Abstract
OBJECTIVE:
To investigate whether women with early pregnancy elevated blood pressure or stage 1 hypertension exhibit increased risk of preeclampsia and maternal or neonatal morbidity.
METHODS:
We conducted a clinical cohort study of 18,162 women who delivered a singleton infant from 2015 to 2018 and attended at least two prenatal appointments before 20 weeks. Data were collected within the Magee Obstetric Maternal and Infant database, an aggregate of prenatal and delivery health records. Early pregnancy blood pressure (BP) was defined as average BP prior to 20 weeks of gestation, and women were classified with normal, elevated BP, stage 1 or 2 hypertension according to current guidelines. The primary outcome was preeclampsia. Secondary outcomes were severe maternal morbidity, placental abruption, gestational diabetes, and composite neonatal morbidity.
RESULTS:
Overall, 75.2% of the women were categorized with normal BP, 13.9% with elevated BP, 5.4% with stage 1 hypertension, and 5.5% with stage 2 hypertension. Risk of preeclampsia increased in a stepwise fashion with increasing BP category, adjusted for covariates (normal BP, 4.7%, referent; elevated BP, 7.3%, aOR 1.29 [95%CI: 1.07, 1.56]; stage 1, 12.3%, aOR 2.35 [95%CI: 1.86, 2.96]), and stage 2, 30.2%, aOR 6.49 [95%CI: 5.34, 7.89]). Results were similar among black and white women. Gestational diabetes was more prevalent among women with stage 1 (11.4%; aOR 1.50 [95%CI: 1.18, 1.91]) and stage 2 hypertension (14.2%; aOR 1.65 [95%CI: 1.30, 2.10]). Severe maternal morbidity and neonatal morbidity were increased only among women with stage 2 hypertension (aOR 2.99 [95%CI: 2.26, 3.99], and aOR 2.67 [95%CI 2.28, 3.12], respectively).
CONCLUSIONS:
Women with elevated BP, stage 1 and 2 hypertension in early pregnancy are at increased risk for preeclampsia. These findings emphasize the importance of applying the 2017 blood pressure guidelines to reproductive-aged women. Strategies to incorporate these guidelines into obstetric care may also be warranted.
Precis
Entering pregnancy with elevated blood pressure, stage 1, or stage 2 hypertension classified by 2017 cardiovascular society guidelines is associated with an increased preeclampsia risk.
INTRODUCTION
The American College of Cardiology (ACC) and the American Heart Association (AHA) Task Force on Clinical Practice guidelines revised the criteria for diagnosis of hypertension in adults in 2017, recommending a downward shift in hypertension classification criteria.(1) Adults who previously would have been diagnosed with prehypertension are now divided into two categories of elevated blood pressure (systolic blood pressure [SBP] 120–129 mmHg and diastolic blood pressure [DBP] < 80 mmHg) or stage 1 hypertension (SBP 130–139 mmHg or DBP 80–89 mmHg). Blood pressure above this range is now considered stage 2 hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg). This new classification of blood pressure was supported by evidence that modest elevations in blood pressure among adult men and non-pregnant adult women increase risk for cardiovascular disease and was designed to encourage clinicians to counsel and intervene early in the life course to improve cardiovascular health.(2) While the evidence supporting the 2017 guidelines was primarily derived from older adult populations, recent evidence suggests that young men and women with elevated blood pressure or stage 1 hypertension by the 2017 ACC and AHA guidelines have a higher risk of subsequent cardiovascular events than normotensive young adults.(3)
The implications of these new criteria for reproductive-age women are unclear. The prevalence of hypertension in young adult women (20–44 years old) increased from 10% to 19% with application of the current hypertension guidelines compared to the former criteria. (1) Therefore, more women will enter pregnancy with a diagnosis of preexisting hypertension. It is well established that pregnant women with what is now defined as stage 2 hypertension have higher rates of maternal, fetal, and neonatal complications than normotensive women, including preeclampsia, cesarean delivery, postpartum hemorrhage, hypertension-induced end-organ damage, gestational diabetes, placental abruption, fetal growth restriction, and perinatal mortality.(4, 5) Moreover, women with pre-existing hypertension and those who develop preeclampsia have an increased risk of future cardiovascular disease.(6–10) What is unknown is whether newly pregnant women with elevated blood pressure or stage 1 hypertension also have increased risks of preeclampsia and maternal or neonatal morbidity. Neither the 2017 ACC and AHA guidelines, nor recent recommendations from the American College of Obstetricians and Gynecologists (ACOG) (11) provide guidance regarding how women with early pregnancy elevated blood pressure or stage 1 hypertension should be managed due to the lack of evidence.
We aimed to investigate whether women with elevated BP or stage 1 hypertension in early pregnancy exhibit an increased risk for preeclampsia and adverse maternal or neonatal outcomes compared to normotensive women. We also investigated whether the relationship between early pregnancy blood pressure and preeclampsia risk was modified by race. The primary outcome was preeclampsia, and secondary outcomes included severe maternal morbidity, placental abruption, gestational diabetes, and a neonatal morbidity composite score. We hypothesized that there will be an increasing, step-wise association between 2017 ACC and AHA blood pressure groups and risk, such that women with elevated BP, stage 1 hypertension, and stage 2 hypertension will have increasing risks of preeclampsia, maternal morbidity, and neonatal morbidity.
METHODS
We conducted a clinical cohort study using data collected from the Magee Obstetric Maternal and Infant database (MOMI, Magee-Womens Research Institute, Pittsburgh, Pennsylvania). Established in 1995, data are aggregated from prenatal and delivery health records for women who give birth at Magee-Womens Hospital (Pittsburgh, Pennsylvania). State-level birth certificate data are also available for each patient in the database. The Magee Obstetric Maternal and Infant database is regularly reviewed by an honest broker for quality control by comparison to patient charts and examination of frequencies for outliers. The study was approved by the University of Pittsburgh Institutional Review Board and supported by grants from the Richard King Mellon Foundation, ID8069, Magee Obstetrical Maternal & Infant (MOMI) Database; the American Heart Association Go Red for Women Strategic Focused Research Network, Contract 1SFRN27810001; and the National Institutes of Health/University of Pittsburgh Clinical and Translational Science Institute, 5UL1TR001857–04. The funding sources had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
The current study included women who delivered a singleton infant at or after 20 weeks of gestation at Magee-Womens Hospital between January 1, 2015, and June 30, 2018 and who attended at least two prenatal appointments before 20 weeks of gestation (n=18,162). Blood pressure elevations recorded prior to 20 weeks of gestation are considered by obstetric guidelines to characterize chronic (preexisting) hypertension, (5, 11) and we required at least two early pregnancy visits to minimize the influence of a single spurious clinic blood pressure measurement. Women were excluded if they had missing blood pressure measurements at prenatal visits, unknown gestational age at delivery, or suspected inaccurate blood pressure measurements (SBP<70 mmHg or DBP<40 mmHg). Additionally, only first births were included for women who had more than one delivery within the study period. In general, women who were included in the final cohort and women who were excluded due to a lack of prenatal record or insufficient blood pressure data had similar baseline characteristics including age, race, body mass index (BMI), and frequency of nulliparity; more excluded women were smokers (Appendix 1).
