Abstract
Background:
Periconceptional diet quality is commonly suboptimal and sociodemographic disparities in diet quality exist. However, it is unknown whether individual periconceptional diet quality is associated with obstetric outcomes.
Objective:
Our objective was to assess differences in maternal and neonatal outcomes according to maternal periconceptional diet quality.
Study Design:
This is a secondary analysis of a large, multicenter prospective cohort study of 10,038 nulliparous women receiving obstetrical care at 8 United States centers. Women underwent three antenatal study visits and had detailed maternal and neonatal data abstracted by trained research personnel. In the first trimester (between 6 and 13 weeks), women completed the modified Block 2005 Food Frequency Questionnaire, a semiquantitative assessment of usual dietary intake for the 3 months around conception. Responses were scored using the Healthy Eating Index-2010, which assesses adherence to the 2010 Dietary Guidelines for Americans. Higher scores on the Healthy Eating Index represent better adherence. Healthy Eating Index scores were analyzed by quartile; quartile 4 represents the highest dietary quality. Bivariable and multivariable analyses were performed to assess associations between diet quality and outcomes. A sensitivity analysis in which markers of socioeconomic status were included in the multivariable Poisson regression models was performed.
Results:
In the cohort of 8,259 women with Healthy Eating Index data, the mean Healthy Eating Index score was 63 (± 13) of 100. Women with the lowest quartile Healthy Eating Index scores were more likely to be younger, non-Hispanic black and Hispanic, publicly insured, low income, and tobacco users. They were more likely to have comorbidities (obesity, chronic hypertension, pregestational diabetes, mental health disorders), a higher pre-pregnancy body mass index, and less education. Women with lowest quartile scores experienced less frequent major perineal lacerations and more frequent postpartum hemorrhage requiring transfusion and hypertensive disorders of pregnancy, which persisted on multivariable analyses (controlling for age, body mass index, tobacco use, chronic hypertension, pregestational diabetes mellitus, and mental health disorders) comparing women in each quartile to quartile 4. Additionally, women in quartiles 1 and 2 experienced greater adjusted relative risk of cesarean delivery compared to women in quartile 4. Neonatal outcomes also differed by dietary quartile, with women in the lowest Healthy Eating Index quartile experiencing greater adjusted relative risk of preterm birth, neonatal intensive care unit admission, small for gestational age infant, and low birthweight, and lower risk of macrosomia; all neonatal findings also persisted in multivariable analyses. The sensitivity analysis with inclusion of markers of socioeconomic status (race/ethnicity, insurance status, marital status) in the multivariable models supported these findings.
Conclusions:
Periconceptional diet quality among women in the United States is poor. Poorer periconceptional dietary quality is associated with adverse maternal and neonatal outcomes even after controlling for potential comorbidities and body mass index, suggesting periconceptional diet may be an important social or biological determinant of health underlying existing health disparities.
Keywords: dietary disparities, dietary quality, healthy eating index, periconceptional diet, pregnancy diet, pregnancy outcome
CONDENSATION:
Poor periconceptional dietary quality, common among US women, is associated with adverse maternal and neonatal outcomes even after controlling for potential comorbidities.
INTRODUCTION
Overall dietary quality is poor for most Americans.1,2 Fewer than 3% of United States (US) adults have ideal diet scores, and ample public health data suggest poor dietary quality is associated with morbidity.1–3 Moreover, racial, ethnic, and socioeconomic disparities in dietary quality are substantial for nearly all measures, including diet scores, individual nutrient sources, and energy intake, and while overall dietary quality in the US may be improving, these disparities are widening.1,2,4,5 Reproductive age women planning pregnancy have similarly poor diets,1,6,7 despite potential fetal health implications.8 Multiple European-based studies show that women planning pregnancy are only marginally more likely to comply with dietary recommendations and that dietary patterns changed little from before pregnancy to early pregnancy.6,9,10 Thus, a woman’s periconceptional diet is highly reflective of her general nutritional patterns and dietary intake later in pregnancy.
In 2017, using data from a large cohort of US nulliparous women, Bodnar et al demonstrated both that periconceptional dietary quality is suboptimal in US women and that racial, ethnic, and sociodemographic disparities in dietary quality exist.11 In this analysis, non-Hispanic white women had the highest quality of periconceptional diet, whereas almost half of non-Hispanic black women had dietary quality in the lowest quintile. Furthermore, although the quality of diet increased with greater maternal education in all racial or ethnic groups, education was most strongly associated with diet quality for white women.11 Top sources of energy, overall, in this study were foods rich in sugars and solid fats and included refined bread, soda, pasta, grain desserts, and alcohol.11
Periconceptional dietary quality has been hypothesized to be an important determinant of maternal and fetal outcomes,8,12 with suboptimal nutrition having a critical negative influence on fetal growth, placentation, inflammation, and maternal metabolic regulation, and possibly leading to differences in outcomes such as livebirth rate or birth weight.11–15 Poor periconceptional dietary quality may affect pregnancy outcomes via mechanisms such as micronutrient deficiency or relationship with gestational weight gain. However, data to confirm this hypothesis are lacking, particularly in the US. Thus, our objective was to assess if there is an association between periconceptional dietary quality and maternal and neonatal outcomes.
