Abstract
Fibrotic posterior capsular opacification (PCO), one of the major complications of cataract surgery, occurs when lens epithelial cells (LCs) left behind post cataract surgery (PCS) undergo epithelial to mesenchymal transition, migrate into the optical axis and produce opaque scar tissue. LCs left behind PCS robustly produce fibronectin, although its roles in fibrotic PCO are not known. In order to determine the function of fibronectin in PCO pathogenesis, we created mice lacking the fibronectin gene (FN conditional knock out-FNcKO) from the lens. While animals from this line have normal lenses, upon lens fiber cell removal which models cataract surgery, FNcKO LCs exhibit a greatly attenuated fibrotic response from 3 days PCS onward as assessed by a reduction in surgery-induced cell proliferation, and fibrotic extracellular matrix (ECM) production and deposition. This is correlated with less upregulation of Transforming Growth Factor b (TGFβ) and integrin signaling in FNcKO LCs PCS concomitant with sustained Bone Morphogenetic Protein (BMP) signaling and elevation of the epithelial cell marker E cadherin. Although the initial fibrotic response of FNcKO LCs was qualitatively normal at 48 h PCS as measured by the upregulation of fibrotic marker protein αSMA, RNA sequencing revealed that the fibrotic response was already quantitatively attenuated at this time, as measured by the upregulation of mRNAs encoding molecules that control, and are controlled by, TGFβ signaling, including many known markers of fibrosis. Most notably, gremlin-1, a known regulator of TGFβ superfamily signaling, was upregulated sharply in WT LCs PCS, while this response was attenuated in FNcKO LCs. As exogenous administration of either active TGFβ1 or gremlin-1 to FNcKO lens capsular bags rescued the attenuated fibrotic response of fibronectin null LCs PCS including the loss of SMAD2/3 phosphorylation, this suggests that fibronectin plays multifunctional roles in fibrotic PCO development.
Keywords: Cataract surgery, Posterior capsular opacification, PCO, Fibronectin, TGFβ signaling, Matrix assembly
Introduction
Fibronectin, a structurally complex extracellular matrix (ECM) protein, is essential for diverse physiological processes such as blood coagulation, opsonization, and embryogenesis [1–7]. Plasma fibronectin is a compact, soluble protein produced by the liver that is present at high levels in body fluids [8]. Tissue fibronectin, which is produced locally in tissues, is an alternatively spliced isoform of fibronectin possessing a more open conformation that allows it to assemble readily into insoluble fibrils [3,8–10]. Fibronectin is known to concentrate at sites of wound healing and tissue repair [11–15], while chronic fibronectin deposition is a feature of numerous fibrotic diseases [3,11,16–19].
Plasma fibronectin complexes with fibrin immediately after cutaneous wounding to form the early provisional ECM necessary for primary wound closure [20]. Later, fibronectin is produced locally at the wound site as part of the late provisional matrix, which is then remodeled to facilitate the assembly of secondary scars rich in collagen I [21]. Fibronectin fibrils also serve as an extracellular depot for numerous growth factors, suggesting that fibronectin could play multifunctional roles in the wound healing response and fibrotic diseases [22,23]. The importance of fibronectin in wound healing and fibrotic diseases has been confirmed in vivo using mice lacking the EDA exon which is often included in tissue fibronectin [24–28]. Many of these in vivo studies suggest that fibronectin deposition drives fibrosis in their system. However, these studies only explore the function of one form of fibronectin produced by wounded tissue, and do not typically explore other fibronectin functions such as its tethering of latent transforming growth factor-beta (TGFβ) to the ECM, which is crucial for subsequent activation of TGFβ, suggesting the need for the comprehensive in vivo study of the role of fibronectin in wound healing [11,29–31].
Cataracts, a major cause of blindness worldwide [32–34], are effectively treated by surgical removal of opaque lens fiber cells followed by implantation of an artificial intraocular lens (IOL) [33]. However, months to years later, a significant proportion of patients experience an apparent recurrence of their cataract as Posterior Capsular Opacification (PCO) [35–38]. PCO occurs when lens epithelial cells (LCs) left behind post cataract surgery (PCS) migrate into the optical axis and transition into a mixture of myofibroblasts embedded in a fibrotic ECM, and aberrant lens fiber cells [35].
Transforming growth factor β (TGFβ) signaling is a major inducer of the epithelial to mesenchymal transition of LCs to myofibroblasts expressing numerous “fibrotic” markers, including fibronectin [39,40]. However, the function of fibronectin in the pathogenesis of fibrotic PCO is unclear. In a mouse cataract surgery model, fibronectin mRNA levels upregulate in remnant LCs by 24 h PCS, and fibronectin fibrils are first detected around LCs expressing fibrotic markers such as αSMA by 48 h PCS [19], coincident with the onset of detectable TGFβ signaling. In vitro studies suggest that fibronectin is a negative regulator of posterior capsular wrinkling in PCO [39] although disruption of fibronectin assembly attenuates LC conversion to myofibroblasts in culture [41]. Most recently, it was reported that exposure of cultured chicken LCs to plasma fibronectin (as would occur after cataract surgery) led to the activation of the latent TGFβ being produced endogenously by cultured cells, indicating that fibronectin plays an important mechanistic role in PCO pathogenesis [42]. However, the function of the cellular fibronectin produced autonomously by remnant LCs in vivo PCS is not well understood.
Here, we deleted the fibronectin gene from the lenses of adult mice and evaluated how this deletion affects the response of LCs to a lens fiber cell removal operation that models cataract surgery. This study reveals, for the first time, the multifunctional roles that cellular fibronectin plays in PCO pathogenesis and adds to our understanding of how fibronectin can contribute to the pathophysiology of fibrotic disease.
Results
Deletion of the fibronectin gene from the lens does not affect the later stages of lens development, while fibronectin protein expression increases during PCO progression
Fibronectin deposition around remnant lens epithelial cells (LCs) has long been a known feature of PCO and thus is often used as a “readout” for the progression of PCO in experimental models [43–45]. We previously reported that fibronectin mRNA levels upregulate in a mouse model of cataract surgery by 24 h after fiber cell removal (post cataract surgery (PCS)), while cell associated fibronectin protein deposition can be detected around the remnant LCs by 48 h PCS [19,46]. Consistent with this, here we found that fibronectin protein (red) is not readily detected around the remnant LCs at either the time of surgery (0 h PCS) or 24 h later (24 h PCS) by immunofluorescence (IF) confocal imaging (Fig. 1A), although some fibronectin is associated with the external surface of the lens capsule as previously reported [19]. Cell-associated fibronectin (highlighted with arrow) is first detectable around the a smooth muscle actin (αSMA) positive remnant LCs (green) at 48 h PCS, and this deposition greatly increases by 5 days PCS (Fig. 1A). In addition to that, IF reveals all the classic features of fibrotic tissue in our cataract surgery model at 5 days PCS such as the absence of a normal cuboidal monolayer of epithelial cells, presence of multilayered spindle-shaped cells and capsular wrinkling as previously described [47].
Fig. 1. Fibronectin protein is not required for lens transparency, but deposits around remnant LCs PCS.

(A) Dynamics of fibronectin protein deposition around remnant LCs PCS. At 0 h PCS, little to no fibronectin is associated with remnant LCs, although the outer surface of the lens capsule is fibronectin positive (red). Fibronectin starts to deposit around αSMA positive remnant LCs by 48 h PCS (arrow), and this deposition is more marked at 5 days PCS as PCO progresses. Fibronectin (red), αSMA (green), and DNA detected by Draq5 (blue). Scale bar-35 μm, C-lens capsule, LC-remnant lens cells. (B) Deletion of the fibronectin gene from the developing lens. Diagram of fibronectin gene locus showing the position of the loxP sites (left), and PCR results from DNA obtained from 9 week old control (wildtype-WT) and FNcKO lenses demonstrating successful deletion of the floxed fibronectin gene fragment in FNcKO lenses (right). (C) FNcKO lenses are morphologically similar to WT lenses. A dark field image showing that 9 week old WT (A) and FNcKO lenses (B) are both transparent; 200-mesh electron microscopy grid analysis of 12 week old WT (C) and FNcKO lenses (D) showing that fibronectin null lenses have refractive properties similar to WT; Hematoxylin and eosin (H&E) staining showing the anterior epithelium of 9 week old WT lens (E) and FNcKO lens (F); H&E staining showing the transition zone of a 9 week old WT lens (G) and FNcKO lens (H) showing that FNcKO lenses are structurally normal although FNcKO fibers may stain more intensely with Eosin than WT. Abbreviations: le-lens epithelium, f - lens fiber cells, tz - transition zone. Scale bar Panels A, B - 1.0 mm; Panels C, D - 0.5 mm; Panels E, F, G, H – 150 μm.
In order to test the function of fibronectin in PCO, we generated mice conditionally lacking a functional fibronectin gene from the lens (FNcKO) by mating mice carrying a floxed fibronectin allele [48] to mice harboring the lens-specific MLR10 CRE transgene (Fig. 1B, left) whose activity is first detected in the lens beginning around embryonic day 10.5 (the lens vesicle stage) [49]. The complete deletion of the floxed region of the fibronectin gene was confirmed by PCR analysis of genomic DNA isolated from adult lenses (Fig. 1B, right). FNcKO lenses are transparent under dark field imaging (Fig. 1C–A, B) and have refractive properties similar to wildtype (WT) lenses ((Fig. 1C–C, D), while hematoxylin and eosin (H&E) staining demonstrated that both WT (Fig. 1C–E, G) and FNcKO lenses (Fig. 1C–F, H) exhibit similar morphology. This overall study suggests that fibronectin does not play a crucial role in regulating the structural properties of the adult lens.
To gain further insight into the role of fibronectin in adult lenses, RNA sequencing (RNAseq) was done on adult WT and FNcKO lenses, and the results submitted to the Gene Expression Omnibus (GEO) under accession number GSE119878. A total of 195 genes exhibited a statistically significant False Discovery Rate (FDR) ≤0.05; Fold change (FC) between adult WT and FNcKO null adult lenses of ≥2 or ≤ −2. However, only 121 of these genes met the criteria we have developed to identify biologically significant differentially expressed genes in the lens (FDR ≤0.05; FC ≥2 or −2; an absolute difference in group means > 2; and an expression level at least 2 Fragments Per Kilobase Million (FPKM) for at least one condition) [50]. Notably, the FN1 (fibronectin1) gene which was deleted in this experiment did not make the list of “significant” differentially expressed genes because it is only expressed at very low levels (0.3 FPKM) in the unoperated adult lens. Analysis of these data for differentially expressed cellular components and pathways using iPathway guide (Advaita Corporation) revealed that the most significant gene ontology (GO) term calculated for the differentially expressed genes was “proteinaceous extracellular matrix” (p ≤ 5.4 × 10 8; data not shown), which included the upregulated genes Col1a2, Col9a1, Col9a2, and Col18a1, and the downregulated genes Col6a2 and Col6a3 (Supplemental Table 2), although in all cases the expression levels are low, and/or the fold changes modest.
Fibronectin is essential for prolonged cell proliferation and fibrotic responses post cataract surgery (PCS), with fibronectin null lenses retaining epithelial characteristics, and undergoing unhindered fiber cell regeneration PCS
To test whether there is any change in the fibrotic response of lenses lacking the fibronectin gene (FNcKO), the expression of the common fibrotic marker a smooth muscle actin (αSMA) was determined at both early and late times PCS (Fig. 2A). As expected, little to no αSMA protein was detected in cells associated with either WT or FNcKO capsular bags at 0 h PCS. By 48 h PCS, both WT and FNcKO remnant lens cells exhibited detectable, but low, levels of αSMA staining which was significantly elevated by 3 days PCS (WT, ***P ≤ 0.001; FNcKO, ***P ≤ 0.001). This fibrotic response is sustained until 5 days PCS in WT LCs (0 h vs 4 days PCS, *P = 0.011; 0 h vs 5 days PCS, *P = 0.013). In contrast, lens capsular bags from FNcKO mice have significantly fewer associated αSMA positive cells compared to WT by the fourth day PCS (***P ≤ 0.001), and this reduction persists at 5 days PCS (***P ≤ 0.001). Overall, FNcKO capsular bags exhibit a significant reduction in αSMA staining between 3 and 4 days PCS (***P ≤ 0.001) which is also true at 5 days PCS (***P ≤ 0.001) (Fig. 2A). The significant reduction in αSMA protein levels in FNcKO LCs compared to WT at 5 days PCS was confirmed by flow cytometry of LCs isolated from dissected lens capsular bags (**P = 0.005) (Fig. 2A and Supplemental Fig. 1). Not only do FNcKO capsular bags have fewer αSMA positive cells than WT controls at 5 days PCS, but the overall size of the capsular plaque appeared smaller.
Fig. 2. The response of LCs lacking the fibronectin gene to lens fiber cell removal.
