Abstract
Background and Aims:
The treatment of submucosal (T1b) esophageal adenocarcinoma (EAC) remains in evolution with some evidence supporting endoscopic management of low-risk lesions. Using a multicenter cohort, we evaluated outcomes of patients with T1b EAC and predictors of survival.
Methods:
Patients diagnosed between 2001 and 2016 with T1b EAC were identified from 3 academic medical centers in the United States. Demographic, clinical and outcome data were collected. Outcomes studied included overall and cancer-free survival. Cox proportional hazards models were constructed to assess independent predictors of survival.
Results:
One hundred forty-one patients were included, of whom 68 (48%) underwent esophagectomy and 73 (52%) were treated endoscopically. Most (85.8%) patients had high-risk histological features. Thirty-day operative mortality was 2.9%. Median follow-up in the esophagectomy and endoscopic cohorts was 49.4 and 43.4 months, respectively. Patients treated endoscopically were older with higher comorbidity scores. 46 (63%) patients treated endoscopically achieved histologic remission. Nineteen (26.0%) patients also received chemoradiation. Five-year overall survival rates in the surgical and endoscopic cohorts were 89% and 59% respectively whereas 5-year cancer free survival rates were 92 and 69%. Presence of high-risk histological features was associated with reduced overall survival.
Conclusions:
In this large multicenter study of patients with T1b EAC, esophagectomy was associated with improved overall but not cancer-free survival. High-risk histological features were associated with poorer survival.
Keywords: Adenocarcinoma, submucosal, survival, endoscopic, surgery
INTRODUCTION
The rapid rise in the incidence of esophageal adenocarcinoma (EAC) in the past decade, coupled with improved recognition of dysplasia and early stage carcinoma has expanded our therapeutic options.1 Mucosal (T1a) EAC is associated with a low risk of metastatic lymphadenopathy and outcomes with endoscopic therapy are comparable with those with surgery.2 However the risk of metastatic lymphadenopathy is considerably higher in submucosal (T1b) EAC ranging from 22% to 28%,3 and hence, esophagectomy continues to be the preferred treatment, given that it allows for regional lymph node resection, more complete staging and institution of adjuvant therapy if needed.4,5 Although surgical outcomes continue to improve with refined surgical approaches, particularly at larger volume centers, by more-experienced operators,6 the procedure continues to carry significant morbidity and some mortality risk. This is particularly relevant in patients with significant comorbidities.
Prevalence of lymph node metastasis in these patients have been described, with earlier studies reporting a greater than 10% risk even in those with superficial submucosal invasion.3 More recently managing low-risk patients with T1b EAC (those with superficial submucosal invasion, well-moderate differentiation, without lymphovascular invasion) have been described in single center studies.7 Recent surgical series also report excellent outcomes in patients with T1b EAC treated surgically with lower mortality risk (3%) and given the risk of metastatic lymphadenopathy, advocate esophagectomy in these patients. In small case series, the recurrence rate in patients with T1b EAC who received chemoradiation postoperatively has been found to be substantially less frequent.8
Risk stratification for lymph node metastasis on the basis of histological variables has also been described.9 Histologic variables such as invasion depth less than 500, well-moderate differentiation and the absence of lymphovascular invasion have been proposed to predict a lower risk of metastatic lymphadenopathy in recent multicenter studies.10 Although attractive, these prediction models are not completely accurate. However, given the mortality and substantial morbidity risk with esophagectomy, interest has persisted in exploring alternative treatment approaches including endoscopic therapy alone and combinations of endoscopic therapy/assessment and chemoradiation for these patients.11 Comparative survival data after surgical and nonsurgical management (with or without chemoradiation) have been published for squamous cell carcinoma.12 The recent ESGE position statement suggest patients with T1b EAC with low-risk histological features can be treated endoscopically.13 There are limited data for T1b EAC with high-risk features. More recently modeling studies have been used given the challenges of prospective large series.14
Endoscopic resection (EMR) continues to be the criterion standard in staging early esophageal carcinoma. In addition to providing accurate depth of invasion, it also provides additional vital prognostic information via degree of differentiation and the presence or absence of lymphovascular invasion.15 These histologic variables have been reported to have prognostic significance in the long term outcomes of patients with T1a EAC.16 However, their impact in the outcomes of those with T1b EAC is not fully understood. In this study we aimed to assess the outcomes (and predictors of survival) of patients with T1b EAC treated endoscopically and surgically, focusing on, overall survival as the primary outcome. Cancer-free survival and recurrence after remission were studied as secondary outcomes.
METHODS
PATIENTS
Pathology reports of all patients with a diagnosis of T1b EAC on endoscopic resection specimens between October 2001 and October 2016 at Mayo Clinic (Rochester, Minn, USA), University of Pennsylvania (Philadelphia, Pa, USA) and Columbia University (New York, NY, USA) were reviewed. Patients who underwent neoadjuvant treatment with chemotherapy and/or radiotherapy before surgery or endoscopic resection were excluded. Tumors invading the muscularis propria (T2) as well as intramucosal (T1a) tumors were also excluded, as were patients with less than 10 days follow-up, unless the lack of follow-up may have been due to early mortality. Finally, specimens with T1b EAC on EMR found to have more advanced T stage disease at esophagectomy were also excluded.
Medical records of all these patients were reviewed and the following data categories were abstracted: demographics, radiographic, endoscopic, endosonographic, pathology, surgical, endo-therapeutics, and oncologic parameters. Outcome parameters included CRIM complete remission of intestinal metaplasia status/date, CRD complete remission of dysplasia status/date, date of last follow-up, date of mortality, and cause of mortality if available.
All esophagectomies were performed by experienced thoracic surgeons using either a transthoracic or a transhiatal approach with lymph node dissection. All EMRs were performed by expert therapeutic endoscopists, using either a cap snare or band ligation technique. (Figure 1) The methods of EMR have been described previously.17 Comorbidities, coded based on International Classification of Diseases, 9th Revision, Clinical Modification coding system, were used to compute the age adjusted Charlson comorbidity index at the time of EAC diagnosis.18 Endoscopic ablative treatments applied after EMR included radiofrequency ablation, spray cryotherapy, thermal therapies (such as argon plasma coagulation) and combination therapy, all performed by experienced endoscopists at the 3 medical centers.
