Abstract
There are over 15 million survivors of cancer in the United States whose rates of frailty, an aging phenotype, range from just under ten to over eighty percent. Frailty impacts not only disease survival, but also long-term function and quality of life in children, adolescents, and in all adults diagnosed and/or treated for cancer. This review explains frailty as a construct and model of physiologic well-being. It also describes how frailty at diagnosis impacts cancer outcomes in adult populations and enumerates the prevalence of frailty in different populations of cancer survivors. Biological mechanisms responsible for aging and potentially for frailty among individuals with or who have been treated for cancer are discussed. Finally, promising pharmaceutical and lifestyle interventions designed to impact aging rather than a specific disease, tested in other populations, but likely applicable in cancer patients and survivors, are discussed.
Introduction
Survival following a diagnosis of cancer has increased over the past seven decades, such that 69.8% percent of persons with a cancer diagnosis will survive five years.[1] This achievement is related to improved screening methods and early detection, to advances in surgical techniques and radiation delivery, and to discovery of new and effective pharmaceutical agents. Cure, however, is not without consequences. Among the over 15 million individuals living in the U.S. today with or after a cancer diagnosis, 66% will have at least one additional chronic health condition.[2] Some of these conditions will predate the cancer diagnosis, and be exacerbated by cancer and/or its therapies, potentially impacting survival. Other conditions will be new, or a direct result of cancer and or exposure to cancer treatment modalities. Either way, individuals with or who have survived cancer are at risk for frail health, a phenotype characterized by reduced physiologic reserve and/or an accumulation of conditions that impact both function and longevity.[3] This review explains frailty as a construct and model of physiologic well-being, describes the impact of frailty on cancer outcomes in adult populations, enumerates the prevalence of frailty in different populations of cancer survivors, discusses potential biological mechanisms responsible for frailty among individuals with or who have been treated for cancer, and briefly outlines promising pharmaceutical and lifestyle interventions for frailty that are likely applicable in cancer patients and survivors.
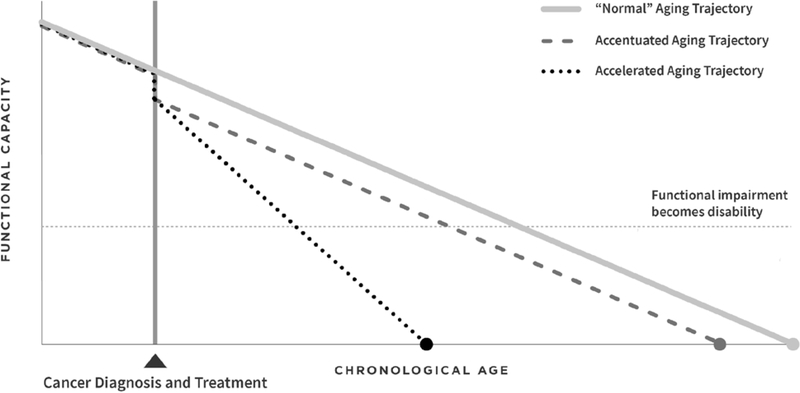
The development of frailty is a consequence of normal aging. However, it is a dynamic construct. Persons move in and out of frailty states, increasing or decreasing their vulnerability to adverse outcomes, the most extreme of which is death (Figure 1).[4] Frailty describes a state of reduced physiologic reserve, and can be modeled in populations, including those with and who have survived cancer, either phenotypically or as an accumulation of deficits. Frailty is used clinically to classify persons with cancer at risk for poor outcomes, and after cancer therapy to identify cancer survivors at risk for early morbidity and mortality.[3] The phenotypic approach (typically referred to as the Fried criteria), originally described in the Cardiovascular Health Study, [5] a sample of 5,317 men and women 65 years of age or older, includes assessment of sarcopenia, decreased muscle strength, poor endurance, slow walking speed, and low physical activity. In this aging population, 7% of cohort members had three or more of these impairments and were classified as “frail”, and 47% had two of these impairments and were classified as “pre-frail.” Adjusted hazard ratios for falls, disability, hospitalization and death after seven years of follow-up ranged from 1.2–1.8 when persons with frailty were compared to those with no frailty. This or a modification of this phenotype is documented in other cohorts of older adults and/or persons with chronic disease.[6–13]
Figure 1.
Hypothesized trajectories of aging-related consequences of cancer and cancer treatment.
Frailty is also characterized using an accumulation of deficits approach, where a frailty index is defined as the proportion of a total number of “things that individuals have wrong with them.”[14] his approach considers symptoms, signs, disabilities, diseases, and abnormal laboratory measurements as deficits. Deficits are tallied, and sometimes weighted, from readily available survey or clinical data, often employing a combination of self-report and directly measured items like those included in a Comprehensive Geriatric Assessment.[14, 15] Rates of frailty are reported to vary from 11% among older adults[16] to a median of 42–43% among cancer survivors.[4, 17] However, accurate enumeration of frailty prevalence on a global level is difficult, as most studies are done in high income countries, and do not always use the same definition of frailty. De Souto Barreto[18] suggests that frailty should be operationalized locally so that definitions can take into account the unique characteristics of a given population. This approach seems reasonable, as in nearly every study examining frailty, no matter what the measure, associations between frailty and adverse outcomes are consistent; individuals who are frail are at higher risk for development of chronic disease and for mortality.[4]
Frailty at diagnosis and acute treatment outcomes
Frailty prior to or concomitant with cancer diagnosis can impact both acute outcomes, long-term function, and perceived health. Acute outcomes are most commonly reported and are summarized among older adults with cancer in a systematic review published in 2015. Twenty-two studies were included in the review with seven reporting adverse outcomes associated with frailty. These include mortality, post-operative treatment complications and poor tolerance to chemotherapy.[17] In more recent studies, focused on cancer survivors, Shahrokni et al. [19] documented frailty using the Memorial Sloan Kettering Frailty-Index (MSK-FI; an 11 point tally of self-reported functional status (impaired or not), and ten co-morbid conditions: diabetes, chronic obstructive pulmonary disease, hypertension, cerebrovascular accident, transient ischemic attack, congestive heart failure, peripheral vascular disease, cognitive dysfunction, myocardial infarction and coronary artery disease) in 1,137 patients with cancer, 51.2% female, who were 75 years of age or older and referred to the geriatric service prior to surgical management. Higher scores on the MSK-FI were associated with increased post-surgical length of stay, increased risk for intensive care unit admission and one-year mortality. Using a similar measure, the modified Frailty Index, Franco et al.,[20] in a cohort of 138 patients with Stage I/II Non-Small-Cell Lung Cancer treated with Stereotactic Body Radiation Therapy, reported a negative association between frailty, prevalent among 72.7% of patients, and overall survival. Frailty was also associated with non-cancer related deaths over three years of follow-up. Using only a single measure of impaired physiologic reserve, gait speed, Pamoukdjian et al.[21] reported a 5.6 (95% Confidence Interval (CI) 1.6–19.7) fold increased hazard of death among 96 older adult outpatients with cancer (mean age in the cohort was 80.6±5.6 years) with gait speeds <0.8 meter/second (m/s) when compared to 94 with gait speeds ≥ 0.8 m/s.
