Abstract
Objective
To estimate the prevalence of microcephaly and central nervous system (CNS) defects during the Zika virus (ZIKV) epidemic in Colombia and proportion attributable to congenital ZIKV infection.
Study design
Clinical and laboratory data for cases of microcephaly and/or CNS defects reported to national surveillance between 2015 and 2017 were reviewed and classified by a panel of clinical subject matter experts. Maternal and fetal/infant biologic specimens were tested for congenital infection and chromosomal abnormalities. Infants/fetuses with microcephaly and/or CNS defects (cases) were classified into broad etiologic categories (teratogenic, genetic, multifactorial, and unknown). Cases classified as potentially attributable to congenital ZIKV infection were stratified by strength of evidence for ZIKV etiology (strong, moderate, or limited) using a novel strategy considering birth defects unique or specific to ZIKV or other infections and laboratory evidence.
Results
Among 858 reported cases with sufficient information supporting a diagnosis of microcephaly or CNS defects, 503 were classified as potentially attributable to congenital ZIKV infection. Of these, the strength of evidence was considered strong in 124 (24.7%) cases; moderate in 232 (46.1%) cases; and limited in 147 (29.2%). Of the remaining, 355 (41.4%) were attributed to etiologies other than ZIKV infection (syphilis, toxoplasmosis, rubella, cytomegalovirus, herpes 1 and herpes 2 viruses only, n = 32 [3.7%]; genetic, n = 16 [1.9%]; multifactorial, n = 42 [4.9%]; unknown, n = 265 [30.9%]).
Conclusions
Fifty-eight percent of cases of microcephaly and/or CNS defects were potentially attributable to congenital ZIKV infection; however, the strength of evidence varied considerably. This surveillance protocol might serve as a model approach for investigation and etiologic classification of complex congenital conditions.
In 2015, the Colombian National Institute of Health (INS) began Zika virus (ZIKV) surveillance, including protocols for testing and management of pregnant women and infants with suspected ZIKV infection.1,2 In 2016, the World Health Organization declared a Public Health Emergency of International Concern prioritizing global efforts to prevent ZIKV transmission and to perform surveillance for ZIKV transmission and for adverse outcomes related to congenital ZIKV infection, including microcephaly and birth defects of the central nervous system (CNS).3–6 Building on its existing birth defects surveillance infrastructure, the INS established an enhanced surveillance protocol focused on microcephaly and CNS defects to monitor potential increases in birth prevalence; identify possible causes, including congenital ZIKV infection; determine proportion of reported cases potentially attributable to ZIKV; and direct appropriate public health intervention.
The first confirmed case of ZIKV-related congenital microcephaly in Colombia was reported in April 2016.7 A preliminary report of surveillance data indicated a significant, almost 4-fold increase in reported cases of microcephaly in Colombia in 2016, compared with the previous year.8 However, the report did not span the entire ZIKV epidemic, excluded other CNS defects, and did not describe the etiologic classification of reported cases.
This report describes the epidemiology and etiologic classification of cases of microcephaly and CNS defects nationwide in Colombia among pregnancies completed between September 1, 2015 and April 30, 2017. In addition, the proportion of cases potentially attributable to congenital ZIKV infection and the strength of the evidence linking cases to ZIKV infection are described in this report.
Methods
INS maintains national public health surveillance for notifiable conditions, including birth defects such as microcephaly and CNS defects. Information that is collected by healthcare centers is compiled and transmitted to the national public health surveillance system, which aggregates and publishes the results. The typical reporting time by healthcare centers to preliminary national reporting is approximately 1.5 weeks after detection.9
National public health surveillance was expanded in 2015 to include monitoring for symptomatic ZIKV infection and enhancement of existing microcephaly and CNS defect surveillance.9–11 Findings of microcephaly and/or CNS defects in a fetus, pregnancy loss, or infant up to 1 year of age (potential cases) were reported voluntarily by health care centers to INS. Clinical data (including maternal history of ZIKV symptoms, infant physical examination and birth measurements, postnatal neuroimaging, ophthalmologic examination, hearing evaluation, and other clinical care) were abstracted from prenatal, delivery, birth hospitalization, and pediatric medical records. Biologic specimens indicating maternal infection (ie, maternal serum, amniotic fluid, placenta, umbilical cord tissue, umbilical cord blood) and fetal/infant biologic specimens (ie, infant serum, urine, cerebrospinal fluid [CSF], fetal/infant tissue) were collected when possible per surveillance protocols and sent to the INS laboratory in Bogotá for testing.1,10 This activity was deemed public health practice (nonresearch) by the Centers for Disease Control and Prevention (CDC) and the INS.
Laboratory Methods
Laboratory procedures for detecting possible ZIKV infection evolved during the epidemic as assays became available. Molecular detection of ZIKV RNA was performed using the singleplex or Trioplex real-time reverse transcription polymerase chain reaction (PCR) assay.12,13 Serologic testing for ZIKV IgM antibodies was performed on infant serum and CSF using ZIKV Detect TM IgM 1.0 Capture enzyme-linked immunosorbent assay kit (Inbios International, Seattle, Washington) and/or ZIKV IgM antibody capture enzyme-linked immunosorbent assay (CDC, Atlanta, Georgia). An immunohistochemical assay for ZIKV was performed using a mouse polyclonal anti-ZIKV antibody and a polymer-based indirect colorimetric immunoalkaline phosphatase detection system with fast red chromogen (Thermo Fisher Scientific, Runcom, Cheshire, United Kingdom). Chromosomal analysis for aneuploidy was performed on amniotic fluid or infant blood specimens. Evaluations for syphilis, toxoplasmosis, rubella, cytomegalovirus, herpes 1 and herpes 2 viruses (STORCH) infections (treponemal and nontreponemal testing for detection of syphilis; PCR for detection of toxoplasmosis; IgM serology for detection of rubella, cytomegalovirus [CMV], herpes simplex virus type 1, and herpes simplex virus type 2) were performed on available fetal/infant biologic specimens.
Case Review and Etiologic Classification
Clinical data and laboratory results for each case were reviewed by a panel of INS and CDC clinical subject matter experts to confirm findings and assign cases into likely etiologic categories. The panel included reviewers with expertise in clinical genetics, obstetrics and gynecology, pediatrics, and epidemiology, in addition to experienced surveillance staff. Discrepant review findings were discussed among clinical subject matter experts to reach agreement. Based on published reports and expert opinion, a schema was developed to categorize confirmed birth defect findings into groups based on the likelihood of the defect occurring in cases of congenital ZIKV infection and/or STORCH infection. For cases determined potentially attributable to congenital ZIKV infection, a schema for the strength of evidence for ZIKV etiology was developed based on expert opinion. These classification schemas were developed for public health surveillance and were not intended for clinical use or for circumstances outside the context of the ZIKV epidemic.