Figure 1:
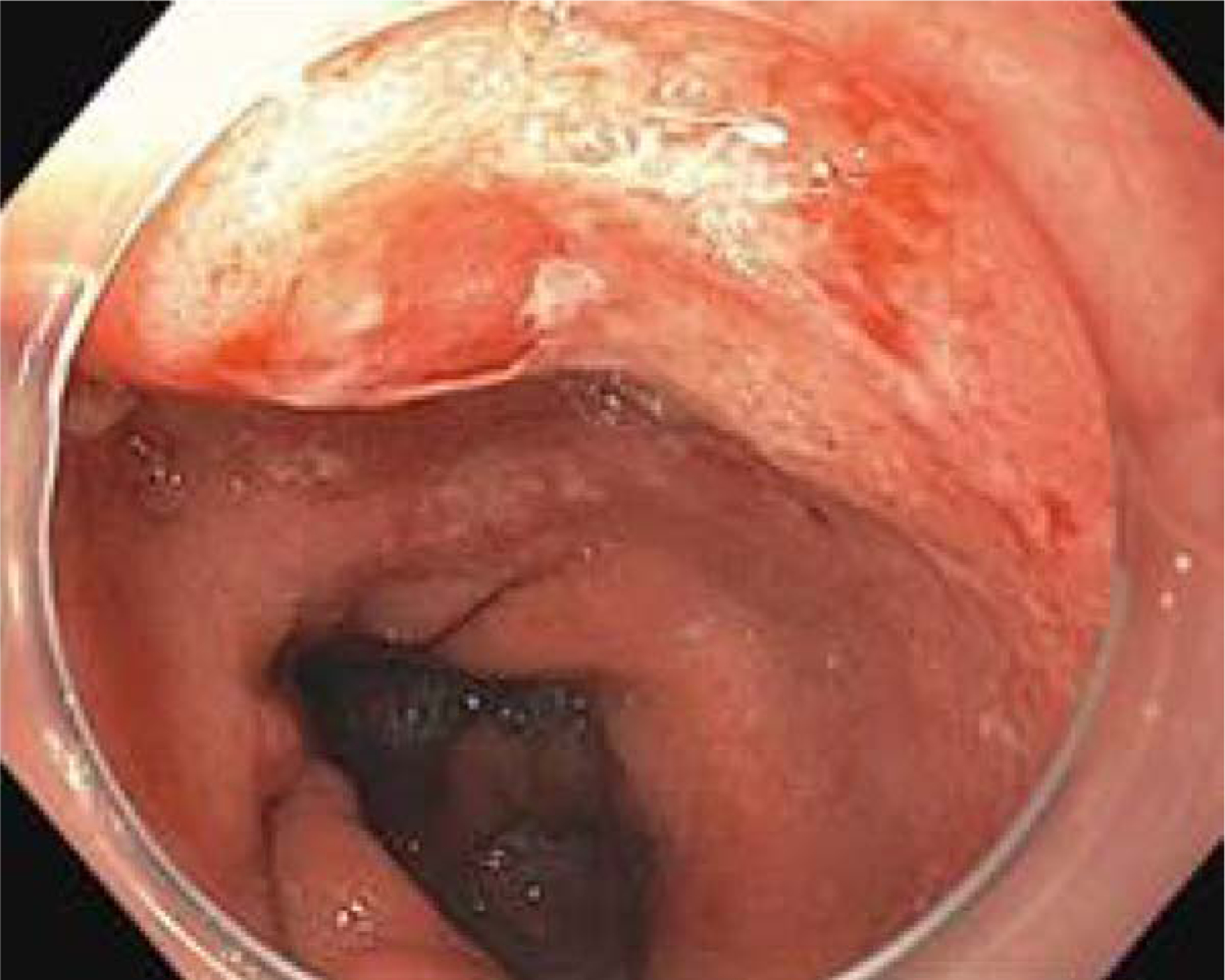
Paris type 2a/2c lesion (with elevated and depressed components) in Barrett’s esophagus segment suspicious for a submucosally invasive cancer.
PATHOLOGICAL ASSESSMENT
Highly experienced GI pathologists at the 3 locations evaluated all histology. Data on margins of resection, grade of differentiation and presence or absence of lymphovascular invasion from EMR and esophagectomy surgical reports were abstracted from clinical records. The presence of any tumor (micro or macrometastasis) in lymph nodes was considered positive for metastasis. Areas of deepest invasion on histological examination were recorded. Deep and lateral margin positivity with dysplasia and/or cancer was documented for each surgical and EMR specimen. Lymphovascular invasion, defined as the presence of clusters of malignant cells within an endothelial-lined vascular channel, and grade of differentiation were also assessed on standard hematoxylin and eosin stained sections. True submucosal invasion was deemed to have occurred only if the cancer extended beyond the duplicated layer of the muscularis mucosa and the sectioned tissue plane contained submucosal features (including gland and large-caliber arterial branches) (Figure 2). An expert pathologist re-reviewed all slides if any of the histological features above were missing from the clinical chart. Depth of invasion into the submucosa, either in micrometers or thirds of the submucosa, was not routinely reported or included in the analysis given that this is not a standard of care at participating institutions due to technical issues limiting accuracy of this assessment.