Frailty at diagnosis and long-term outcomes
Associations between frailty at diagnosis and health care utilization, long-term functional outcomes and perceived health are reported in survivors of breast and colorectal cancer. Williams et al. conducted geriatric assessments on 125 older adults with cancer at diagnosis and followed them for five years to assess hospital or long-term care admissions. In this group of older adults (mean age 74 years), prefrailty/frailty were associated with a 2.5 fold increased risk of hospital and a 1.9 fold increased risk of long term care admissions.[22] Mandelblatt et al. evaluated associations between frailty at diagnosis and cognition in two separate cohorts (N=344, N=1,280) of women with non-metastatic breast cancer, and found that frailty was negatively associated with measured cognition at baseline[23] and self-reported cognitive decline over 24 months[23] and 7 years.[24] Using the European Organization for Research and Treatment Cancer Quality of Life Questionnaire Core 30 (EORTC_QLQC30), Ronning et al. evaluated the association between frailty and quality of life (QOL) over time in 68 persons with colorectal cancer referred for surgery. QOL scores were lower among frail (35% of the cohort) than among non-frail survivors at baseline, and at three- and eighteen-months post surgically.
Prevalence estimates of frailty among survivors
The prevalence of frailty among cancer survivors ranges from 7.9% in adult survivors of childhood cancer in their fourth decade of life[25] to 59% among older adult survivors of adult onset cancer in their seventies and eighties (Table).[26] In addition to sex and age, frailty in cancer survivors is associated with cancer type, treatment exposures, chronic disease, lifestyle and access to care. Rates in childhood cancer survivors in their 30s and 40s (7.9–47.0%) approximate those of community dwelling older adults in their sixties, seventies and eighties.[25, 27–32] Rates in survivors of adult cancers are even higher, ranging from 9.1–59.0%, often double and sometime nearly quadruple the rates of frailty in age, sex and race matched populations.[19–21, 23, 24, 33–40] Potentially, because in the general population, females have less capacity to regenerate muscle than do males, [41] female cancer survivors are more likely to be frail than male cancer survivors.[25, 29] As estrogen deficiency also influences muscle recovery, [42] females whose cancer therapy impacts estrogen production are at particular risk.[27] Among childhood cancer survivors, those with brain tumors, bone tumors and Hodgkin lymphoma (HL) are at greatest risk for frailty.[25, 29] Treatment related risk factors for frailty among cancer survivors include radiation to the brain, abdomen and pelvis, extremity amputation, lung surgery, platinum exposure,[25, 29] hematopoietic stem cell transplantation, [28, 31, 33] and among males with prostate cancer, androgen deprivation therapy.[36, 40] Growth hormone deficiency,[30, 32] surgical or chemotherapy induced premature ovarian insufficiency,[27] graft versus host disease,[28, 33] and the presence of severe, disabling or life threatening neurologic or cardiopulmonary conditions[25, 29, 36] are also associated with frailty or its’ components. Smoking, obesity and sedentary behavior are also identified risk factors for frailty in cancer survivors.[25, 29, 32, 34, 43]
Table 1.
Frailty estimates in cancer survivors
| Author | Year published | Population | Study design | N | Age | Frailty measure | Prevalence or “Average” Frailty score | Predictors of frailty | Other health outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Childhood, adolescent and young adult cancer | |||||||||
| Hayek | 2019 | Childhood cancer survivors and siblings; 48% male; 86% white | Retrospective cohort | 10,899 survivors; 2.097 siblings | Mean 37.6±9.6y survivors; 42.9±9.4y siblings | Self-report, modified Fried criteria | 6.4% survivors; 2.2% siblings | Amputation; cranial, pelvic or abdominal radiation, platinum exposure, lung surgery | |
| Van lersel | 2019 | Childhood cancer survivors | Cross-sectional | 3,141 survivors | Median 31.7y | Performance-based; modified Fried Criteria | 5.7% | Growth hormone deficiency | |
| Smitherman | 2018 | Adolescent and young adult cancer survivors (≥1y post treatment) | Cross-sectional | 271 | 15–39y | Self-report of fatigue, difficulty climbing stairs, activities of daily living, comorbid conditions, weight loss - frailty characterized as endorsing ≥3 | 10% | Smoking, obesity, anxiety or depression, delayed care because of lack of insurance | |
| Eissa | 2017 | Survivor of leukemia or lymphoma treated with or without HCT during childhood | Retrospective cohort | 112 treated with HCT/1106 treated without HCT | 28.4 ± 5.9y HCT; 29.3 ± 6.2y conventional therapy | Performance-based; modified Fried Criteria | 7.1% among those treated with HCT; 1.6% among those treated with conventional therapy | HCT | Severe, disabling, life threatening chronic conditions |
| Chemaitilly | 2017 | Childhood cancer survivors, female | Cross-sectional | 921 | Median 31.7y | Performance-based; modified Fried Criteria | 4.9% among those without premature ovarian insufficiency, 16.0% among those with premature ovarian insufficiency | Premature ovarian insufficiency | |
| Vatanen | 2017 | Survivors of childhood high-risk neuroblastoma | Cross Sectional | 19 survivors, 20 age and sex-matched controls | Median 22 (16–30)y | 47% survivors, 0% controls | telomere length, higher levels of C-reactive protein | ||
| Wilson | 2016 | Survivors of Acute Lymphoblastic Leukemia treated during childhood | Cross Sectional | 862 | Median 31.3 (18.4–59.7)y | Performance-based; modified Fried Criteria | 18.6% were pre-frail or frail | GHD, smoking | |
| Ness | 2013 | Adult survivors of childhood cancer | Retrospective cohort - prospective follow-up | 1,922 | Mean age 33.6±8.1y | Performance-based; modified Fried Criteria | 7.90% | Chronic conditions, cranial radiation, pelvic radiation, smoking | Frailty was associated with new onset chronic conditions and with mortality |
| Hematopoietic stem cell transplant | |||||||||
| Arora | 2016 | Survivors (≥2y) of HCT 18–64 y and siblings | Retrospective cohort | 998 survivors, 297 siblings | 42.5±11.6y survivors. 43.8±10.9y siblings | Self-report, modified Fried criteria | 8.4% survivors, 0.7% siblings | Graft versus host disease; severe, disabling or life-threatening chronic conditions | Frailty was associated with mortality |
| Breast cancer | |||||||||
| Mandelblatt | 2018 | Non-metastatic breast cancer patients and matched controls aged ≥60y | Prospective cohort | 344 patients; 347 controls | 60–98y | Searle’s deficits accumulation index (pre-frail defined as 0.2–0.35; frail as ≥0.35) | 25.6% patients; 18.1% controls prefrail/frail | Frailty was used as a predictor of cognitive outcomes | Prefrailty/frailty associated with attention, processing speed, executive function at baseline |
| Mandelblatt | 2016 | Non-metastatic breast cancer patients, 41% treated with chemotherapy | Prospective cohort | 1,280 | 65–91y (mean 74.1) | Searle’s deficits accumulation index (pre-frail defined as 0.2–0.35; frail as ≥0.35) | 5.1% frail, 18.3% pre-frail | Frailty status was used as a predictor of cognition | Self-reported cognitive function from the EORTC QLQ-30 |
| Bennett | 2013 | Breast cancer survivors not participating in exercise | Cross-sectional | 216 | 53–87 | Fried Criteria | 18% frailty among 70–79y old; 50% of cohort pre-frail |
Obesity, sedentary behavior | Data were compared to published data from the Cardiovascular Health Study and the Women’s Health and Aging Study |