Microcephaly was defined as head circumference less than the 3rd percentile for gestational age and sex by the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH21st) standards for measurements collected ≤2 weeks after birth or by World Health Organization standards for measurements collected >2 weeks after birth.14,15 Results from prenatal ultrasound, pathology examination (in cases of pregnancy loss), and from postnatal neuroimaging (in cases of live birth) were reviewed for the presence of CNS defects. Results of ophthalmologic examination were reviewed for the presence of eye defects. Potential cases without sufficient information (eg, missing for any: head circumference measurement; gestational age at delivery; infant sex; neuroimaging, pathology examination, and/or ophthalmologic examination) to support a diagnosis of microcephaly or CNS defects were excluded from etiologic classification.
Cases with sufficient evidence of microcephaly and/or CNS defects were assigned to broad etiologic categories: teratogenic (including congenital infection), genetic (chromosomal or recognized genetic phenotype), multifactorial (including neural tube defects), and unknown (including holoprosencephaly and cases of multiple congenital anomalies), all based on clinical and laboratory findings (Figure 1; available at www.jpeds.com). A similar schema has been used to classify birth defects by etiology; however, no one classification schema has been universally accepted.16,17 Cases with laboratory findings of a STORCH infection but clinical findings more consistent with congenital ZIKV infection were classified as potentially attributable to ZIKV infection.
Figure 1.
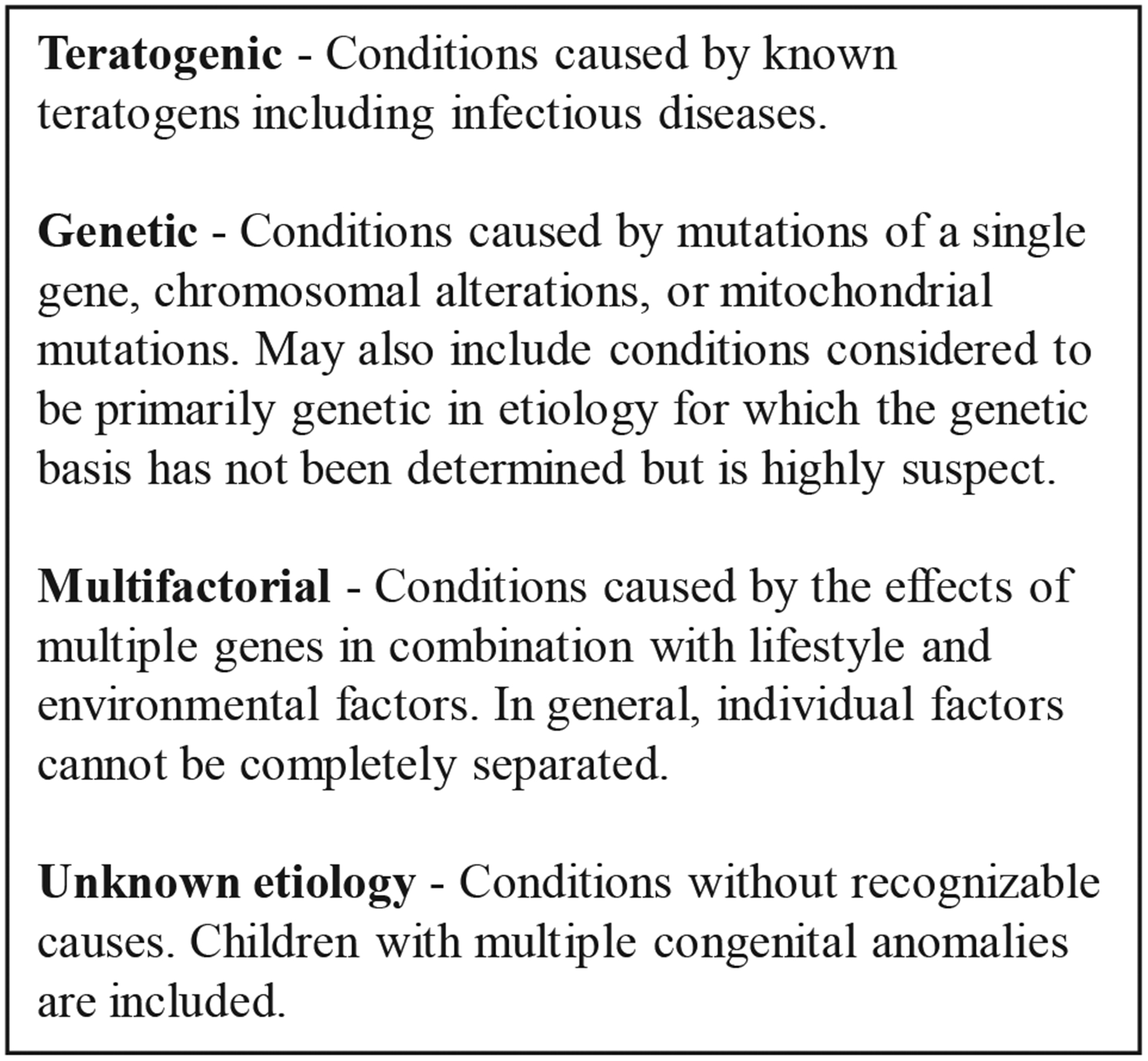
Etiologic categories for classification of cases of microcephaly and/or CNS defects.
ZIKV infection during pregnancy can cause microcephaly and CNS defects of the brain and eye.18 Congenital Zika syndrome is a recognizable pattern of structural anomalies and functional disabilities secondary to CNS injury.19 Findings unique to congenital ZIKV infection or rarely seen with other congenital infections include severe microcephaly with partially collapsed skull, thin cerebral cortices with subcortical calcifications, macular scarring and focal pigmentary retinal mottling, arthrogryposis, and marked early hypertonia with symptoms of extrapyramidal involvement.
Additional brain abnormalities among infants affected by ZIKV infection in pregnancy include periventricular, thalamic and basal ganglia calcifications; absent or hypoplastic corpus callosum; neuronal migration abnormalities such as polymicrogyria, schizencephaly, and gray-white matter heterotopia; and cerebellar hypoplasia and absent or hypoplastic cerebellar vermis.18–22 Eye abnormalities among affected infants also include microphthalmia, anophthalmia, congenital cataracts, glaucoma, and hypoplasia of the optic nerve. The spectrum of structural findings among infants with congenital ZIKV infection also includes those described among infants with other congenital STORCH infections and with rare single gene genetic disorders.19
Birth defect characteristics and laboratory results were used to distinguish birth defects likely due to ZIKV infection from those that might have been caused by STORCH infections. Birth defects were assigned to 1 of 3 groups based on the likelihood of the defect occurring in various congenital infection scenarios (Figure 2). Group A includes birth defects reported with congenital ZIKV infection but rarely reported in STORCH infections. Birth defects such as cortical/subcortical calcifications and arthrogryposis are highly characteristic of ZIKV infection. Group B includes birth defects commonly seen in, but are less specific to, congenital ZIKV infection and can occur in STORCH infections. Defects such as cortical atrophy and corpus callosum anomalies fall into this group. Group C includes birth defects such as microcephaly and hydrocephaly, which are nonspecific to and equally frequent in congenital ZIKV infection and STORCH infections and cannot be used to differentiate among infections.