Figure 2:
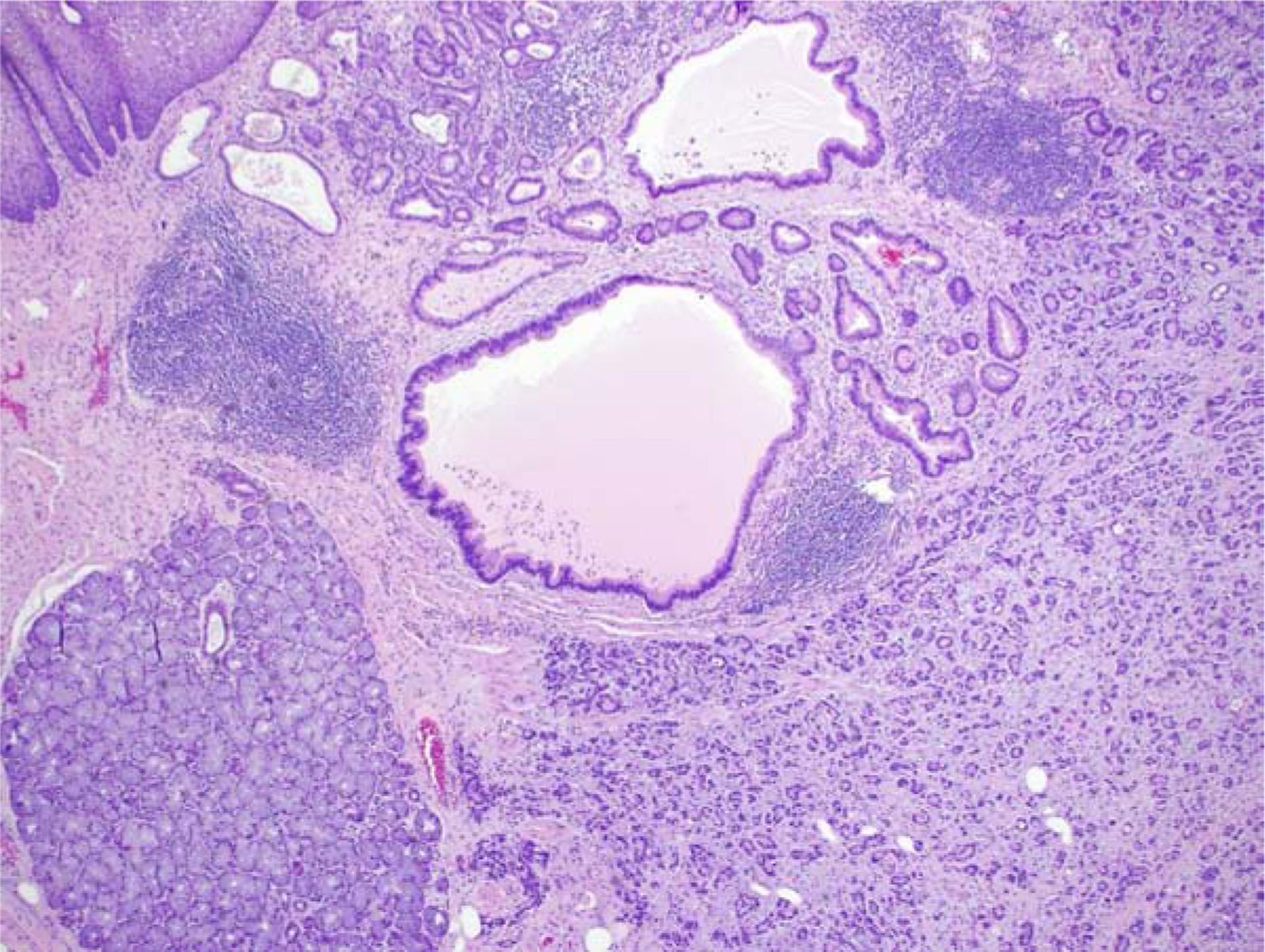
Low-power H&E view of T1b EAC. Benign squamous epithelium is in the upper left portion of the field, and benign submucosal mucous glands occupy the lower left area of the image. EAC is present as sheets of smaller glands extending next to the submucosal glands.
TUMOR STAGING
Staging was performed by using EUS and positron emission tomography (PET)/CT fusion scans. Lymph nodes with worrisome appearance on EUS were sampled by FNA, except if sampling involved traversing the tumor given risk of false positives.
SURVIVAL DATA
Survival (vital status and death date) information for both groups was assessed by using an institutionally approved internet research and location service (www.accurint.com). Cause of death was obtained from either the medical records or each center’s prospective database. In addition, patients at Mayo Clinic who had not been seen for more than 12 months were contacted via telephone using an institutional review board–approved telephone script to obtain information on care received elsewhere and evaluation for esophageal carcinoma recurrence.
STATISTICAL ANALYSIS
Descriptive statistics were used to summarize patients treated endoscopically and surgically with respect to demographics, clinical parameters, endoscopic findings, histological characteristics, and adjuvant chemo/radiation therapy. Baseline continuous data were compared between the groups using the 2-sample t-test or the Wilcoxon rank sum test depending on the data normality. Categorical data were compared using the Pearson chi-square test.
The distribution of overall survival time after EAC diagnosis was estimated for each therapy group (endoscopy and surgery) using the Kaplan-Meier method, where patients were followed until death or last follow-up date. The distribution of cancer-free survival time, defined as the time without a cancer recurrence after remission, was estimated for each therapy group using the competing risk extension of the Kaplan-Meier method, where death was the competing risk and patients were followed until recurrence, death, or last follow-up date. Survival estimates at 1, 3, and 5 years using the log-rank were reported for each treatment arm. In the endoscopic group, the date of cancer remission was defined as the first endoscopic surveillance without carcinoma on surveillance histology, whereas the date of esophagectomy was the remission date in the surgical group. The distributions of time to remission, CRIM, and CRD after EAC diagnosis were also estimated using the Kaplan-Meier method accounting for the competing risk of death. Baseline clinical and demographic variables were analyzed as factors affecting overall and cancer free survival for each of the endoscopic esophagectomy groups using univariate Cox models. Multivariable Cox models including all factors were fit for survival outcomes with sufficient event totals. A p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
RESULTS
361 patients with T1b EAC were identified. After exclusion of patients who received neoadjuvant therapy before surgery or endoscopic resection, patients found to have more extensive disease than T1b EAC at esophagectomy, patients lacking local staging EMR, and patients with no clinical follow-up after staging EMR, 141 patients were included in the analysis. Initial cancer staging was performed with EUS and PET/CT in 90.1% and 84% of patients, respectively.