| Prostate cancer | |||||||||
| Winters-Stone | 2017 | Prostate cancer survivors | Cross-sectional | 280 | Mean 72±8y | Self-report of fatigue, difficulty climbing stairs, activities of daily living, comorbid conditions, weight loss or obesity) where frailty characterized as endorsing ≥3 of these | 59% among those on androgen deprivation therapy; 62% among those who previously used ADT therapy and 25% among those who never used ADT | ADT | |
| Bylow | 2011 | Men with prostate cancer | Case-control | 63 with biochemical recurrence of PC on ADT; 60 without recurrence | Cases 72.1±7.0y Controls 70.5±6.3y | Performance-based; modified Fried Criteria (replacing weight loss with obesity (BMI≥30 kg/m2) | 8.7% with ADT frail; 2.9% without ADT Frail | Comorbidities, ADT | Frailty was associated with falls and lower performance on the Short Physical Performance Battery |
| Colorectal cancer | |||||||||
| Ronning | 2016 | Persons with colorectal cancer referred for surgery | Prospective cohort | 68 | mean 79y (range 70–94) | Frailty classified as one of physical dependence in basic ADL measured by the Barthel index, >1 grade 4 comorbidity/>2 grade 3 comorbidities on the cumulative rating scale for geriatrics, a score of <17 on the mini nutritional assessment, score <24 on the mini-mental status examination, score of >13 on the geriatric depression scale, or daily use of ≥8 medications | 35% | Frailty was used as a predictor of QOL | Quality of Life - EORTC-QLQ-C30 - Frailty was associated with QOL at all time points (baseline, 3 months, 18 months) |
| Ronning | 2014 | Patients undergoing elective surgery for colorectal cancer | Prospective follow-up | 38–84 depending on outcome | Median 82 (72–95)y | Frailty classified as one of: physical dependence in basic ADL measured by the Barthel index, >1 grade 4 comorbidity/>2 grade 3 comorbidities on the cumulative rating scale for geriatrics, a score of <17 on the mini nutritional assessment, score <24 on the mini-mental status examination, score of >13 on the geriatric depression scale, or daily use of ≥8 medications | Overall prevalence not reported | Frailty used as a predictor of decline in physical function | Frailty was not associated with performance outcomes |
| Lung cancer | |||||||||
| Franco | 2018 | Patients with stage I/II non-small-cell lung cancer treated with stereotactic body radiation therapy | Retrospective review | 139 | Median 74y | Modified frailty index (11-point tally of performance status ≥2, impaired sensorium, diabetes mellitus, chronic/acute lung disease, myocardial infarction in past ≤6 months, hospitalization for congestive heart failure in past ≤6 months, coronary or cardiac disease, hypertension on medications, history of transient ischemic attack, cerebrovascular accident or stroke with neurological deficits, and peripheral vascular disease. Frailty status was defined as non-frail (score 0–1) and frail (score ≥2) | 72.7% | Frailty was used to predict overall and cause specific survival | Frailty associated with three-year overall survival and non-cancer death |
| Head and neck cancer | |||||||||
| Johnson | 2014 | Patients undergoing tracheostomy, 100% male | Retrospective chart review | 99 (38 with head and neck cancer) | Median 64 (35–89)y | Risk Analysis Index - a modification to the Revised Minimum Data Set Mortality Rating Index (11 weighted items) | Score (out of 100%) 44.9% for those with head and neck cancer; 30.2% for those without head and neck cancer | Frailty used to predict mortality | This instrument did not differentiate between head and neck cancer patients who survived <6 months compared to those who survived ≥6 months |
| Mixed diagnoses | |||||||||
| Shahrokni | 2019 | Cancer patients ≥75y; 51.2% female | Prospective cohort | 1,137 cancer patients | Median 80y | Memorial-Sloan Kettering Frailty Index (tally of comorbid disease at admission) | 41.2% had MSK-FI score ≥3 | Score on the MSK-FI was evaluated as the predictor of post-surgical adverse outcomes | Higher MSK-FI associated with increased length of stay, risk for intensive care unit admission, mortality |
| Moore | 2018 | REGARDS Cohort (population-based study of adults ≥45y, 45% male, 41% black) | Prospective cohort | 2773 cancer survivors; 25.289 without cancer history | 69y survivors, 64y those without cancer history | Self-report where frailty characterized as 2 of any of weakness, exhaustion, or low physical activity | 23% survivors, 20% those without cancer history | Frailty was evaluated as a mediator of the association between cancer history and hospital admission for community acquired sepsis | Frailty was a weak mediator of the association between cancer history and community acquired sepsis |
| Pamoukdjian | 2017 | Outpatients with cancer. 50.5% male | Prospective cohort | 190 | Mean 80.6±5.6y | Gait speed <0.8 meter/second | 50.5% | Gait speed was used to predict mortality | Gait speed was independently associated with mortality |
| Perez-Zepeda | 2016 | Mexican older adults enrolled in the Mexican Health and Aging Study | Nested Case-Control Study | 8022 (288 with cancer history) | Median age 70.6y | 55 item frailty index where incident frailty defined as >0.25 and worsening frailty defined as negative residual value from frailty index scores 11 years apart | 29.9% incident frailty, 53.8% worsening frailty | Shorter time <10y from diagnosis associated with both incident and worsening frailty | |
| Brown | 2015 | National Health and Nutrition Examination Survey III Population Based Sample of Older Adults with a Non-Skin Cancer History | Mortality Linked Prospective Cohort Study | 416 | Median 72.2y | Performance-based; modified Fried Criteria | 37.3% Pre-frail, 9.1% frail | Frailty used as predictor of all- cause mortality | Prefrailty and frailty were associated with mortality |
y=years, %=percent, MSK-FI=Memorial Sloan Kettering Frailty Index, EORTC QLQ-30= European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, HCT= hematopoietic stem cell transplant, QOL=Quality of Life, PC=Prostate cancer, ADT=Androgen deprivation therapy, ADL=Activities of daily living, GHD=Growth hormone deficiency
Frailty, chronic conditions, and mortality in survivors
Frailty among cancer survivors is associated with increased risk for adverse events, including hospitalization, new onset chronic disease and mortality. In a cohort of over 27,000 community dwelling adults 45 years of age and older, Moore et al. reported that frailty is a mediator of the association between cancer history and hospital admission for community acquired sepsis.[38] In the St. Jude Lifetime Cohort, we documented a 2.6 (95% CI 1.2–6.2) fold increased hazard for death and a 2.2 (95% CI 1.2–4.2) fold increased hazard for new onset chronic conditions among childhood cancer survivors (mean age 33.6±8.1 years, 50.3% male) who were frail when compared to those who were not frail. Using a similar performance based phenotype (where 2 of 5 criteria indicate prefrailty, and 3 or more of 5 criteria indicate frailty), Brown et al.[35] evaluated associations between prefrailty, frailty and mortality among 416 older adult (median age 72.2 years) cancer survivors who participated in the Third National Health and Nutrition Examination Survey. Mortality varied by frailty status with median survival of 13.9 years among non-frail (53.6% of total), 9.5 years among pre-frail (37.3% of total) and 2.5 years among frail (9.1% of total) survivors. Others have used a single measure of impaired physiologic reserve, specifically, sarcopenia, to predict outcomes in cancer survivors, Lee et al, using data from the Korean National Health and Nutrition Examination Survey, reported increased cardiovascular disease risk in male cancer survivors with compared to those without sarcopenia (RR 2.67, 95% CI 1.18–6.56),[44] and increased risk for metabolic syndrome among both male and female survivors with sarcopenia when compared to those without sarcopenia (RR 2.76, 95% CI 1.92–3.97). Villasenor et al.[45], followed 471 breast cancer survivors for a median 9.2 years, and found that the hazard of death was 2.86 (95% CI 1.67–4.89) times higher in women with sarcopenia compared to those without sarcopenia.[46]