MATERIALS AND METHODS
This is a secondary analysis of data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-To-Be (nuMoM2b), which was a large, multicenter observational cohort study conducted at 8 US medical centers from 2010 to 2013.16 In this study, over 10,000 nulliparous women with singleton pregnancies were enrolled for prospective study. Recruitment was conducted at geographically diverse locations and was designed to sample a population reflective of the general US population. Women were eligible for enrollment if they had a live singleton pregnancy, had no previous pregnancy that progressed beyond 20 weeks of gestation, and were between 6 weeks 0 days and 13 weeks 6 days of gestation at recruitment. Exclusion criteria included maternal age younger than 13 years, history of three or more spontaneous abortions, current pregnancy complicated by suspected fatal fetal malformation or known fetal aneuploidy, assisted reproduction with a donor oocyte, multifetal reduction, or plan to terminate the pregnancy. Data were collected via multiple sources, including in-person interviews, surveys completed by participants, and medical record review. Participants completed three study visits with trained research personnel, with Visit 1 occurring between 6 weeks 0 days and 13 weeks 6 days of gestation. At least 30 days after delivery, trained and certified chart abstractors reviewed the medical records of all participants and recorded final birth outcomes.16 Full details of the study protocol previously have been published.16
This analysis specifically addresses periconceptional dietary quality as the exposure of interest. At Visit 1, women completed the modified Block 2005 Food Frequency Questionnaire, a semiquantitative assessment of usual dietary intake for the 3 months around conception. The Block questionnaire assesses 52 nutrients and 35 food groups from approximately 120 food and beverage items. The questionnaire includes serial adjustment items to estimate portion size, and the instrument has been validated in many populations. Details of the Block questionnaire have previously been reported by Bodnar et al.11
Answers to the Block questionnaire were scored using the Healthy Eating Index 2010 (HEI-2010), or the HEI.17,18 The HEI, which is a measure used to assess how well a set of foods aligns with key recommendations of the 2010 Dietary Guidelines for Americans, evaluates 12 key aspects of dietary quality, including adequacy of intake of specific food groups and moderation of intake of less nutritious foods. Higher scores represent better adherence to national guidelines, and an ideal score of 100 indicates that the reported food intake is consistent with the Dietary Guidelines recommendations.17 The mean HEI-2010 score for adult Americans in 2007–2008 was 54.3 out of 100, which indicated that the average diet of adult Americans did not align with dietary recommendations.17 This analysis is restricted to women with available HEI data.
We a priori selected 5 maternal and 5 neonatal outcomes of interest, each of which was chosen based on the plausible relationship of these outcomes with periconceptional food quality.4,15,19–22 Maternal outcomes included gestational diabetes mellitus (GDM), major perineal laceration (defined as 3rd or 4th degree perineal laceration), cesarean delivery, postpartum hemorrhage requiring a blood transfusion, and hypertensive disorder of pregnancy. GDM diagnosis was based on clinical record review using each site’s local protocol for diagnosis. Postpartum hemorrhage was restricted to women who required a transfusion in order to assess associations with the most severe version of this outcome. Hypertensive disorder of pregnancy included antepartum gestational hypertension, or antepartum, intrapartum, or postpartum (up to 14 days) preeclampsia, eclampsia, or superimposed preeclampsia, as defined by the American College of Obstetricians and Gynecologists (ACOG).23 Neonatal outcomes of interest included preterm birth (<37 weeks of gestation), admission to the neonatal intensive care unit (NICU), small-for-gestational age infant (defined as <10%ile by Alexander criteria24), low birthweight (defined as <2500g), and macrosomia (defined as >4000g).
Multiple maternal demographic and clinical characteristics were assessed as potentially confounding factors. Demographic factors included maternal age, insurance status (public versus non-public), marital status, household income (<200% or ≥200% of the poverty line), educational attainment (some college or greater versus no college), and self-reported race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, and other). Clinical factors included body mass index (BMI, kg/m2 at visit 1, tobacco use currently or before pregnancy, chronic hypertension (regardless of medication status), pregestational diabetes mellitus, and any mental health disorder.
We examined differences between maternal baseline demographic and clinical characteristics by HEI quartile using chi-squared and ANOVA tests, as appropriate. We then assessed differences between maternal and neonatal outcomes by HEI quartile using chi-squared tests. HEI scores were analyzed by quartile because such groupings best reflect clinically relevant categories of dietary quality and are most consistent with existing literature. Analyses for the outcome of GDM excluded women with pregestational diabetes mellitus. Using multivariable Poisson regression models, adjusted relative risks were constructed to estimate the independent associations of HEI quartile with each outcome, with HEI quartile 4 (highest level of food quality) as the referent, and each HEI quartile individually compared to the referent. The multivariable model included potentially confounding variables that were associated with HEI quartile on bivariable models with a p-value of <0.05. Although markers of socioeconomic status differed by HEI quartile, these factors were (a priori) not used in multivariable models because of likely collider bias related to the potential causal relationship between socioeconomic factors, periconceptional dietary quality, and maternal and neonatal outcomes. Thus, final models did not include race/ethnicity, insurance status, marital status, and educational attainment. However, in order to confirm the primary findings, we performed a sensitivity analysis in which race/ethnicity, insurance status, and marital status were included in the multivariable Poisson models.