Figure 2.
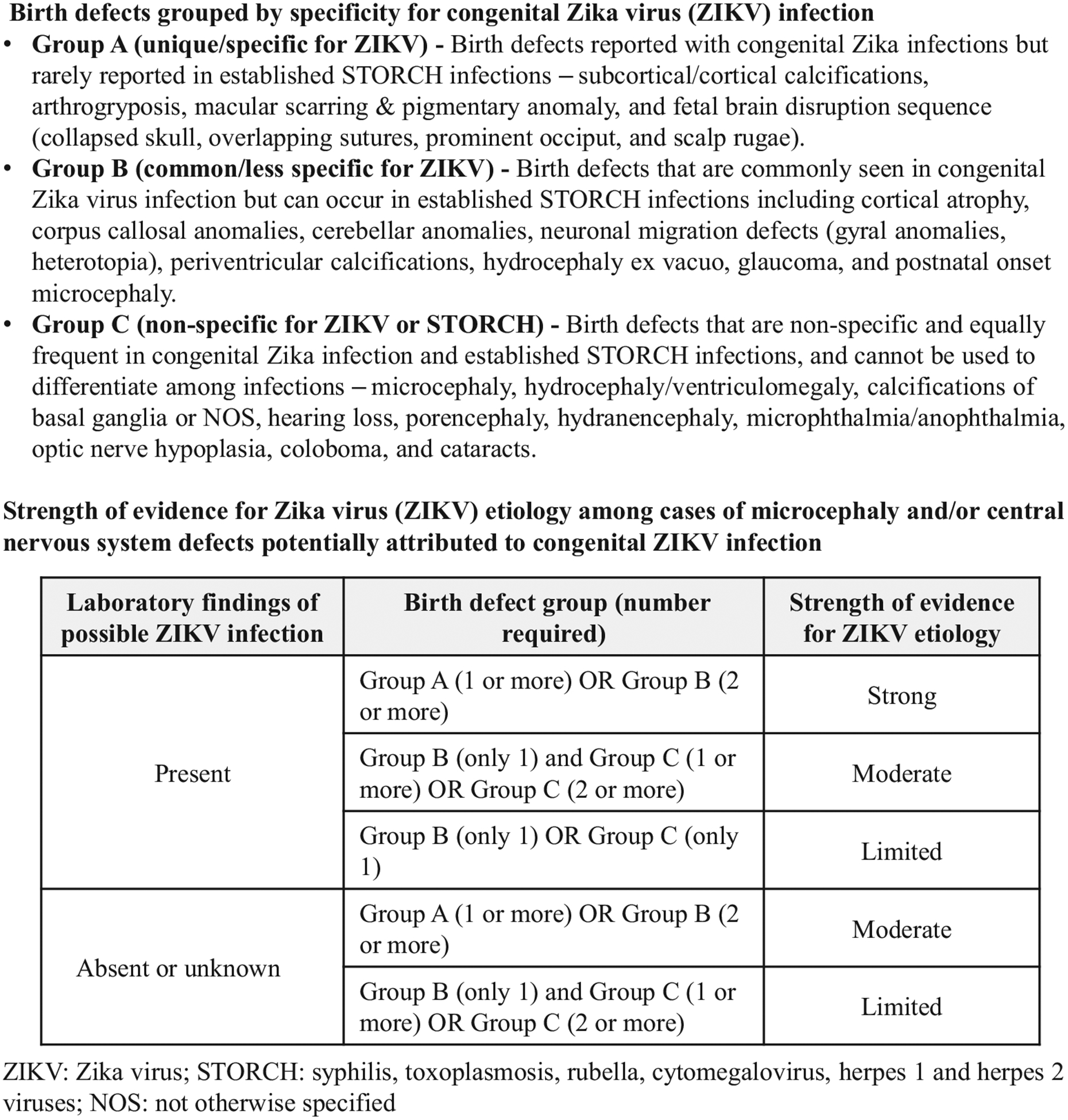
Birth defects grouped by specificity for congenital ZIKV infection and strength of evidence for potential ZIKV etiology.
Laboratory findings of possible maternal ZIKV infection include ZIKV RNA detected by PCR from any maternal serum, maternal urine, placenta, umbilical cord, and umbilical cord blood specimen, ZIKV IgM detected by serological testing of maternal serum, and ZIKV antigen detected by immunohistochemistry (IHC) of placenta. Laboratory findings of possible ZIKV infection in a fetus/infant include ZIKV RNA detected by PCR from any fetal/infant specimen including serum, urine, CSF, and fetal/infant tissues. ZIKV IgM detected by serological testing of infant serum or CSF, and ZIKV antigen detected by IHC testing of fetal/infant tissue.
Cases attributed to congenital ZIKV infection were sub-classified by strength of supporting evidence for ZIKV etiology (strong, moderate, or limited) determined by the birth defect group (group A, B, or C) and number of birth defects within each group, and laboratory findings of possible ZIKV infection in the fetus/infant or mother (Figure 2). Strong evidence of congenital ZIKV etiology was characterized by 1 or more group A birth defects or 2 or more group B birth defects with laboratory findings in the mother or fetus/infant of possible ZIKV infection. The same combination of group A and B birth defects with indeterminate or negative laboratory findings for ZIKV was considered moderate evidence of congenital ZIKV etiology, as were combinations of group B and C birth defects with laboratory findings of possible ZIKV infection. Other combinations of group B and C birth defects with and without laboratory findings of possible ZIKV infection were considered limited evidence for ZIKV etiology. Cases with laboratory findings of both congenital ZIKV infection and STORCH infection and accompanied by birth defects consistent with these infections were classified as potentially attributable to ZIKV-STORCH coinfection.
Analytic Methods
The cumulative incidence of cases of microcephaly and/or CNS defects was calculated per 1000 live births from September 1, 2015 to April 30, 2017, overall and by territorial department of residence (area of residence analogous to a US state). The proportion of cases of microcephaly and/or CNS defects potentially attributable to each etiologic category was calculated. Analyses were conducted using SAS v 9.4. (SAS Institute, Cary, North Carolina).
Results
A total of 1239 potential cases of microcephaly and/or CNS defects were reported to national surveillance. The remainder of these results describe findings among 858 cases with sufficient information to support a diagnosis of microcephaly and/or CNS defects (Figure 3; available at www.jpeds.com). The cumulative incidence of microcephaly and/or CNS defects from September 1, 2015 to April 30, 2017 for all of Colombia was 0.80 cases per 1000 live births (range: 0.28–7.86). Among 653 cases reported as alive at the time of data collection and with nonmissing data, 318 of 651 (48.8%) were female, 141 of 646 (21.8%) were born earlier than 37 weeks of gestation, and 135 of 437 (30.9%) weighed <10th percentile for age and sex by INTERGROWTH21st standards. Among 205 cases reported as deceased at the time of data collection (including pregnancy losses and death after birth) and with nonmissing data, 107 of 204 (53.2%) were female, 147 of 201 (73.1%) were born earlier than 37 weeks of gestation, and 26 of 94 (27.7%) weighed <10th percentile for age and sex by INTERGROWTH21st standards. By territorial department of residence (not necessarily place of infection), cases of microcephaly and CNS defects were reported nationwide, including areas at an elevation above 2200 meters where local ZIKV transmission because of mosquitos is not likely (Figure 4; available at www.jpeds.com).