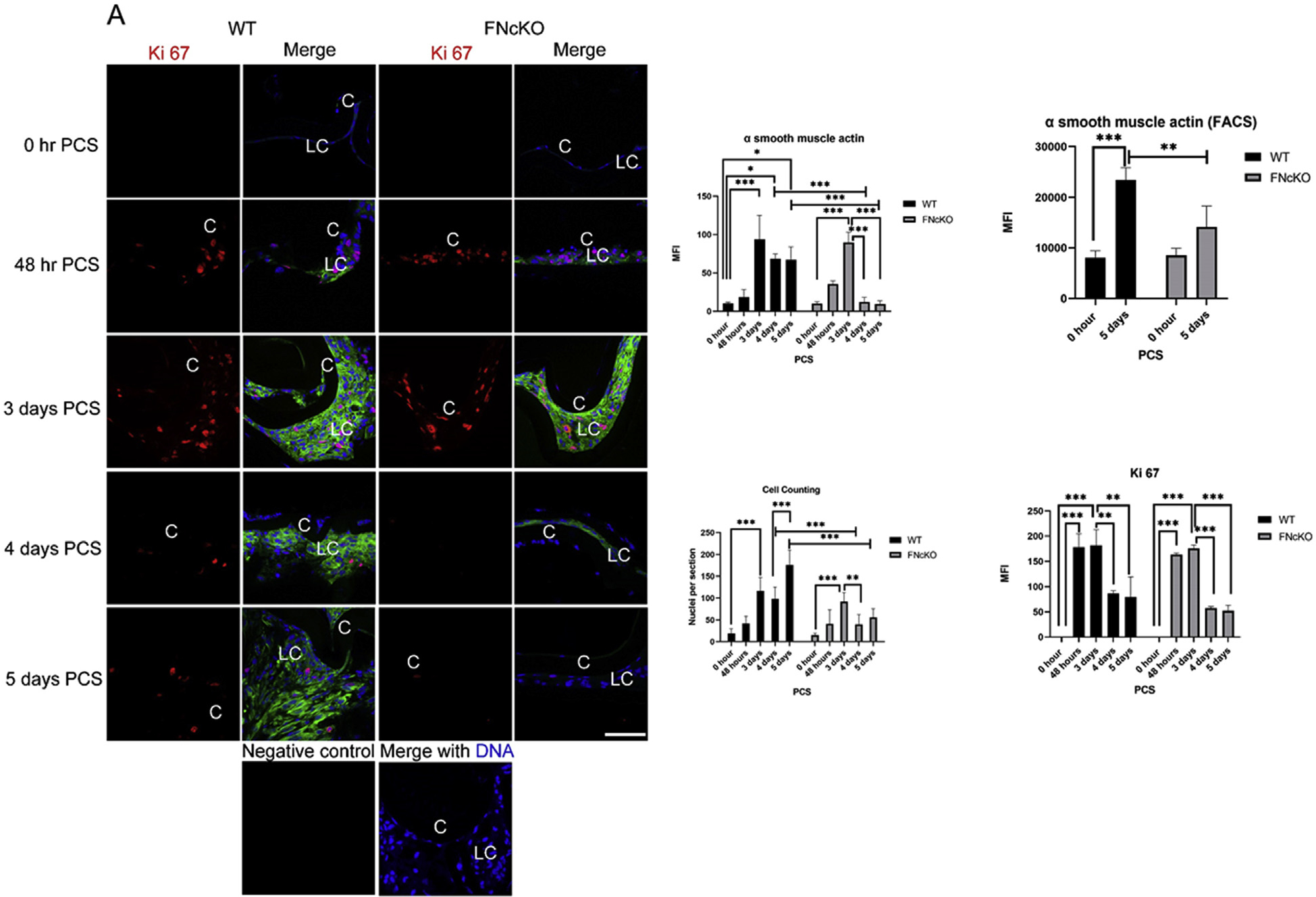

(A) Neither WT nor FNcKO LCs express detectable levels of the proliferation marker Ki 67 or the common fibrotic marker a smooth muscle actin (αSMA) immediately PCS. By 48 h PCS, appreciable numbers of Ki 67 positive LCs are detected in both WT and FNcKO capsular bags (WT ***P ≤ 0.001; FNcKO ***P ≤ 0.001), and this is sustained at 3 days PCS (WT ***P ≤ 0.001; FNcKO ***P ≤ 0.001). However, while the number of Ki 67 positive WT LCs was qualitatively attenuated by 4 days PCS, this effect is more prominent in FNcKOs which exhibit few to no Ki 67 positive LCs at either 4 or 5 days PCS [3 days vs 4 days PCS, WT **P = 0.007; FNcKO ***P ≤ 0.001) (3 days vs 5 days PCS, WT **P = 0.004; FNcKO ***P ≤ 0.001)] although mean fluorescence intensity (MFI) of Ki 67 staining determined by Image J is not statistically significant (T = 4 PCS, P = 0.308; T = 5 days PCS, P = 0.310) between WT and FNcKO. A sharp increase in the average number of cell nuclei associated with capsular bags is seen at 48 h which becomes statistically significant at 3 days PCS in both WT LCs (***P ≤ 0.001) and FNcKO LCs (***P < 0.001). However, while the average number of nuclei associated with capsular bags is reduced in both WT (**P = 0.003) and FNcKO LCs beginning at 4 days PCS, this decrease is more pronounced in FNcKO LCs (***P < 0.001). At 5 days PCS, while WT capsular bags have an increase in the average number of nuclei detected compared to 4 days PCS (***P ≤ 0.001), FNcKO capsular tissue fail to greatly expand the average number of nuclei between 4 and 5 days PCS (P = 0.712), leading 5 day PCS FNcKO capsular bags to have significant reductions in total nuclei count (***P ≤ 0.001) compared to control. Similarly, both WT and FNcKO LCs begin expressing elevated amounts of αSMA protein by 48 h PCS, and this becomes quite prominent by 3 days PCS (WT ***P ≤ 0.001; FNcKO ***P ≤ 0.001). However, while αSMA positive cells persist through 5 days PCS in WT capsular bags (*P = 0.013), few to no αSMA positive cells are detected in FNcKO capsular bags at either 4 days (***P ≤ 0.001), or 5 days PCS (***P ≤ 0.001). FACS analysis further supports the finding that FNcKO LCs express less αSMA protein at 5 days PCS (**P ≤ 0.005) than controls. Ki 67 (red), αSMA (green), DNA detected by Draq5 (blue). Scale bars: 35 μm; LC, remnant lens cells; C, lens capsule. All experiments had and N = 3 except the cell counting analysis where N = 6. Values are expressed as mean ± SEM. Asterisks (*) indicate statistically significant changes between WT and FNcKO LCs at a time PCS or between two PCS time points. (B) Neither WT nor FNcKO LCs express detectable protein for the fiber cell marker cMaf immediately PCS, however, by 48 h PCS, some of the remnant cells found in both WT and FNcKO capsular bags PCS are strongly cMaf positive which is maintained until 5 days PCS. Although the remnant cells of FNcKO qualitatively express more cMaf protein both at 48 h and 5 days PCS compared to WT, this is not statistically significant (T = 48 h PCS, P = 0.269; 5 days PCS P = 0.851). cMaf (red), DNA detected by Draq5 (blue). In contrast to cMaf, both WT and FNcKO lens cells express appreciable levels of E cadherin, an epithelial cell marker at 0 h PCS. However, by 48 h PCS, E cadherin protein levels are downregulated in both WT and FNcKO capsular bags (WT, *P = 0.019, FNcKO, ***P ≤ 0.001) and this downregulation continues at 5 days PCS in WT lens cells (**P = 0.004). In contrast to WT, E cadherin protein levels upregulate in FNcKO capsular bags between 48 h and 5 days PCS (***P ≤ 0.001). mean All experiments had N = 3. are expressed as ± SEM. Asterisks (*) indicate statistically Values significant MFI between WT and FNcKO at a PCS or between two PCS time points..
Next, we determined whether the qualitative reduction in plaque size observed in FNcKO eyes at 5 days PCS reflected differences in cell number by quantitating the number of cell nuclei associated with capsular bags at different times PCS and comparing that with the number of cells in the cell cycle as measured by staining for the proliferation marker, Ki67 (Fig. 2A) which is present at all stages of the cell cycle except G0 [51]. At 0 h PCS, remnant LCs exhibit little to no cell proliferation. However, a sharp increase in the Average Number of Nuclei (ANN)/section is seen at 48 h. PCS which becomes statistically significant at 3 days PCS for both WT (***P ≤ 0.001) and FNcKO (***P ≤ 0.001) (Fig. 2A) capsular bags. This finding correlates with a significant upregulation of Ki67 staining in LCs between 0 h PCS and 48 h PCS in both WT and FNcKO LCs (***P ≤ 0.001 for both) which is sustained at 3 days PCS (***P ≤ 0.001 for both). However, while the average number of nuclei detected per section remains steady in WT eyes at 4 days PCS (P = 0.717), it is significantly decreased in FNcKO capsular bags at (**P = 0.003) leading FNcKO capsular bags to have significantly fewer associated cell nuclei at 4 days PCS compared to WT (***P ≤ 0.001). This phenomenon correlates with the significant attenuation of LC proliferation between 3 and 4 days PCS (**P = 0.007) that appears more pronounced in FNcKO LCs (***P ≤ 0.001). At 5 days PCS, WT capsular bags have significantly more associated cell nuclei than 4 days PCS (***P ≤ 0.001), while this was not seen in FNcKO capsular bags (P = 0.712), leading the FNcKO capsular bags to have significantly fewer associated cell nuclei than controls at 5 days PCS (***P ≤ 0.001]. However, quantification of the Ki67 staining did not reveal a statistically significant difference between WT and FNcKO capsular bags (4 days PCS, P = 0.308; 5 days PCS, P = 0.310) largely due to the small numbers of Ki67 positive cells associated with capsular bags after 3 days PCS leading to variability in the measurements. However, these data in aggregate suggest that fibronectin is essential for the long-term, but the not initial, fibrotic response of LCs to cataract surgery.
As fewer αSMA positive myofibroblastic cells were observed in FNcKO capsular bags compared to controls at later time PCS, we attempted to determine the fate of the αSMA expressing LCs that were detected in FNcKO capsular bags prior to 3 days PCS. First, we determined if these cells are lost by apoptosis as this has been seen in the lens under some pathological conditions such as TGFβ induced cataract [52]. However, staining with cleaved caspase 3, a marker of conventional apoptosis, did not reveal any apoptotic cell death in either WT or FNcKO LCs at any time PCS tested, while tissue samples known to exhibit apoptosis stained appropriately (data not shown) suggesting that the loss of αSMA positive cells from the FNcKO capsular bag after 3 days PCS was not caused by apoptotic cell death.
After cataract surgery, some remnant LCs are known to differentiate into structurally aberrant lens fiber cells which contribute to the development of “pearl-like” PCO when present in the visual axis, and Soemmering’s ring when restricted to the ocular periphery [35]. Thus, it is possible that the myofibroblasts formed in FNcKO capsular bags may trans-differentiate into the lens fiber cells after 3 days PCS. Remnant LCs from both wildtype (WT) and FNcKO mice express little protein for either the transcription factor cMaf, which controls lens fiber cell differentia tion [53] or aquaporin 0, a lens fiber cell preferred membrane protein [54], immediately PCS (Fig. 2B and Supplemental Fig. 2). By 48 h PCS, some remnant LCs express cMaf and aquaporin 0 in both WT and FNcKO eyes, and the expression of these lens fiber cell markers become more robust by 5 days PCS (aquaporin 0, WT **P = 0.002; FNcKO *P = 0.032) suggesting that fiber cell differentiation is unhindered in FNcKO capsular bags (Fig. 2B and Supplemental Fig. 2).
Finally, we followed the expression of a classic epithelial cell marker, E cadherin [55], to determine if some of the myofibroblasts convert back to an epithelial phenotype upon the deletion of fibronectin PCS. As expected, both WT and FNcKO lens cells express appreciable amounts of E cadherin at 0 h PCS (Fig. 2B). However, by 48 h PCS, E cadherin protein levels are significantly downregulated in both WT and FNcKO capsular bags (WT *P = 0.019; FNcKO ***P ≤ 0.001). However, while this downregulation is sustained through 5 days PCS in WT lens cells (0 h vs 5 days PCS **P = 0.004), E cadherin protein levels significantly upregulate in FNcKO capsular bags between 48 h and 5 days PCS (**P = 0.003). This results in E cadherin levels being significantly higher in FNcKO LCs than WT controls at 5 days PCS (***P = 0.001) (Fig. 2B). Overall, these data suggest that mesenchymal to epithelial transition [56], perhaps associated with reductions in cell proliferation and increases in lens fiber cell differentiation, may lead to the observed lack of sustained fibrotic response in FNcKO capsular bags at 5 days PCS.
RNAseq analysis revealed that WT LCs exhibit elevated mRNA levels for genes known to play roles in fibrosis and inflammation, and reduced expression of lens markers, at 48 h PCS, while only a small subset of these expression differences is altered in FNcKO LCs
In order to elucidate the mechanisms by which fibronectin mediates the prolonged fibrotic response PCS, RNAseq was used as a global and unbiased approach to identify all genes whose expression levels change in WT lens epithelial cells (LCs) by 48 h post cataract surgery-PCS (the time point when canonical TGFβ signaling is first detectable in LCs PCS) [46], and which of those genes require fibronectin for their differential expression PCS (data deposited into the Gene Expression Omnibus (GEO) under accession number GSE119879). This analysis revealed that 2507 genes are expressed at significantly different levels in WT LCs at 48 h PCS compared to 0 h PCS (1569 genes upregulated, 938 genes downregulated) based on criteria that we have previously found to filter for biologically significant gene expression changes in lens cells (False Discovery Rate (FDR) ≤0.05; Fold Change (FC) in mRNA levels greater than 2; an absolute difference between group means > 2 RPKM (Reads Per Kilobase Million); expressed higher than 2 RPKM either immediately PCS or 48 h later) [50]. As expected, these differentially expressed genes (DEGs) included many fibrotic genes that are known to upregulate in LCs undergoing EMT (Table 1A) as well as other genes known to be involved in fibrosis in other systems, but unreported or poorly described in PCO (Table 1B). Further, consistent with our recent report describing gene expression changes observed in LCs at 24 h PCS [46], the most enriched biological pathway in WT LCs at 48 h PCS identified by iPathway guide corresponds to cytokine-cytokine receptor interactions which included numerous known inflammatory proteins (Table 1C), many of which are also upregulated at 24 h PCS [46]. Finally, the expression of many genes important for lens structure and function downregulate in LCs by 48 h PCS as well as would be expected in LCs undergoing EMT (Table 1D).
Table 1A.
Known markers of LC EMT upregulated in remnant LCs at 48 h PCS.
| Gene ID | Gene description | Fold change (FC) | False discovery rate (FDR) | WT RPKM 0 h | WT RPKM 48 h | Reference |
|---|---|---|---|---|---|---|
| Tnc | Tenascin C | 116 | 7.8E-44 | 1 | 156 | [19] |
| Col1a1 | Collagen, type I, alpha 1 | 83 | 2.1E-42 | 0.82 | 79 | [139,140] |
| MMP9 | Matrix metallopeptidase 9 | 70 | 7.4E-14 | 0.47 | 40 | [141] |
| Fn1 | Fibronectin 1 | 53 | 1.3E-44 | 2 | 135 | [19] |
| Tgfβi | TGFβ induced protein | 42 | 5.7E-52 | 7 | 359 | [19,142] |
| Itga5 | Integrin alpha 5 | 7 | 5.1E-24 | 6 | 50 | [111] |
| Acta2 | Alpha smooth muscle actin | 4 | 3.3E-08 | 74 | 380 | [139] |
| Tgfβr2 | Transforming growth factor, beta receptor II | 3 | 3.4E-07 | 3 | 10 | [143] |
| Tgfβ1 | Transforming growth factor, beta 1 | 3 | 6.1E-08 | 32 | 99 | [144] |
| Mylk | Myosin light chain kinase | 3 | 0.001 | 1.75 | 7 | [145] |
| Grem1 | Gremlin-1 | 380 | 1.6E-40 | 1.4 | 642 | [81] |
Table 1B.