All analyses were carried out in STATA release 15.0 (StataCorp, College Station, TX). All statistical tests were two-tailed and considered significant at the p < 0.05 level. Each site’s local governing institutional review board approved the study and all women provided written informed consent prior to participation.
RESULTS
The nuMoM2b cohort included 10,038 women, of whom 82% (N=8259) were eligible for inclusion in this analysis. The mean HEI score was 63 with a standard deviation of 13 (Figure 1). Women in the lowest quartile had scores less than 53.7, whereas quartile 2 included 53.8 to 63.7, quartile 3 included 63.8 to 72.7, and quartile 4 included women with scores 72.8 and greater. Women in the lowest HEI quartile, representing poorest dietary quality, were younger, and more likely to be non-Hispanic black or Hispanic, have public insurance, use tobacco, and have a lower household income (Table 1). They were less likely to be married and have at least some college education. Women in the lowest HEI quartile additionally had a higher mean pre-pregnancy BMI and were more likely to have comorbidities, including chronic hypertension, pregestational diabetes, and mental health disorders.
Figure 1:
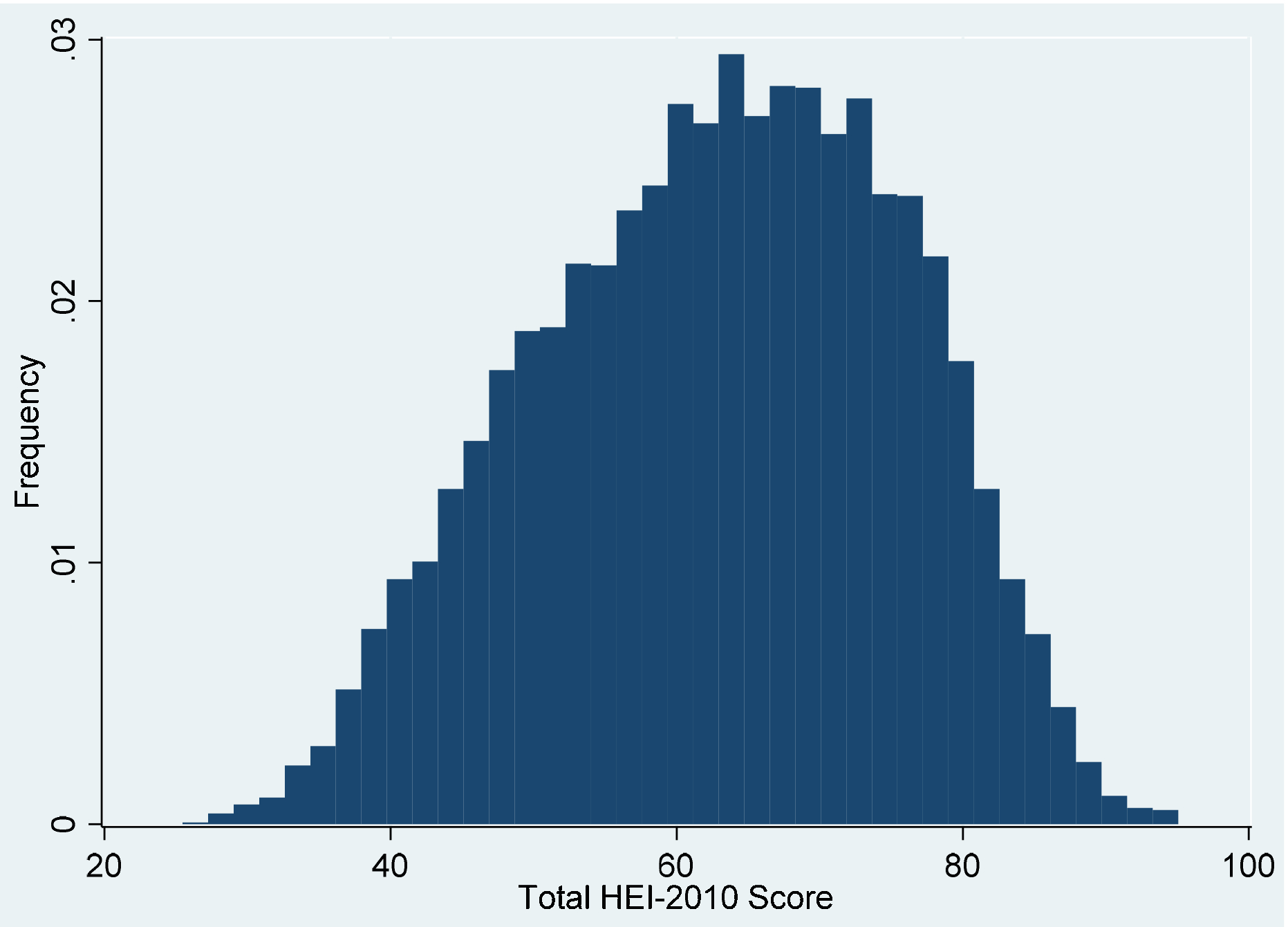
Healthy Eating Index-2010 Score Distribution
Table 1:
Demographic and clinical characteristics associated with Healthy Eating Index quartile
| HEI quartile 1 (N=2065) | HEI quartile 2 (N=2065) | HEI quartile 3 (N=2065) | HEI quartile 4 (N=2064) | P value* | |
|---|---|---|---|---|---|
| Maternal age, years | 23.9 (±5.2) | 26.6 (±5.5) | 28.7 (±5.1) | 29.9 (±4.5) | <0.001 |
| Race/ethnicity | <0.001 | ||||
| Non-Hispanic white | 987 (47.8) | 1198 (58.1) | 1472 (71.3) | 1536 (74.4) | |
| Non-Hispanic black | 496 (24.0) | 277 (13.4) | 113 (5.5) | 58 (2.8) | |
| Hispanic | 420 (20.3) | 421 (20.4) | 287 (13.9) | 246 (11.9) | |
| Asian | 31 (1.5) | 68 (3.3) | 107 (5.2) | 142 (6.9) | |
| Other | 131 (6.3) | 99 (4.8) | 85 (4.1) | 82 (4.0) | |
| Public insurance | 1037 (50.7) | 604 (29.5) | 313 (15.2) | 174 (8.4) | <0.001 |
| Household income <200% poverty line | 782 (55.7) | 567 (33.8) | 341 (18.5) | 241 (12.4) | <0.001 |
| Married | 630 (30.5) | 1201 (58.2) | 1571 (76.2) | 1795 (87.0) | <0.001 |
| Some college education or greater | 1581 (82.0) | 1532 (90.7) | 1384 (96.5) | 1182 (98.8) | <0.001 |
| Body mass index, kg/m2 | 27.1 (±7.3) | 26.9 (±6.6) | 25.9 (±5.6) | 24.9 (±4.9) | <0.001 |