Potential biological mechanisms for frailty among survivors
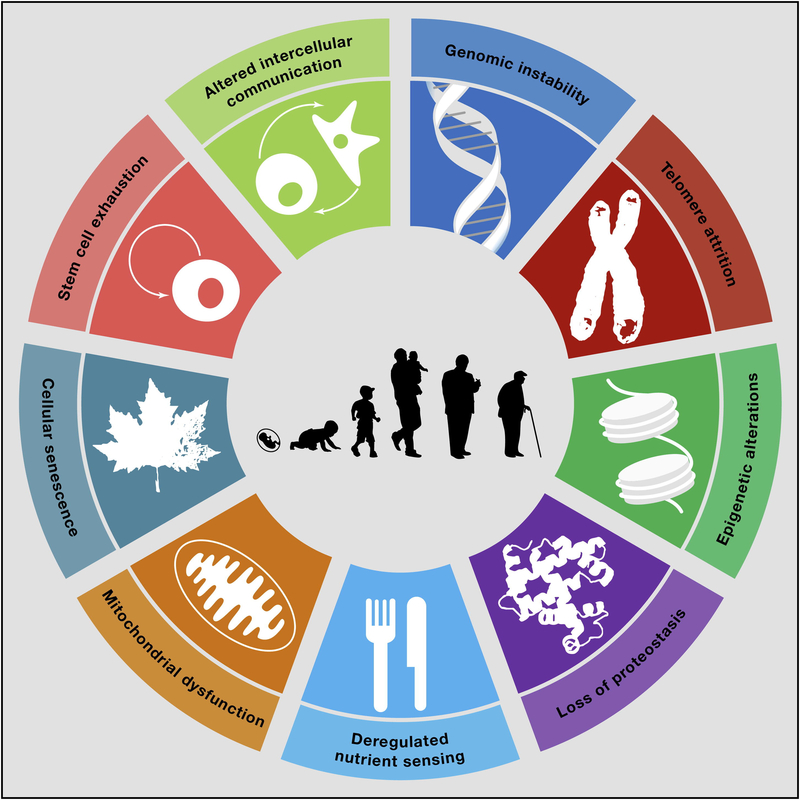
Recent research advances document that the rate of aging in the general population is controlled by genetic pathways and biochemical processes conserved in evolution. Lopez-Odin et al.[47] describe potential hallmarks that explain aging across organisms. These include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication (Figure 2). These processes are likely interconnected and have different relative contributions to aging and related functional states. In patients with cancer, where cure requires interventions that do not spare normal tissue (or cellular function), intrinsic damage is possible and may accelerate one or more of these processes (Table 2).[48] Aging processes are also likely to vary among cancer survivors, based on germline genetic variations in both the genes that control longevity,[49] and those associated with biological response to cancer therapies. [50–58] DNA integrity and stability are challenged by administration of chemotherapy and radiation. Chemotherapy agents interfere with DNA synthesis, replication and transcription, tubulin polymerization and mitotic spindle formation. Chromosomal deletions, insertions, inversions, translocations, aneuploidy, polyploidy and endoreduplication are possible. Ionizing radiation breaks DNA molecules by generating single and double strand breaks. Lesions are repaired by non-homologous end joining, resulting in both numerical and structural chromosomal damage.[59] Evidence to support the persistence of chromosomal damage in cancer survivors is provided by studies evaluating HL survivors whose lymphocytes show evidence of chromosomal abnormalities years following treatment,[60–63] and studies demonstrating relative telomere shortening in young adult survivors of childhood acute lymphoblastic leukemia[64], high risk neuroblastoma,[31] and in a cohort of childhood cancer survivors with mixed diagnoses.[65] Epigenetic alterations are also evident in cancer survivors where DNA methylation patterns in blood among adult survivors of adolescent and young adult onset HL differ from those of their unaffected monozygotic twin,[66] DNA methylation signatures in T-cells of childhood cancer survivors treated with total body radiation and hematopoietic stem cell transplant are altered and associated with over-activation of cytokine signaling pathways,[67] and DNA methylation loci that are associated with obesity in the general population are also associated with obesity in survivors of childhood onset ALL.[68]
Figure 2.
Hallmarks of Aging.
Table 2.
Specific hallmarks of aging and evidence in cancer survivors
| Hallmark of Aging | Evidence in cancer survivors |
|---|---|
| Genomic instability | Chromosomal abnormalities are documented in Hodgkin Lymphoma survivors [59–63] |
| Telomere attrition | Relative telomere shortening is documented in survivors of childhood leukemia, high risk neuroblastoma and is associated with chronic disease in survivors of childhood cancer [31, 64, 65] |
| Epigenetic alterations | DNA methylation patterns differ between survivors of Hodgkin Lymphoma and their monozygotic twins, are altered in T-cells and associated with over-activated cytokine signaling pathways of survivors of childhood cancer treated with hematopoietic stem cell transplantation, and are associated with obesity in survivors of childhood onset acute lymphoblastic leukemia. [66–68] |
| Loss of proteostasis | Differences in mitochondrial protein content are documented in survivors of childhood acute lymphoblastic leukemia when compared to healthy controls.[69] |
| Dysregulated nutrient sensing | Growth hormone deficiency is common in cancer survivors whose hypothalamic-pituitary axis is exposed to radiation.[70, 71] Downregulation of metabolism in cancer survivors is thought to be a protective mechanism to spare cellular damage.[47] |
| Mitochondrial dysfunction | Differential expression of genes related to mitochondrial function are reported in breast cancer survivors with peripheral neuropathy and cardiomyopathy.[75, 112]Children treated with anthracyclines dexrazoxane, a cardio protectant) have higher mitochondrial content per cell than those who had anthracycline exposure with no dexrazoxane.[76] |
| Cellular senescence | Markers of cellular senescence and associated inflammatory cytokines are evident in breast cancer survivors, survivors of childhood acute lymphoblastic leukemia, and those treated with hematopoietic stem cell transplant.[77–80] |
| Altered intercellular communication | Increased tumor cell levels of advanced glycation end products (AGEs), reactive metabolites produced during normal metabolism, are documented among women with estrogen receptor positive (ER+) breast cancer when compared to those with ER- breast cancer.[82] |
Impaired protein homeostasis is not yet well documented in cancer survivors, although one group of investigators report differences in mitochondrial protein content in a small group of childhood ALL survivors when compared to healthy controls. These authors indicate that their findings suggest changes in regulation of mitochondrial processes associated with inflammation, oxidative stress and apoptosis, and that these disordered processes are associated with post-cancer therapy increased risk for cardiometabolic disorders.[69] More data is needed to confirm these findings. However, dysregulated nutrient sensing is a well-known problem in cancer survivors, particular those whose somatotropic axis is directly impacted by cranial radiation.[30] Growth hormone insufficiency is not uncommon,[70, 71] with resultant dysfunction in the insulin and insulin-like-growth-factor signaling (IIS) pathway and the ability of cells to detect glucose. While downregulation of this pathway in animal and human models of dietary restriction increases longevity, presumably because involved cells have slower rates of growth and metabolism, such is not the case in aging, and among cancer survivors, where downregulation appears to be a protective mechanism to lower rates of cellular damage.[47] Cancer survivors with disordered metabolism are at greater risk for cardiovascular disease,[72] cancer recurrence[73] and mortality.[74]
Evidence for mitochondrial dysfunction in cancer survivors is reported in several studies. Kober et al. studied gene-expression in peripheral blood among 50 breast cancer survivors treated with paclitaxel, 25 who had peripheral neuropathy (PN) and 25 who did not. When compared to those without PN, those with PN had differential expression of four genes related to mitochondrial dysfunction, and perturbation of ten pathways identified in the Kyoto Encyclopedia of Genes and Genomes database.[74] Ruiz-Pinto et al. also found genetic evidence of mitochondrial dysfunction in breast cancer survivors with anthracycline induced cardiomyopathy using exome array analysis. Survivors with a minor T-allele in electron transfer flavoprotein beta subunit (EFTB) had a higher risk of cardiomyopathy. EFT is located in the inner membrane of mitochondria and acts as an electron acceptor of energy production from amino and fatty acids.[75] Finally, Lipshultz et al. reported higher mitochondrial copy number per cell in childhood cancer survivors exposed to doxorubicin without compared to those exposed to doxorubicin with the cardio-protectant dexrazoxane. These authors suggest that this may represent persistent clonal expansion of mitochondria in response to early damage during doxorubicin administration.[76]