Genes upregulated in LCs at 48 h PCS that are known to be involved in fibrosis in other systems, but are unreported, or only poorly described, in PCO.
| Gene ID | Gene description | Fold change (FC) from WT 0 h to WT 48 h PCS in LCs | False discovery rate (FDR) | WT Mean RPKM 0 h | WT Mean RPKM 48 h | Reference |
|---|---|---|---|---|---|---|
| Arg1 | Arginase | 411 | 8.2E-81 | 0.38 | 185 | [146] |
| Spp1 | Osteopontin | 126 | 9.2E-76 | 3 | 461 | [147] |
| ECM1 | extracellular matrix protein 1 | 85 | 8.9E-68 | 4 | 425 | [148] |
| Lox | Lysyl oxidase | 44 | 5.0E-78 | 0.47 | 24 | [149] |
| Thbs1 | Thrombospondin 1 | 23 | 9.4E-22 | 3 | 85 | [62] |
| Tagln | Transgelin | 17 | 5.2E-44 | 6 | 119 | [150] |
| Postn | Periostin | 5 | 3.8E-10 | 3 | 19 | [151] |
| Osmr | Oncostatin M Receptor | 5 | 9.4E-18 | 5.5 | 30 | [152] |
| Ltbp1 | Latent transforming growth factor beta binding protein 1 | 3 | 1.894E-07 | 30 | 98 | [71] |
Table 1C.
Genes known to be involved in inflammation are upregulated by LCs at 48 h PCS.
| Gene ID | Gene description | Fold change (FC) | False discovery rate (FDR) | WT Mean RPKM 0 h | WT Mean RPKM 48 h | Reference |
|---|---|---|---|---|---|---|
| Tnfrsf11b | Tumor necrosis factor receptor superfamily, member 11b | 1587 | 8.0E-21 | 0 | 8 | [153] |
| Cxcl1 | Chemokine (C-X-C motif) ligand 1 | 1288 | 2.6E-19 | 0 | 20 | [154] |
| S100a9 | S100 calcium binding protein A9 | 643 | 1.8E-23 | 0.06 | 68 | [155] |
| Cxcl3 | Chemokine (C-X-C motif) ligand 3 | 213 | 6.7E-19 | 0.2 | 55 | [156] |
| Igfbp3 | Insulin-like growth factor binding protein 3 | 151 | 1.0E-102 | 0.45 | 80 | [157] |
| Slfn4 | Schlafen 4 | 110 | 1.7E-32 | 0.1 | 17 | [158] |
| Ccl7 | Chemokine (C-C motif) ligand 7 | 108 | 9.3E-44 | 0.4 | 56 | [159] |
| S100a8 | S100 calcium binding protein A8 | 102 | 4.8E-16 | 0.4 | 47 | [160] |
| Lcn2 | Lipocalin 2 | 60 | 1.9E-53 | 98 | 6715 | [161] |
| Hmox1 | Heme oxygenase 1 | 7 | 3.3E-08 | 15 | 123 | [162] |
Table 1D.
Genes that are preferentially expressed in the lens that downregulate in LCs by 48 h PCS.
| Gene ID | Gene description | Fold change (FC) | False discovery rate (FDR) | WT Mean RPKM 0 h | WT Mean RPKM 48 h | Reference |
|---|---|---|---|---|---|---|
| Crygd | Crystallin, gamma D | −71 | 1.1E-06 | 306 | 5 | [163] |
| Crygb | Crystallin, gamma B | −32 | 5.3E-05 | 650 | 24 | [164] |
| Dnase2b | Deoxyribonuclease II beta | −24 | 1.6E-06 | 2.4 | 0.11 | [165] |
| Crygc | Crystallin, gamma C | −20 | 1.5E-05 | 368 | 21 | [164] |
| Cryba4 | Crystallin, beta A4 | −8 | 0.0002 | 2242 | 342 | [164] |
| Bfsp1 | Beaded filament structural protein 1 | −7 | 8.3E-06 | 202 | 33 | [163] |
| Mip | Major intrinsic protein of lens fiber | −7 | 7.9E-10 | 1264 | 215 | [166] |
| Cryba1 | Crystallin, beta A1 | −6 | 3.9E-06 | 18405 | 3522 | [164] |
| Lenep | Lens epithelial protein | −6 | 0.004 | 191 | 38 | [167] |
| Crybb1 | Crystallin, beta B1 | −6 | 2.2E-05 | 1735 | 348 | [164] |
| Crygs | Crystallin, gamma S | −5 | 6.7E-06 | 9090 | 2003 | [164] |
| Cryba2 | Crystallin, beta A2 | −5 | 1.0E-05 | 5950 | 1415 | [164] |
| Lim2 | Lens intrinsic membrane protein 2 | −5 | 1.3E-05 | 409 | 100 | [168] |
| Crybb2 | Crystallin, beta B2 | −4 | 0.0004 | 31276 | 10027 | [164] |
Comparison of RNA expression profiles between WT and FNcKO LCs at 48 h PCS revealed that the expression levels of 89 genes that meet the criteria for likely biological significance (False Discovery Rate (FDR) corrected p-value ≤ 0.05, Fold Change (FC) ≥ 2, Reads Per Kilobase Million (RPKM) ≥ 2) [50] were significantly different. Fifteen DEGs over lapped with the list of genes that were differentially expressed between unoperated WT and FNcKO lenses, leaving 74 DEGs differentially expressed in FNcKO lens cells at 48 h PCS (Supplemental Table 3). Of these, 4 were genes that normally downregulate in WT LCs by 48 h PCS but do not in FNcKO lenses, while 59 were genes that normally upregulate in remnant LCs whose upregulation was attenuated in FNcKO LCs (Supplemental Table 4). Further, consistent with the muted fibrotic response that LCs from FNcKO lenses undergo PCS, the mRNA levels of several genes associated with fibrotic disease exhibit attenuated upregulation in FNcKO LCs at 48 h PCS (Table 2A), while another notable subset of attenuated DEGs plays known roles in inflammatory responses (Table 2B) PCS.
Table 2A.
Genes known to be involved in fibrosis are less upregulated in remnant LCs of FNcKOs at 48 h PCS.
| Gene ID | Gene description | Fold change (FC) from WT to FNcKO at 48 h PCS in LCs (attenuated upregulation) | False discovery rate (FDR) | WT Mean RPKM At 48 h | FNcKO Mean RPKM At 48 h | Reference |
|---|---|---|---|---|---|---|
| Grem1 | Gremlin 1 | −7 | 0.0001 | 642 | 87 | [81] |
| Col1a1 | Collagen, type I, alpha 1 | −6.5 | 1.5E-10 | 79 | 11 | [139,140] |
| Mylk | Myosin, light polypeptide kinase | −5.6 | 7.4E-06 | 7 | 1 | [145] |
| Postn | Periostin | −4.6 | 2.7E-09 | 19 | 4 | [151] |
| Lox | Lysyl oxidase | −4.5 | 2.1E-09 | 24 | 5 | [149] |
Table 2B.
Genes known to be involved in inflammation are less upregulated by FNcKO LCs at 48 h PCS.
| Gene ID | Gene description | Fold change (FC) from WT to FNcKO at 48 h PCS in LCs (attenuated upregulation) | False discovery rate (FDR) | WT Mean RPKM At 48 h | FNcKO Mean RPKM At 48 h | Reference |
|---|---|---|---|---|---|---|
| Serpina3f | Serine (or cysteine) peptidase inhibitor, clade A, member 3F | −157 | 1.3E-39 | 20 | 0.11 | [169] |
| Serpina3m | Serine (or cysteine) peptidase inhibitor, clade A, member 3 M | −94 | 4.1E-42 | 33 | 0.32 | [169] |
| Serpina3c | Serine (or cysteine) peptidase inhibitor, clade A, member 3C | −60 | 2.9E-20 | 13 | 0.2 | [169] |
| Serpina3h | Serine (or cysteine) peptidase inhibitor, clade A, member 3H | −14 | 1.4E-18 | 21 | 1.5 | [169] |
| Lbp | Lipopolysaccharide binding protein | −11 | 2.4E-15 | 16 | 1.4 | [170] |
| Slfn4 | Schlafen 4 | −7 | 1.1E-07 | 17 | 2.3 | [158] |
| Crlf1 | Cytokine receptor-like factor 1 | −3 | 0.008 | 26 | 8 | [171] |
| Slfn5 | Schlafen 5 | −3 | 0.01 | 5 | 1.5 | [172] |
Fibronectin is required for the expression and assembly of a subset of fibrotic ECM molecules produced by lens cells undergoing EMT post cataract surgery
EMT of LCs produces myofibroblasts that synthesize a “fibrotic” extracellular matrix (ECM) which provides a scaffold for cell attachment, stiffens the tissue, and contributes to the light scatter caused by fibrotic PCO [35]. In other tissues/cell types, fibronectin is the initial scaffold that allows for fibrotic extracellular matrix (ECM) assembly [13,20]; however, its role in the formation of the fibrotic matrix associated with PCO has not been described. Notably, intact adult FNcKO lenses exhibit a 2 fold increase in the expression of the Col1a1 and Col1a2 genes (Supplemental Table 2) which encode the “pro-fibrotic” matrix molecule, collagen I [57]. However, at 48 h PCS, Col1a1 levels upregulate over 80 fold in WT LCs compared to time 0 (Table 1A), while 48 h PCS FNcKO LCs express significantly lower levels of Col1a1 mRNA compared to WT (Table 2A). Similarly, the mRNA encoding lysyl oxidase (Lox), an enzyme required for collagen I cross-linking [58,59], upregulates over 40 fold in WT LCs by 48 h PCS (Table 1B), while this response is also attenuated in FNcKO LCs (Table 2A). Consistent with the RNAseq data, we have found that while FNcKO LCs produce collagen I and Lox proteins at 48 h PCS, their levels are significantly attenuated compared to WT LCs (collagen I **P = 0.009; Lox ***P ≤ 0.001). This result is more dramatic at 5 days PCS as FNcKO LCs are not associated with collagen I fibrils at this time (WT vs FNcKO ***P ≤ 0.001), while the levels of Lox protein are still attenuated (WT vs FNcKO ***P ≤ 0.001) (Fig. 3). Overall, this suggests that cellular fibronectin produced by LCs PCS is required not just as a scaffold for collagen I assembly, but also triggers signal transduction cascades that regulate genes required for collagen I and Lox production during EMT.
Fig. 3. The production and assembly of fibrotic ECM PCS require fibronectin expression by LCs.
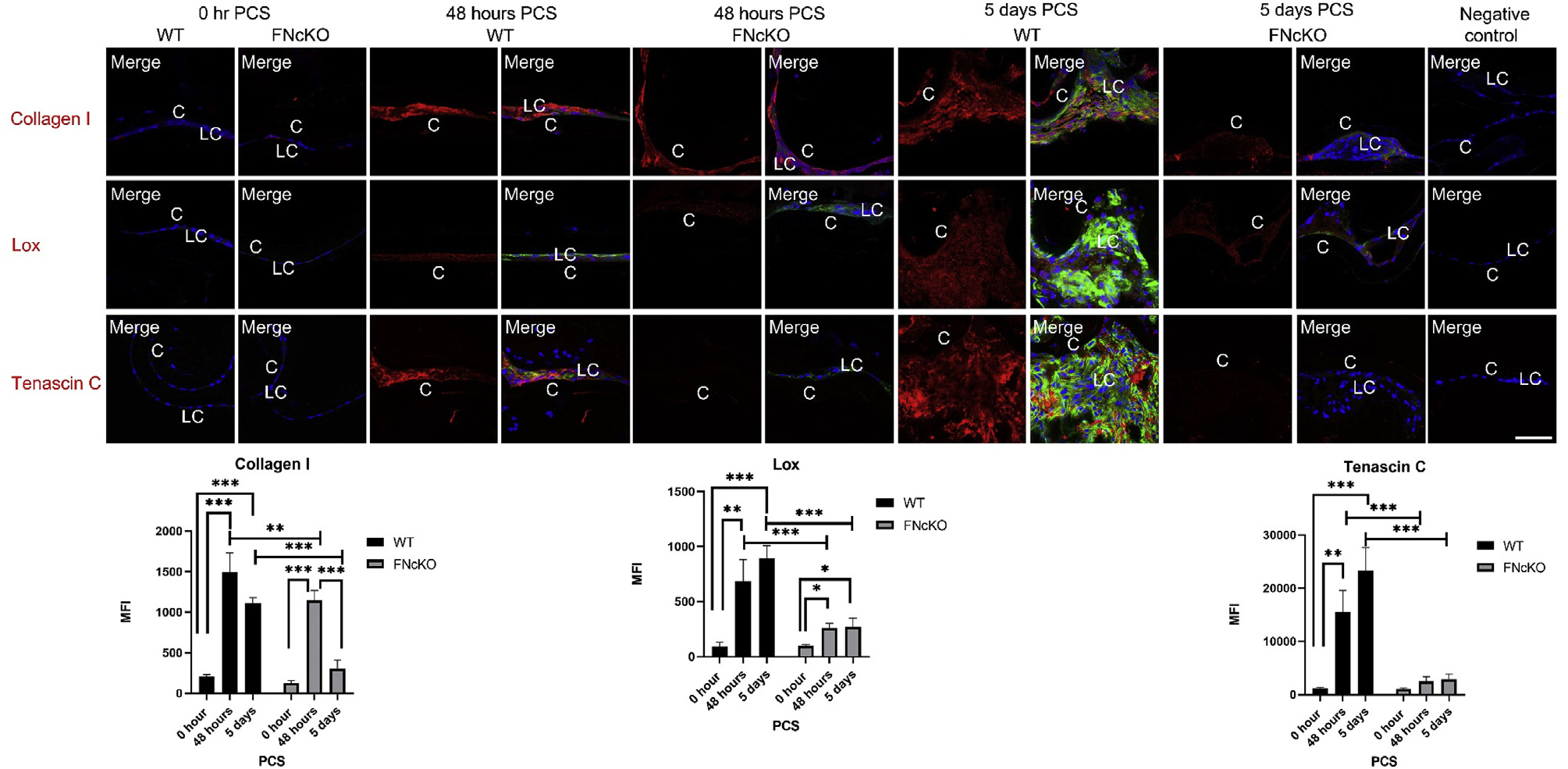
At 0 h PCS, only low levels of fibrotic ECM proteins (collagen I and tenascin C) and the enzymatic ECM crosslinker Lysyl oxidase (Lox) are detected in both WT and FNcKO LCs. Both WT and FNcKO LCs significantly upregulate collagen I protein expression by 48 h PCS (WT ***P ≤ 0.001; FNcKO ***P ≤ 0.001) although FNcKO LCs exhibit less association with collagen I fibrils compared to WT (**P ≤ 0.009). By 5 days PCS, WT LCs expressing αSMA are associated with a robust matrix of collagen I (***P ≤ 0.001) while this is absent in the area surrounding FNcKO lens cells (WT vs FNcKO ***P ≤ 0.001). Similarly, Lox protein upregulates in both WT and FNcKO LCs at 48 h PCS (WT **P = 0.004; FNcKO *P = 0.020) while this signal is much less pronounced in FNcKO LCs compared to WT (***P ≤ 0.001). There is a significant increase in tenascin C fibrils surrounding WT LCs both at 48 h (**P = 0.005) and 5 days (***P ≤ 0.001) PCS compared to 0 h PCS. However, FNcKO lens cells are associated with significantly less tenascin C fibrils both at 48 h PCS (***P ≤ 0.001) and 5 days PCS (***P ≤ 0.001) compared to WT. Collagen I, Tenascin C, and Lox (red), αSMA (green), are merged with DNA detected by Draq5 (blue). Scale bars: 35 μm. LC, remnant lens epithelial cells/lens cells; C, lens capsule. All experiments had N = 3. Values are expressed as mean ± SEM. Asterisks (*) indicate statistically significant MFI between WT and FNcKO at a PCS or between two PCS time points.