| Ever used tobacco | 1047 (50.7) | 864 (41.9) | 788 (38.3) | 756 (36.6) | <0.001 |
| Chronic hypertension | 64 (3.3) | 60 (3.0) | 43 (2.2) | 24 (1.2) | <0.001 |
| Pregestational diabetes mellitus | 39 (2.0) | 33 (1.7) | 29 (1.5) | 16 (0.8) | 0.018 |
| Mental health disorder | 433 (22.0) | 356 (17.9) | 339 (17.0) | 289 (14.6) | <0.001 |
Data displayed as N (%) or mean (±standard deviation).
HEI, Healthy Eating Index; quartile 4 represents the best quality of periconceptional diet
P-value for chi-squared or ANOVA test.
Women in the lowest HEI quartile (quartile 1) experienced a greater frequency of postpartum hemorrhage requiring transfusion (p=0.02) and hypertensive disorder of pregnancy (p<0.001), but a significantly lower frequency of major perineal laceration (p<0.001) (Table 2). There were no differences in frequency of GDM or cesarean delivery by HEI quartile on bivariable analyses. These findings largely persisted on multivariable analyses (Table 3). For postpartum hemorrhage requiring transfusion and hypertensive disorders, women in quartile 1 had greater relative risk of both outcomes (hemorrhage: aRR 3.33, 95% CI 1.47–7.52; hypertension: aRR 1.16, 95% CI 1.02–1.31) compared to women in quartile 4. Women in HEI quartile 1 also had lower relative risk of major perineal laceration (aRR 0.68, 95% CI 0.47–0.98) compared to women in quartile 4. The adjusted relative risk of cesarean delivery was greater for women with HEI quartile 1 (aRR 1.20, 95% CI 1.07–1.34) and quartile 2 (aRR 1.11, 95% CI 1.00–1.23) than women in quartile 4. Women in quartile 3 of HEI did not differ from quartile 4 with respect to any outcome, and risk of GDM was unassociated with HEI quartile.
Table 2:
Maternal outcomes by Healthy Eating Index quartile
| HEI quartile 1 (N=2065) | HEI quartile 2 (N=2065) | HEI quartile 3 (N=2065) | HEI quartile 4 (N=2064) | P value* | |
|---|---|---|---|---|---|
| Gestational diabetes mellitus | 89 (4.6) | 92 (4.7) | 84 (4.3) | 80 (4.1) | 0.758 |
| Cesarean delivery | 536 (27.2) | 559 (28.1) | 559 (28.1) | 521 (26.3) | 0.539 |
| Major perineal laceration | 47 (4.7) | 83 (7.5) | 102 (8.6) | 113 (9.3) | <0.001 |
| Postpartum hemorrhage requiring transfusion | 28 (1.4) | 18 (0.9) | 15 (0.7) | 10 (0.5) | 0.02 |
| Hypertensive disorder of pregnancy | 510 (25.9) | 481 (24.1) | 445 (22.4) | 401 (20.3) | <0.001 |
Data displayed as N (%)
HEI, Healthy Eating Index; quartile 4 represents the best quality of periconceptional diet
P-value for chi-squared test.