Blood pressure was measured at the patients’ prenatal visits according to the protocol of each individual obstetric practice and was not regulated by a research protocol. Women were classified as having normal blood pressure, elevated blood pressure, stage 1 hypertension, or stage 2 hypertension based on the 2017 ACC and AHA guidelines using the average of all available clinic blood pressure measurements prior to 20 weeks of gestation (2.9 ± 0.9 prenatal visits). The normal BP group determination required women to have both an average SBP < 120 mmHg and average DBP < 80 mmHg in early pregnancy. The elevated blood pressure group included women with average SBP between 120 and 129 mmHg and average DBP < 80 mmHg. Stage 1 hypertension was identified by an average SBP between 130–139 mmHg or an average DBP between 80–89 mmHg. If average SBP or DBP exceeded either of the stage 1 hypertension ranges, women were considered to have stage 2 hypertension. Women with chronic (pre-pregnancy) hypertension identified using diagnostic codes were grouped into the stage 2 hypertension category, regardless of early pregnancy measurements.
Primary and secondary outcomes were identified using ICD-9 and ICD-10 diagnostic and procedure codes, admitting and discharge data, and other medical record features (Appendix 2). Preterm preeclampsia was defined as mild or severe preeclampsia, HELLP, or eclampsia, and having delivered before 37 weeks of gestation; term cases delivered at or after 37 weeks. Severe maternal morbidity was defined according to Main et al (12) if women met one of three conditions: one of 21 Centers for Disease Control and Prevention (CDC)-determined indicators (Appendix 3),(13) admittance to the intensive care unit, or prolonged postpartum length-of-stay (Appendices 2 and 3). Prolonged postpartum length-of-stay was defined as a stay longer than three standard deviations above the mean postpartum length-of-stay for the study time period, stratified by mode of delivery (mean length-of-stay 1.93 ± 0.73 days for vaginal delivery and 3.11 ± 0.95 days for Cesarean delivery). Gestational diabetes was diagnosed according to Carpenter-Coustan criteria (at least two values > 95, 180, 155, or 140 mg/dL at fasting, one, two, and three hours, respectively, after a 100-g glucose load; a 3-hour glucose tolerance test was performed for a 1-hour glucose screening test value > 135 mg/dL). The neonatal composite outcome included at least one of the following: intrauterine fetal death after 20 weeks of gestation, neonatal death within 28 days of life, 5-minute Apgar less than 7, neonatal intensive care unit (NICU) admission, small-for-gestational age according to national birth weight standards (14), or preterm birth (gestational age < 37 weeks at delivery).
Clinical and demographic characteristics of the analysis cohort are described by mean and standard deviation for continuous variables and by frequency and percentages for categorical variables. Patient characteristics across hypertension groups are compared using ANOVA for continuous variables and Pearson chi-square or its exact alternative when cell frequencies were small for categorical variables. Incidence of preeclampsia across hypertension groups was compared using logistic regression with pair-wise comparisons conducted using linear contrasts. We reported the results as odds ratios and corresponding 95% confidence intervals (CI). We also fit a multivariable logistic regression model with preeclampsia as the binary outcome and hypertension categories, age, race, BMI, smoking status, pre-existing diabetes, parity, number of visits before 20 weeks, gestational age at first prenatal visit and average time between prenatal visits were covariates. Results from the model are presented as adjusted odds ratio (aOR) and corresponding 95% CI. We have a small proportion (at maximum <6%) of participants having missing data on BMI (n=1073 missing), current smoking (n=1083 missing), gestational weight gain (n=1220 missing) and parity (n=752 missing). We do not anticipate that the likelihood of missing these covariates is associated with the outcome. Therefore, a missing at random assumption was reasonable. Likelihood-based methods using all available data such as the logistic regression model used in this paper provide valid estimates.
Considering the elevated BP and stage 2 hypertensive groups had higher percentages of African American women, we also fit the above logistic regression models stratified by race. Incidences of maternal complications (gestational diabetes, placental abruption, severe maternal morbidity) are reported as frequencies and percentages and compared across hypertension categories using Pearson chi-square test or its exact version as appropriate. We then repeated the above modeling strategy for the following: preterm preeclampsia, term eclampsia, severe maternal morbidity, placental abruption, gestational diabetes, and composite neonatal morbidity.
All p-values reported were two sided. The analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
There were 30,244 deliveries during the study period. Of these, 12,082 were excluded due to: lack of prenatal record (n=5570, largely due to transfers from community hospitals), non-singleton pregnancy (n=538), fewer than two prenatal visits prior to 20 weeks of gestation (n=5323), second delivery within study interval (n=630), and missing or indeterminate blood pressure data (n=21; Figure 1). The final study population included 18,162 women. To ensure we did not introduce selection bias by excluding women who did not receive prenatal care (based on the lack of prenatal record), we matched the 5570 women with no prenatal record in our database with their state-level birth record data to ascertain gestational age at initiation of prenatal care. Of those 5570 women, 4631 (83.1%) reported initiation of prenatal care at or before 12 weeks of gestation, and therefore we concluded that these women received care outside of the hospital system used for this study. The remaining women reported no prenatal care (n=902; 16.2%) or prenatal care initiated at or after 16 weeks of gestation (n=37; 0.7%).
Figure 1.
