. Prevalence of Obesity Among Adults From Rural and Urban Areas of the United States: Findings From NHANES (2005-2008). J Rural Heal. 2012;28(4):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dis J Where We Live: Health Care in Rural vs Urban America. JAMA. 2002;287(1):108. [PubMed] [Google Scholar]
- 8.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168(21):2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perri MG, Limacher MC, von Castel-Roberts K, et al. Comparative effectiveness of three doses of weight-loss counseling: two-year findings from the rural LITE trial. Obesity. 2014;22(11):2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle Risk Factors and New-Onset Diabetes Mellitus in Older Adults. Arch Intern Med. 2009;169(8):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laaksonen MA, Knekt P, Rissanen H, et al. The relative importance of modifiable potential risk factors of type 2 diabetes: a meta-analysis of two cohorts. Eur J Epidemiol. 2010;25(2):115–124. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson S, Hammar N, Grill V. Alcohol consumption and type 2 diabetes. Diabetologia. 2005;48(6):1051–1054. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrecher A, Morimoto Y, Heak S, et al. The Preventable Proportion of Type 2 Diabetes by Ethnicity: The Multiethnic Cohort. Ann Epidemiol. 2011;21(7):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28(11):845–858. [DOI] [PubMed] [Google Scholar]
- 15.Dow C, Balkau B, Bonnet F, et al. Strong adherence to dietary and lifestyle recommendations is associated with decreased type 2 diabetes risk in the AusDiab cohort study. Prev Med. 2019;123:208–216. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan RSM, Woo J. Prevention of Overweight and Obesity: How Effective is the Current Public Health Approach? Int J Environ Res Public Health. 2010;7(3):765–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14 Suppl 5:S1–85. [PubMed] [Google Scholar]
- 19.American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuo X, Zhang P, Barker L, Albright A, Thompson TJ, Gregg E. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care. 2014;37(9):2557–2564. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, You W, Almeida F, Estabrooks P, Davy B. The Effectiveness and Cost of Lifestyle Interventions Including Nutrition Education for Diabetes Prevention: A Systematic Review and Meta-Analysis. J Acad Nutr Diet. 2017;117(3):404–421.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Agriculture. Cooperative Extension System Homepage. USDA webpage. https://nifa.usda.gov/cooperative-extension-system Accessed May 8, 2019.
- 24.Braun B, Bruns K, Cronk L, Fox LK, Koukel S, LeMenestrel S, & Warren T Cooperative Extension’s national framework for health and wellness. http://www.aplu.org/members/commissions/food-environment-and-renewable-resources/CFERR_Library/national-framework-for-health-and-wellness/file Published 2014. Accessed August 10, 2019.
- 25.US Department of Agriculture. National Institute of Food and Agriculture Health Homepage. USDA webpage. https://nifa.usda.gov/topic/health Accessed May 8, 2019.
- 26.Radcliff TA, Bobroff LB, Lutes LD, et al. Comparing Costs of Telephone vs Face-to-Face Extended-Care Programs for the Management of Obesity in Rural Settings. J Acad Nutr Diet. 2012;112(9):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. Value Heal. 2013;16(2):e1–e5. [DOI] [PubMed] [Google Scholar]
- 28.Ramsey SD, Willke RJ, Glick H, et al. Cost-Effectiveness Analysis Alongside Clinical Trials II—An ISPOR Good Research Practices Task Force Report. Value Heal. 2015;18(2):161–172. [DOI] [PubMed] [Google Scholar]
- 29.Bandura A Social Foundations of Thought and Cognition: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 30.Bandura A Self-Efficacy: The Exercise of Control. New York, NY: Freeman and Co.; 1997. [Google Scholar]
- 31.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33(Supplement 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Dall TM, Chen Y, et al. Medical Cost Associated with Prediabetes. Popul Health Manag. 2009;12(3):157–163. [DOI] [PubMed] [Google Scholar]
- 33.Francis BH, Song X, Andrews LM, et al. Progression to type 2 diabetes, healthcare utilization, and cost among pre-diabetic patients with or without comorbid hypertension. Curr Med Res Opin. 2011;27(4):809–819. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan PW, Ghushchyan VH. EQ-5D Scores for Diabetes-Related Comorbidities. Value Health. 2016;19(8):1002–1008. [DOI] [PubMed] [Google Scholar]
- 35.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 36.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 37.Excel [computer program] MSO 2016. Redmond, WA: Microsoft, Inc.; 2016. [Google Scholar]
- 38.Finkelstein EA, Kruger E. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity. 2014;22(9):1942–1951. [DOI] [PubMed] [Google Scholar]