Of the 141 patients included in the study, 68 (48%) underwent esophagectomy. The remaining 73 (52%) were managed with endoscopic therapy. Twenty-four (17%) received adjuvant chemoradiation after initial ER: 19 in the endoscopic group and 5 in the surgical group. The median interval between first EMR and surgery was 49 days and the median follow-up for patients in the surgical and endoscopic groups was 49.4 and 43.4 months, respectively. As seen in Table 1, the vast majority of patients in both groups were older males. Patients in the surgical group were younger, more likely to be male and had a lower comorbidity score. Patients in the endoscopic group had longer Barrett’s esophagus segments and were more likely to receive chemoradiation.
Table 1:
Baseline Demographics
| Surgical Group (N=68) | Endoscopic Group (N=73) | P value | |
|---|---|---|---|
| Age, median (IQR) | 64.1 (59.8, 71.9) | 73.4 (65.8, 80.5) | <0.001 |
| Male, n (%) | 63 (92.6) | 56 (76.7) | 0.009 |
| BMI (kg2/m), median (IQR)* | 29.3 (25.7, 32.3) | 28.8 (24.9, 31.8) | 0.595 |
| Charlson Comorbidity Index, median (IQR)§ | 4.0 (4.0, 6.0) | 5.0 (4.0, 6.0) | 0.060 |
| Lymphovascular invasion, n (%)♩ | 25 (37.9%) | 20 (29.4%) | 0.300 |
| Well-moderately (G1/2) Differentiated, n (%) | 44 (64.7%) | 48 (65.8%) | 0.896 |
| EMR Deep Margin Positive, n (%) | 41 (60.3%) | 42 (57.5%) | 0.739 |
| Lymph node metastasis, n (%) | 10 (14.7%) | ||
| Adjuvant Chemo-radiation, n (%) | 5 (7.4%) | 19 (26.0%) | 0.003 |
| BE segment length (cm), median (IQR) | 3 (0, 6) | 4 (2, 7) | 0.027 |
BE = Barrett’s esophagus, BMI = body mass index, EMR = endoscopic mucosal resection, IQR = interquartile range
missing 10 in the endoscopic group and 3 in the surgical group
missing in 2 in the endoscopic group
missing in 5 in the endoscopic group and 2 in the surgical group
Surgical consultation was offered after confirmation of submucosal tumor involvement. Of the 73 patients treated nonsurgically, 16 (21.9%) refused surgery, in 31 (42.5%) surgery was thought to be high risk by the consulting surgeons, and remaining 26 (35.6%) did not receive additional follow-up at the referral medical centers.
Forty-three esophagectomies were performed via the Ivor Lewis transthoracic approach (63.2%) whereas the remainder (36.8%) were performed using a transhiatal approach. Two patients (2.9%) died of postoperative adverse events within 90 days of surgery. All specimens had negative resection margins. 29 (42.6%) patients who underwent esophagectomy after EMR had no residual tumor in the surgical specimen. 10 patients (14.7%) had metastatic lymphadenopathy. In 6 of these 10 patients, this was detected by preoperative EUS or PET/CT. 9 of 10 patients had 1 to 2 lymph nodes involved (N1 per AJCC criteria). The presurgical EMR histology in patient’s found to have metastatic lymphadenopathy had cancer involving the deep margin in 8 (80%) patients, whereas 3 (30%) patients were LVI positive, and 3 (30%) patients had poorly/undifferentiated cancers.
In the endoscopic group (n=73), 19 (26.0%) received adjuvant chemoradiation, and 30 (41.1%) received additional ablative therapy. Of these 30 patients, 11 received radiofrequency ablation, 6 received cryotherapy, 4 received photodynamic therapy, 4 received argon plasma coagulation, and 5 received multimodal therapy (combination of thermal therapies and radiofrequency ablation). In the endotherapy group, 46 subjects (63%) achieved cancer remission defined as histology negative for carcinoma. Twenty-four (32.9%) of the endotherapy patients went on to achieve CRD and 19 (12.2%) achieved CRIM.
Survival outcomes are summarized in Table 2. Forty-eight patients died during follow-up in the entire cohort, 34 in the endoscopic group, and 14 in the surgical group. Causes of death are listed in Table 2. Cause of death was unfortunately not available in 68% and 43% of patients in the endoscopic and surgical groups, respectively, despite review of medical records and attempts at contacting patient relatives via telephone. Additionally, several states in the country do not release death certificates to nonfamily members. Only a minority of patients in the entire cohort (8, 17%) had documented esophageal cancer related mortality. Although we may assume that a greater proportion of patient mortality in the endoscopic cohort was non-EAC related given the older age and greater comorbidities, yet we are unable to draw any strong conclusions given the lack of autopsy results or detailed clinical records. The overall 5-year survival was significantly higher at 89% in the surgical compared with 59% in the endoscopic group (Figure 3). However, cancer-free survival 5 years after remission, although numerically higher in the surgical than the endoscopic group (92.3% vs 68.8%), was not statistically significant (p=0.09). (Figure 4).