Table 3:
Multivariable analysis of maternal outcomes by Healthy Eating Index quartile
| HEI Q1 aRR (95% CI) | HEI Q2 aRR (95% CI) | HEI Q3 aRR (95% CI) | HEI Q4 | |
|---|---|---|---|---|
| Gestational diabetes mellitus | 1.20 (0.86–1.65) | 1.11 (0.82–1.49) | 1.01 (0.75–1.36) | Ref |
| Cesarean delivery | 1.20 (1.07–1.34) | 1.11 (1.00–1.23) | 1.07 (0.96–1.18) | Ref |
| Major perineal laceration | 0.68 (0.47–0.98) | 0.97 (0.73–1.28) | 0.97 (0.75–1.26) | Ref |
| Postpartum hemorrhage requiring transfusion | 3.33 (1.47–7.52) | 2.07 (0.94–4.52) | 1.59 (0.71–3.58) | Ref |
| Hypertensive disorder of pregnancy | 1.16 (1.02–1.31) | 1.11 (0.98–1.25) | 1.05 (0.94–1.19) | Ref |
Data displayed as adjusted relative risk (95% confidence interval), estimated through a Poisson regression model.
HEI, Healthy Eating Index; quartile 4 represents the best quality of periconceptional diet and is the referent.
Adjusted for age, body mass index, tobacco use, chronic hypertension, pregestational diabetes mellitus, and mental health disorder.
Neonatal outcomes additionally differed by HEI quartile (Table 4). Women with lower HEI quartiles experienced greater frequency of preterm birth (p=0.014), NICU admission (p=0.009), small-for-gestational-age status (p<0.001), and low birthweight (p=0.002). Women with lower HEI quartiles also experienced lower frequency of macrosomia (p=0.025). On multivariable analyses, all relationships persisted for women in quartile 1 compared to quartile 4 (Table 5). Further, women in quartiles 1 and 2 had lower risk of macrosomia than women in quartile 4. The risk of NICU admission was elevated for women in all quartiles compared to quartile 4.
Table 4:
Neonatal outcomes by Healthy Eating Index quartile
| HEI quartile 1 (N=2065) | HEI quartile 2 (N=2065) | HEI quartile 3 (N=2065) | HEI quartile 4 (N=2064) | P value* | |
|---|---|---|---|---|---|
| Preterm birth (<37 weeks) | 197 (9.5) | 171 (8.3) | 155 (7.5) | 143 (6.9) | 0.014 |
| NICU admission | 350 (18.0) | 362 (18.3) | 345 (17.5) | 288 (14.6) | 0.009 |
| Small for gestational age (<10%ile) | 252 (12.8) | 218 (11.0) | 174 (8.8) | 187 (9.5) | <0.001 |
| Low birth weight <2500g | 158 (7.7) | 129 (6.2) | 105 (5.1) | 111 (5.4) | 0.002 |
| Macrosomia >4000g | 214 (10.4) | 226 (10.9) | 244 (11.8) | 273 (13.2) | 0.025 |
Data displayed as N (%).
HEI, Healthy Eating Index; quartile 4 represents the best quality of periconceptional diet. NICU, neonatal intensive care unit
P-value for chi-squared test.
Table 5:
Multivariable analysis of neonatal outcomes by Healthy Eating Index quartile
| HEI Q1 aRR (95% CI) | HEI Q2 aRR (95% CI) | HEI Q3 aRR (95% CI) | HEI Q4 | |
|---|---|---|---|---|
| Preterm (<37 weeks) | 1.27 (1.01–1.60) | 1.12 (0.90–1.40) | 1.02 (0.81–1.27) | Ref |
| NICU admission | 1.22 (1.04–1.42) | 1.23 (1.06–1.42) | 1.19 (1.03–1.38) | Ref |
| Small for gestational age (<10%ile) | 1.24 (1.02–1.51) | 1.11 (0.92–1.34) | 0.91 (0.4–1.11) | Ref |
| Low birth weight <2500g | 1.32 (1.02–1.71) | 1.10 (0.85–1.42) | 0.89 (0.68–1.16) | Ref |
| Macrosomia >4000g | 0.60 (0.47–0.76) | 0.78 (0.63–0.96) | 0.85 (0.70–1.03) | Ref |
Data displayed as adjusted relative risk (95% confidence interval), estimated through a Poisson regression model.
HEI, Healthy Eating Index; quartile 4 represents the best quality of periconceptional diet and is the referent. NICU, neonatal intensive care unit
Adjusted for age, body mass index, tobacco use, chronic hypertension, pregestational diabetes mellitus, and mental health disorder.
Results of the sensitivity analysis with inclusion of race/ethnicity, insurance status, and marital status in the multivariable models confirmed the primary analysis, in that the direction and magnitude of associations remained consistent. Specifically, all point estimates for the relative risks in the sensitivity analysis remained within 15% of the primary analysis with the exception of quartile 1 comparisons for small-for-gestational-age status and low birthweight, in which the risks both decreased by 17% (Table 6).