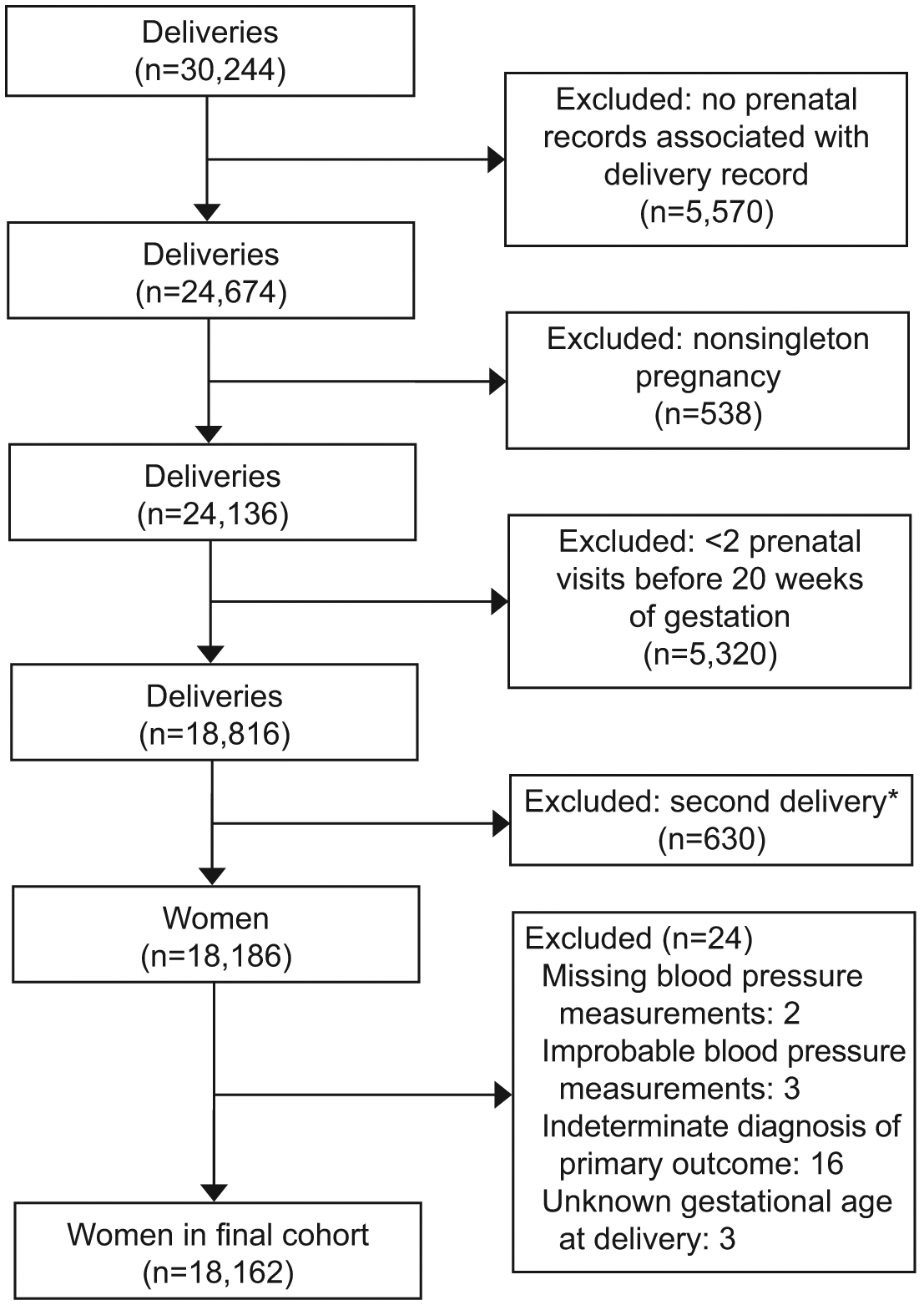
Flow chart of the final study cohort from the Magee Obstetric Maternal and Infant Database (January 2015–June 2018). *In the case of women delivering twice during the study period, only the first delivery was included.
By the 2017 ACC and AHA blood pressure guidelines, 75.2% (n=13,640) of women were categorized with early pregnancy normal BP, 13.9% (n=2,529) with elevated BP, 5.4% (n=988) with stage 1 hypertension, and 5.5% (n=1,005) with stage 2 hypertension. Per the guidelines, women only needed to meet the SBP or the DBP criterion for each BP category. For example, 101/988 (10%) of women met both systolic and diastolic BP criteria for stage 1 hypertension, while 216/988 (22%) met only the systolic criterion, and 670/988 (68%) met only the diastolic criterion (Appendix 4). Most women in the stage 2 hypertension group (836/1005; 83%) were classified based on diagnostic codes rather than BP criteria (Appendix 4). No data were available in our dataset on antihypertensive medication use.
As severity of blood pressure classification increased (elevated < stage 1 hypertension < stage 2 hypertension), women tended to be older, more obese, and more likely to have preexisting diabetes (Table 1). Women with elevated BP and stage 2 hypertension were more likely to be of Black race (Table 1).
Table 1.
Characteristics of participants
| Characteristic | Normal BP | Elevated BP | Stage 1 Hypertension | Stage 2 Hypertension | P value* |
|---|---|---|---|---|---|
| N (%) | 13640 (75.1) | 2529 (13.9) | 988 (5.4) | 1005 (5.5) | |
| Age (years) | 29.7 ± 5.4 | 29.0 ± 5.7† | 30.2 ± 5.2† | 31.4 ± 5.3† | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 25.7 ± 5.6 | 30.7 ± 7.2† | 32.6 ± 7.8† | 35.1 ± 9.3† | <0.001 |
| Gestational weight gain category‡ | † | † | <0.001 | ||
| Race | † | † | † | <0.001 | |
| Ethnicity | 0.62 | ||||
| Current smoker | 1217 (9.5) | 295 (12.4)† | 81 (8.8) | 122 (13.1)† | <0.001 |
| Pre-existing diabetes | 142 (1.0) | 34 (1.3) | 32 (3.2)† | 102 (10.1)† | <0.001 |
| First delivery | 5971 (45.6) | 1208 (49.8)† | 471 (49.6)† | 379 (39.7)† | <0.001 |
| Gestational age at first prenatal visit (weeks) | 9.7 ± 2.5 | 9.5 ± 2.4† | 10.0 ± 2.5† | 9.2 ± 2.5† | <0.001 |
| Number of visits before 20 weeks of gestation | 2.9 ± 0.9 | 3.0 ± 0.9† | 2.8 ± 0.8† | 3.3 ± 1.1† | <0.001 |
| Average SBP before 20 weeks of gestation (mmHg) | 109.7 ± 6.2 | 123.1 ± 2.6† | 124.8 ± 6.9† | 128.3 ± 10.2† | <0.001 |
| Average DBP before 20 weeks of gestation (mmHg) | 66.6 ± 5.9 | 70.8 ± 5.9† | 79.7 ± 5.0† | 77.1 ± 8.7† | <0.001 |
Blood pressure group was assigned based on the highest eligible category for either average systolic or average diastolic blood pressure before 20 weeks of gestation; stage 2 hypertension includes women with diagnostic codes for chronic hypertension in addition to women who met blood pressure criteria.