Figure 3.
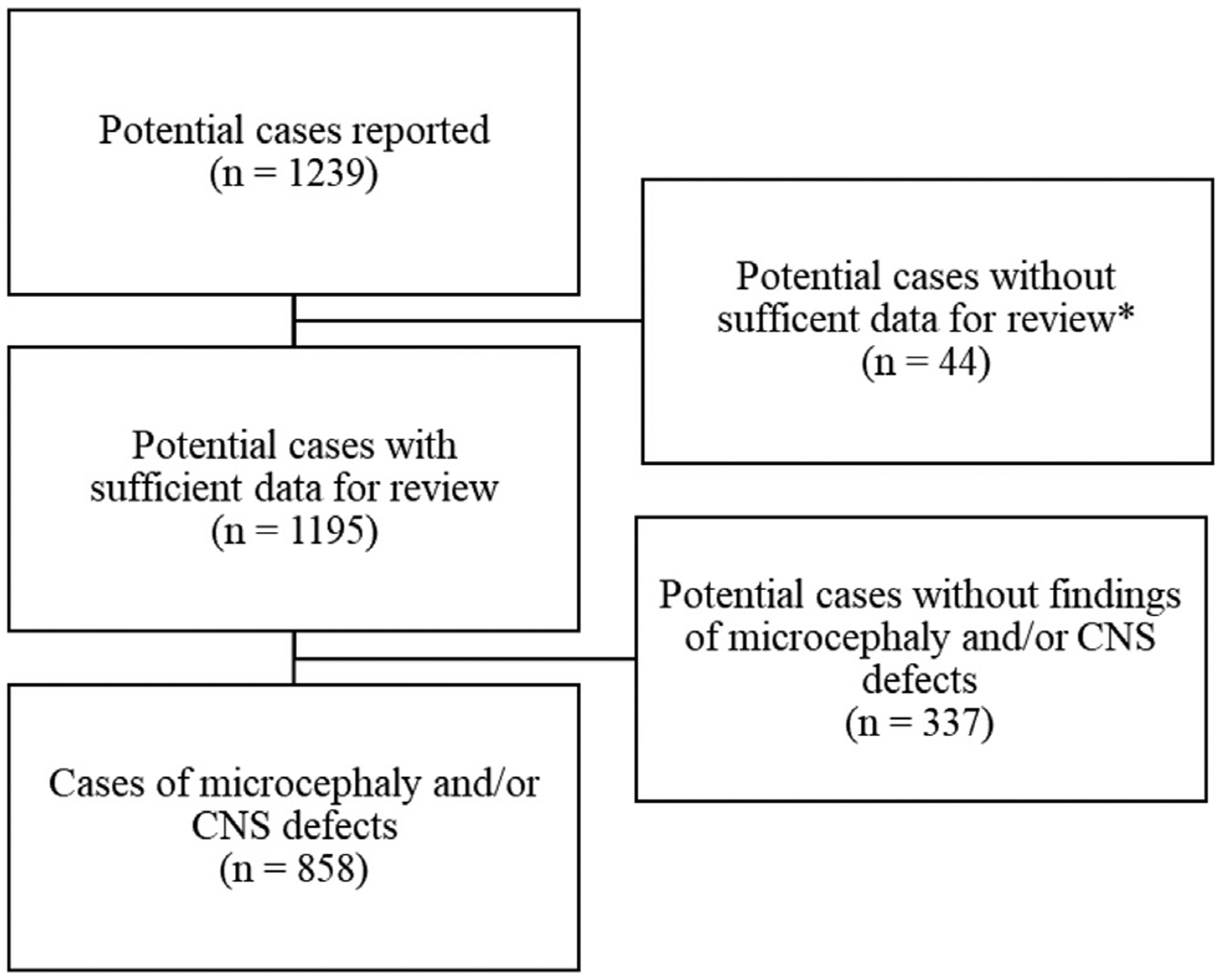
Potential cases of microcephaly and/or CNS defects, Colombia, September 2015-April 2017; *Among 44 potential cases, the following types of missing data precluded review for microcephaly and/or CNS defects: missing head circumference (n = 42); missing gestational age at delivery (n = 1); indeterminate or missing fetal/infant sex (n = 3); missing results of neuroimaging, pathology examination, and/or ophthalmologic evaluation (n = 14).
Figure 4.