Table 6:
Sensitivity analyses including markers of socioeconomic status
| HEI Q1 aRR (95% CI) | HEI Q2 aRR (95% CI) | HEI Q3 aRR (95% CI) | HEI Q4 | |
|---|---|---|---|---|
| Maternal outcomes | ||||
| Gestational diabetes mellitus | 1.09 (0.78–1.53) | 1.07 (0.79–1.35) | 1.00 (0.74–1.35) | Ref |
| Cesarean delivery | 1.12 (1.00–1.25) | 1.07 (0.96–1.19) | 1.06 (0.96–1.17) | Ref |
| Major perineal laceration | 0.78 (0.53–1.14) | 1.02 (0.77–1.35) | 0.99 (0.77–1.28) | Ref |
| Postpartum hemorrhage requiring transfusion | 3.32 (1.48–7.44) | 1.98 (0.91–4.31) | 1.57 (0.70–3.52) | Ref |
| Hypertensive disorder of pregnancy | 1.13 (1.00–1.29) | 1.10 (0.98–1.24) | 1.05 (0.93–1.19) | Ref |
| Neonatal outcomes | ||||
| Preterm (<37 weeks) | 1.11 (0.88–1.42) | 1.07 (0.8501.33) | 0.99 (0.79–1.23) | Ref |
| NICU admission | 1.18 (1.00–1.39) | 1.21 (1.04–1.41) | 1.18 (1.02–1.37) | Ref |
| Small for gestational age (<10%ile) | 1.03 (0.83–1.27) | 1.01 (0.83–1.23) | 0.88 (0.72–1.07) | Ref |
| Low birth weight <2500g | 1.09 (0.83–1.44) | 1.01 (0.78–1.31) | 0.86 (0.66–1.13) | Ref |
| Macrosomia >4000g | 0.63 (0.49–0.81) | 0.81 (0.65–0.99) | 0.85 (0.70–1.04) | Ref |
Data displayed as adjusted relative risks (95% confidence interval), estimated through a Poisson regression model.
HEI, Healthy Eating Index; quartile 4 represents the best quality of periconceptional diet and is the referent. NICU, neonatal intensive care unit
Adjusted for age, body mass index, tobacco use, chronic hypertension, pregestational diabetes mellitus, mental health disorder, race/ethnicity, insurance status, and marital status.
COMMENT
Principal findings
Periconceptional dietary quality is associated with differences in demographic characteristics among US pregnant women, but previous work had not addressed associations of dietary quality with obstetric and perinatal outcomes. We identified that poor periconceptional dietary quality is associated with multiple adverse maternal and neonatal outcomes, including postpartum hemorrhage, hypertensive disorders of pregnancy, cesarean delivery, preterm birth, NICU admission, small-forgestational-age status, and low birthweight, even when accounting for comorbidities and BMI. In contrast, women with poor dietary quality experienced lower risk of macrosomia. There is a dose-response effect, such that women with the lowest dietary quality had the strongest associations with adverse outcomes, whereas outcomes for women in the third quartile of dietary quality were similar to those of women in the highest quartile.
Results in Context
There are several postulated mechanisms that may underlie these findings. First, poor periconceptional dietary quality may lead to micronutrient deficiency, potentially interfering with clotting factors that allow normal recovery in the context of obstetrical hemorrhage or other factors that alter risk of placentally-mediated diseases. This hypothesis has been explored in small studies where obese women had lower amounts of micronutrients despite energy-rich diets.25 Second, greater intake of low-quality foods has been previously associated with excessive weight gain.26 Thus, periconceptional dietary quality may affect outcomes via its influence on gestational weight gain.27 For example, in an Italian cohort, women with “prudent” dietary patterns before pregnancy had improved gestational weight gain outcomes than women with worse dietary quality.28 Third, food insecurity, or sufficient access by all people at all times to enough food to lead an active, healthy life, may also play an important role.29 It is plausible that women in the lowest quartiles of periconceptional dietary quality experienced poor quality due to food insecurity.
Although the landscape of racial, ethnic, and socioeconomic inequities in the US differ from those of Western European countries, some of our findings mirror theirs. For example, in a Spanish cohort of 787 women, early pregnancy HEI scores in the lowest quartile were associated with greater odds of fetal growth restriction; the effect was most pronounced for the first versus fourth quartiles.15 Work from the Norwegian Mother and Child Cohort Study found that better quality mid-pregnancy diet was associated with more optimal fetal growth outcomes and lower odds of preeclampsia, preterm birth, and postpartum weight retention.19,20,22
Clinical and research implications
These data suggest that health care providers who care for pregnant and preconception women should include a basic assessment of dietary quality as a component of counseling about lifestyle factors that may promote maternal and fetal health. Ample evidence suggests pregnancy is an opportunity for improvement of healthy behaviors, that nutrition and lifestyle modification advice are well received by women who seek preconception care, and that some interventions in this period may have long-lasting maternal and child health benefits.12,30 ACOG addresses the importance of discussing diet in the context of caring for women who are overweight or obese and additionally includes food access as one of several social determinants of health to be screened.31,32 We propose that further attention to dietary quality in the obstetric context may be worthwhile for clinical practice and future research.