Table 2:
Outcome Summaries
| Surgical group N=68 | Endoscopic group N=73 | |
|---|---|---|
| Follow-up (months) median, [IQR] | 49.4 [21.8–82.7] | 43.4 [12.8–79.2] |
| Follow-up (person years) | 287.8 | 189.1 |
| Total number of deaths | 14 | 34 |
| Overall mortality (incidence rate) | 4.9 per 100 person years | 18.0 per 100 person years |
| Nonesophageal cancer | 1 (7.1%) | 1 (3.0%) |
| Number of recurrent cancers / Number achieving remission | 4 / 68 | 6 / 46 |
| Follow-up after remission (person years) | 277.4 | 89.5 |
| Recurrent carcinoma (incidence rate) | 1.4 per 100 person years | 6.7 per 100 person years |
IQR = interquartile range
Figure 3:
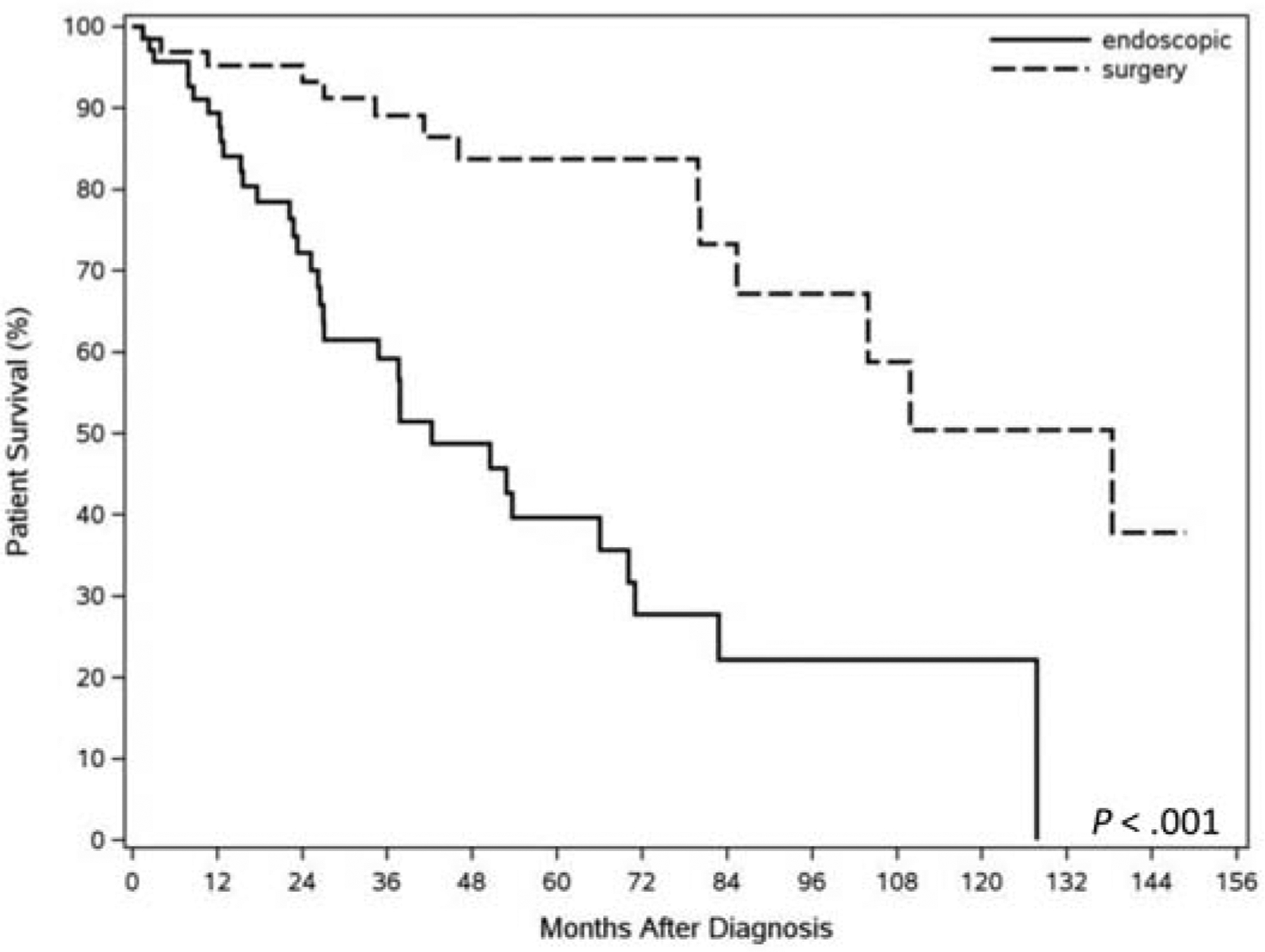
Kaplan Meier curve displaying overall patient survival in subjects treated surgically and endoscopically.
Figure 4:
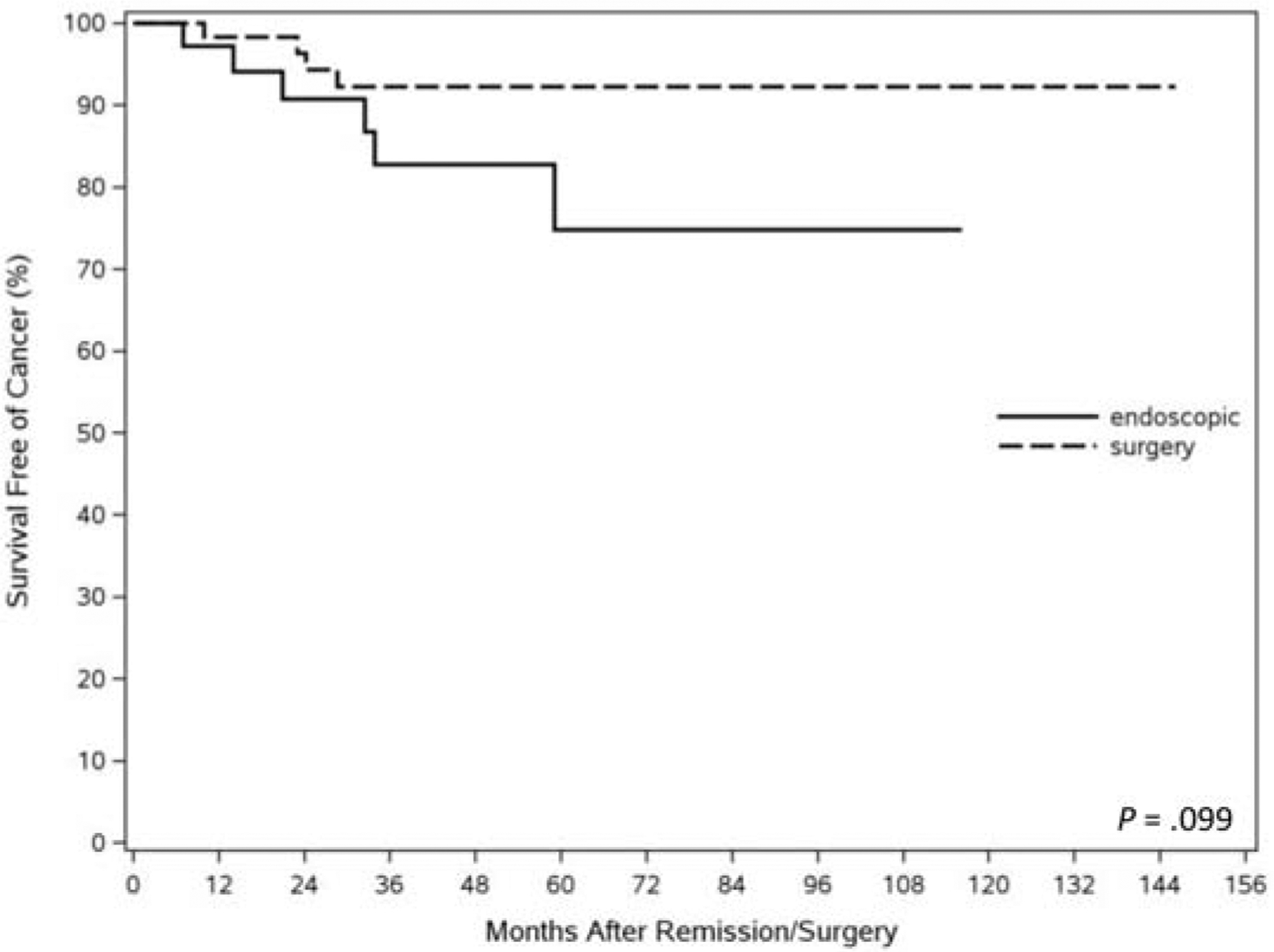
Kaplan Meier curve representing cancer free survival in subjects treated surgically and endoscopically.