Markers of cellular senescence and associated inflammatory markers are evident in survivors of breast cancer, survivors of childhood cancer, and among survivors of hematopoietic stem cell transplant (HCT). Sanoff et al. report increased levels of senescence markers p16INK4a, ARF mRNA, and senescence associated cytokines VEGFA and MCP1 among 33 women with stage I-III breast cancer immediately after treatment and 12-months later. Independent assessment of p16INK4a expression in another cohort of 176 breast cancer survivors 3.4 years after treatment show an average of 10.4 years of additional biological on top of chronological aging.[77] Alfano et al. also reported increased levels of inflammatory cytokines in breast cancer survivors (N=209) compared to non-cancer controls (N=106). Cytokine levels were associated with comorbidities in this population who were on average 55 years of age.[78] Survivors of childhood ALL (N=10) treated with radiation,[79] and HCT survivors (N=63) also demonstrate increased expression of p16INK4a, associated with radiation exposure, with higher doses of chemotherapy prior to transplant, and with autologous versus allogeneic HCT.[80] Evidence of stem cell exhaustion is provided by a study of 14 of 15 long-term survivors of HCT, whose lymphocytes demonstrate a significant decrease in ability to differentiate and proliferate, which is, along with telomere shortening, phenotypically consistent with an aged immune system.[81]
All of these alterations in cellular integrity and behavior that occur with aging culminate by affecting intercellular communication. Disrupted intracellular communication alters neurohormonal signaling, with resultant chronic inflammation, declining immunosurveillance, and a compromised intercellular environment. This milieu is not benign and influences the structure and function of all of the body’s tissues.[47] This hypothesis is supported in a preliminary study by Walter et al.,[82] who document increased tumor cell levels of advanced glycation end products (AGEs), reactive metabolites produced during normal metabolism, among women with estrogen receptor positive (ER+) breast cancer when compared to those with ER- breast cancer. AGEs are thought to modify the genome, interfere with protein function/crosslinking, resulting in aberrant cell-signaling.[83] These processes are relevant in aging and disease,[84] and likely contribute to the potential accelerated aging landscape in cancer survivors after treatment, although this is difficult to disentangle in survivors of adult onset cancers. Adults have chronic disease and may have elevated levels of inflammatory markers or excessive accumulation of lifestyle associated reactive metabolites prior to cancer diagnosis, which in some cases may have contributed to the development of the malignancy.[85] Adult survivors also have a lifetime of exposure to chronic psychological stressors, including a cancer diagnosis, which also contribute to increased levels of systemic inflammation and an impaired immune response.[86] Survivors of childhood onset cancer offer a unique opportunity in this research arena, as most of them do not have an accumulation of somatic damage or chronic disease when they are diagnosed with cancer. Mechanisms triggered by exposure to cancer and cancer related therapies may be easier to isolate in younger cohorts. Preliminary work in this area is scarce, but the published work indicates that inflammatory markers are present in groups of childhood cancer survivors and are related to other aging biomarkers as discussed above.[31, 64, 67] Nevertheless, there has been little work done to comprehensively characterize the complex associations between the multiple and interrelated hallmarks of aging in childhood cancer survivors. Almost no work has been done to correlate biomarkers of aging to function or frailty in children or young survivors of childhood cancer. Research to elucidate these mechanisms in cancer survivors is needed.
Interventions to prevent or remediate frailty
Frailty is a construct that includes many components, thus, interventions to prevent or remediate frailty in cancer survivors need to either be multi-focal, or those that globally address aging and its multiple biologic processes. There are not yet any published clinical trials in cancer survivors where preventing or remediating frailty, either as a phenotype or an accumulation of disease, is the primary outcome. Certainly, chronic disease in cancer survivors is managed medically, with appropriate pharmaceutical agents, and with lifestyle counseling. However, in aging populations, pharmaceutical interventions that specifically target aging appear to be more effective in preventing or remediating age-related disorders compared to treatments that target a specific disease. This approach has potential in cancer survivors, particularly for prevention.
Recent data from the aging literature describe a number of promising pharmaceutical approaches (Table 3). These include molecular compounds that mimic caloric restriction (CRM), that increase nicotinamide adenine dinucleotide (NAD+) levels, that eliminate senescent cells, and that inhibit the receptor for advanced glycation end-products (RAGE). One study documents the feasibility and safety of rapamycin, an inhibitor of mTOR (mechanistic target of rapamycin) that prolongs life in animal models without requiring a reduction in food intake, for short periods in older adults who are otherwise healthy,[87] and another study is underway evaluating the effects of metformin on aging.[88] Metformin is a powerful modulator of glucose metabolism, decreases insulin levels and insulin like growth factor type I (IGF-1) signaling, inhibits mTOR and mitochondrial complex 1, activates AMP-activated kinase (AMPK) and reduces DNA damage.[88] Human data supporting the efficacy of resveratrol and SRT2104, sirtuin-activating compounds, to treat age related conditions, including type 2 diabetes, loss of physical abilities and cognition are mixed.[89–93] However, a recent study indicates that chronic nicotinamide riboside supplementation is tolerated, elevates NAD+ levels in healthy middle aged and older adults, and appears to lower blood pressure in persons with pre-hypertension.[94] NAD+ is an enzyme cofactor that declines with aging, critical for cellular energy metabolism, DNA repair and cellular response to oxidative stress.[95, 96] Justice et al., [97] reporting first in human results, indicate that intermittent administration of dasatinib and quercetin (DQ) to clear senescent cells is feasible, safe and improved physical function in persons with idiopathic pulmonary fibrosis, a progressive fatal cellular senescence associated disease. Finally, several studies in humans have utilized RAGE inhibitors (TTP488) in an attempt to slow cognitive decline in persons with Alzheimer’s Disease. [98–100] Pharmaceutical trials like these are needed in cancer survivors.
Table 3.