BP, blood pressure; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure
Data are n (%) or mean ± standard deviation
P values for continuous and categorical variables were generated from ANOVA F test and Pearson chi-square/Exact chi-square test, respectively.
Pairwise comparison with normal BP group significant at p<0.05 level. For multi-category variables (race, gestational weight gain), p value presented is for overall test of differences between groups.
Gestational weight gain category was defined according to Institute of Medicine Criteria(31) Due to missing data (n=1220), subgroup totals do not add up to column total
A total of 1,249 cases of preeclampsia (6.9%) occurred among our cohort. The incidence of preeclampsia increased in stepwise fashion with increasing BP category from 4.7% in the normal BP group to 7.3% in the elevated BP group, 12.3% in the stage 1 hypertension group, and 30.2% in the stage 2 hypertension group (Figure 2). Compared to women with normal BP, the odds of preeclampsia increased from 1.59 (95% CI 1.35, 1.89) in the elevated BP group to 2.86 (95% CI 2.33, 3.52) in the stage 1 hypertension group to 8.77 (95% CI 7.50, 10.25) in the stage 2 hypertension group (Table 2). In adjusted analyses accounting for baseline differences among groups in age, race, BMI, smoking status, preexisting diabetes, parity, number of prenatal visits prior to 20 weeks, gestational age at first prenatal visit, and average time between visits, the odds of preeclampsia increased from 1.29 (95% CI 1.07, 1.56) to 2.35 (95% CI 1.86, 2.96) to 6.49 (95% CI 5.34, 7.89) among women with elevated BP, stage 1 hypertension, and stage 2 hypertension, respectively, compared to normotensive women (Table 2). Among women with elevated BP, this increase in the odds of preeclampsia was driven by an increase in preterm preeclampsia. Among women with stage 1 and stage 2 hypertension, increased odds of both term and preterm preeclampsia were observed, albeit with a greater increased odds of risk for preterm compared to term preeclampsia (Table 2).
Figure 2.
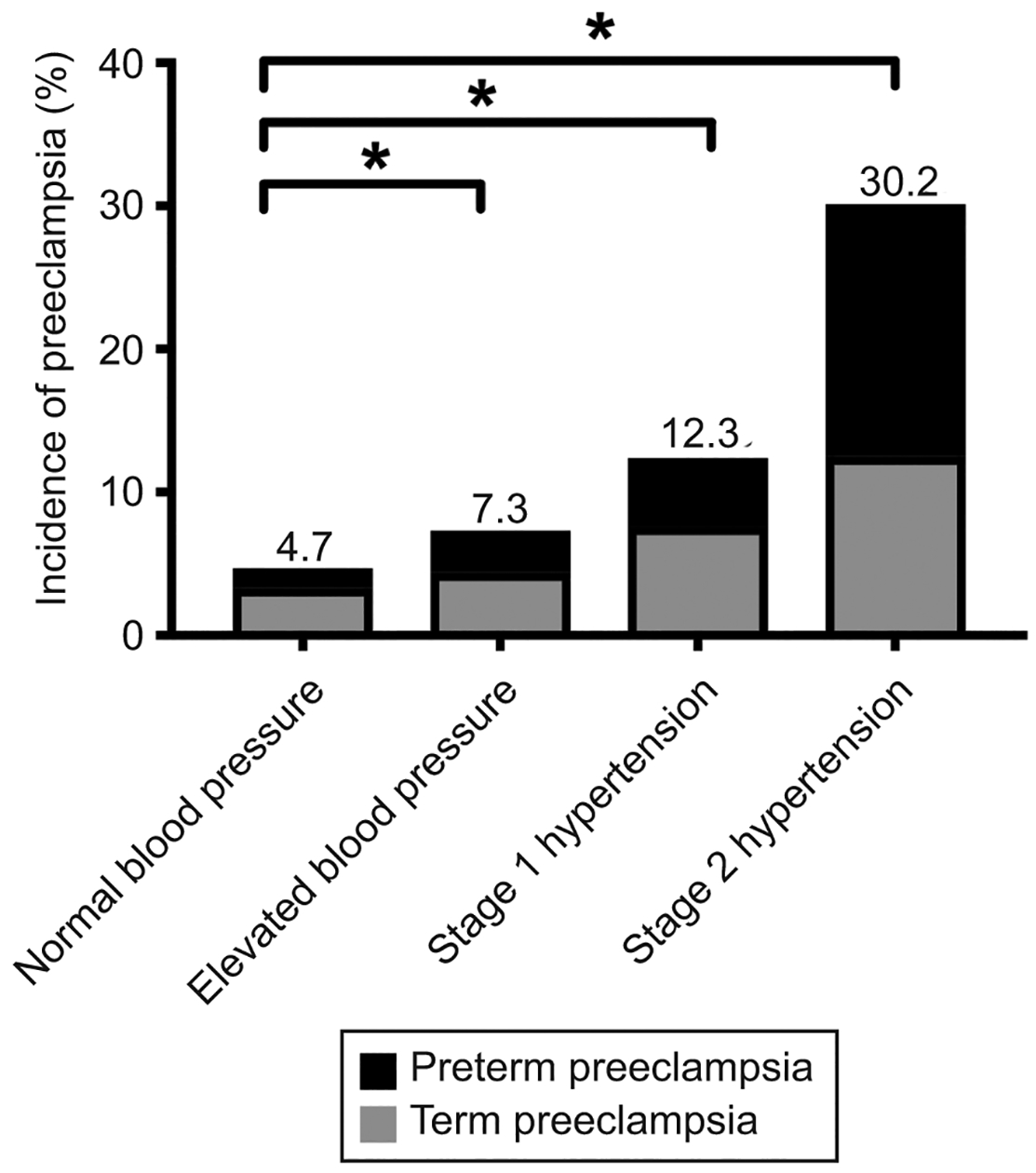
Incidence of preeclampsia among blood pressure groups as determined by early pregnancy average clinic blood pressure measurements. *Horizontal bars indicate P<.001 in pairwise comparison with normal blood pressure group.