In contrast, while RNAseq analysis reveals that the mRNA levels for tenascin C, which encodes another common fibrotic ECM protein [19,60] upregulate 116 fold at 48 h PCS in WT capsular bags, this gene still upregulates to a similar level in FNcKO capsular bags, suggesting that tenascin C gene expression is not under the control of fibronectin-induced signaling. However, as tenascin C also has been reported to be dependent on fibronectin for its incorporation into ECM [61] and colocalizes with cell-associated fibronectin PCS (Supplemental Fig. 3B), we investigated the fate of tenascin C fibril formation PCS in the absence of fibronectin. While tenascin C mRNA levels increase in FNcKO LCs similar to wildtype at 48 h PCS, tenascin C protein fibril deposition around FNcKO LCs is significantly reduced at both 48 h (***P ≤ 0.001) and 5 days PCS (***P ≤ 0.001) compared to WT LCs (Fig. 3) suggesting that fibronectin regulates tenascin C fibril formation but not tenascin C gene expression PCS.
Interestingly, thrombospondin-1 and extracellular matrix protein 1 are two other fibrotic extracellular matrix proteins [62,63] whose mRNAs upregulate similarly in WT and FNcKO LCs PCS. While the matrix deposition of both is also proposed to be under fibronectin regulation [64,65], both are still deposited around FNcKO LCs at 5 days PCS in a pattern similar to that seen in WT LCs (Supplemental Fig. 4) although quantitation shows that significantly less thrombospondin is deposited around FNcKO LCs at 5 days PCS (**P = 0.003) while ECM1 deposition is unaffected in FNcKO lenses (P = 0.925). This suggests that fibronectin is essential for the deposition of only a subset of fibrotic ECM molecules PCS.
In aggregate, these data suggest that fibronectin is both critical for the assembly of the fibrotic ECM during PCO, as well as the activation of signal transduction cascades that elevate the expression of some fibrotic ECM genes in LCs PCS.
Deletion of fibronectin from the lens alters integrin expression and downstream signaling PCS
Notably, LCs elevate the protein expression of the fibronectin receptors α5β1-integrin and several αV class integrins PCS [19,45,66]. At 48 h PCS, WT LCs upregulate the protein expression of α5 integrin (**P = 0.005), αV integrin (P = 0.070) and β1-integrin (P ≤ 0.001), and this upregulation remains robust at 5 days PCS (α5 integrin **P = 0.008; αV integrin **P = 0.001; b1 integrin ***P ≤ 0.001) (Fig. 4). However, compared to WT LCs, α5 and β1-integrin protein levels fail to upregulate in FNcKO LCs at either 48 h (α5-integrin ***P ≤ 0.001; β1-integrin, ***P ≤ 0.001) or 5 days PCS (α5-integrin, ***P ≤ 0.001; β1-integrin, ***P ≤ 0.001). In contrast, FNcKO LCs still upregulate the expression of the αV-integrin subunit by 48 h PCS (**P = 0.004) at levels similar to WT (P = 0.168). However, αV integrin levels are significantly attenuated in FNcKO LCs at 5 days PCS (***P ≤ 0.001) compared to WT LCs (Fig. 4). These data are suggesting that fibronectin expression in LCs is necessary for the upregulation of its integrin receptors PCS.
Fig. 4. Fibronectin expression by LCs is necessary for the upregulation of some integrin subunits and integrin signaling PCS.
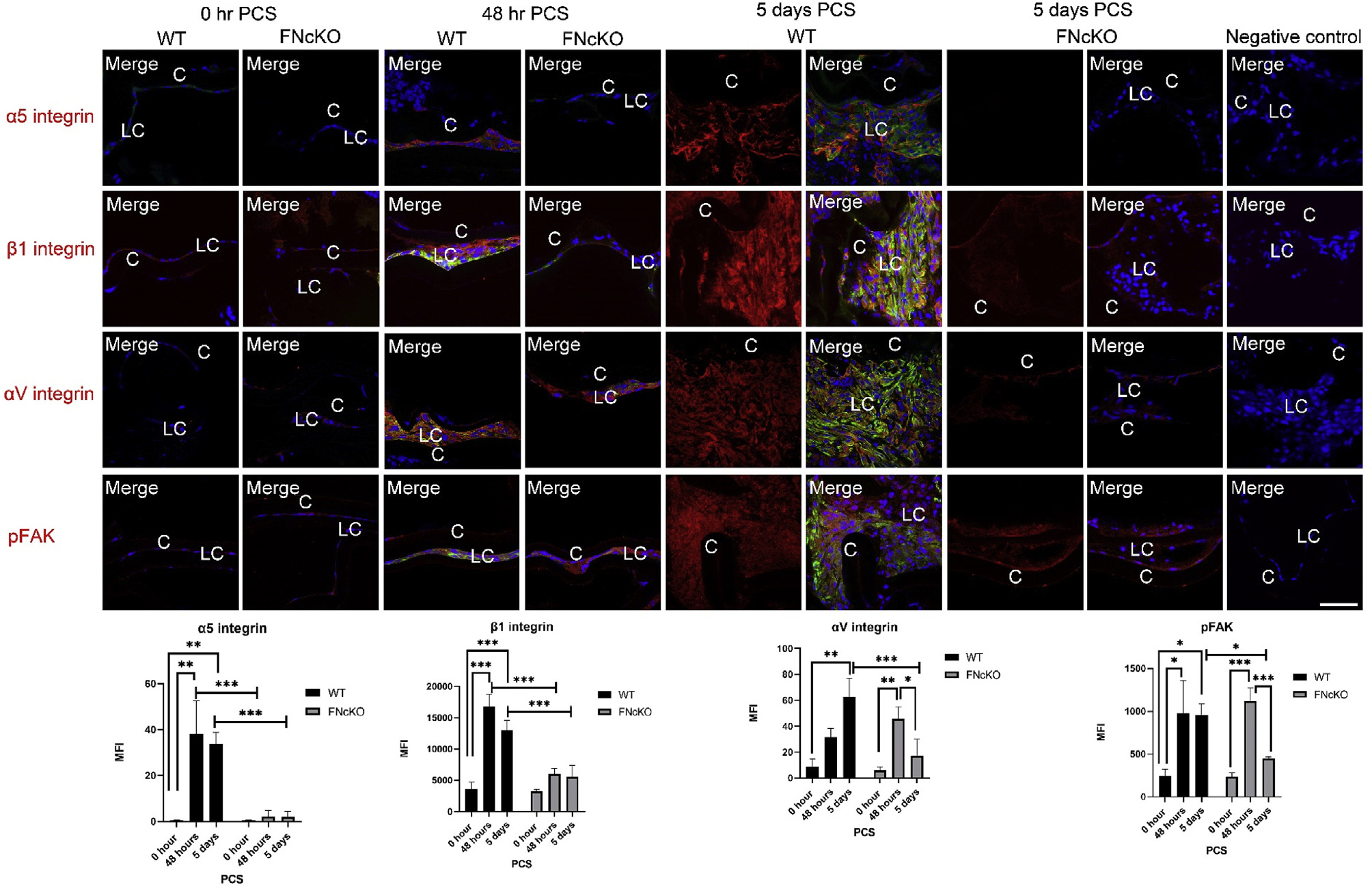
At 0 h PCS, both WT and FNcKO LCs exhibit low protein expression for all three integrins (α5-integrin, β1-integrin and αV-integrin) while pFAK levels are also low. However, by 48 h PCS, WT LCs significantly upregulate the protein levels of α5 integrin (**P = 0.005) and β1 integrin (***P ≤ 0.001) while the upregulation of αV integrin did not reach 95% confidence of upregulation. (P = 0.070). However, the expression of all three proteins becomes quite robust by 5 days PCS (α5 integrin **P = 0.008; αV integrin **P = 0.001; b1 integrin ***P ≤ 0.001). Concomitant with the detected elevation in integrin expression, pFAK levels are significantly elevated in WT LCs by 5 days PCS (*P = 0.022). However, FNcKO LCs fail to upregulate α5- and β1-integrin protein levels at both 48 h and five days PCS compared to WT leading FNcKO LCs to have significantly less integrin staining than control at 48 h(α5-integrin ***P ≤ 0.001; β1-integrin, ***P ≤ 0.001) and 5 days PCS (α5-integrin ***P ≤ 0.001; β1-integrin ***P ≤ 0.001) compared to WT. In contrast, FNcKO LCs also initially upregulate aV-integrin (**P = 0.004) and pFAK levels (**P ≤ 0.004) at 48 h PCS, at levels not significantly different from WT 48 h (αV-integrin, P = 0.168; pFAK P = 0.576). Notably, this is not sustained as αV-integrin and pFAK upregulation is attenuated at 5 days PCS (αV-integrin ***P ≤ 0.001; pFAK *P = 0.013) compared to WT. α5-integrin, β1-integrin, αV integrin, and pFAK (red) are merged with αSMA (green) and DNA detected by Draq5 (blue). Scale bars: 35 μm; LC, remnant lens epithelial cells/lens cells; C, lens capsule. All experiments had N = 3. Values are expressed as mean þ SEM. Asterisks (*) indicate statistically significant difference in MFI between WT and FNcKO at a time PCS or between two PCS time points..
Since integrin expression fails to upregulate in FNcKO capsular bags PCS and the assembly of ECM around LCs is altered PCS (see Fig. 3), next we sought to determine the levels of phosphorylated focal adhesion kinase (pFAK) which is the activated form of an important signaling molecule that transmits integrin signals [67]. Although pFAK levels upregulate to a similar extent in WT and FNcKO LCs at 48 h PCS (P = 0.576), WT αSMA expressing LCs sustain elevated pFAK levels at 5 days PCS (*P = 0.022), while pFAK levels are significantly lower in FNcKO LCs compared to controls (*P = 0.013) at this time (Fig. 4). These data show that fibronectin expression is required for LCs to sustain FAK activation post cataract surgery.
Late PCS elevations in TGFβ signaling are attenuated in FNcKO LCs
As it is established that transforming growth factor beta (TGFβ) signaling is critical for sustained fibrotic PCO [45], and fibronectin plays a role in the regulation of the latent TGFβ complex in other systems [68,69], we next determined the extent of canonical TGFβ pathway activation in WT and FNcKO LCs PCS by following pSMAD2/3 levels. Activation of TGFβ signaling is seen both in WT and FNcKO lens cells at 48 h PCS (WT *P = 0.037; FNcKO *P = 0.014) and this was not significantly different between WT and FNcKO LCs (P = 0.216) (Fig. 5) which supports the idea that fibronectin is not a major driver of the early fibrotic response PCS (Fig. 2A). However, while WT LCs exhibit enhanced activation of canonical TGFβ signaling at 5 days PCS (***P ≤ 0.001), pSMAD2/3 is barely detected in FNcKO LCs at 5 days PCS which is significantly different from WT (***P ≤ 0.001) suggesting that the upregulation of fibronectin by LCs is required for sustained TGFβ signaling PCS (Fig. 5).
Fig. 5. TGFβ signaling is attenuated in FNcKO lens cells at later times PCS.

At 0 h PCS, pSMAD2/3 is not detected in either WT or FNcKO LCs. However, at 48 h PCS, pSMAD2/3 is first detected in WT LCs (*P = 0.037) which becomes robust at 5 days PCS (***P ≤ 0.001). FNcKO LCs also upregulate pSMAD2/3 levels at 48 h PCS (*P = 0.014) at levels quantitatively similar levels to WT (P = 0.216), while these levels do not continue to upregulate 5 days PCS and are significantly reduced (***P ≤ 0.001) compared to WT. pSMAD2/3 (downstream effector of canonical TGFβ signaling) (red), αSMA (green), and DNA detected by Draq5 (blue). Scale bars: 35 μm. LC, remnant lens epithelial cells/lens cells; C, lens capsule. All experiments had N = 3. Values are expressed as mean + SEM. Asterisks (*) indicate statistically significant difference in MFI between WT and FNcKO at a time PCS or between two PCS time points.