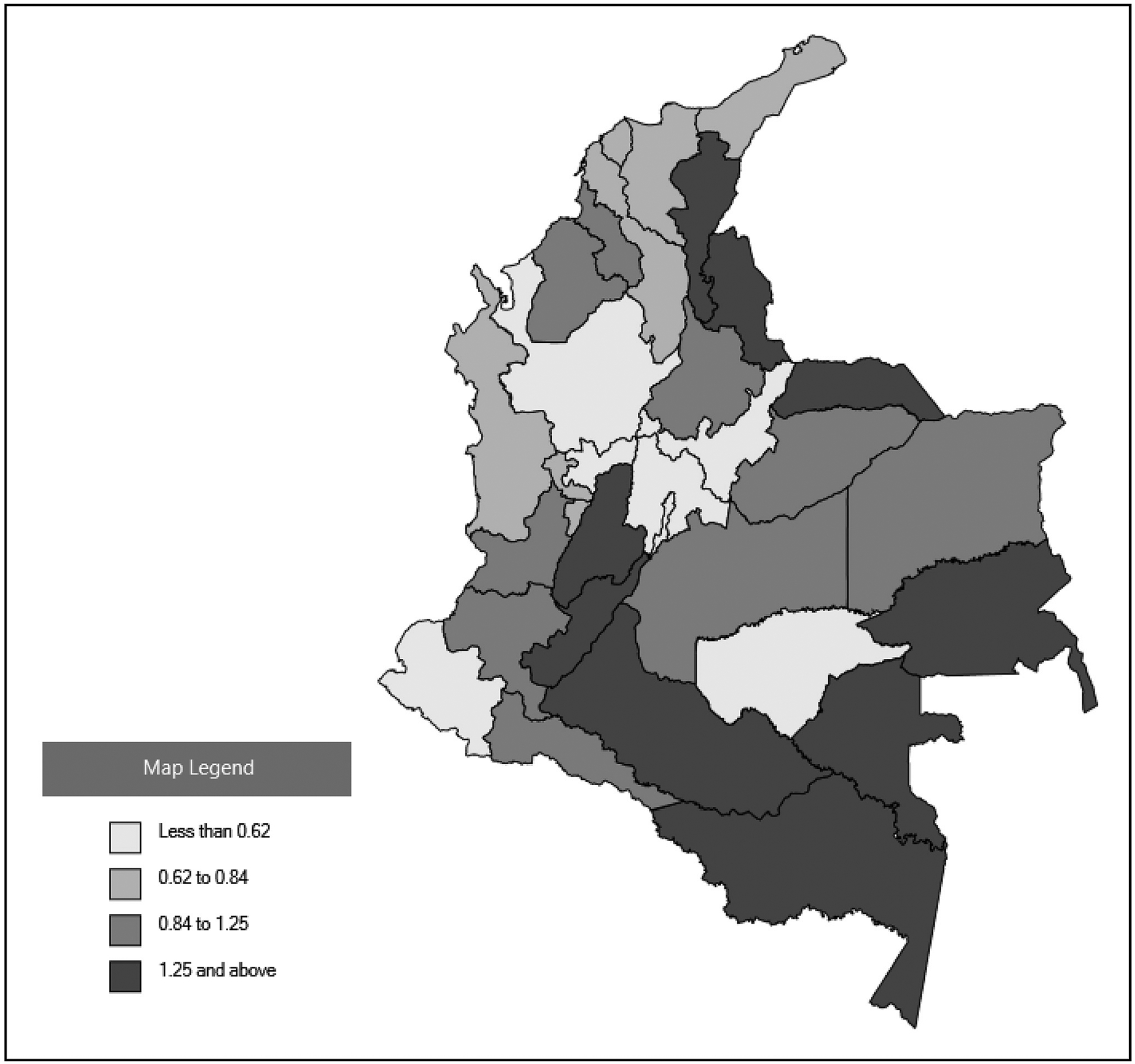
Cumulative prevalence of cases of microcephaly and/or CNS defects per 1000 live births by Colombian territorial department of residence, Colombia, September 2015-April 2017 (n = 858).
Among 858 cases, 492 received ZIKV testing (maternal specimen only [n = 134]; fetal/infant specimen only [n = 228]; both maternal and fetal/infant specimens [n = 130]) (Table I; available at www.jpeds.com). A total of 366 cases of microcephaly and/or CNS defects had no maternal or fetal/infant specimen tested for ZIKV infection. Laboratory findings of possible ZIKV infection were found in 278 (56.5%) cases (103 with only maternal testing performed; 87 with only fetal/infant testing performed; and 88 cases with both maternal and fetal/infant testing performed). ZIKV IgM antibodies were not detected consistently among cases assigned to ZIKV etiology (Table II; available at www.jpeds.com).
Table I.
Maternal and fetal/infant ZIKV testing results among cases of microcephaly and/or CNS defects, Colombia, September 2015-April 2017 (n = 858)
| Laboratory findings consistent with possible ZIKV infection in a maternal specimen* | Laboratory findings consistent with possible ZIKV infection in a fetal/infant specimen† | Reported cases, n (%) |
|---|---|---|
| Yes | Yes | 52 (6.1) |
| Yes | No | 17 (2.0) |
| Yes | Not tested | 103 (12.0) |
| No | Yes | 19 (2.2) |
| No | No | 42 (4.9) |
| No | Not tested | 31 (3.6) |
| Not tested | Yes | 87 (10.1) |
| Not tested | No | 141 (16.4) |
| Not tested | Not tested | 366 (42.7) |
Laboratory findings consistent with possible ZIKV infection in a maternal specimen include (1) ZIKV RNA detected by PCR from any maternal serum, maternal urine, placenta, umbilical cord, or umbilical cord blood; (2) ZIKV IgM detected by serologic testing of maternal serum; and (3) ZIKV antigen detected by immunohistochemistry of placenta.
Laboratory findings consistent with possible ZIKV infection in a fetal/infant specimen include (1) ZIKV RNA detected by PCR from any fetal/infant specimen including serum, urine, CSF, and fetal/infant tissues; (2) ZIKV IgM detected by serologic testing of infant serum or CSF, and (3) ZIKV antigen detected by immunohistochemistry testing of fetal/infant tissue; confirmatory plaque reduction neutralization testing was not conducted.
Table II.
Fetal/infant ZIKV serology results among cases assigned to ZIKV etiology, by strength of evidence, Colombia, September 2015-April 2017 (n = 503)
| Strength of evidence* | Serology positive† n (%) | Serology negative n (%) | Serology not tested n (%) |
|---|---|---|---|
| Strong evidence of congenital ZIKV (n = 124) | 16 (12.9) | 15 (12.1) | 93 (75.0) |
| Moderate evidence of congenital ZIKV (n = 232) | 8 (3.4) | 25 (10.8) | 199 (85.8) |
| Limited evidence of congenital ZIKV (n = 147) | 6 (4.1) | 14 (9.5) | 127 (86.4) |
Figure 2 provides more information. Strength of evidence for ZIKV etiology among cases of microcephaly and/or CNS defects potentially attributed to congenital ZIKV infection.
ZIKV IgM detected by serologic testing of fetal/infant serum or CSF; confirmatory plaque reduction neutralization testing was not conducted. Data were not collected in a manner that permits interpretation of negative ZIKV serology results relative to timing of testing. Test results from specimens collected outside of the window of detection might not be informative, and results among cases that did not receive testing cannot be inferred. For this analysis, absent or unknown laboratory findings of possible ZIKV infection do not rule out possible congenital ZIKV etiology.
Among cases classified as potentially attributable to congenital ZIKV infection, strength of evidence was considered strong in 124 (24.7%), moderate in 232 (46.1%), and limited in 147 (29.2%) cases (Table III), including 142 with moderate evidence of congenital ZIKV infection and 117 with limited evidence based on clinical findings alone (Table IV). Among cases classified as potentially attributable to ZIKV-STORCH coinfection, CMV (n = 8) and toxoplasmosis (n = 11) were the most frequently detected STORCH coinfections, followed by syphilis (n = 1) and 1 case of coinfection with ZIKV and multiple STORCH infections (n = 1). Among 858 cases, 355 (41.4%) were classified as potentially attributable to etiologies other than ZIKV infection (STORCH only, n = 32 [3.7%]; genetic, n = 16 [1.9%]; multifactorial, n = 42 [4.9%]; unknown etiology, n = 265 [30.9%]) (Table III).
Table III.