Predictors of overall mortality on univariate and multivariable analyses, are listed in Table 3. Esophagectomy was associated with lower overall mortality, whereas older age, comorbidity, and deep margin positivity on EMR were associated with increased overall mortality and on multivariable analysis. Ten patients had cancer recurrence after remission (6 in the endoscopic group, and 4 in the surgical group). Predictors of cancer recurrence after remission on univariate analyses are listed in Table 4 for the entire cohort. Esophagectomy was associated with a lower risk of cancer recurrence. Predictors of survival varied slightly when the endoscopic only versus multimodal care were separated (Supplementary Table 1).
Table 3:
Univariate and multivariable Cox regression models to predict overall mortality in the entire cohort
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Esophagectomy | 0.24 | 012–0.45 | <0.0001 | 0.22 | 0.11–0.46 | <0.0001 |
| Age at diagnosis, per year | 1.05 | 1.02–1.09 | 0.001 | 1.03 | 0.99–1.07 | 0.13 |
| Charlson Comorbidity Index | 1.17 | 1.05–1.30 | 0.006 | 1.18 | 1.03–1.35 | 0.01 |
| Male sex | 0.89 | 0.41–1.91 | 0.76 | 1.7 | 0.69–4.24 | 0.25 |
| Poor/Undifferentiated tumors | 0.81 | 0.45–1.46 | 0.49 | 0.77 | 0.40–1.46 | 0.42 |
| LVI | 1.19 | 0.64–2.22 | 0.58 | 1.80 | 0.93–3.49 | 0.08 |
| Deep margin positivity | 1.93 | 1.05–3.57 | 0.036 | 2.46 | 1.25–4.84 | 0.009 |
Table 4:
Univariate Cox regression models to predict cancer recurrence in the entire cohort
| HR | 95% CI | P value | |
|---|---|---|---|
| Age at diagnosis, per year | 1.05 | 0.98–1.12 | 0.161 |
| Charlson Comorbidity Index | 1.29 | 1.02–1.63 | 0.036 |
| Male sex | 0.46 | 0.10–2.15 | 0.321 |
| Poorly/undifferentiated tumors | 0.61 | 0.16–2.38 | 0.480 |
| LVI | 1.13 | 0.28–4.54 | 0.86 |
| Deep margin cancer positivity | 0.40 | 0.10–1.55 | 0.18 |
All 6 recurrences in the endoscopic group were T1a cancers, and all were managed endoscopically, 4 of which did not have evidence of cancer on their most recent endoscopy. 3 of 6 recurrences in the endoscopic cohort subsequently died and the cause of death in 1 patient was from esophageal cancer, whereas the cause of the death in the remaining 2 was unknown. 4 out of 9 recurrences in the surgical group were found to be extra esophageal invasion or metastases. Among the 10 patients who recurred, the median time to recurrence was 23.6 months (IQR 14 – 32.4 months).
121 (85.8%) patients had at least one high-risk histological attributes in the original endoscopic resection specimen―deep margin cancer positivity, presence of LVI, or poorly/undifferentiated tumors. Having one more high-risk histological features was associated with decreased survival, compared with the group without any high-risk histological features (3-year survival of 72.3% vs 93.8%; 5-year survival of 59.7% vs 83.3% as seen in Supplementary Table 3). Additionally, the presence of any high-risk histological feature was also associated with increased overall mortality in the entire cohort (HR, 2.20; 95% CI, 0.78–6.17; p=0.014). When stratified into the surgical and endoscopic cohorts, this association was present only in the surgical cohort (Supplementary Table 2).
Although this study was not powered to address the question of a survival benefit of adjuvant chemoradiation after endoscopic resection in the small subset of patients treated in this manner, there did not appear to be any survival benefit (Supplementary Tables 3 and 4).
DISCUSSION
In this large multicenter study from 3 tertiary care centers, patients who underwent esophagectomy for submucosal EAC had better overall survival, but not cancer-free survival than a cohort that was managed nonsurgically. The endoscopic cohort was older and had a higher comorbidity scores than the surgical cohort. Predictors of decreased overall survival included age and deep margin positivity on the endoscopic resection specimen. 63% of patients in the nonsurgical group achieved cancer remission (endoscopic evaluations negative for carcinoma).