Extracellular matrix deposition of the latent TGFβ complex around LCs PCS is dependent on fibronectin
Latent TGFβ is secreted from cells bound to latent TGFβ binding proteins (LTBPs) and is incorporated into the extracellular matrix prior to the activation needed for TGFβ to initiate signaling transduction upon injury [68,70]. In other systems, fibronectin binds LTBPs directly or indirectly to tether the latent TGFβ to the ECM [71–73]. Out of four LTBPs, LTBP1–3 are all abundantly expressed in adult LCs at the mRNA level (30, 83, and 70 RPKM, respectively). Notably, the mRNA for LTBP1, which uniquely binds to cell-associated fibronectin [71], upregulates 3 fold in WT LCs by 48 h PCS. (Table 1B). Consistent with this, immunolocalization found that significant LTPB1 protein was associated with fibronectin deposits surrounding αSMA positive LCs at 5 days PCS (Fig. 6A and B). In contrast, this LTBP1 deposition was significantly reduced around FNcKO LCs at 5 days PCS (Fig. 6B) compared to WT (**P = 0.006) suggesting that fibronectin influences TGFβ signaling in LCs PCS, at least in part, at the level of matrix deposition of the latent TGFβ complex.
Fig. 6. LCs are associated with latent TGFβ binding protein at 5 days PCS, and this is highly attenuated in FNcKO LCs.
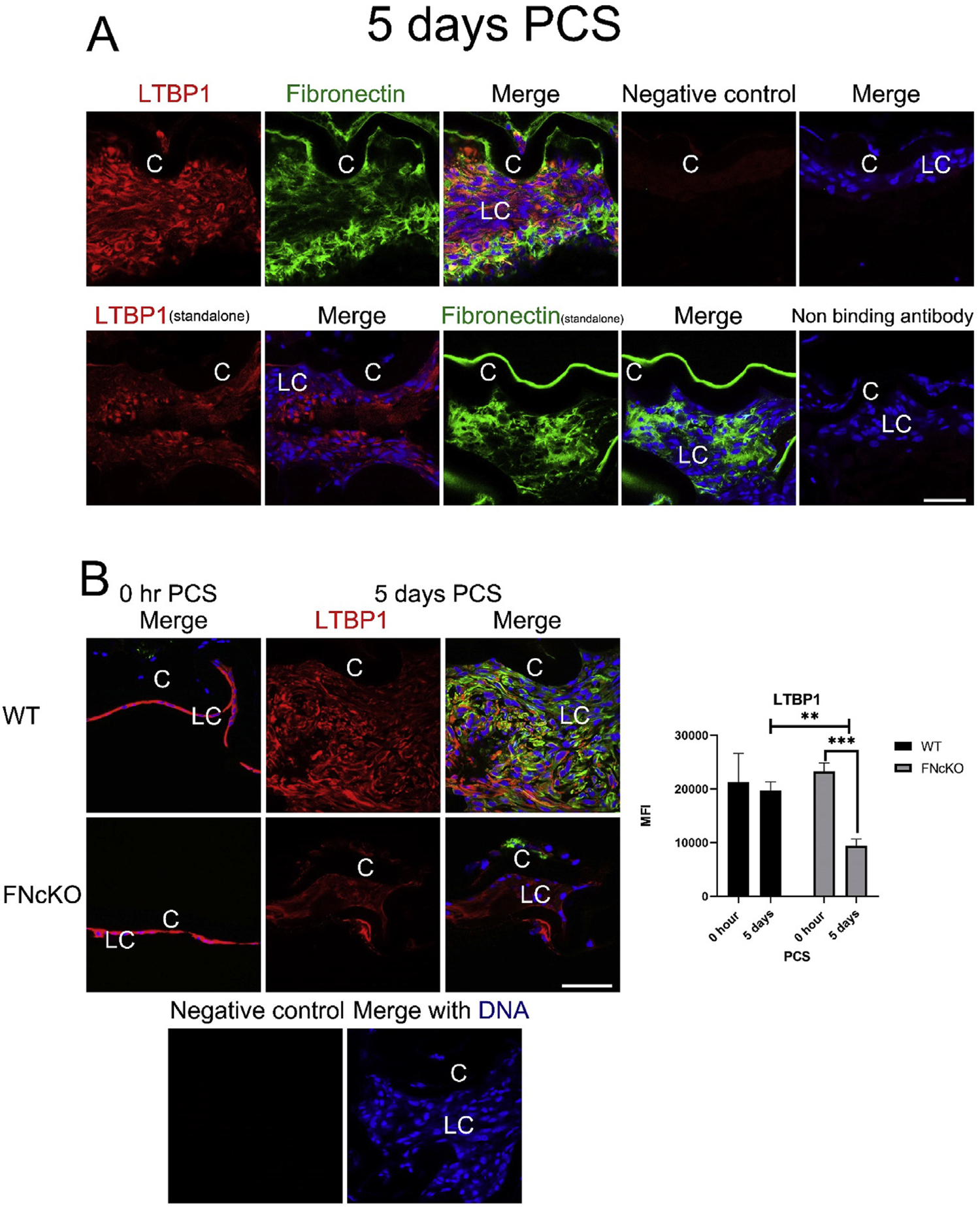
(A) At 5 days PCS, WT LCs are associated with robust levels of cell-associated fibronectin and LTBP1. Fibronectin (green) and LTBP1 (red) merged with DNA detected by Draq5 (blue). (B) At 0 h PCS, appreciable levels of LTBP1 protein are detected in both WT and FNcKO LCs whereas αSMA protein levels are low. However, by 5 days PCS, WT LCs maintain the robust levels of ECM-associated LTBP1 whereas extracellular deposition of LTBP1 around FNcKO LCs is greatly attenuated (**P = 0.006) compared to WT and is even reduced compared to 0 h PCS (***P ≤ 0.001). αSMA (green) and LTBP1 (red) merged with DNA detected by Draq5 (blue). Scale bars: 35 μm. LC, remnant lens epithelial cells/lens cells; C, lens capsule. All experiments had N = 3. Values are expressed as mean ± SEM. Asterisks (*) indicate statistically significant difference in MFI between WT and FNcKO at a time PCS or between two PCS time points.
The attenuation of canonical BMP signaling in LCs PCS requires fibronectin
Canonical Bone Morphogenetic Protein (BMP) signaling is required for normal lens development [74–76], while it has been proposed that BMP signaling can counterbalance TGFβ signaling in fibrotic diseases [77]. Intact adult lenses have easily detectable levels of pSMAD1/5/8 (Supplemental Fig. 5) in the lens epithelium, and this is not affected by the deletion of the fibronectin gene (Fig. 7). After surgery, remnant LCs from WT mice significantly downregulate pSMAD1/5/8 signaling by 24 h PCS (*P = 0.013), while both qualitatively and quantitatively, more FNcKO than WT LCs retain pSMAD1/5/8 at this time (P = 0.390). pSMAD1/5/8 levels continue to fall in WT LCs through 48 h PCS (**P = 0.006) and by 5 days PCS, pSMAD1/5/8 staining is largely absent from WT LCs (0 h vs 5 days PCS; (**P = 0.004). In contrast, pSMAD1/5/8 staining remains prominent in FNcKO LCs at all times PCS investigated, and is significantly elevated at 5 days PCS compared to WT (**P = 0.003) (Fig. 7) suggesting that fibronectin is necessary for the sustained suppression of canonical BMP signaling in LCs PCS.
Fig. 7. The dynamics of BMP signaling in PCS LCs upon deletion of the fibronectin gene.
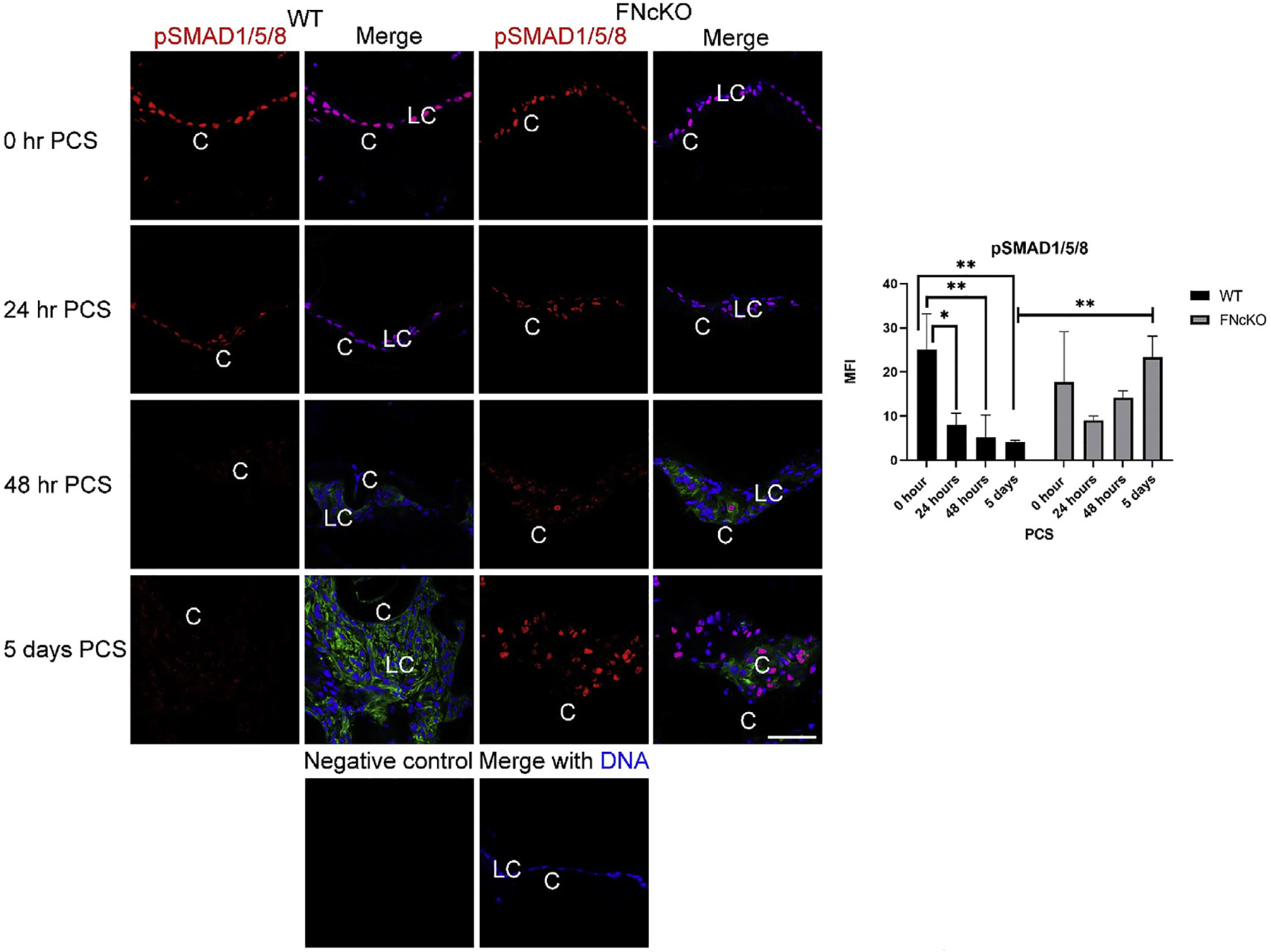
Immediately following lens fiber cell removal, both WT and FNcKO remnant lens cells stain robustly for pSMAD1/5/8, while this signaling begins to decrease at 24 h PCS in WT LCs (P = 0.013) although this does not occur in FNcKO LCs (P = 0.390). pSMAD1/5/8 levels continue to downregulate in WT LCs at 48 h PCS (**P = 0.006), and this reduction of pSMAD1/5/8 levels persists through 5 days PCS in WT LCs expressing αSMA (**P = 0.004). In contrast, pSMAD1/5/8 levels never significantly downregulate in FNcKO LCs, so they have elevated levels of pSMAD1/5/8 at 5 days PCS (**P = 0.003) compared to WT LCs and do not express elevated levels of αSMA. pSMAD1/5/8 (downstream of BMP signaling)=(red), αSMA (green), and DNA detected by Draq5 (blue). Scale bars: 35 μm. LC, remnant lens epithelial cells/lens cells; C, lens capsule. All experiments had N = 3. Values are expressed as mean ± SEM. Asterisks (*) indicate statistically significant change in MFI between WT and FNcKO at a PCS or between two PCS time points.
Fibronectin production by LCs is required for the upregulation of gremlin-1 expression PCS
In order to obtain further mechanistic insight into the function of fibronectin PCS, we investigated the list of genes differentially expressed at the mRNA level in FNcKO LCs at 48 h PCS for those with the potential to mechanistically regulate BMP and TGFβ signaling. Gremlin-1, a secreted BMP antagonist [77] and profibrotic factor [78–80], is upregulated 379 fold in WT LCs at 48 h PCS and this upregulation was attenuated 7 fold in FNcKO LCs (Table 2A). As gremlin-1 has been reported to regulate TGFβ signaling in different fibrotic conditions [78–82], we sought to determine the expression dynamics of gremlin-1 at the protein level PCS. As expected, based on the RNAseq data, no gremlin-1 protein was detected in either WT or FNcKO LCs immediately following surgery. Consistent with the upregulation of gremlin-1 detected at the mRNA level, WT LCs begin to express detectable levels of gremlin-1 protein by 24 h PCS (***P ≤ 0.001) and this upregulation is also seen in FNcKO LCs (*P = 0.015). WT LCs continue to upregulate gremlin-1 protein levels through 48 h PCS (***P ≤ 0.001) and these levels remain quite high through 5 days PCS (***P ≤ 0.001). In contrast, while gremlin-1 protein levels also continue to elevate in FNcKO LCs at 48 h PCS (***P ≤ 0.001), gremlin-1 levels are significantly lower than seen in WT 48 h PCS (**P = 0.004) consistent with the RNAseq results (Table 2A), resulting in FNcKO LCs exhibiting greatly reduced gremlin 1 staining compared to WT at 5 days PCS (***P ≤ 0.001) (Fig. 8A).
Fig. 8. Exogenous gremlin-1 treatment of FNcKO capsular bags rescues the defect in TGFβ signaling and fibrotic marker expression PCS.