Table 2.
Risk of Preeclampsia among Patients by Blood Pressure Group and Race
| Outcome | Normal BP | Elevated BP | Stage 1 Hypertension | Stage 2 Hypertension | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | OR | aOR* | N (%) | OR | aOR* | N (%) | OR | aOR* | N (%) | OR | aOR* | |
| All participants | n=13640 | n=2529 | n=988 | n=1005 | ||||||||
| Preeclampsia | 640 (4.7) | REF | REF | 184 (7.3) | 1.59 [1.35–1.89] | 1.29 [1.07–1.56] | 122 (12.3) | 2.86 [2.33–3.52] | 2.35 [1.86–2.96] | 303 (30.1) | 8.77 [7.50–10.25] | 6.49 [5.34–7.89] |
| Preterm preeclampsia | 191 (1.4) | REF | REF | 74 (2.9) | 2.12 [1.62–2.79] | 1.82 [1.34–2.48] | 47 (4.8) | 3.52 [2.54–4.87] | 3.15 [2.17–4.56] | 177 (17.6) | 15.05 [12.13–18.69] | 10.88 [8.23–14.39] |
| Term preeclampsia | 449 (3.3) | REF | REF | 110 (4.3) | 1.34 [1.08–1.65] | 1.08 [0.85–1.36] | 75 (7.6) | 2.41 [1.87–3.11] | 1.93 [1.45–2.56] | 126 (12.5) | 4.21 [3.42–5.19] | 3.25 [2.51–4.21] |
| Black participants | n=2565 | n=729 | n=176 | n=362 | ||||||||
| Preeclampsia | 150 (5.8) | REF | REF | 67 (9.2) | 1.63 [1.21–2.20] | 1.47 [1.05–2.06] | 21 (11.9) | 2.18 [1.34–3.54] | 1.98 [1.14–3.46] | 122 (33.7) | 8.18 [6.23–10.76] | 8.12 [5.77–11.41] |
| Preterm preeclampsia | 45 (1.8) | REF | REF | 31 (4.3) | 2.49 [1.56–3.96] | 2.49 [1.50–4.12] | 9 (5.1) | 3.02 [1.45–6.28] | 2.86 [1.23–6.65] | 69 (19.1) | 13.19 [8.89–19.57] | 11.23 [6.88–18.33] |
| Term preeclampsia | 105 (4.1) | REF | REF | 36 (4.9) | 1.22 [0.83–1.79] | 1.00 [0.64–1.56] | 12 (6.8) | 1.71 [0.92–3.18] | 1.49 [0.74–3.01] | 53 (14.6) | 4.02 [2.83–5.71] | 4.41 [2.87–6.78] |
| White participants | n=9588 | n=1663 | n=764 | n=611 | ||||||||
| Preeclampsia | 434 (4.5) | REF | REF | 108 (6.5) | 1.47 [1.18–1.82] | 1.18 [0.93–1.51] | 94 (12.3) | 2.96 [2.34–3.75] | 2.25 [1.71–2.94] | 175 (28.6) | 8.47 [6.93–10.34] | 5.66 [4.41–7.25] |
| Preterm preeclampsia | 136 (1.4) | REF | REF | 42 (2.5) | 1.80 [1.27–2.56] | 1.45 [0.97–2.17] | 36 (4.7) | 3.44 [2.36–5.00] | 2.87 [1.87–4.41] | 103 (16.9) | 14.09 [10.75–18.48] | 9.76 [6.87–13.83] |
| Term preeclampsia | 298 (3.1) | REF | REF | 66 (4.0) | 1.29 [0.98–1.69] | 1.08 [0.80–1.45] | 58 (7.6) | 2.56 [1.91–3.43] | 1.91 [1.37–2.66] | 72 (11.8) | 4.17 [3.17–5.46] | 2.76 [1.97–3.85] |
BP, blood pressure; OR, odds ratio; aOR, adjusted odds ratio; REF, referent group
Data are n(%) or odds ratio [95% confidence interval]
Adjusted for age, race, body mass index, current smoking, pre-existing diabetes, parity, number of visits before 20 weeks, gestational age at first prenatal visit, and average time between prenatal visits
Blood pressure group was assigned based on the highest eligible category for either average systolic or average diastolic blood pressure before 20 weeks of gestation; stage 2 hypertension includes diagnostic women with diagnostic codes for chronic hypertension in addition to women who met blood pressure criteria.
Data for Other/Not specified race not shown in race-stratified analysis, thus subgroup totals do not add up to column totals.
Black race was associated with higher risk of preeclampsia (OR 1.34, 95% CI 1.15, 1.57), but there was no statistical evidence of effect modification by race in our overall model or in analyses stratified by gestational age at delivery (p=0.56 for multiplicative interaction between race and hypertension in total preeclampsia; p=0.25 for preterm preeclampsia; p=0.38 for term preeclampsia). When stratified by race, a similar pattern of increasing odds of preeclampsia with higher blood pressure category was seen in both black and white women (Table 2).
To evaluate for other sequelae of elevated BP and hypertension, we determined the incidences of severe maternal morbidity, placental abruption, and gestational diabetes. While there was a stepwise increase in incidence of severe maternal morbidity with increasing BP category (from 2.6% to 2.9% to 3.5% to 12.4% among women with normal BP, elevated BP, stage 1 hypertension, and stage 2 hypertension, respectively; Table 3), the unadjusted and adjusted odds of severe maternal morbidity were only increased among women with stage 2 hypertension (OR 5.33 [95% CI 4.30, 6.61]; aOR 2.99 [95% CI 2.26, 3.99]). Women with elevated BP (OR 1.55, 95% CI 1.32, 1.82), stage 1 hypertension (OR 2.32, 95% CI 1.88, 2.86), and stage 2 hypertension (OR 2.98, 95% CI 2.46, 3.61) had a stepwise increase in the odds of gestational diabetes, compared to normotensive women (Table 3). Accounting for covariates including BMI, the adjusted odds of gestational diabetes were more modestly increased across groups (elevated BP aOR 1.20, 95% CI 1.00–1.45; stage 1 hypertension aOR 1.50, 95% CI 1.18, 1.91; stage 2 hypertension aOR 1.65, 95% CI 1.30, 2.10). The overall incidence of placental abruption in the total cohort was low (1.4%), and the odds of placental abruption were not increased for any group.