There are several potential areas for future investigation. This analysis only addresses total HEI scores as a reflection of adherence to national nutrition guidelines. Future work can also assess specific dietary sources of nutrients, dietary sources of energy, components of the HEI, and the role of nutrient supplementation. Additional methods of examining diet may include measures of food group diversity, which has been shown to reflect micronutrient intake in a study of pregnant women.33 Future work also should investigate food security and the mechanisms between inequity and food quality. Future investigations may also address whether interventions that improve dietary quality during pregnancy are associated with improvements in perinatal outcomes. Finally, we must also understand the dietary quality issues unique to women with comorbidities such as diabetes.
Importantly, race and ethnicity are socially mediated concepts that have previously been associated with food quality. For this reason, we opted to not adjust for race and markers of socioeconomic status in the primary analysis, due to the possibility of collider bias and the obscuring of the potential effects of periconceptional food quality on outcomes. Moreover, results of the sensitivity analysis supported the main analysis; in some cases the confidence intervals crossed unity, but given the overall consistency of the adjusted relative risk point estimates, this appears to be largely a result of reduced degrees of freedom once more variables are added into the regression. The etiologies of race and socioeconomic status as drivers of adverse perinatal outcomes have not fully been elucidated, but we theorize that suboptimal periconceptional and pregnancy food quality may be one mechanism. Future work on dietary quality needs to address disparities by race, ethnicity, education, and socioeconomic status in more depth in attempt to understand their role in contributing to differences in adverse outcomes.
Strengths and limitations
A major strength of this study is the use of a large and diverse sample of US women that is representative of the population at large. Moreover, the nuMoM2b cohort is extraordinarily well characterized and includes detailed assessments that enhance the granularity and quality of data, in contrast to data from vital statistics databases. The direct questioning of food quality via the HEI only a short amount of time after the period of interest also enhances the quality and fidelity of dietary recall, in contrast to investigations that use more generalized assessments, less standardized measurement approaches, or require longer periods of recall.
However, there are several limitations to consider. This is an observational analysis, as are most studies of dietary quality, and as such, findings can be affected by unmeasured confounding. Second, although much data suggest pregnancy diet is likely to be very similar to periconceptional diet, the association may be imprecise. Third, all estimates of typical dietary intake have inherent imperfections due to misreporting or recall bias, although self-reported dietary data have sufficient fidelity to inform policy and dietary guidelines.11 Finally, nuMoM2b participants were interested in a longitudinal research investigation that began in early pregnancy and were recruited from a large academically-affiliated medical centers, and thus findings may not be fully generalizable.
Conclusions
In summary, US women have very poor dietary quality prior to pregnancy. Dietary quality remains an important public health issue in the US and internationally, and is a major contributor to morbidity and overall population health.3 Additionally, dietary inequities are pervasive and may have an impact on perinatal health, which is an important area for ongoing study. These data demonstrate that periconceptional dietary quality may be associated with adverse maternal and child health outcomes, which can have both short- and long-term implications for the health of the family, including potential intergenerational or epigenetic effects. These findings emphasize the critical nature of preconception care, food-focused public health policies, and systems-level changes that promote healthy food choices, particularly during important windows of opportunity such as pregnancy.
AJOG AT A GLANCE:
Why was the study conducted? Although disparities in periconceptional dietary quality exist, it is unknown whether individual periconceptional diet quality is associated with obstetric outcomes.
What are the key findings? Poor periconceptional dietary quality is associated with greater relative risk of cesarean delivery, hypertensive disorders, postpartum hemorrhage, NICU admission, preterm birth, and low birthweight, whereas it is associated with lower risk of major perineal laceration and macrosomia.
What does the study add to what we already know? Poor periconceptional dietary quality is associated with adverse perinatal outcomes even after controlling for body mass index and potential comorbidities.
FUNDING:
LMY was supported by the NICHD K12 HD050121-11 at the time the study was performed. Support for the NuMoM2b study was provided by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10 HD063036, RTI International; U10 HD063072, Case Western Reserve University; U10 HD063047, Columbia University; U10 HD063037, Indiana University; U10HD063041, University of Pittsburgh; U10 HD063020, Northwestern University; U10 HD063046, University of California Irvine; U10 HD063048, University of Pennsylvania; and U10 HD063053, University of Utah. In addition, support was provided by respective Clinical and Translational Science Institutes to Indiana University (UL1TR001108) and University of California Irvine (UL1TR000153).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors report no conflicts of interest.
PRESENTATION: Presented in oral format at the 2019 39th Annual Meeting of the Society for Maternal-Fetal Medicine (February 11–16, 2019).