(A) At 0 h PCS, little expression of gremlin-1 protein is detected in both WT and FNcKO LCs. However, by 48 h PCS, gremlin-1 protein expression is elevated in both WT (***P ≤ 0.001) and FNcKO LCs (***P ≤ 0.001) although the expression is significantly less in FNcKO LCs (**P = 0.004) compared to WT. In contrast, gremlin-1 levels are greatly attenuated in FNcKO LCs by 5 days PCS (***P ≤ 0.001) compared to WT whereas WT LCs maintain the robust expression of gremlin-1 (***P ≤ 0.001). Gremlin-1 (red) is merged with αSMA (green) and DNA detected by Draq5 (blue). Scale bars: 35 μm. LC, remnant lens epithelial cells/lens cells; C, lens capsule. All experiments had N = 3. Values are expressed as mean ± SEM. Asterisks (*) indicate statistically significant MFI between WT and FNcKO at a PCS or between two PCS time points. (B) Administration of exogenous gremlin-1 to FNcKO capsular bags elevates the levels of the fibrotic proteins αSMA (*P = 0.015), tenascin C (**P = 0.002), and collagen I (*P = 0.017) concomitant with elevated levels of pSMAD2/3 levels (*P = 0.013) at 5 days PCS. In contrast, exogenous gremlin-1 treatment did not reduce pSMAD1/5/8 levels in FNcKO capsular bags (P = 0.440). Collagen I, Tenascin C, pSMAD2/3 (downstream of TGFβ signaling), pSMAD1/5/8 (downstream of BMP signaling) (red), αSMA (green) and DNA detected by Draq5 (blue). Scale bars: 35 μm. LC, remnant lens epithelial cells/lens cells; C, lens capsule. All experiments had N = 3. Values are expressed as mean ± SEM. Asterisks (*) indicate statistically significant MFI between WT and/or FNcKO and/or FNcKO (gremlin-1) at 5 days PCS..
Since gremlin-1 can function as both an antagonist of BMP signaling [77,81] and an agonist of canonical TGFβ signaling [78–80], we next investigated if exogenous administration of gremlin-1 can rescue the defects in the fibrotic response and alterations in BMP and TGFβ signaling observed in FNcKO LCs PCS. Notably, exogenous gremlin-1 restored the ability of FNcKO LCs to upregulate the fibrotic marker αSMA (*P = 0.015) and deposit the fibrotic ECM proteins tenascin C (**P = 0.002) and collagen I (*P = 0.017) at 5 days PCS (Fig. 8B) suggesting that the attenuation of gremlin-1 expression in FNcKO LCs plays a major role in the FNcKO phenotype. Thus, the effect of exogenous gremlin-1 on TGFβ and BMP signaling in FNcKO LCs was then determined. Consistent with the restoration of αSMA upregulation and collagen I/tenascin C distribution, FNcKO LCs treated with gremlin-1 exhibited robust pSMAD2/3 immunostaining at 5 days PCS compared to untreated FNcKO LCs (*P = 0.013) suggesting that gremlin-1 is working via its effects on the TGFβ pathway. Surprisingly though, in light of literature defining gremlin-1 as a BMP antagonist [79,80], gremlin-1 treated FNcKO LCs still exhibited sustained BMP signaling at 5 days PCS (P = 0.440), suggesting that gremlin-1 was largely acting via its effects on the TGFβ pathway (Fig. 8B).
Fibronectin mediates sustained fibrotic PCO via TGFβ dependent pathway
Exogenous treatment of FNcKO lens capsular bags with gremlin-1 can rescue many aspects of the FNcKO phenotype including the defect in canonical TGFβ signaling as measured by pSMAD2/3 levels. As active TGFβ induces lens cells to convert to myofibroblasts [45], we then determined whether exogenous active TGFβ could also rescue the FNcKO phenotype. We found that active TGFβ1 treated FNcKO capsular bags show robust activation of pSMAD2/3 at 5 days PCS(***P ≤ 0.001) as well as robust expression of the fibrotic markers αSMA (**P = 0.001) and collagen I (*P = 0.034), and the profibrotic factor gremlin-1(**P = 0.004) 5 days PCS (Fig. 9A). Interestingly, like gremlin-1, active TGFβ1 treated FNcKO LCs still retain elevated pSMAD1/5/8 levels at 5 days PCS (P = 0.286) (Fig. 9A) suggesting that TGFβ signaling may not inhibit BMP signaling in LCs.
Fig. 9. (A & B): Treatment of FNcKO LCs with exogenous active TGFβ1 restores the fibrotic response.


(A) Active TGFβ1 treated FNcKO capsular bags exhibit robust pSMAD2/3 levels (a measure of active TGFβ signaling) (***P ≤ 0.001), as well as robust expression of the fibrotic markers αSMA (**P = 0.001) and collagen I (**P ≤ 0.034) along with the profibrotic factor gremlin- (**P = 0.004) at 5 days PCS. In contrast, tenascin C deposition is not increased in FNcKO capsular bags after TGFβ1 treatment (P = 0.979) and the robust pSMAD1/5/8 levels indicative of active BMP signaling are also not affected at 5 days PCS (P = 0.286). (B) Treatment of FNcKO capsular bags with exogenous active TGFβ1 induces the upregulation of α5-integrin(***P ≤ 0.001), β1-integrin(**P 0.001), and αV-integrin expression (*P = 0.018), as well as pFAK levels (*P = 0.025), in FNcKO LCs at 5 days PCS. Collagen I, tenascin C, gremlin-1, pSMAD2/3 (downstream of TGFβ signaling), pSMAD1/5/8 (downstream of BMP signaling), α5-integrin, β1-integrin, αV-integrin, and pFAK (red) merged with αSMA (green) and DNA detected either by Draq5 or DAPI (blue). Scale bars: 35 μm; C, lens capsule; LC, remnant lens epithelial cells. All experiments had N = 3. Values are expressed as mean ± SEM. Asterisks (*) indicate statistically significant difference in MFI between WT and/or FNcKO and/or FNcKO (TGFβ1) at 5 days PCS..
Exogenous active TGFβ1 treatment was also able to induce the expression α5 −integrin (***P ≤ 0.001), β1-integrin (**P = 0.001) and αV-integrin (*P = 0.018) LCs in FNcKO by 5 days PCS, consistent with previously described feedforward mechanisms between integrins and TGFβ signaling [83]. These upregulated integrins are likely engaging with their ligands as pFAK levels are also increased in active TGFβ1 treated FNcKO LCs at 5 days PCS (*P = 0.025) compared to untreated capsular bags (Fig. 9B); In contrast, active TGFβ1 treatment did not rescue the defect in tenascin C deposition observed in FNcKO LCs (P = 0.979) (Fig. 9A) while exogenous gremlin-1 treatment did (Fig. 8B) suggesting that gremlin-1 and TGFβ1 are not fully redundant. This is supported by the observation that gremlin-1 is more potent in rescuing the defect in periostin deposition observed in FNcKO capsular bags than TGFβ1 (Supplemental Fig. 6). Notably, the precocious elevation of either the fibrotic response or activation of TGFβ signaling was not detected at 24 h PCS after treatment of WT capsular bags with either exogenous active TGFβ1 or gremlin-1 PCS (Supplemental Fig. 7).
Fibronectin fibrils are detected in FNcKO capsular bags upon addition of active TGFβ1 and gremlin-1 PCS
In our study, exogenous addition of TGFβ1 and gremlin-1 rescue the sustained fibrotic response in FNcKO capsular bags PCS (Figs. 8B and 9) including the deposition of (at least some) fibrotic ECM proteins and fibronectin binding integrins. Notably, a recent study has suggested that plasma fibronectin, which is abundant in aqueous humor [84] plays a critical role in sustained fibrotic PCO by regulating TGFβ and integrin signaling [42] as a small proportion of plasma fibronectin molecules are in the open conformation necessary for RGD presentation to integrins. Thus, we immunostained 5 day PCS WT or FNcKO eyes which have been treated with either vehicle, active TGFβ1 or gremlin-1 for fibronectin deposition. As expected, large numbers of fibronectin fibrils were detected around WT LCs, while this was not seen in FNcKO LCs (Fig. 10) consistent with the proposal that cell autonomous fibronectin production is needed for fibronectin deposition PCS. However, treatment of FNcKO mice with exogenous TGFβ1 or gremlin-1 restored the deposition of fibronectin fibrils around LCs at 5 days PCS (Fig. 10). As these LCs do not have the ability to produce their own fibronectin, this suggests that these fibrils are produced from fibronectin present in the aqueous humor. Overall, this result suggests that active TGFβ1 and gremlin-1 rescue the sustained fibrotic response of FNcKO LCs PCS by acting as agonists of the TGFβ signaling pathway which may allow for the upregulation of the integrins necessary for the assembly of plasma fibronectin into a matrix that allows for the assembly of fibrotic ECM PCS.
Fig. 10. Fibronectin fibrils are detected in FNcKO capsular bags upon treatment with either active TGFβ1 or gremlin-1 at 5 days PCS.
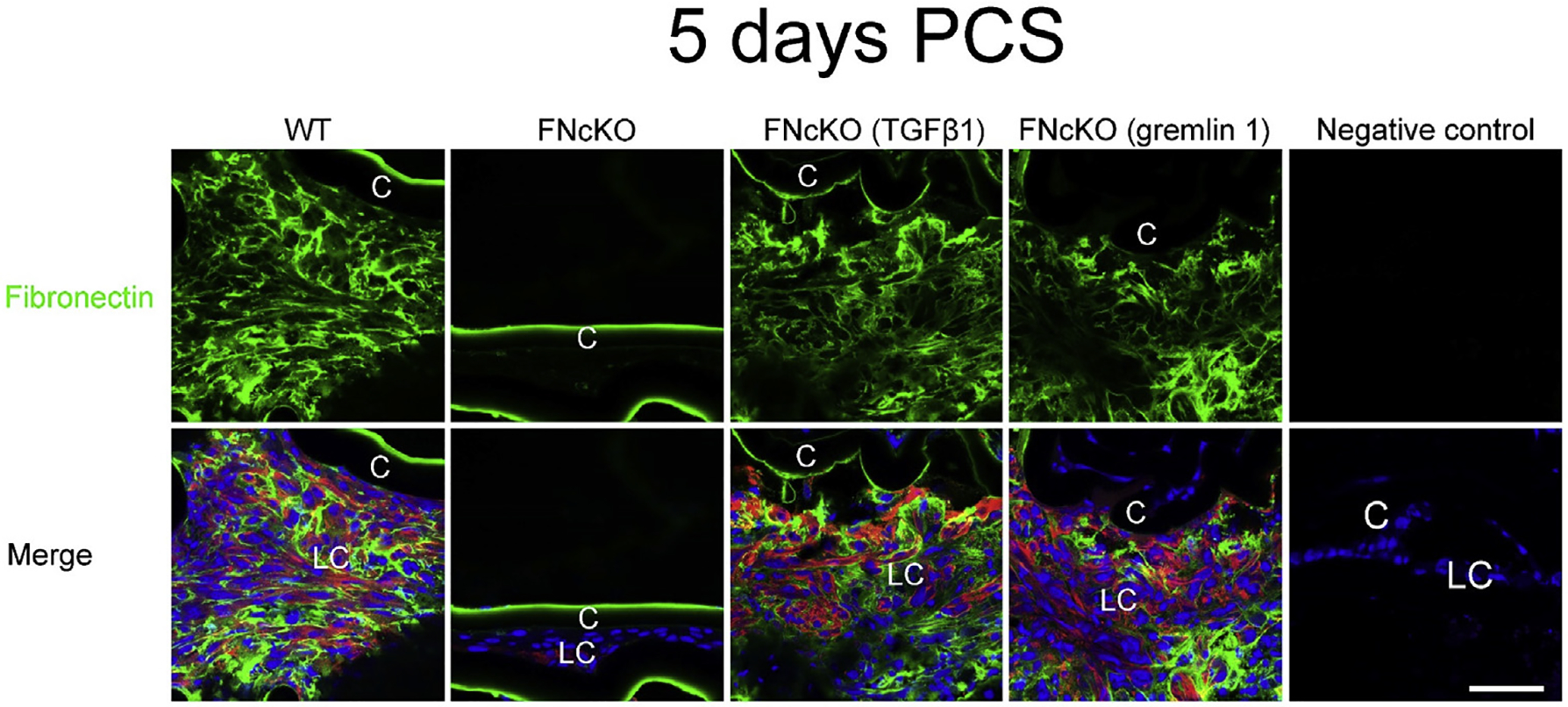
Active TGFβ1 and gremlin-1 treated FNcKO capsular bags are positive for fibronectin fibrils similar to WT capsular bags (vehicle-treated) at 5 days PCS. As expected, vehicle treated FNcKO capsular bags are not positive for fibronectin fibrils. Fibronectin (green), αSMA (red) and DNA detected by Draq5 (blue). Scale bars: 35 μm. LC, remnant lens epithelial cells/lens cells; C, lens capsule.
Discussion
Cellular fibronectin, a multifunctional protein that regulates cellular behavior at diverse levels [8,15,22,23,27,28,88], has been long associated with lens development and fibrotic PCO pathogenesis [89–91]. However, its functions in the adult lens and PCO pathogenesis have been elusive. Here we deleted the fibronectin gene from the lens and used these conditional knockouts animals to characterize the role of fibronectin in adult lens homeostasis and the response of lens cells (LCs) to lens fiber cell removal which models cataract surgery. This work provides insight into the multifunctional roles of cellular fibronectin in the pathophysiology of fibrotic PCO as well as the multitude of other fibrotic conditions that feature fibronectin rich extracellular matrices (ECM).
Fibronectin is dispensable in the adult lens
Fibronectin is produced by the embryonic lens [89,92], and its deposition in the ECM underlying the lens placode is required for placode invagination [90]. However, the role of fibronectin in the later stages of lens development was not known. Here we generated mice conditionally lacking a functional fibronectin gene from the lens (FNcKO) using MLR10 CRE, which has the potential to delete the FN1 gene from the lens as early as the lens vesicle stage although characterization of FNcKO animals indicated that FN1 deletion from LCs was not complete until after birth. These observations suggest that fibronectin plays little to no role in the uninjured adult lens as adult FNcKO lenses are transparent and structurally normal. RNAseq revealed that the 121 genes differentially expressed in FNcKO lenses were enriched in those encoding ECM proteins suggesting that FNcKO lenses may be compensating for fibronectin loss, although the expression of all of these genes, even after upregulation, was still quite low. This is consistent with the observation that uninjured adult lenses express levels of fibronectin mRNA (FPKM 0.3) that may be too low to be biologically significant to lens function [50]. Notably, none of the DEGs included genes known to be important for lens homeostasis, although 27 of the 121 FNcKO lens DEGs exhibit lens enriched expression as defined by iSyte analysis of the P56 lens (not shown), so would be bioinformatically predicted to regulate lens biology[93].