A large Japanese retrospective series compared outcomes of a similar multicenter cohort. Unlike our study it combined mucosal and submucosal adenocarcinoma and the outcomes was the presence of lymph node metastasis. Treatment strategies included esophagectomy, and endoscopic resection (EMR and submucosal dissection). Other notable differences were the exclusion of patients with less than 5 years of follow-up, patients that received endoscopic therapy and chemoradiotherapy, and patients who died from nonesophageal causes. In this study, there was no evidence of lymph node metastasis in patients with submucosal adenocarcinoma without high-risk histological risk features.19
Recent smaller reports of the surgical management of T1b EAC report results similar to ours, with a retrospective esophagectomy series of 32 patients reporting a 5-year overall survival of 70% and disease specific survival rate of 90% respectively, in the background of a lymph node positivity rate of 22%.9 In our surgical cohort, the overall and cancer-free survival rates at 5 years (overall 89% and cancer free 92%, respectively) are similar. Nine percent of patients in this surgical series died of EAC, compared with 6% of the surgical patients in our series, though the cause of death was unknown in almost 40% in our series.
In contrast, the overall survival in the endoscopic cohort in our study was lower, likely due to older age and higher comorbidity scores. EAC related survival was numerically lower than the surgical group, but the difference was not statistically significant. Of note, 86% of the patients in our endoscopic cohort were relatively high risk (with at least one histological risk factor; deep margin cancer positivity, presence of LVI, or poorly/undifferentiated tumor). In univariate analyses, these high-risk features were associated with poorer outcomes. The relatively favorable survival of this high-risk cohort managed endoscopically may be attributed to the administration of chemoradiation in some (26%), along with additional endoscopic therapy (ablation) in 41%.
In a smaller cohort study from the Netherlands, patients with either deep margins positive for cancer or those with high-risk histological characteristics (similar to those managed in our series) had poorer outcome than those with low-risk features.20 Similarly, in our multivariable model, the presence of LVI and deep margin cancer positivity were significantly associated with decreased survival in the endoscopic cohort. Similar to other reports, patients with T1b EAC without any high-risk features appear to do very well with endoscopic therapy alone.
Sixty-three percent of patients treated endoscopically had at least one endoscopic evaluation negative for carcinoma, reflective of the success of endoscopic therapy, which is less than that reported by Manner and Schölvinck who reported a higher rate of endoscopic tumor eradication of 92.4%, and 93%, respectively.7,20 Thirteen patients in the endoscopic cohort had only clinical follow-up without endoscopy. Of the remaining 60 patients, 76.7% were carcinoma free on their last endoscopy. It also should be noted that 25.7% of our nonsurgical cohort received additional chemoradiation therapy, which was an exclusion criteria for the other studies listed above. However, many of the patients in the endoscopic cohort in this study had high-risk histological factors. This is likely responsible for the lower rate of remission in our cohort and the somewhat higher rates of recurrence and cancer related deaths in our endoscopic cohort. Most notably, all the recurrences in our endoscopic cohort were mucosal adenocarcinomas and managed endoscopically. Only 1 had esophageal cancer related mortality. A more-recent publication evaluating the experience of 2 large Dutch academic centers included 18 high-risk patients, based on similar criteria, within a total cohort of 35 T1b EAC patients. In their strict endoscopic and endosonographic surveillance protocol, with only 2 patients being excluded, none of the 35 patients developed LNM within a median follow-up 23 months. In fact, of the 5 total recurrences, none occurred in the high-risk group.21
Compared with our endoscopic cohort, our surgical cohort had significantly longer follow-up. This was likely due to the later acceptance of nonsurgical means to manage patients with T1b EAC. Forty-one percent of the endoscopically managed cohort was diagnosed between 2011 and 2016, compared with 29% of the surgical cohort.
Our study has several strengths. This is the largest multicenter study of patients with T1b EAC comprising patients managed endoscopically and surgically. All endoscopic, surgical and histological care was provided by a subset of highly trained physicians, surgeons and pathologists with extensive experience in the management of Barrett’s related esophageal cancer. Comparatively, our endoscopic cohort had a a greater proportion of high-risk histological features. With a median follow-up approximately 4 years in our surgical and endoscopic cohorts, respectively, this study adds additional information to previous studies.20,7 Various attempts to verify survival status were made. These include the use of external databases (Accurint®) and prospectively contacting patients to assess their cancer and survival status. Not only were our pathology specimens carefully reviewed by GI pathologists, each pathology report was reviewed by the authors and cases with ambivalent depth of tumor penetration (shallower or deeper invasion) were excluded. Although there is a growing literature on the increased risk of metastasis associated with depth of invasion into the submucosa,10,22,23 we were unable to include submucosal depth in micrometers (Paris classification) or tertiles (Pragmatic classification). This is not part of the standard of practice at our 3 tertiary care centers. Other reasons include the infrequent identification of the muscularis propria on EMR specimens, making precise measurement of depth of invasion challenging, occasional distorted plane of sectioning, specimen curling (particularly in the absence of specimen pinning), artefactual alterations in muscularis due to thermal injury or endoscopic lifting injectate, and tissue preservation artifacts.24 Morphological variability including duplication and hypertrophy of the muscularis have also been cited. Last, there are data to suggest moderate agreement between pathologists in measuring submucosal invasion.25
We recognize that despite being a large cohort, the study might be underpowered to detect improved cancer-free survival in the surgery group. As a retrospective study, multiple biases will persist despite our adjustment, notably referral bias given the nature of the 3 study centers. Although this study was nonrandomized it provides the opportunity to comparatively assess the outcomes of T1b EAC managed by these 2 approaches (one of which is being increased proposed as an alternative). There is also a growing body of literature regarding the use of endoscopic submucosal dissection in this group of patients, which has its own set of advantages―most notably en bloc resection―and limitations.19 Nearly half of our patients who underwent esophagectomy after EMR had no evidence of residual disease on pathological review. Further studies may identify a subset of T1b EAC patients that are overtreated by esophagectomy and may benefit from conservative multimodal therapy. Last, although the data from our study might not be generalizable, we feel strongly that such complex patients should be managed in a multidisciplinary manner in larger academic centers.