REFERENCES
- 1.Wang D, Leung C, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med 2014;174:1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehm C, Penalvo J, Afshin A, Mozaffarian D. Dietary Intake Among US Adults, 1999–2012. JAMA 2016;315:2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Li Y, Afshin A, et al. Global Improvement in Dietary Quality Could Lead to Substantial Reduction in Premature Death. J Nutr 2019;149:1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orr C, Keyserling T, Ammerman A, Berkowitz S. Diet quality trends among adults with diabetes by socioeconomic status in the U.S.: 1999–2014. BMC Endocr Disord 2019;19:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Zhang F, Liu J, Rehm C, Wilde P, Mande J, Mozaffarian D. Trends and Disparities in Diet Quality Among US Adults by Supplemental Nutrition Assistance Program Participation Status. JAMA Netw Open 2018;1:e180237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inskip H, Crozier S, Godfrey K, et al. Women’s compliance with nutrition and lifestyle recommendations before pregnancy: general population cohort study. BMJ 2009;338:b481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramage S, McCargar L, Berglund C, Harber V, Bell R, Team ftAS. Assessment of Pre-Pregnancy Dietary Intake with a Food Frequency Questionnaire in Alberta Women. Nutrients 2015;7:6155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson J, Heslehurst N, Hall J, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018;391:1830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundqvist A, Johansson I, Wennberg A, et al. Reported dietary intake in early pregnant compared to non-pregnant women - a cross-sectional study. BMC Pregnancy Childbirth 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crozier S, Robinson S, Godfrey K, Cooper C, Inskip H. Women’s dietary patterns change little from before to during pregnancy. J Nutri 2009;139:1956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodnar L, Simhan H, Parker C, et al. Racial or ethnic and socioeconomic inequalities in adherence to National Dietary Guidance in a large cohort of US pregnant women. J Acad Nutri Diet 2017;117:867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baird J, Jacob C, Barker M, et al. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare (Basel) 2017;5:pii: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver M, Jaquiery A, Bloomfield F, Harding J. The effects of maternal nutrition around the time of conception on the health of the offspring. Soc Reprod Fertil Steril 2007;64:397–410. [DOI] [PubMed] [Google Scholar]
- 14.Gaskins A, Nassan F, Chiu Y, et al. Dietary patterns and outcomes of assisted reproduction. Am J Obstet Gynecol 2019;220:567.e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Bernal C, Rebagliato M, Iniguez C, et al. Diet quality in early pregnancy and its effects on fetal growth outcomes: the Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr 2010;91:1659–66. [DOI] [PubMed] [Google Scholar]
- 16.Haas D, Parker C, Wing D, et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b). American Journal of Obstetrics and Gynecology 2015;212:539.e1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healthy Eating Index (HEI). 2019. (Accessed Accessed September 30, 2019, at https://www.fns.usda.gov/resource/healthy-eating-index-hei.)
- 18.Guenther P, Kirkpatrick S, Reedy J, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 2014;144:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torjusen H, Brantsaeter A, Haugen M, et al. Reduced risk of pre-eclampsia with organic vegetable consumption: results from the prospective Norwegian Mother and Child Cohort Study. BMJ Open 2014;4:e006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englund-Ogge L, Brantsaeter A, Sengpiel V, et al. Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ 2014;348:g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal S, Fledderjohann J, Vellakkal S, Stuckler D. Adequately diversified dietary intake and iron and folic acid supplementation during pregnancy is associated with reduced occurrence of symptoms suggestive of pre-eclampsia or eclampsia in Indian women. PLOS One 2015;10:e0119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Ruesten A, Brantsaeter A, Haugen M, et al. Adherence of pregnant women to Nordic dietary guidelines in relation to postpartum weight retention: results from the Norwegian Mother and Child Cohort Study. BMC Public Health 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 24.Alexander G, Himes J, Kaufman R, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–8. [DOI] [PubMed] [Google Scholar]
- 25.Mohd-Shukri N, Duncan A, Denison F, et al. Health Behaviours during Pregnancy in Women with Very Severe Obesity. Nutrients 2015;7:8431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boggs D, Rosenberg L, Rodriguez-Bernal C, Palmer J. Long-term diet quality is associated with lower obesity risk in young African American women with normal BMI at baseline. J Nutr 2013;143:1636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uusitalo U, Arkkola T, Ovaskainen M, et al. Unhealthy dietary patterns are associated with weight gain during pregnancy among Finnish women. Public Health Nutr 2009;12:2392–9. [DOI] [PubMed] [Google Scholar]
- 28.Maugeri A, Barchitta M, Favara G, et al. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino” Cohort. Nutrients 2019;11:1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman-Jensen A, Rabbitt M, Gregory C, Singh A, for the United States Department of Agriculture. Household food security in the United States in 2018 Economic Research Report Number 270. US Department of Agriculture Economic Research Service; 2019. [Google Scholar]
- 30.Stephenson J, Patel D, Barrett G, et al. How do women prepare for pregnancy? Preconception experiences of women attending antenatal services and views of health professionals. PLOS One 2014;9:e103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American College of Obstetricans and Gynecologists. Challenges for Overweight and Obese Women, Committee Opinion No 591. Obstet Gynecol 2014;123:726–30. [DOI] [PubMed] [Google Scholar]
- 32.American College of Obstetricans and Gynecologists. Importance of social determinants of health and cultural awareness in the delivery of reproductive health care, Committee Opinion No. 729. Obstet Gynecol 2018;131. [DOI] [PubMed] [Google Scholar]
- 33.Komatowski B, Comstock S. Dietary diversity is inversely correlated with pre-pregnancy body mass index among women in a Michigan pregnancy cohort. PeerJ 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]