Fibronectin is essential for the pathogenesis of fibrotic PCO
Fibronectin mRNA and protein levels upregulate sharply in lens epithelial cells (LCs) during the progression of anterior subcapsular cataract [44] and after lens fiber cell removal modeling cataract surgery [19]. Fibronectin has been used as a fibrotic marker in PCO for years [43–45,94–96] and was implicated in PCO pathogenesis [91,97,98]. However, exogenous fibronectin, such as that present in blood/aqueous humor, has been proposed as both a positive and negative regulator of growth factors involved in PCO pathology [39,41,42]. Due to both conflicting literature on fibronectin function in PCO, and the dearth of studies on cellular fibronectin in this condition, we took advantage of the mouse cataract surgery model [99] to comprehensively characterize the role of endogenous tissue fibronectin in fibrotic PCO.
Here, we show that cellular fibronectin protein is robustly produced by LCs starting around 48 h PCS, although fibronectin does not seem to be required for the early fibrotic response of LCs PCS as this was qualitatively and quantitatively normal in FNcKO LCs at this time, and fewer than 100 genes were differentially expressed between WT and FNcKO LCs at 48 h PCS. The modest role that fibronectin produced by LCs plays in the initial response of LCs to cataract surgery is not surprising as its basal expression in the adult lens is very low, and its mRNA expression does not elevate in lens cells until 24 h PCS [19]. However, fibronectin deposition around LCs does become more robust at later times PCS, and this study found that it is critical for the maintenance of fibrotic PCO at 5 days PCS as little evidence of LC fibrosis was apparent in FNcKO mice at this time.
However, FNcKO LCs did initiate the fibrotic response PCS as both RNAseq and immunofluorescence revealed the upregulation of numerous fibrotic markers at 48 h PCS, and this was maintained through 3 days PCS, although these cells diminish in number by 4 days PCS, and were largely absent by 5 days PCS. Our study found no evidence that these cells expressing fibrotic markers are lost via traditional apoptosis, although they may still be lost through one of the several known nonapoptotic cell death pathways [100]. However, this study provides some evidence that LC derived FNcKO myofibroblasts may be returning to a lens epithelial cell phenotype and/or differentiating into lens fiber cells in the absence of cellular fibronectin. A definitive understanding of the fate of FNcKO myofibroblasts at later times PCS will require future cell lineage tracing experiments.
Fibronectin influences the pathogenesis of fibrosis via multiple mechanisms
Due to the critical role of autocrine fibronectin production in the maintenance of fibrotic PCO, we attempt to address the underlying molecular mechanisms by integrating RNAseq analysis of FNcKO lenses PCS with previous reports on fibronectin function in other systems.
Fibronectin and fibrotic matrix production and assembly
Tissue fibronectin is produced locally in tissues, where it assembles into insoluble fibrils, often in response to injury [8,101]. Later, fibronectin is remodeled to facilitate the assembly of secondary scars rich in collagen I and other fibrotic ECM proteins [21,23,86,102]. Numerous prior cell culture studies suggest that fibronectin is a master regulator of ECM assembly because of its ability to regulate a wide range of ECM molecules [31]. However, these findings had not been corroborated in vivo [11,31,103]. Here we fill this knowledge gap by discovering that fibronectin is required for lens cells to upregulate both the mRNA expression and matrix assembly of some major fibrotic ECM components PCS including collagen I, tenascin C and Periostin (Figs. 11–1). Interestingly, contrary to some reports [65,104], we found that fibronectin production by LCs was not required for the matrix deposition of the extracellular matrix proteins (ECM1) and thrombospondin-1 during the progression of LC fibrosis although thrombospondin-1 deposition was attenuated. This suggests either that the small amounts of exogenous fibronectin from aqueous humor that may deposit around FNcKO LCs is sufficient for ECM1 and thrombospondin-1 assembly or that LCs produce other mediators of their assembly [105,106]. Overall, this study suggests that cellular fibronectin plays a previously unappreciated dual role in matrix formation in fibrotic disease in vivo as it is required for both the expression of fibrotic ECM genes and the assembly of their protein products.
Fig. 11. Multifunctional roles of fibronectin in PCO pathogenesis.
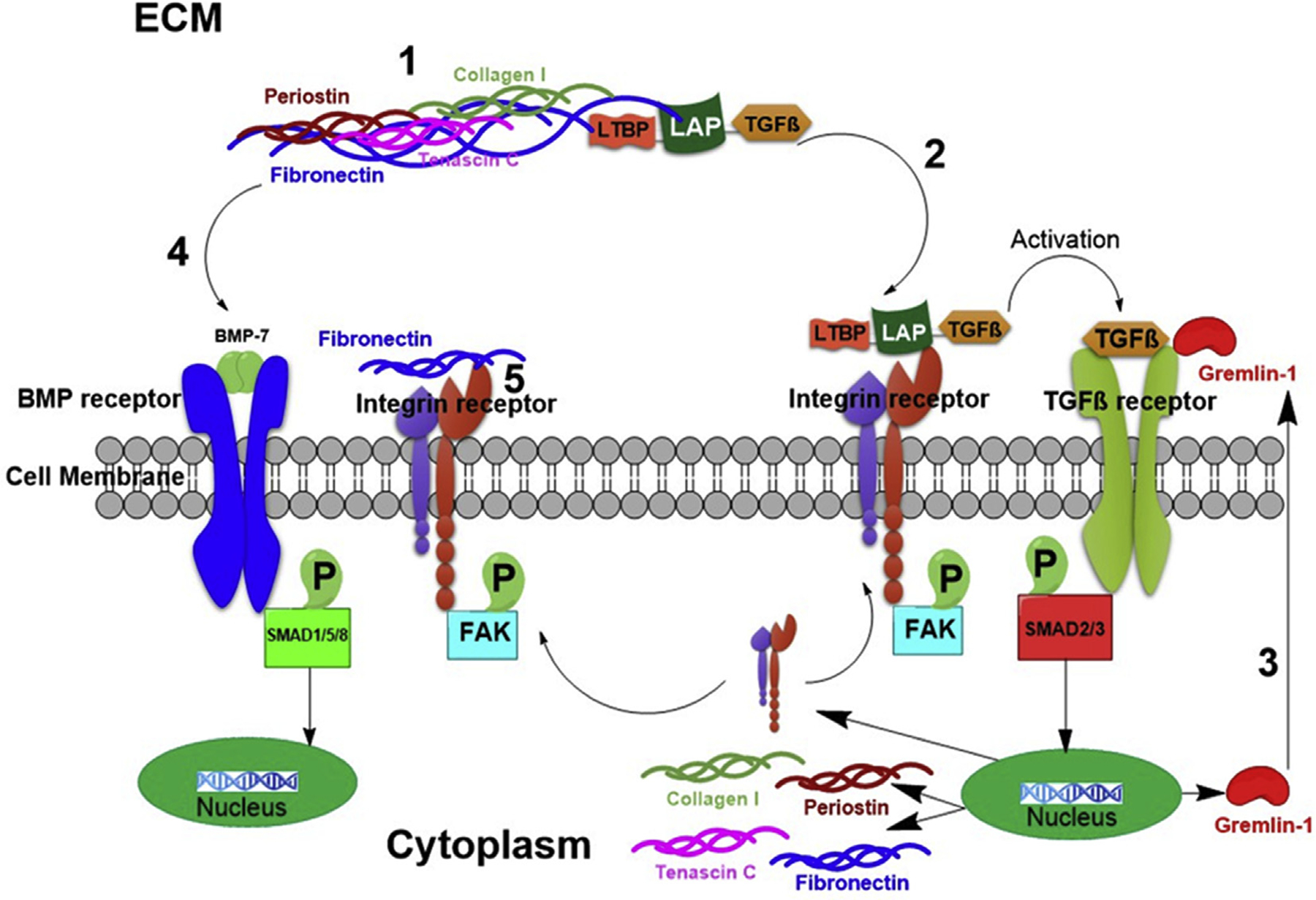
This diagram depicts the multifunctional roles of cellular fibronectin in fibrotic PCO and potentially other fibrotic like conditions. (1) Regulation of fibrotic extracellular matrix protein assembly; (2) Extracellular deposition of latent TGFβ complex needed for its subsequent activation; (3) Regulation of the expression of the TGFβ signaling agonist, gremlin-1; (4) Regulation of BMP signaling; (5) Modulation of integrin signaling.
Fibronectin and TGFβ superfamily signaling
It is well-established that TGFβ signaling mediates fibrotic PCO [45] while plating dissociated embryonic lens cells on plasma fibronectin can activate TGFβ signaling [42]. Fibronectin is also crucial for the incorporation of the latent TGFβ complex into the ECM in other in vitro cell models [22,107]. However, the relationship between the production of endogenous cellular fibronectin and the induction of TGFβ signaling in fibrotic conditions like PCO had not been explored in vivo.
In this study, we show that cellular fibronectin’s role in driving TGFβ signaling is a major reason that endogenous expression of fibronectin by remnant LCs PCS is critical for sustained fibrotic PCO. In vitro studies have previously revealed that fibronectin interactions with latent TGFβ binding protein 1 (LTBP1) are critical to tether latent TGFβ to the ECM; a process necessary for its activation. In vivo, we found the LTBP1 normally associates with the fibrotic ECM that assembles around LCs PCS, while this does not occur around FNcKO LCs PCS, suggesting that the tethering of latent TGFβ (and its subsequent activation) cannot occur in the absence of cellular fibronectin expression by LCs (Figs. 11–2).
However, the RNAseq analysis of FNcKO LCs at 48 h PCS revealed that fibronectin is playing multifunctional roles in the regulation of the TGFβ pathway PCS. The expression of gremlin-1, a known activator of TGFβ signaling and antagonist of BMP signaling that has been implicated in the pathogenesis of fibrotic diseases including PCO [77–82,108,109], is highly upregulated in LCs by 48 h PCS, while its mRNA and protein levels are markedly attenuated in FNcKO LCs. Notably, the addition of exogenous gremlin-1 can also rescue the defects in TGFβ signaling, and fibrotic ECM production, observed in FNcKO LCs PCS, and its effects on tenascin C and periostin expression are qualitatively and quantitatively more potent than TGFβ1 (Figs. 8B and 11). However, further study of the role of periostin in the assembly of tenascin C and other fibrotic ECM matrix components is required to understand these relationships better. Overall, this suggests that fibronectin could be playing multifunctional roles in regulating TGFβ pathway activation PCS which include both the regulation of the gene expression of a TGFβ pathway agonist (Figs. 11–3) and the activation of latent TGFβ.
Notably, gremlin-1 is also well known to be an antagonist of BMP signaling [77] which was particularly interesting as BMP signaling plays a critical role in lens development [74,76] while BMP signaling can play anti-fibrotic roles in epithelia [77] as it can counterbalance TGFβ signaling [77]. A prior in vitro study on primary LCs suggested that the BMP signaling agonist BMP-7 can suppress TGFβ mediated epithelial mesenchymal transition (EMT) [110] and we show in this study that BMP signaling rapidly decreases in LCs PCS, a process that is attenuated in FNcKO LCs (Figs. 11–4). To further understand how fibronectin regulates BMP signaling PCS, we tested whether the rescue of the fibrotic phenotype of FNcKO LCs by either gremlin-1 or TGFβ included the downregulation of BMP signaling and found that BMP signaling remained high in both cases. This was surprising as it shows that it was possible for both BMP and TGFβ signaling to be high in the same cell even though the fibrotic phenotype is qualitatively rescued suggesting that 1) BMP signaling is not sufficient to protect LCs from fibrotic transformation in the presence of gremlin-1 or TGFβ-induced Smad2/3 activation and 2) fibronectin’s effect on BMP signaling PCS is not mediated by its effects on TGFβ pathway activation.
The rescue experiments performed by adding active TGFβ1 and gremlin-1 to FNcKO capsular bags revealed that plasma fibronectin can participate in sustained fibrotic PCO when TGFβ signaling is ectopically activated as fibronectin fibrils assembled around FNcKO LCs treated with either active TGFβ1 or gremlin-1. Based on previous studies, it is likely that this fibronectin is coming from the aqueous humor (an important source of plasma fibronectin [84]) as FNcKO LCs are unable to produce cell derived fibronectin and we did not observe any elevations in fibronectin expression by any other ocular structures besides LCs in active TGFβ1 or gremlin-1 treated eyes (data not shown). Overall, this study suggests that TGFβ1 and gremlin-1, by acting as agonists of the TGFβ signaling pathway, may allow for the integrin upregulation necessary for the assembly of plasma fibronectin into a matrix that allows for the assembly of fibrotic ECM PCS. This finding is consistent with a recent study that identified a critical role for plasma fibronectin in `sustained PCO [42].
Fibronectin and integrin signaling
Integrins have been proposed as therapeutic targets for PCO due to their roles in cell/ECM attachment, cell migration, and transmission of tractional forces [41,66,111–113]. As fibronectin is a well-known ligand for several integrin receptors that are upregulated by LCs PCS [19,45,66], we investigated the effect of autocrine fibronectin on integrin pathways. Notably, our data revealed that cellular fibronectin is not just important for downstream integrin signaling PCS but is also necessary for the enhanced protein expression of several integrin receptors by LCs PCS. Notably, active TGFβ1 can rescue both the defects in integrin expression and downstream integrin signaling seen in FNcKO LCs PCS. As integrins can mediate the activation of latent TGFβ [19,114] whereas TGFβ signaling can upregulate their expression, this finding further supports a model by which the diverse functions of fibronectin, including its interaction with integrins, drives the epithelial mesenchymal transition of LCs in PCO, and potentially the pathogenesis of other disorders of EMT such as cancer and some fibrotic conditions (Figs. 11–5).
Implications for the role of fibronectin in wound healing and fibrotic diseases
This comprehensive study shows that fibronectin production by LCs is required for the persistence of myofibroblasts PCS and we have laid out several possible mechanisms by which fibronectin mediates this response (Fig. 11). This provides the first insight into why myofibroblasts, which are lost after initial wound healing responses in normal healing [115], are maintained at such extended times after surgery to cause fibrotic PCO in humans, an intractable complication PCS. Overall our study will provide important insights towards improving the outcome of cataract surgery [116].