Likely etiology among cases of microcephaly and/or CNS defects, Colombia, September 2015-April 2017 (n = 858)
| Etiologic classifications | N (%) |
|---|---|
| Teratogenic—infectious | 535 (62.4) |
| ZIKV (n = 503) | |
| Strong evidence of congenital ZIKV (n = 124) | |
| ZIKV only (n = 117) | |
| ZIKV-STORCH coinfection (n = 7) | |
| Cytomegalovirus (n = 2) | |
| Toxoplasmosis (n = 5) | |
| Moderate evidence of congenital ZIKV (n = 232) | |
| ZIKV only (n = 224) | |
| ZIKV-STORCH coinfection (n = 8) | |
| Cytomegalovirus (n = 3) | |
| Toxoplasmosis (n = 4) | |
| Syphilis (n = 1) | |
| Limited evidence of congenital ZIKV (n = 147) | |
| ZIKV only (n = 141) | |
| ZIKV-STORCH coinfection (n = 6) | |
| Cytomegalovirus (n = 3) | |
| Toxoplasmosis (n = 2) | |
| Multiple STORCH (n = 1) | |
| STORCH (n = 32) | |
| Cytomegalovirus (n = 17) | |
| Toxoplasmosis (n = 10) | |
| Herpes simplex virus 1 or 2 (n = 2) | |
| STORCH-STORCH coinfection (n = 3) | |
| Genetic | 16 (1.9) |
| Multifactorial—neural tube defects | 42 (4.9) |
| Unknown etiology | 265 (30.9) |
| Holoprosencephaly (n = 10) | |
| Multiple congenital anomalies (n = 38) | |
| Other unknown etiology (n = 217) |
Table IV.
Strength of evidence for ZIKV etiology among cases of microcephaly and/or CNS defects potentially attributable to congenital ZIKV infection
| Laboratory findings of possible ZIKV infection | Strength of evidence for ZIKV etiology | Birth defect group* (number required) | ZIKV only (n) | ZIKV-STORCH coinfection (n) |
|---|---|---|---|---|
| Present | Strong | Group A (1 or more) | 78 | 6 |
| Group B (2 or more) | 39 | 1 | ||
| Moderate | Group B (only 1) AND Group C (1 or more) | 72 | 6 | |
| Group C (2 or more) | 10 | 2 | ||
| Limited | Group B (only 1) | 5 | 0 | |
| Group C (only 1) | 19 | 6 | ||
| Absent or unknown | Moderate | Group A (1 or more) | 84 | 0 |
| Group B (2 or more) | 58 | 0 | ||
| Limited | Group B (only 1) AND Group C (1 or more) | 97 | 0 | |
| Group C (2 or more) | 20 | 0 |
Figure 2 provides more information.
The number of cases classified as potentially attributable to congenital ZIKV increased beginning in December 2015, peaked in July 2016, and decreased through the end of April of 2017 (Figure 5). When stratified by strength of evidence for congenital ZIKV etiology, incidence curves for cases with strong, moderate, or limited evidence had similar shape. By comparison, incidence curves for other known etiologies were generally flat over the same period, although the incidence curve for cases of unknown etiology was more consistent with the curve for congenital ZIKV etiology.
Figure 5.
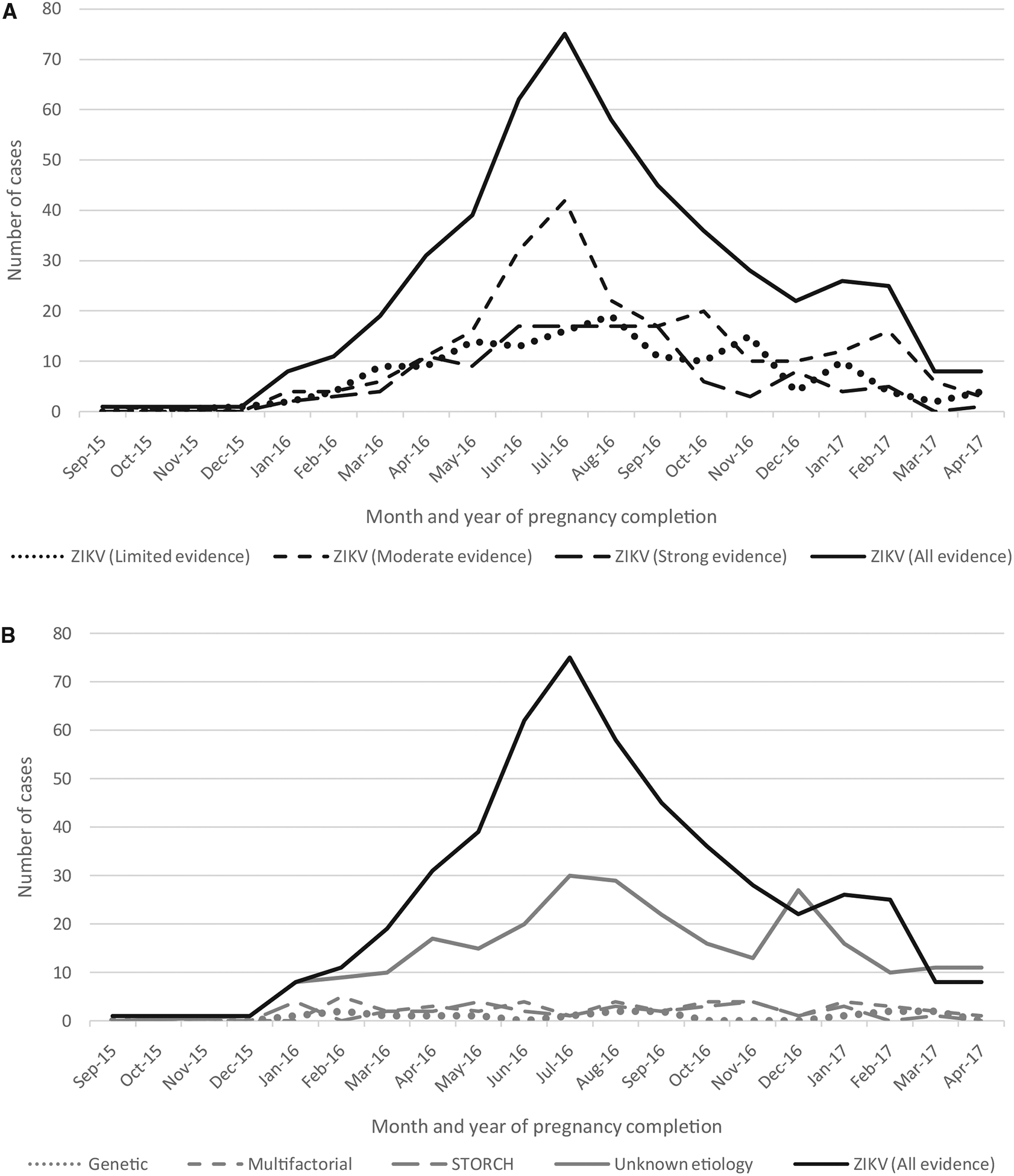
A, Month and year of pregnancy completion among cases of microcephaly and/or CNS defects potentially attributable to ZIKV infection in pregnancy, by strength of evidence for congenital ZIKV, (n = 503); B, Month and year of pregnancy completion among cases of microcephaly and/or CNS defects by etiology (n = 858).