Table 3.
Risk of Maternal Morbidity and Neonatal Morbidity among Patients by Blood Pressure Group
| Outcome | Normal BP (n=13640) | Elevated BP (n=2529) | Stage 1 Hypertension (n=988) | Stage 2 Hypertension (n=1005) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | OR | aOR* | N (%) | OR | aOR* | N (%) | OR | aOR* | N (%) | OR | aOR* | |
| Severe maternal morbidity | 354 (2.6) | REF | REF | 74 (2.9) | 1.13 [0.88–1.46] | 1.00 [0.76–1.33] | 35 (3.5) | 1.38 [0.97–1.96] | 0.98 [0.65–1.49] | 125 (12.4) | 5.33 [4.30–6.61] | 2.99 [2.26–3.99] |
| Placental abruption | 186 (1.4) | REF | REF | 44 (1.7) | 1.28 [0.92–1.78] | 1.37 [0.95–1.99] | 8 (0.8) | 0.59 [0.29–1.2] | 0.76 [0.35–1.64] | 16 (1.6) | 1.17 [0.7–1.96] | 1.07 [0.58–1.97] |
| Gestational Diabetes | 720 (5.3) | REF | REF | 201 (8.0) | 1.55 [1.32–1.82] | 1.20 [1.00–1.45] | 113 (11.4) | 2.32 [1.88–2.86] | 1.50 [1.18–1.91] | 143 (14.2) | 2.98 [2.46–3.61] | 1.65 [1.30–2.10] |
| Composite neonatal morbidity† | 3087 (22.6) | REF | REF | 650 (25.7) | 1.18 [1.07–1.30] | 1.06 [0.95–1.19] | 237 (24.0) | 1.08 [0.93–1.26] | 1.11 [0.93–1.31] | 446 (44.4) | 2.73 [2.39–3.11] | 2.67 [2.28–3.12] |
BP, blood pressure; OR, odds ratio; aOR, adjusted odds ratio; REF, referent group
Data are n (%) or odds ratio [95% confidence interval]
Adjusted for age, race, body mass index, current smoking, pre-existing diabetes, parity, number of visits before 20 weeks, gestational age at first prenatal visit, and average time between prenatal visits
Composite neonatal morbidity includes any of the following: intrauterine fetal demise after 20 weeks of gestation, neonatal death within 28 days of life, 5-minute Apgar < 7, neonatal intensive care unit admission, small-for-gestational-age birthweight, or preterm birth.
The incidence of neonatal morbidity (a composite of intrauterine fetal death after 20 weeks of gestation, neonatal death within 28 days of life, 5-minute Apgar less than 7, NICU admission, SGA, and preterm birth) was 22.6% in the normotensive group, 25.7% in the elevated BP group, 24.0% in the stage 1 hypertension group, and 44.4% in the stage 2 hypertension group (Table 3). The rates of NICU admission, 5-minute Apgar <7, SGA, and both spontaneous and indicated preterm birth were each associated with severity of maternal blood pressure group (p<0.007, Table 4). In both unadjusted and adjusted analyses, only stage 2 hypertension was associated with increased odds of neonatal morbidity (OR 2.73, 95% CI 2.39, 3.11; aOR 2.67, 95% CI 2.28, 3.12) (Table 3).
Table 4.
Incidence of Neonatal Morbidity Composite Score Components by Hypertension Classification Group
| Outcome | Normal Blood Pressure | Elevated Blood Pressure | Stage 1 Hypertensive | Stage 2 Hypertensive | P value* |
|---|---|---|---|---|---|
| Neonatal Morbidity Composite | 3,087 (22.6) | 650 (25.7) | 237 (24.0) | 446 (44.4) | <0.001 |
| IUFD after 20 weeks gestation | 4 (0.03) | 2 (0.08) | 0 (0.00) | 1 (0.10) | 0.274 |
| Neonatal death within 28 days of life | 44 (0.3) | 13 (0.5) | 3 (0.3) | 3 (0.30) | 0.502 |
| 5-minute Apgar < 7 | 201 (1.5) | 50 (2.0) | 17 (1.7) | 32 (3.2) | <0.001 |
| NICU admission | 1,724 (12.6) | 382 (15.1) | 153 (15.5) | 296 (29.5) | <0.001 |
| Small-for-gestational-age | 1,391 (10.3) | 262 (10.5) | 88 (9.0) | 159 (16.0) | <0.001 |
| Spontaneous preterm birth < 37 weeks | 521 (5.0) | 108 (5.8) | 32 (4.7) | 43 (8.1) | 0.007 |
| Indicated preterm birth < 37 weeks | 154 (1.5) | 57 (3.2) | 32 (4.7) | 103 (17.4) | <0.001 |
IUFD, intrauterine fetal demise; NICU, neonatal intensive care unit
Data are n (%)
P values determined using Pearson chi-squared test or exact chi-squared test
DISCUSSION
In this large clinical cohort, elevated BP, stage 1 hypertension, and stage 2 hypertension in early pregnancy were associated with an increased risk for preeclampsia, with the severity of hypertension associated with higher risks. The risk of preeclampsia associated with stage 2 hypertension in early pregnancy has been well established, and our study adds to the accumulating evidence that a new population of women – those with SBP ranging from 120–139 mmHg and DBP ranging from 80–89 mmHg in early pregnancy – are also at increased risk for preeclampsia. Our findings highlight the importance of early pregnancy blood pressure elevations, which may reflect pre-pregnancy blood pressure status, and suggest that the revised 2017 ACC and AHA hypertension guidelines can identify women early in pregnancy who may benefit from increased surveillance for signs and symptoms of preeclampsia.