Pharmaceutical agents with potential to target aging processes
| Agent | Mechanism | Targets |
|---|---|---|
| Rapamycin | Inhibits mammalian target of rapamycin (mTOR). | • Loss of proteostasis • Dysregulated nutrient sensing • Mitochondrial dysfunction • Stem cell exhaustion |
| Metformin | Modulates glucose metabolism, decreases insulin and insulin like growth factor signaling, inhibits mTOR and mitochondrial complex 1. activates AMP-Kinase and reduces DNA damage | • Genomic instability • Loss of proteostasis • Dysregulated nutrient sensing • Mitochondrial dysfunction |
| Nicotinamide Riboside | Precursor for NAD+ (NAD regulates the energy balance, stress response, and cellular homeostasis through sirtuins, PARPs and various redox enzymes) | • Genomic instability • Dysregulated nutrient sensing • Mitochondrial dysfunction |
| Dasatinib and Quercetin | Senolytic | • Cellular senescence |
| TTP448 | Receptor for advanced glycation end-products inhibitor | • Intracellular communication • Genomic instability |
Successful exercise, physical activity and dietary interventions that preserve or improve physical function in middle aged and older adult cancer survivors, like the pharmaceutical trials described above, also address an overall aging phenotype. A review of 38 randomized trials published in 2016 indicates structured exercise intervention improves parameters of physical function (e.g. increased distance walked in six minutes, more chair rises in a set time) and fatigue, and that these improvements are related to better quality of life in middle-aged and older adult cancer survivors.[101] This review in cancer survivors is consistent with a another systematic review that includes a meta-analysis of 66 randomized trials targeting prevention or treatment of frailty as a primary outcome in adult populations with and without chronic disease. Across 21 randomized trials including 5,262 participants and eight interventions (physical activity, physical activity with protein or nutritional supplementation, psychosocial or cognitive training, pharmacotherapy, multifactorial, geriatric comprehensive assessments, nutrition only), physical activity, when compared to standard of care or placebo, was associated with reduction in frailty (standardized mean difference −0.92, 95% CI −1.55–0.29), and both physical activity and physical activity with nutritional supplementation were the two most likely interventions (using the surface under the cumulative ranking curve technique: 100%, 71% respectively) to reduce frailty.[102]
Lifestyle interventions among survivors also show promise when targeting biomarkers of aging. Caloric restriction (500–1000 kilocalorie/day deficit) and moderate physical activity among survivors of solid tumors and hematological malignancies (N=60) randomized to intervention reduced blood insulin concentration of −7.7±3.5 μU/mL compared to controls. Similar results are reported in rural breast cancer survivors (N=34), where a 1000 kcal/day deficit combined with progression to 225 minutes of moderate intensity exercise per week resulted in greater than 10% weight loss and a 16.7% reduction in fasting insulin levels.[103] Caloric restriction also impacts telomerase activity in cancer survivors. Ornish et al., in a study that implemented lifestyle changes (calorie restriction, moderate intensity aerobic exercise, stress management activities and increased social support) in men with low grade prostate cancer (N=30) report an increase in telomerase activity from 8.05±3.5 to 10.38±6.01 standard arbitrary units after the initial three month intervention period,[104] and over five years of follow-up.[105] In another study, breast cancer survivors (N=125) with body mass index values ≥25 kilograms per square meter who were a mean age of 58±8 years, those randomized to a six-month weight loss intervention (lifestyle, exercise and nutrition components) had a mean increase in relative leukocyte telomere length (LTL) of 3% compared to a 5% decrease in the usual care group.[106] Two randomized studies report changes in DNA methylation patterns among survivors of breast cancer after short term diet/lifestyle interventions when compared to controls,[107, 108] and several studies report improvements in biomarkers/pathways associated with nutrient sensing,[109] metabolism,[110] inflammation[111], cellular signaling[82] among breast cancer and colon cancer survivors after diet and physical activity interventions.
Summary
There are over 15 million survivors of cancer in the United States whose rates of frailty, an aging phenotype, range from just under ten to over eighty percent. Frailty impacts not only disease survival, but also long-term function and quality of life in children, adolescents, and in all adults diagnosed and/or treated for cancer. Biological evidence to support frailty as an aging construct is strong; biomarkers of aging are prevalent in cancer survivors, where identification of responsible biological pathways is extremely important, because it provides targets for pharmaceutical or nutraceutical interventions, while also providing intermediate markers of eventual disease. Earlier markers of disease onset are employed in screening to identify persons at high risk for future adverse outcomes, and in research, where interventions are delivered not after disease appearance, but before to either prevent or delay disease onset. Data from the aging literature identifies potential pharmaceutical strategies to address frailty in cancer survivors, however, clinical trials are needed to test safety and efficacy (Figure 3). Lifestyle interventions that include physical activity, exercise and/or caloric restriction have been evaluated in some cancer survivors, are associated with both improvements in physical function and with moderation of specific biomarkers of aging. Multiple delivery approaches and intervention doses are employed, making it difficult to provide survivors and clinicians with clear direction in survivors at risk for, or who develop frailty. More research is needed to identify the most effective intervention modalities and doses. Nevertheless, while the clinical community awaits the results of ongoing pharmaceutical and lifestyle based clinical trials, it seems prudent that at-risk survivors would be referred to professionals with expertise in diet and exercise prescription.
Figure 3.
Future Research Directions.
Acknowledgements
The authors would like to acknowledge Tracie Gatewood for her assistance with article preparation. Both authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. Financial support at St. Jude Children’s Research Hospital was provided to both authors by the National Institutes of Health (P30CA021765) and the American Lebanese and Syrian Associated Charities.
References
- [1].Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- [2].Guy GP Jr, Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC. Economic Burden of Chronic Conditions Among Survivors of Cancer in the United States. J Clin Oncol. 2017;35:2053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guida JL, Ahles TA, Belsky D, et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst. 2019;111:1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–75. [DOI] [PubMed] [Google Scholar]
- [5].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- [6].Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. [DOI] [PubMed] [Google Scholar]
- [7].Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–71. [DOI] [PubMed] [Google Scholar]
- [8].Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–30. [DOI] [PubMed] [Google Scholar]
- [9].Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–9. [DOI] [PubMed] [Google Scholar]
- [11].Forti P, Rietti E, Pisacane N, et al. A comparison of frailty indexes for prediction of adverse health outcomes in an elderly cohort. Arch Gerontol Geriatr. 2012;54:16–20. [DOI] [PubMed] [Google Scholar]
- [12].Freiheit EA, Hogan DB, Eliasziw M, et al. Development of a frailty index for patients with coronary artery disease. J Am Geriatr Soc. 2010;58:1526–31. [DOI] [PubMed] [Google Scholar]
- [13].Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–9. [DOI] [PubMed] [Google Scholar]
- [14].Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7. [DOI] [PubMed] [Google Scholar]
- [15].Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–33. [DOI] [PubMed] [Google Scholar]
- [16].Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92. [DOI] [PubMed] [Google Scholar]
- [17].Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26:1091–101. [DOI] [PubMed] [Google Scholar]