In conclusion, esophagectomy was associated with improved overall, but not cancer free, survival in the management of patients with T1b adenocarcinoma. Although possibly underpowered, our collective 15-year experience is the largest such series of confirmed submucosal cancer. Relative to other studies, we present the outcomes of endoscopic therapy in a relatively higher risk endoscopic cohort. Moreover, the role of chemoradiotherapy in this group warrants further studies. The care of such patients continues to be served by centers of excellence that are able to tailor multidisciplinary therapy depending on various predictors.
Supplementary Material
Funding:
Supported in part by NIH grant U54 CA163004 and R01 CA241164 (to PGI)
Acronyms:
- CRD
complete remission of dysplasia
- CRIM
complete remission of intestinal metaplasia
- EAC
esophageal adenocarcinoma
- EMR
endoscopic mucosal resection
- PET
positron emission tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
| Cadman L Leggett: | Research Funding from Nine Point Medical |
| Denis Wigle: | None |
| Kenneth K Wang: | Research funding from Nine Point Medical, C2 Therapeutics, CSA Medical |
| Julian A Abrams: | Consulting C2 Therapeutics |
| Charles J Lightdale: | Consultant C2 Therapeutics, CDX Diagnostics, Boston Scientific |
| Prasad G Iyer: | Research funding from C2 Therapeutics, Nine Point Medical, Exact Sciences, Consultant: Medtronic, CSA Medical, Symple Surgical |
REFERNCES:
- 1.Rustgi AK, El-Serag HB. Esophageal Carcinoma. New England Journal of Medicine. 2014;371:2499–2509. [DOI] [PubMed] [Google Scholar]
- 2.Dunbar KB, Spechler SJ. The Risk of Lymph-Node Metastases in Patients With High-Grade Dysplasia or Intramucosal Carcinoma in Barrett’s Esophagus: A Systematic Review. The American Journal of Gastroenterology. 2012;107:850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson TJ. Esophagectomy for Superficial Esophageal Neoplasia. Gastrointestinal Endoscopy Clinics of North America. 2017;27:531–546. [DOI] [PubMed] [Google Scholar]
- 5.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and Surgical Treatment of Mucosal (T1a) Esophageal Adenocarcinoma in Barrett’s Esophagus. Gastroenterology. 2009;137:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markar SR, Karthikesalingam A, Thrumurthy S, Low DE. Volume-Outcome Relationship in Surgery for Esophageal malignancy: Systematic Review and Meta-analysis 2000–2011. Journal of Gastrointestinal Surgery. 2012;16:1055–1063. [DOI] [PubMed] [Google Scholar]
- 7.Manner H, Pech O, Heldmann Y, et al. Efficacy, Safety, and Long-term Results of Endoscopic Treatment for Early Stage Adenocarcinoma of the Esophagus With Low-risk sm1 Invasion. Clinical Gastroenterology and Hepatology. 2013;11:630–635. [DOI] [PubMed] [Google Scholar]
- 8.Ballard DD, Choksi N, Lin J, et al. Outcomes of submucosal (T1b) esophageal adenocarcinomas removed by endoscopic mucosal resection. World J Gastrointest Endosc. 2016;8:763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwameis K, Green KM, Worrell SG, et al. Outcome with Primary En-bloc Esophagectomy for Submucosal Esophageal Adenocarcinoma. Ann Surg Oncol. 2017;24:3921–3925. [DOI] [PubMed] [Google Scholar]
- 10.Boys JA, Worrell SG, Chandrasoma P, et al. Can the Risk of Lymph Node Metastases Be Gauged in Endoscopically Resected Submucosal Esophageal Adenocarcinomas? A Multi-Center Study. Journal of Gastrointestinal Surgery. 2016;20:6–12. [DOI] [PubMed] [Google Scholar]
- 11.Blevins CH, Physician R, Iyer PG, Physician R, Iyer PG. Endoscopic therapy for Barrett’s oesophagus. Best Practice & Research Clinical Gastroenterology. 2015;29:167–177. [DOI] [PubMed] [Google Scholar]
- 12.Min YW, Lee H, Song BG, et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched analysis. Gastrointest Endosc. 2018;88:624–633. [DOI] [PubMed] [Google Scholar]
- 13.Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191–198. [DOI] [PubMed] [Google Scholar]
- 14.Chu JN, Choi J, Tramontano A, et al. Surgical vs Endoscopic Management of T1 Esophageal Adenocarcinoma: a Modeling Decision Analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leggett CL, Lewis JT, Wu TT, et al. Clinical and histologic determinants of mortality for patients with Barrett’s esophagus-related T1 esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2015;13:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Namasivayam V, Wang KK, Prasad GA. Endoscopic mucosal resection in the management of esophageal neoplasia: current status and future directions. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:743–754; quiz e796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara R, Oyama T, Abe S, et al. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol. 2017;52:800–808. [DOI] [PubMed] [Google Scholar]
- 20.Schölvinck D, Künzli H, Meijer S, et al. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surgical Endoscopy. 2016;30:4102–4113. [DOI] [PubMed] [Google Scholar]
- 21.Kunzli HT, Belghazi K, Pouw RE, et al. Endoscopic management and follow-up of patients with a submucosal esophageal adenocarcinoma. United European Gastroenterol J. 2018;6:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manner H, Pech O, Heldmann Y, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc. 2015;29:1888–1896. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez Herrero L, Pouw R, van Vilsteren F, et al. Risk of lymph node metastasis associated with deeper invasion by early adenocarcinoma of the esophagus and cardia: study based on endoscopic resection specimens. Endoscopy. 2010;42:1030–1036. [DOI] [PubMed] [Google Scholar]
- 24.Leers JM, DeMeester SR, Oezcelik A, et al. The Prevalence of Lymph Node Metastases in Patients With T1 Esophageal Adenocarcinoma. Annals of Surgery. 2011;253:271–278. [DOI] [PubMed] [Google Scholar]
- 25.Gotink AW, Ten Kate FJ, Doukas M, et al. Do pathologists agree with each other on the histological assessment of pT1b oesophageal adenocarcinoma? United European Gastroenterol J. 2019;7:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