Discussion
We found that 58% of cases of microcephaly and/or CNS defects were potentially attributable to congenital ZIKV infection. However, strength of the evidence varied, and one-third had limited evidence. Considering only cases with strong or moderate evidence, 41.5% (n = 356) were classified as potentially attributable to congenital ZIKV infection. Because of the enhanced surveillance protocol and detailed review processes implemented as part of the ZIKV response in Colombia, these findings cannot be easily compared with estimates from surveillance in these regions conducted outside the context of this ZIKV epidemic. These findings differ from previous estimates due to differences in study timeframe, additional data collection that allowed careful classification beyond initial reports, and case classification methods developed for this specific analysis.23
Many cases with clinical findings highly characteristic of ZIKV infection did not have laboratory findings of possible ZIKV infection, because samples were either not tested or tested negative for ZIKV. Although absent testing might be due to evolving ZIKV testing recommendations, there are limitations even when ZIKV testing is performed.24 ZIKV RNA is present transiently in body fluids, and negative PCR results do not rule out infection, particularly given the narrow window to detect the virus.25 In addition, the sensitivity of infant testing for ZIKV is unknown. Birth defects from each of the 3 groups (A, B, and C) were observed among cases with and without supporting laboratory evidence. These findings are consistent with reports of infants with clinical findings suggestive of possible congenital Zika syndrome but with negative laboratory results.3,24–27 In 7 cases, birth defect findings were inconsistent with laboratory findings that suggested STORCH infection; however, the birth defects met criteria for strong or moderate evidence of congenital ZIKV infection, and they were classified as potentially attributable to ZIKV infection.
The full spectrum of birth defects caused by congenital ZIKV infection continues to expand. The existing case definitions for congenital Zika syndrome developed by the Pan American Health Organization (PAHO) (https://www.paho.org/hq/index.php?option=com_content&view=article&id=11117:zika-resources-case-definitions&Itemid=41532&lang=en) and the Council of State and Territorial Epidemiologists (CSTE) (https://wwwn.cdc.gov/nndss/conditions/zika/case-definition/2016/06/) use nonspecific terms, such as congenital malformation of the CNS or structural brain or eye abnormalities, to describe birth defects. These definitions were developed early in the epidemic before studies were able to more fully describe the congenital ZIKV phenotype.28
Few studies have attempted to describe the range of structural and developmental findings in congenital ZIKV phenotype.29 One review focused on identifying birth defects that are unique to congenital Zika syndrome or rarely seen in other congenital infections.19 A prospective cohort study developed major and minor criteria to define signs and symptoms of congenital ZIKV infection at birth; however, this classification schema did not provide sufficient detail to distinguish ZIKV-affected infants from those with other congenital infections such as CMV.30 The classification schema in this investigation considers the totality of findings for each case to differentiate those attributable to ZIKV from those more likely attributable to other etiologies based on existing birth defects literature. Of note, the incidence curve for cases potentially attributable to congenital ZIKV mirrored that for national ZIKV incidence whereas curves for other known etiologies remained flat by comparison, providing additional support to this classification schema.23 Given the large epidemic in Colombia and the possibility of case misclassification, it is not surprising that the incidence curve for cases of unknown etiology closely resembled that of congenital ZIKV.
The study is subject to limitations. First, laboratory testing was conducted on the presence of symptoms consistent with ZIKV disease in the pregnant woman or findings of birth defects in the fetus or infant, and in 42.7% of reviewed cases, neither maternal nor fetal/infant test results were available. In addition, results of ZIKV testing depend on timing of infection relative to specimen collection. Data were not collected to assess laboratory findings relative to timing from symptom onset to specimen collection; test results from specimens collected outside of the window of detection might not have been informative. Second, birth defect descriptions varied in quality and completeness between medical records. Third, results of postnatal neuroimaging, ophthalmologic examination, and hearing evaluation were not available for all infants. CNS defects might have gone undetected without neuroimaging or ophthalmologic evaluation, especially among infants without microcephaly or other abnormalities at the time of birth; therefore, voluntary reporting might be biased toward cases with more severe findings, resulting in an underestimate of total number of reported cases. For pregnancy losses, prenatal diagnoses of microcephaly could not be confirmed postnatally and were not included among birth defects for classification. Limited data suggest that when present, abnormal findings detected on prenatal ultrasound are associated with abnormal findings on physical examination and in postnatal neuroimaging; however, the absence of abnormal prenatal findings is not a predictor of a normal neonatal outcome.31 True cases of microcephaly and/or CNS defects detected prenatally alone might not have been ascertained. Finally, differentiating birth defects associated with ZIKV infection from those associated with other congenital infections is complicated due to continued evolving data on the congenital ZIKV phenotype, resulting in classification bias.
This enhanced surveillance activity and classification schema demonstrate a comprehensive approach to investigating complex congenital conditions of unclear etiology. Surveillance methods might be improved by efforts to detect asymptomatic ZIKV infection (such as ZIKV testing in each trimester of pregnancy during an epidemic), to emphasize immediate ZIKV testing and standard infant evaluations soon after birth for infants with risk of congenital exposure and for infants with abnormal findings, and to improve timeliness of data collection and reporting (such as rapid data collection focused on a limited number of birth defects reported within the first month of life). This study provides a large population-based sample assessing the etiology of cases of microcephaly and/or CNS defects during the Colombian ZIKV epidemic. Etiological classification took into account situations of limited clinical and laboratory information. Identification, classification, and follow-up care of reported cases are essential to fully characterize the impact of congenital ZIKV infection and to quantify the number of ZIKV-associated birth defects. These methods might be useful for mitigating the impact of ZIKV by aligning correct case identification with public health prevention efforts.
Acknowledgments
We thank the many collaborators who participated in this work as part of an interinstitutional agreement between the INS and CDC. We thank Sarah Mulkey, Roberta L. Debiasi, Panagiotis Maniatis, Jessica Ricaldi Camahuali, and Sheila Dollard for their clinical expertise and technical assistance.
Supported by the Instituto Nacional de Salud, Centers for Disease Control and Prevention, and US Agency for International Development. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or US Agency for International Development. The authors declare no conflicts of interest.
Glossary
- CDC
Centers for Disease Control and Prevention
- CMV
Cytomegalovirus
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- INS
Colombian National Institute of Health
- PCR
Polymerase chain reaction
- STORCH
Syphilis, toxoplasmosis, rubella, cytomegalovirus, herpes 1 and herpes 2 viruses
- ZIKV
Zika virus
Footnotes
Portions of this study were presented at the Teratology Society Annual Meeting, June 26, 2018, Clearwater, Florida; the International Conference on Emerging Infectious Diseases, August 28, 2018, Atlanta, Georgia; and the American Society of Tropical Medicine and Hygiene 67th Annual Meeting, October 29, 2018, New Orleans, Lousiana.