Our results are consistent other studies evaluating the 2017 ACC and AHA definition of stage 1 hypertension in pregnancy. In two secondary analyses of prophylactic low-dose aspirin trials to reduce risk for preeclampsia conducted in the 1990s, Sutton et al. and Hauspurg et al. demonstrated increased risk of preeclampsia as well as preterm delivery and gestational diabetes in women newly classified with stage 1 hypertension using the 2017 ACC and AHA guideline.(15, 16) A recent secondary analysis of the Nulliparous Pregnancy Outcome Study: Monitoring Mother-to-Be cohort (NuMoM2b study) also found elevated blood pressure and stage 1 hypertension categorized in first trimester with the 2017 ACC and AHA guidelines were associated with an increased risk of preeclampsia.(17) Finally, a large retrospective cohort study of low-risk patients from China found increased rates of hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, and low birth weight among women with stage 1 hypertension, compared to normotensive controls.(18) This study categorized women by BP assessed at a single time point prior to 20 weeks of gestation.
We extend these findings in several ways. Our study examines a large and contemporary clinical cohort in the United States considering multiple clinical blood pressure measurements, evaluating both term and preterm disease, including nulliparous and parous patients, and evaluating race differences and maternal and neonatal morbidity. We demonstrate that women with elevated BP in early pregnancy have excess preeclampsia risk compared to those with normal BP, and that this association is driven by black women with preterm preeclampsia. Further, as the early pregnancy blood pressure category increases, the risk for gestational diabetes increases in a monotonic fashion.
Our findings may have immediate relevance for primary care providers caring for reproductive age women. Both pharmacologic and non-pharmacologic interventions to lower blood pressure and to address other risk factors for cardiovascular disease, such as obesity, glucose intolerance, and hyperlipidemia, have the potential to improve future maternal and fetal health. Considering that our results demonstrated increased rates of obesity and gestational diabetes among women with elevated BP and stage 1 hypertension, implementing lifestyle interventions to address cardiovascular and metabolic risk factors could be beneficial in affected women.(19)
Our findings also provoke questions regarding first-time diagnosis of elevated blood pressure or stage 1 hypertension during early pregnancy. How such early pregnancy diagnosis may translate to prenatal management is not yet known, and randomized trials will be required to identify prevention and management strategies among women with these early pregnancy blood pressure elevations. In the meantime, lifestyle modifications such as dietary improvements and exercise programs that improve cardiometabolic health during pregnancy are safe and effective, and may be relevant when blood pressure is elevated.(20) During pregnancy, low-dose aspirin is currently the only intervention with demonstrated efficacy for reducing the risk of preterm preeclampsia in high-risk women,(21–25) including those entering pregnancy with chronic hypertension.(26) Aspirin is a cost-effective intervention(27, 28) with an excellent risk-benefit profile in pregnancy,(21, 22, 25) and its widespread use for preeclampsia prevention has been advocated.(27–29) Thus, it may be reasonable to recommend this low-cost(28) medication for the newly identified population of at-risk women, although trials are needed to characterize its efficacy among these women. Medical tests routinely employed in pregnant women with stage 2 hypertension (additional laboratory studies, ultrasounds, and antenatal fetal surveillance) are of unknown utility in women with lower-range hypertension. Our study did not find higher rates of small-for-gestational-age infants or adverse neonatal outcomes among women with elevated BP and stage 1 hypertension. Therefore, our data are not sufficient to recommend increased antenatal fetal surveillance for these women. Moreover, limiting increased fetal surveillance would likely help avoid iatrogenic morbidity in this population.
Our study has limitations that should be considered. First, blood pressure measurements in our study were clinical measures collected during the routine care of women at many medical offices. It is likely that many measurements were collected without full adherence to all recommendations regarding BP assessment methodology. This lack of protocol is a limitation but, on balance, does reflect medical practice outside of research protocols. As recommended by the ACC and AHA guidelines (1), diagnostic classification was based on the average of at least two measures. The use of routinely measured blood pressure is a pragmatic approach and potentially enhances the generalizability of our findings. Consideration should also be given to the absence of information about antihypertensive medication use in our database. Given that 83% of women in the stage 2 hypertension group had blood pressures below the range of stage 2 hypertension (i.e., SBP < 140 mmHg and DBP < 90 mmHg), we speculate that misclassification bias may be modest, as these women were still identified by their diagnostic codes. The lower measured blood pressures in this group might be due to a combination of pharmacologic and nonpharmacologic treatment and to the physiologic lowering of blood pressure in early pregnancy. It is notable that despite such a small proportion of women in the stage 2 hypertension group having measured blood pressures in the stage 2 hypertension range, this group still had the highest risk of preeclampsia and maternal and neonatal morbidity.
Restricting our analysis to women who had at least two prenatal visits prior to 20 weeks of gestation might introduce selection bias, as women with medical comorbidities might be more likely to establish early prenatal care. Indeed, we noted slightly more prenatal visits prior to 20 weeks in the stage 2 hypertension group, and we controlled for the number of prenatal visits in our adjusted analyses. In comparing the baseline characteristics and rate of outcomes between patients included and excluded in our final cohort, we noted many similarities (Appendix 1), and thus the influence of selection bias is likely minimal. The data in this study are from a single institution, which might limit generalizability. Yet, Magee-Womens Hospital provides care for a diverse population of both low- and high-risk patients from a large geographical area, and providers range from certified nurse midwives to family medicine physicians to general obstetricians and maternal-fetal medicine specialists.
Another limitation is our reliance on diagnostic codes which may misclassify preeclampsia and other obstetric conditions. We cannot confirm the diagnostic criteria that were used to diagnose preeclampsia in each patient in our cohort. However, use of diagnostic codes is a routine epidemiologic and clinical cohort approach, and data in the Magee Obstetric Maternal and Infant database are regularly evaluated to assure accuracy and completeness. Additionally, a validation study in the subset of women with chronic hypertension with superimposed preeclampsia diagnoses (the subgroup with the most complex medical records) demonstrated that among these 322 women, 95% had a clinical diagnosis of preeclampsia in the medical record. The primary strength of this study is the large, contemporary cohort of women with prenatal, delivery, and neonatal data and our ability to evaluate maternal morbidity.