- [18].de Souto Barreto P One operational definition by population: the need for local evaluations of frailty. J Physiol Anthropol. 2011;30:259–62. [DOI] [PubMed] [Google Scholar]
- [19].Shahrokni A, Tin A, Alexander K, et al. Development and Evaluation of a New Frailty Index for Older Surgical Patients With Cancer. JAMA Netw Open. 2019;2:e193545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Franco I, Chen YH, Chipidza F, et al. Use of frailty to predict survival in elderly patients with early stage non-small-cell lung cancer treated with stereotactic body radiation therapy. J Geriatr Oncol. 2018;9:130–7. [DOI] [PubMed] [Google Scholar]
- [21].Pamoukdjian F, Levy V, Sebbane G, et al. Slow Gait Speed Is an Independent Predictor of Early Death in Older Cancer Outpatients: Results from a Prospective Cohort Study. J Nutr Health Aging. 2017;21:202–6. [DOI] [PubMed] [Google Scholar]
- [22].Williams GR, Dunham L, Chang Y, et al. Geriatric Assessment Predicts Hospitalization Frequency and Long-Term Care Use in Older Adult Cancer Survivors. J Oncol Pract. 2019;15:e399–e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mandelblatt JS, Small BJ, Luta G, et al. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol. 2018:JCO1800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mandelblatt JS, Clapp JD, Luta G, et al. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer. 2016;122:3555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Degesys N, Klein C, Binner M, Browner I, Shapiro G. Fitness screening in older cancer patients. J Am Geriatr Soc. 2011;59:S141. [Google Scholar]
- [27].Chemaitilly W, Li Z, Krasin MJ, et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J Clin Endocrinol Metab. 2017;102:2242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eissa HM, Lu L, Baassiri M, et al. Chronic disease burden and frailty in survivors of childhood HSCT: a report from the St. Jude Lifetime Cohort Study. Blood Adv. 2017;1:2243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hayek S, Gibson TM, Leisenring WM, et al. Prevalence and Predictors of Frailty in Childhood Cancer Survivors and Siblings: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2019:Jco1901226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van Iersel L, Li Z, Srivastava DK, et al. Hypothalamic-Pituitary Disorders in Childhood Cancer Survivors: Prevalence, Risk Factors and Long-Term Health Outcomes. J Clin Endocrinol Metab. 2019;104:6101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vatanen A, Hou M, Huang T, et al. Clinical and biological markers of premature aging after autologous SCT in childhood cancer. Bone Marrow Transplant. 2017;52:600–5. [DOI] [PubMed] [Google Scholar]
- [32].Wilson CL, Chemaitilly W, Jones KE, et al. Modifiable Factors Associated With Aging Phenotypes Among Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34:2509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arora M, Sun CL, Ness KK, et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bennett JA, Winters-Stone KM, Dobek J, Nail LM. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol Nurs Forum. 2013;40:E126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brown JC, Harhay MO, Harhay MN. The Prognostic Importance of Frailty in Cancer Survivors. J Am Geriatr Soc. 2015;63:2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bylow K, Hemmerich J, Mohile SG, et al. Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: a case-control study. Urology. 2011;77:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Johnson MS, Bailey TL, Schmid KK, Lydiatt WM, Johanning JM. A frailty index identifies patients at high risk of mortality after tracheostomy. Otolaryngol Head Neck Surg. 2014;150:568–73. [DOI] [PubMed] [Google Scholar]
- [38].Moore JX, Akinyemiju T, Bartolucci A, et al. Mediating Effects of Frailty Indicators on the Risk of Sepsis After Cancer. J Intensive Care Med. 2018:885066618779941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ronning B, Wyller TB, Jordhoy MS, et al. Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol. 2014;5:26–32. [DOI] [PubMed] [Google Scholar]
- [40].Winters-Stone KM, Moe E, Graff JN, et al. Falls and Frailty in Prostate Cancer Survivors: Current, Past, and Never Users of Androgen Deprivation Therapy. J Am Geriatr Soc. 2017;65:1414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Phung LA, Karvinen SM, Colson BA, Thomas DD, Lowe DA. Age affects myosin relaxation states in skeletal muscle fibers of female but not male mice. PLoS One. 2018;13:e0199062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Collins BC, Laakkonen EK, Lowe DA. Aging of the musculoskeletal system: How the loss of estrogen impacts muscle strength. Bone. 2019;123:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Smitherman AB, Anderson C, Lund JL, et al. Frailty and Comorbidities Among Survivors of Adolescent and Young Adult Cancer: A Cross-Sectional Examination of a Hospital-Based Survivorship Cohort. J Adolesc Young Adult Oncol. 2018;7:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lee SJ, Park YJ, Cartmell KB. Sarcopenia in cancer survivors is associated with increased cardiovascular disease risk. Support Care Cancer. 2018;26:2313–21. [DOI] [PubMed] [Google Scholar]
- [45].Villasenor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee SJ, Kim NC. Association Between Sarcopenia and Metabolic Syndrome in Cancer Survivors. Cancer Nurs. 2017;40:479–87. [DOI] [PubMed] [Google Scholar]
- [47].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67:212–5. [DOI] [PubMed] [Google Scholar]
- [49].Dato S, Soerensen M, Rose G. Untangling the Genetics of Human Longevity-A Challenging Quest. Genes (Basel). 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Boldrin E, Rumiato E, Fassan M, et al. Genetic risk of subsequent esophageal cancer in lymphoma and breast cancer long-term survival patients: a pilot study. Pharmacogenomics J. 2016;16:266–71. [DOI] [PubMed] [Google Scholar]
- [51].Hildebrandt MAT, Reyes M, Wu X, et al. Hypertension Susceptibility Loci are Associated with Anthracycline-related Cardiotoxicity in Long-term Childhood Cancer Survivors. Sci Rep. 2017;7:9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Krajinovic M, Elbared J, Drouin S, et al. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2016;16:530–5. [DOI] [PubMed] [Google Scholar]
- [53].Nadeau G, Ouimet-Grennan E, Aaron M, et al. Identification of genetic variants associated with skeletal muscle function deficit in childhood acute lymphoblastic leukemia survivors. Pharmgenomics Pers Med. 2019;12:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rajic V, Aplenc R, Debeljak M, et al. Influence of the polymorphism in candidate genes on late cardiac damage in patients treated due to acute leukemia in childhood. Leuk Lymphoma. 2009;50:1693–8. [DOI] [PubMed] [Google Scholar]
- [55].Richard MA, Lupo PJ, Morton LM, et al. Genetic variation in POT1 and risk of thyroid subsequent malignant neoplasm: A report from the Childhood Cancer Survivor Study. PLoS One. 2020;15:e0228887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schack LMH, Petersen SE, Nielsen S, et al. Validation of genetic predictors of late radiation-induced morbidity in prostate cancer patients. Acta Oncol. 2017;56:1514–21. [DOI] [PubMed] [Google Scholar]
- [57].van der Kooi ALF, Clemens E, Broer L, et al. Genetic variation in gonadal impairment in female survivors of childhood cancer: a PanCareLIFE study protocol. BMC Cancer. 2018;18:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].van Dorp W, van den Heuvel-Eibrink MM, Stolk L, et al. Genetic variation may modify ovarian reserve in female childhood cancer survivors. Hum Reprod. 2013;28:1069–76. [DOI] [PubMed] [Google Scholar]
- [59].Frias S, Ramos S, Salas C, et al. Nonclonal Chromosome Aberrations and Genome Chaos in Somatic and Germ Cells from Patients and Survivors of Hodgkin Lymphoma. Genes (Basel). 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bilban-Jakopin C, Bilban M. Genotoxic effects of radiotherapy and chemotherapy on circulating lymphocytes in patients with Hodgkin’s disease. Mutat Res. 2001;497:81–8. [DOI] [PubMed] [Google Scholar]
- [61].M’Kacher R, Girinsky T, Koscielny S, et al. Baseline and treatment-induced chromosomal abnormalities in peripheral blood lymphocytes of Hodgkin’s lymphoma patients. Int J Radiat Oncol Biol Phys. 2003;57:321–6. [DOI] [PubMed] [Google Scholar]
- [62].Salas C, Niembro A, Lozano V, et al. Persistent genomic instability in peripheral blood lymphocytes from Hodgkin lymphoma survivors. Environ Mol Mutagen. 2012;53:271–80. [DOI] [PubMed] [Google Scholar]
- [63].Smith LM, Evans JW, Mori M, Brown JM. The frequency of translocations after treatment for Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1992;24:737–42. [DOI] [PubMed] [Google Scholar]