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.Ministerio de Salud y Proteccion Social. Circular conjunta externa No 00000043 de 2015. Bogotá, Colombia: Ministerio de Salud y Proteccion Social; 2015. [updated 2015 Oct 14; cited 2019 Feb 20]. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/circular-conjunta-externa-0043.pdf. Accessed February 20, 2019. [Google Scholar]
- 2.Mercado-Reyes M, Acosta-Reyes J, Navarro-Lechuga E, Corchuelo S, Rico A, Parra E, et al. Dengue, chikungunya and Zika virus coinfection: results of the national surveillance during the Zika epidemic in Colombia. Epidemiol Infect 2019;147:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Araujo TV, de Alencar Ximenes RA, de Barros Miranda-Filho D, Souza WV, Montarroyos UR, de Melo AP, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis 2018;18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krow-Lucal ER, de Andrade MR, Canan ea JN, Moore CA, Leite PL, Biggerstaff BJ, et al. Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraiba, Brazil, in 2015–16: a case-control study. Lancet Child Adolesc Health 2018;2: 205–13. [DOI] [PubMed] [Google Scholar]
- 5.Ministério da Saúde. Situação epidemiológica de ocorrência de microcefalias no Brasil, 2015. Brasilia, Brazil: Ministério da Saúde; 2015. [updated 11 Nov 2015; cited 20 Feb 2019]. http://portalarquivos2.saude.gov.br/images/pdf/2015/novembro/19/Microcefalia-bol-final.pdf. Accessed February 20, 2019. [Google Scholar]
- 6.Heymann DL, Hodgson A, Freedman DO, Staples JE, Althabe F, Baruah K, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet 2016;387:719–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan American Health Organization/World Health Organization. Zika suspected and confirmed cases reported by countries and territories in the Americas Cumulative cases, 2015–2016. Washington, DC: Pan American Health Organization/World Health Organization; 2016. [updated 2016 Oct 6; cited 20 Feb 2019]. https://www.paho.org/hq/dmdocuments/2016/2016-October-6-PHE-ZIKV-cases.pdf. Accessed February 20, 2019. [Google Scholar]
- 8.Cuevas EL, Tong VT, Rozo N, Valencia D, Pacheco O, Gilboa SM, et al. Preliminary report of microcephaly potentially associated with Zika virus infection during pregnancy–Colombia, January-November 2016. MMWR Morb Mortal Wkly Rep 2016;65:1409–13. [DOI] [PubMed] [Google Scholar]
- 9.Pacheco O, Beltr an M, Nelson CA, Valencia D, Tolosa N, Farr SL, et al. Zika virus disease in Colombia–Preliminary Report. N Engl J Med. 2016. https://www.nejm.org/doi/10.1056/NEJMoa1604037. Accessed February 20, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Ministerio de Salud y Proteccion Social. Circular externa 1000–00020. Bogotá, Colombia: Ministerio de Salud y Proteccion Social; 2015. [updated 2016 Apr 13; cited 2019 Feb 20]. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/IA/INS/ins-circular-externa-0020-de-2016.pdf. Accessed February 20, 2019. [Google Scholar]
- 11.Ministerio de Salud y Proteccion Social. Circular externa 1000–0004. Bogotá, Colombia: Ministerio de Salud y Proteccion Social; 2016. [updated 2016 Jan 13; cited 2019 Feb 20]. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/circular-004-de-2016.pdf. Accessed February 20, 2019. [Google Scholar]
- 12.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Trioplex real-time RT-PCR assay. Atlanta, GA: Centers for Disease Control and Prevention; 2017. [updated 2017 Apr 6; cited 2019 Feb 20]. https://www.fda.gov/downloads/medicaldevices/safety/emergencysituations/ucm491592.pdf. Accessed February 20, 2019. [Google Scholar]
- 14.Villar J, Papageorghiou AT, Pang R, Ohuma EO, Ismail LC, Barros FC, et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: the fetal growth longitudinal study and newborn cross-sectional study. Lancet Diabetes Endocrinol 2014;2:781–92. [DOI] [PubMed] [Google Scholar]
- 15.Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH. INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016;387:844–5. [DOI] [PubMed] [Google Scholar]
- 16.Toufaily MH, Westgate MN, Lin AE, Holmes LB. Causes of congenital malformations. Birth Defects Res 2018;110:87–91. [DOI] [PubMed] [Google Scholar]
- 17.Feldkamp ML, Carey JC, Byrne JLB, Krikov S, Botto LD. Etiology and clinical presentation of birth defects: population based study. BMJ 2017;357:j2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med 2016;374:1981–7. [DOI] [PubMed] [Google Scholar]
- 19.Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017;171: 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017;317:59–68. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, et al. Vital Signs: update on Zika virus-associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure–U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep 2017;66:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro-Mendoza CK, Rice ME, Galang RR, Fulton AC, VanMaldeghem K, Prado MV, et al. Pregnancy outcomes after maternal Zika virus infection during pregnancy–U.S. territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 2017;66:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan American Health Organization/World Health Organization. Zika–epidemiological report Colombia. Washington, DC: Pan American Health Organization/World Health Organization; 2017. [updated 2017 Feb 20; cited 2019 Feb 20]. https://www.paho.org/hq/dmdocuments/2017/2017-phe-zika-situation-report-col.pdf. Accessed February 20, 2019. [Google Scholar]
- 24.Ribeiro BG, Werner H, Lopes FP, Hygino da Cruz LC Jr, Fazecas TM, Daltro PA, et al. Central nervous system effects of intrauterine Zika virus infection: a pictorial review. Radiographics 2017;37:1840–50. [DOI] [PubMed] [Google Scholar]
- 25.Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, et al. Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection–United States, October 2017. MMWR Morb Mortal Wkly Rep 2017;66:1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaney A, Mai C, Smoots A, Cragan J, Ellington S, Langlois P, et al. Population-based surveillance of birth defects potentially related to Zika virus infection–15 states and U.S. territories, 2016. MMWR Morb Mortal Wkly Rep 2018;67:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira Melo AS, Aguiar RS, Amorim MM, Arruda MB, de Oliveira Melo F, Ribeiro ST, et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol 2016;73:1407–16. [DOI] [PubMed] [Google Scholar]
- 28.Del Campo M, Feitosa IM, Ribeiro EM, Horovitz DD, Pessoa AL, França GV, et al. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet A 2017;173:841–57. [DOI] [PubMed] [Google Scholar]
- 29.Nithiyanantham SF, Badawi A. Maternal infection with Zika virus and prevalence of congenital disorders in infants: systematic review and meta-analysis. Can J Public Health 2019;110:638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pomar L, Vouga M, Lambert V, Pomar C, Hcini N, Jolivet A, et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: prospective cohort study in French Guiana. BMJ 2018;363:k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira JP, Nielsen-Saines K, Sperling J, Maykin MM, Damasceno L, Cardozo RF, et al. Association of prenatal ultrasonographic findings with adverse neonatal outcomes among pregnant women with Zika virus infection in Brazil. JAMA Netw Open 2018;1:e186529. [DOI] [PMC free article] [PubMed] [Google Scholar]