Application of new blood pressure criteria in early pregnancy may identify women at risk for preeclampsia, particularly preterm preeclampsia, and gestational diabetes. While the excess risk identified in our study is modest, particularly among women with elevated blood pressure, there is now accumulating observational evidence from both research and clinical cohorts that women with elevated BP and stage 1 hypertension are at increased risk of obstetric and maternal morbidity. The magnitudes of increased risk noted in our study are comparable to those previously reported. Thus, we are assured that the observed associations between early pregnancy blood pressure elevations and adverse outcomes are real and that there is now sufficient observational evidence to consider how to incorporate these lower blood pressure thresholds into obstetrical care. For example, newly designed trials of interventions for preeclampsia risk reduction in women with elevated blood pressure and stage 1 hypertension in early pregnancy are warranted. Such trials could drive changes in risk identification, improved prenatal maternal monitoring, and perhaps improved maternal outcomes. Our results are also relevant to general clinicians caring for reproductive age women, as the new blood pressure guidelines may identify risks that could impact future pregnancies. Given the well-described association between hypertensive disorders of pregnancy and future cardiovascular risk, interventions for reproductive-age women may have lifelong and multigenerational cardiovascular benefit.
Supplementary Material
Sources of Funding:
Supported by grants from the Richard King Mellon Foundation, ID8069, Magee Obstetrical Maternal & Infant (MOMI) Database; the American Heart Association Go Red for Women Strategic Focused Research Network, Contract 1SFRN27810001; and the National Institutes of Health/University of Pittsburgh Clinical and Translational Science Institute, 5UL1TR001857-04. The funding sources had no role in the design of the study; the collection, analysis, and interpretation of the data; and the decision to approve publication of the finished manuscript.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018. May 15;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 2.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014. May 31;383(9932):1899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline With Cardiovascular Events Later in Life. JAMA 2018. November 6;320(17):1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankumah NE, Sibai BM. Chronic Hypertension in Pregnancy: Diagnosis, Management, and Outcomes. Clin Obstet Gynecol 2017. March;60(1):206–14. [DOI] [PubMed] [Google Scholar]
- 5.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013. November;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007. November 10;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circulation Cardiovascular quality and outcomes 2017. February;10(2). [DOI] [PubMed] [Google Scholar]
- 8.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008. November;156(5):918–30. [DOI] [PubMed] [Google Scholar]
- 9.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive Pregnancy Disorders and Subsequent Cardiovascular Morbidity and Type 2 Diabetes Mellitus in the Mother. Hypertension 2009. June 1, 2009;53(6):944–51. [DOI] [PubMed] [Google Scholar]
- 10.Mannisto T, Mendola P, Vaarasmaki M, Jarvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013. February 12;127(6):681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy . Obstet Gynecol 2019. January;133(1):e26–e50. [DOI] [PubMed] [Google Scholar]
- 12.Main EK, Abreo A, McNulty J, Gilbert W, McNally C, Poeltler D, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol 2016. May;214(5):643.e1–.e10. [DOI] [PubMed] [Google Scholar]
- 13.US Centers for Disease Control and Prevention (CDC). Severe maternal morbidity indicators and corresponding ICD codes during delivery hospitalization,. 2019;https://www.cdc.gov/reproductivehealth/MaternalInfantHealth/SevereMaternalMorbidity.htm.
- 14.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996. February;87(2):163–8. [DOI] [PubMed] [Google Scholar]
- 15.Sutton EF, Hauspurg A, Caritis SN, Powers RW, Catov JM. Maternal Outcomes Associated With Lower Range Stage 1 Hypertension. Obstet Gynecol 2018. October;132(4):843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauspurg A, Sutton EF, Catov JM, Caritis SN. Aspirin Effect on Adverse Pregnancy Outcomes Associated With Stage 1 Hypertension in a High-Risk Cohort. Hypertension 2018. July;72(1):202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauspurg A, Parry S, Mercer BM, Grobman W, Hatfield T, Silver RM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol 2019. September;221(3):277.e1–.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu DD, Gao L, Huang O, Ullah K, Guo MX, Liu Y, et al. Increased Adverse Pregnancy Outcomes Associated With Stage 1 Hypertension in a Low-Risk Cohort: Evidence From 47 874 Cases. Hypertension 2020. March;75(3):772–80. [DOI] [PubMed] [Google Scholar]
- 19.Mohsenzadeh-Ledari F, Taghizadeh Z, Motaghi Z, Keramat A, Moosazadeh M, Najafi A. Appropriate Interventions for Pregnant Women with Indicators of Metabolic Syndrome on Pregnancy Outcomes: A Systematic Review. International journal of preventive medicine 2019;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalle Grave R, Calugi S, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Lifestyle modification in the management of the metabolic syndrome: achievements and challenges. Diabetes, metabolic syndrome and obesity : targets and therapy 2010. November 2;3:373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. The Cochrane database of systematic reviews 2007. April 18(2):Cd004659. [DOI] [PubMed] [Google Scholar]
- 22.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2014. May 20;160(10):695–703. [DOI] [PubMed] [Google Scholar]
- 23.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017. February;216(2):110–20.e6. [DOI] [PubMed] [Google Scholar]
- 24.Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 2010. August;116(2 Pt 1):402–14. [DOI] [PubMed] [Google Scholar]
- 25.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007. May 26;369(9575):1791–8. [DOI] [PubMed] [Google Scholar]
- 26.LeFevre ML. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014. December 2;161(11):819–26. [DOI] [PubMed] [Google Scholar]
- 27.Mone F, O’Mahony JF, Tyrrell E, Mulcahy C, McParland P, Breathnach F, et al. Preeclampsia Prevention Using Routine Versus Screening Test-Indicated Aspirin in Low-Risk Women. Hypertension 2018. December;72(6):1391–6. [DOI] [PubMed] [Google Scholar]
- 28.Werner EF, Hauspurg AK, Rouse DJ. A Cost-Benefit Analysis of Low-Dose Aspirin Prophylaxis for the Prevention of Preeclampsia in the United States. Obstet Gynecol 2015. Dec;126(6):1242–50. [DOI] [PubMed] [Google Scholar]
- 29.Ayala NK, Rouse DJ. A Nudge Toward Universal Aspirin for Preeclampsia Prevention. Obstet Gynecol 2019. Apr;133(4):725–8. [DOI] [PubMed] [Google Scholar]
- 30.ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol 2013. January;121(1):210–2. [DOI] [PubMed] [Google Scholar]
- 31.Institute of M, National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: Reports funded by National Institutes of Health In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US) National Academy of Sciences.; 2009. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