- [64].Ariffin H, Azanan MS, Abd Ghafar SS, et al. Young adult survivors of childhood acute lymphoblastic leukemia show evidence of chronic inflammation and cellular aging. Cancer. 2017;123:4207–14. [DOI] [PubMed] [Google Scholar]
- [65].Song N, Li Z, Qin N, et al. Shortened Leukocyte Telomere Length Associates with an Increased Prevalence of Chronic Health Conditions among Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort. Clin Cancer Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang J, Van Den Berg D, Hwang AE, et al. DNA methylation patterns of adult survivors of adolescent/young adult Hodgkin lymphoma compared to their unaffected monozygotic twin. Leuk Lymphoma. 2019;60:1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Daniel S, Nylander V, Ingerslev LR, et al. T cell epigenetic remodeling and accelerated epigenetic aging are linked to long-term immune alterations in childhood cancer survivors. Clin Epigenetics. 2018;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lupo PJ, Brown AL, Arroyo VM, et al. DNA methylation and obesity in survivors of pediatric acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Genes Chromosomes Cancer. 2019;58:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Leahy J, Spahis S, Bonneil E, et al. Insight from mitochondrial functions and proteomics to understand cardiometabolic disorders in survivors of acute lymphoblastic leukemia. Metabolism. 2018;85:151–60. [DOI] [PubMed] [Google Scholar]
- [70].Chemaitilly W, Cohen LE, Mostoufi-Moab S, et al. Endocrine Late Effects in Childhood Cancer Survivors. J Clin Oncol. 2018;36:2153–9. [DOI] [PubMed] [Google Scholar]
- [71].Mostoufi-Moab S, Seidel K, Leisenring WM, et al. Endocrine Abnormalities in Aging Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34:3240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].DeFilipp Z, Duarte RF, Snowden JA, et al. Metabolic syndrome and cardiovascular disease following hematopoietic cell transplantation: screening and preventive practice recommendations from CIBMTR and EBMT. Bone Marrow Transplant. 2017;52:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Coughlin SS, Smith SA. The Insulin-like Growth Factor Axis, Adipokines, Physical Activity, and Obesity in Relation to Breast Cancer Incidence and Recurrence. Cancer Clin Oncol. 2015;4:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Calip GS, Malone KE, Gralow JR, et al. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat. 2014;148:363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ruiz-Pinto S, Pita G, Martin M, et al. Exome array analysis identifies ETFB as a novel susceptibility gene for anthracycline-induced cardiotoxicity in cancer patients. Breast Cancer Res Treat. 2018;167:249–56. [DOI] [PubMed] [Google Scholar]
- [76].Lipshultz SE, Anderson LM, Miller TL, et al. Impaired mitochondrial function is abrogated by dexrazoxane in doxorubicin-treated childhood acute lymphoblastic leukemia survivors. Cancer. 2016;122:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106:dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Alfano CM, Peng J, Andridge RR, et al. Inflammatory Cytokines and Comorbidity Development in Breast Cancer Survivors Versus Noncancer Controls: Evidence for Accelerated Aging? J Clin Oncol 2017;35:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Marcoux S, Le ON, Langlois-Pelletier C, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiat Oncol. 2013;8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wood WA, Krishnamurthy J, Mitin N, et al. Chemotherapy and Stem Cell Transplantation Increase p16(INK4a) Expression, a Biomarker of T-cell Aging. EBioMedicine. 2016;11:227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lewis NL, Mullaney M, Mangan KF, et al. Measurable immune dysfunction and telomere attrition in long-term allogeneic transplant recipients. Bone Marrow Transplant. 2004;33:71–8. [DOI] [PubMed] [Google Scholar]
- [82].Walter KR, Ford ME, Gregoski MJ, et al. Advanced glycation end products are elevated in estrogen receptor-positive breast cancer patients, alter response to therapy, and can be targeted by lifestyle intervention. Breast Cancer Res Treat. 2019;173:559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. [DOI] [PubMed] [Google Scholar]
- [84].Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhang X, Meng X, Chen Y, Leng SX, Zhang H. The Biology of Aging and Cancer: Frailty, Inflammation, and Immunity. Cancer J 2017;23:201–5. [DOI] [PubMed] [Google Scholar]
- [86].Lacourt TE, Heijnen CJ. Mechanisms of Neurotoxic Symptoms as a Result of Breast Cancer and Its Treatment: Considerations on the Contribution of Stress, Inflammation, and Cellular Bioenergetics. Curr Breast Cancer Rep. 2017;9:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kraig E, Linehan LA, Liang H, et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp Gerontol. 2018;105:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab. 2016;23:1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis. Mol Nutr Food Res. 2015;59:147–59. [DOI] [PubMed] [Google Scholar]
- [90].McCubrey JA, Lertpiriyapong K, Steelman LS, et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany NY). 2017;9:1477–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Baksi A, Kraydashenko O, Zalevkaya A, et al. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br J Clin Pharmacol. 2014;78:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Libri V, Brown AP, Gambarota G, et al. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 2012;7:e51395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Venkatasubramanian S, Noh RM, Daga S, et al. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc. 2013;2:e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Fang EF, Bohr VA. NAD(+): The convergence of DNA repair and mitophagy. Autophagy. 2017;13:442–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Fang EF, Lautrup S, Hou Y, et al. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med. 2017;23:899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Burstein AH, Grimes I, Galasko DR, et al. Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurol. 2014;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Galasko D, Bell J, Mancuso JY, et al. Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology. 2014;82:1536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sabbagh MN, Agro A, Bell J, et al. PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Daum CW, Cochrane SK, Fitzgerald JD, Johnson L, Buford TW. Exercise Interventions for Preserving Physical Function Among Cancer Survivors in Middle to Late Life. J Frailty Aging. 2016;5:214–24. [DOI] [PubMed] [Google Scholar]
- [102].Negm AM, Kennedy CC, Thabane L, et al. Management of Frailty: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. J Am Med Dir Assoc. 2019;20:1190–8. [DOI] [PubMed] [Google Scholar]
- [103].Befort CA, Klemp JR, Austin HL, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132:631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–57. [DOI] [PubMed] [Google Scholar]
- [105].Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112–20. [DOI] [PubMed] [Google Scholar]
- [106].Sanft T, Usiskin I, Harrigan M, et al. Randomized controlled trial of weight loss versus usual care on telomere length in women with breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. Breast Cancer Res Treat. 2018;172:105–12. [DOI] [PubMed] [Google Scholar]
- [107].Delgado-Cruzata L, Zhang W, McDonald JA, et al. Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and Afro-Caribbean breast cancer survivors. J Nutr. 2015;145:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Greenlee H, Ogden Gaffney A, Aycinena AC, et al. Long-term Diet and Biomarker Changes after a Short-term Intervention among Hispanic Breast Cancer Survivors: The ¡Cocinar Para Su Salud! Randomized Controlled Trial. Cancer Epidemiol Biomarkers Prev. 2016;25:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Saxton JM, Scott EJ, Daley AJ, et al. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: a randomised controlled trial. Breast Cancer Res. 2014;16:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Baxter BA, Oppel RC, Ryan EP. Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Harrigan M, Cartmel B, Loftfield E, et al. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol. 2016;34:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kober KM, Mazor M, Abrams G, et al. Phenotypic Characterization of Paclitaxel-Induced Peripheral Neuropathy in Cancer Survivors. J Pain Symptom Manage. 2018;56:908–19 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]