Further, the destruction of tissue architecture by fibrosis has been estimated to cause at least one-third of natural deaths worldwide [117]. While numerous studies have identified important pathways driving fibrosis [118–120], much less is known about the mechanisms by which activated fibro-blasts/myofibroblasts inappropriately persist after the initial injury/stress is removed [115,121]. Notably, fibronectin has been extensively studied due to its important roles in the wound healing response and fibrosis [11]. However, most of the studies done on fibronectin are cell culture based and thus are difficult to correlate to wound healing in vivo [11,31,103]. The few in vivo studies on the function of cellular fibronectin [24–28] mostly address only a single aspect of fibronectin’s role in wound healing as most tissues consist of many cell types with complex interactions. This study has taken advantage of the lens’s relative simplicity, as the cells left behind after cataract surgery consist of a monolayer of epithelial cells that undergo epithelial to mesenchymal transition (EMT) to form myofibroblasts that behave similarly to the myofibroblasts responsible for other fibrotic diseases [122]. This cellular simplicity has allowed for the dissection of the complex regulatory roles that cellular fibronectin plays in fibrosis (Fig. 11). This work provides a new understanding of PCO pathogenesis and identifies new targets for the treatment/prevention of both fibrotic PCO [116] and numerous other fibrotic conditions resulting in death and disability [16,123–125].
Methods
Animals
All animal experiments for this study were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Delaware Institutional Animal Care and Use Committee. All mice were maintained under pathogen-free conditions at the University of Delaware animal facility under a 14/10-h light/dark cycle.
Mice lacking the fibronectin gene from the lens (FNcKO mice) were created by mating mice harboring a FN1 allele in which exon 1 is flanked by lox P sites (B6;129-Fn1tm1ref, originally created in Dr. Reinhard Fassler’s lab [48] and obtained from Dr. David Beebe, Washington University, St. Louis, St. Louis, Missouri) with MLR10-cre mice which express Cre recombinase in all lens cells from the lens vesicle stage onward [49] (mice on an FVB/N background obtained from Dr. Michael Robinson (Miami University, Oxford, Ohio) and backcrossed to C57Bl/6<har> for over 10 generations at the University of Delaware). Mice were genotyped for the presence of the floxed FN1 allele, and MLR10-Cre transgene by PCR using the following primers-FN1 flox/flox, 5′-GTA CTG TCC CAT ATA AGC CT CTG-3′ and 5′-CTG AGC ATC TTG AGT GGA TGG GA-3′; MLR10-Cre, 5′-CCT GTT TTG CAC GTT CAC CG-3′ and 5′-ATG CTT CTG TCC GTT TGC CG-3′. Deletion of exon1 of the FN1 gene was confirmed by PCR analysis of genomic DNA isolated from adult lenses using the following primers-Forward primer 5′-CTG GAG TCA AGC CAG ACA CA −3′, Reverse primer 5′-CGA GGT GAC AGA GAC CAC AA-3′. Surgical removal of lens fiber cells to mimic human cataract surgery was performed in adult mice as previously described [19,46,99,126,127]. Rescue experiments were performed by instilling either active recombinant human TGFβ1 protein (5 ml of 0.1 ng/ml TGFβ1 in balanced saline solution (BSS); R&D systems, Minneapolis, MN, USA; catalog no-240-B) or recombinant human gremlin 1 protein (5 μl of 1 ng/μl gremlin-1 in balanced saline solution (BSS); R&D systems, Minneapolis, MN, USA; catalog no-5190-GR) into the lens capsular bags of FNcKO mice immediately following removal of the lens fibers.
Morphological analysis
Lens clarity was determined by viewing isolated lenses using darkfield optics while lens optical properties were assessed by placing lenses on a 200-mesh electron microscopy grid as described previously [128,129]. For histological analysis, eyes were isolated and immediately fixed in Pen-Fix (Richard Allan Scientific, Kalamazoo, Michigan) for 2 h, then stored in 70% ethanol until paraffin embedding by the Histology Core Laboratory, College of Agriculture, University of Delaware. Six-micrometer sections were stained with hematoxylin and eosin (H&E) and photographed on a Zeiss Axiophot microscope fitted with a Nikon digital camera.
RNA sequencing
RNA sequencing of intact mouse lenses was performed by isolating RNA from 8 weeks old FNcKO and C57BL/6NHsd (wildtype) lenses (three biological replicates for each condition, two lenses per replicate) using the SV Total RNA Isolation System (Promega-Catalog number-Z3100, Madison, Wisconsin, USA). Sequencing libraries were produced using SMARTer Stranded Total RNA-Seq - Pico Input Mammalian (Takara Bio USA, Inc., Mountain View, CA, USA) and sequenced by DNA Link, USA (901 Morena Blvd. Ste 730 San Diego CA92117, USA) on an Illumina NextSeq500 (San Diego, CA, USA). Paired end 101 nucleotide reads were processed using the Tuxedo Suite tools TopHat and Cuffdiff for alignment and differential expression analysis [130]. The UCSC Genome version GRCm38/mm10, and RefSeq GRCm38.p5 annotations were used as the reference for alignment and feature abundance estimates. Read pairs corresponding to RNA fragments were enumerated as FPKM (fragments per kilobase million) by Cuffdiff.
RNA sequencing of lens cells (LCs) isolated from operated lenses was performed by removing the lens fiber cells from one eye of C57BL/6NHsd (WT) or FNcKO mice, then 48 h later, the other eye was operated upon, followed by the immediate sacrifice of mice. Lens capsular bags with attached cells were isolated, and samples from five individual mice were pooled, and flash frozen on dry ice, to create one, 0 h, and one, 48-h, post cataract surgery (PCS) biological replicate. RNA was isolated using the RNeasy Mini Kit (50) from Qiagen (Cat No./ID: 74104, Germantown, MD, USA). One microgram of total RNA was processed using the Illumina TruSeq RNA Sample Prep Kit (Cat#FC-122–1001) to produce sequencing libraries. Three biological replicates from both WT and FNcKO LC at 0 h and 48 h PCS were analyzed on an Illumina HiSeq 2500 by the Genotyping and Sequencing Center, Delaware Biotechnology Institute, the University of Delaware as previously described [46]. Single end 51 nucleotide reads were processed using a modified MAPRseq pipeline. Read alignments were generated with Tophat against the UCSC mm10 genome build and annotations [131]. HTseq was used to quantify reads aligning to genomic features, and edgeR was used for differential expression analysis. Reads per kilobase million (RPKM) was calculated for each gene from count data based on library size and exon-union transcript length. Biologically significant differentially expressed genes (DEGs) are defined as those exhibiting statistically significant changes (False Discovery Rate-FDR ≤ 0.05), a change in mRNA level greater than 2 RPKM (Reads Per Kilobase Million) or FPKM (Fragments per kilobase million) between conditions, Fold Change (FC) greater than 2 in either the positive or negative direction, and expression levels in either condition that were 2 RPKM/FPKM or greater [50,126,132].
Pathway analysis
Pathway analysis was performed on all genes whose expression was called “present” (>1 cpm (counts per million) in at least two samples) with DEGs defined as those exhibiting FC ≥ |2| and FDR < 0.05 using iPathwayGuide (Advaita Bioinformatics, Plymouth Michigan, USA). This software package uses Impact Analysis, an approach that considers both whether DEGs participating in a particular pathway (as defined by the Kyoto Encyclopedia of Genes and Genomes, KEGG [133], analysis performed with KEGG release 84.0+/10–26, Oct 17) are overrepresented in the gene list and their directional interactions within the pathway [134].
Immunofluorescence
Immunofluorescence was performed to assay protein expression at the cellular level as described previously [135]. Briefly, eyes were embedded in Optimum Cutting Temperature Media (Tissue Tek, Torrance, CA, USA) immediately after harvest, and stored at −80 °C. Frozen sections (16 mm) were obtained with a Leica CM3050 cryostat (Leica Microsystems, Buffalo Grove, IL, USA), and mounted on Color Frost plus slides (Fisher Scientific, Hampton, NH, USA). Sections were fixed either in 1:1 acetone-methanol for 15 min at −20 °C or 4% paraformaldehyde (PFA) for 15 min at room temperature (RT). After washing with PBS, slides were blocked for 1 h at RT, then incubated with either a primary antibody diluted in blocking buffer (see Supplemental Table 1 for specifics of the primary antibodies, blocking buffer compositions, incubation times and dilutions used in this study) or just blocking buffer to serve as a negative control to exclude nonspecific staining by the secondary antibodies or channel bleed through as previously described [135]. Following primary antibody treatment, slides were washed, then incubated for 1 h at room temperature with 1:200 dilution of species appropriate Alexa Fluor 488/568/633 labeled secondary antibody (Thermofisher Scientific, Waltham, MA, USA) in PBS. DNA/cell nuclei were detected by adding either a 1:2000 dilution of Draq-5 (Biostatus Limited, Shepshed, Leicestershire, UK) or a 1:1000 dilution of DAPI (Fluoropure D21490, Thermofisher Scientific, Waltham, MA, USA) to the secondary antibody solution. Some experiments also included a 1:250 dilution of fluorescein-labeled anti-αSMA (Sigma-Aldrich, St. Louis, MO, USA) in the secondary detection solution to visualize myofibroblasts. During imaging, the negative control was used [135] to set the baseline so that the low levels of nonspecific binding of the secondary antibody are subtracted from the images.
Co-localization of two proteins (Fig. 6A- fibronectin and LTBP1 and Supplemental Fig. 4A- fibronectin and collagen I) when the requisite antibodies were raised in the same species was performed using a three step blocking procedure as previously described [136]. Blocking step 1 was performed as described above, blocking step 2 used a 1:100 dilution of a rabbit polyclonal antibody against phospho FAK-Tyr 397 (cat-3283, Cell signaling, Danvers, MA, USA) that is unable to detect its target in indirect immunofluorescence assays, and blocking step 3 utilized a 1:20 dilution of an unlabeled pre-adsorbed goat F(ab’)2 anti-rabbit IgG - (Fab)’2 (catab6107, Abcam, Cambridge, United Kingdom). The co-localized samples were compared with samples where each primary antibody, as well as the non-binding blocking antibodies, were stained separately to ensure that the blocking was complete.
Each staining experiment/time point was replicated using at least three biologically independent specimens (3–5 mice, at least 2 sections per mouse). Fluorescently labeled slides were visualized either using Zeiss LSM780 or Zeiss LSM880 confocal microscopes (Carl Zeiss Inc., Gottingen, Germany), and comparisons of images were made between slides imaged using identical imaging parameters. In some cases, the brightness and contrast were adjusted to allow viewing on diverse computer screens; however, these adjustments were made identically for all images within a particular time course.
Flow cytometric analysis of α smooth muscle actin (αSMA) post cataract surgery (PCS)
Single cell suspensions of the cells associated with the capsular bag PCS (three replicates of 0 h and 5 days PCS from WT and FNcKO, 4 capsular bags per replicate) were prepared by modifying an established protocol [137]. Briefly, the PCS capsular bags were isolated from the eye and then treated with 0.25% trypsin at 37 °C for 30 min, and cells were dissociated every 10 min using fine-tipped pipettes. Cells were fixed for 15 min at room temperature with 100 μl Medium A from a FIX & PERM™ Cell Permeabilization Kit (GAS-003, Invitrogen, Carlsbad, CA, USA). Cells were washed in 2 ml cold DPBS (Dulbecco’s phosphate-buffered saline, Invitrogen, Carlsbad, CA, USA), supernatant aspirated. 100 μl Medium B from the FIX & PERM™ Cell Permeabilization Kit was added to each tube. αSMA (dilution 1: 200, cat-F3777, Sigma Aldrich, St. Louis, MO, USA) and isotopic control-IgG2a (FITC Mouse IgG2a, κ Isotype Ctrl Antibody, cat-400207, Biolegend, San Diego, CA) were added to the appropriate tubes. Samples were stained in the dark at 4 °C for 15 min and then washed in 2 ml D-PBS. Samples were aspirated and 500 μl D-PBS was added to each tube. 5 μl of 1 mg/ml propidium iodide (cat-P4170, Sigma Aldrich, St. Louis, MO, USA) was added 10 min prior to acquisition for DNA content analysis. Data were acquired using a BD FACSAria Fusion 15-color flow cytometry with FACSDiva software (V8.0.3) and analyzed using FCS Express (V5.0, research version).
ImageJ quantification and statistical analysis
Immunofluorescence images were quantified by determining the mean fluorescence intensity (MFI) of lens capsule associated tissue viewed in three randomly chosen confocal images from biological independent samples using ImageJ (v1.52P, NIH). Average Number of Nuclei (ANN)/section at different PCS of WT and FNcKO (six randomly chosen immunofluorescence images from each time point of PCS of biologically independent samples) was counted by ImageJ as described [138]. All statistics were assessed using either Student’s t-test (correct for multiple comparisons using the Holm-Sídák method) or one-way ANOVA with Tukey’s post hoc test performed using GraphPad Prism 8.3.0 software. Data are presented as mean ± SE (SEM) and differences were considered significant at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank members of the Duncan lab for their critical reading of this manuscript and the staff of the University of Delaware Bioimaging, Sequencing and Bioinformatics core facilities for technical support. We also thank Mr. Richard West, Associate Scientist of Flow Cytometry Core, Center for Biomedical & Brain Imaging (CBBI), The University of Delaware for helping us with flow cytometry experiments and analysis. We dedicate this paper to Dr. David Beebe, for his many discussions about this project and for providing us with the mice carrying the fibronectin flox allele used in this study prior to his untimely death.
Grant information
This study was supported by National Institutes of Health grant (EY015279) to MKD; MHS received a Fight for Sight Summer Fellowship, and Sigma Xi Grant-in-Aid of Research; MK received a Sigma Xi Grant-in-Aid of Research; EJ received research fellowships from the University of Delaware Undergraduate Research program and Delaware INBRE (P20 GM103446); INBRE program grant P20 GM103446 and the State of Delaware supported the University of Delaware Core Imaging and flow cytometry facilities; a core facility access award from Delaware INBRE to MKD supported the RNA sequencing, and 1S10 (RR027273-01) funded the acquisition of the confocal microscope used in this study.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.matbio.2020.02.004.
References
- [1].Blumenstock FA, Saba TM, Cardarelli P, Dayton CH, Opsonic activity of fibronectin, Methods Enzymol. 132 (1986) 334–349. [DOI] [PubMed] [Google Scholar]
- [2].Eriksen HO, Espersen F, Clemmensen I, Opsonic activity of fibronectin in the phagocytosis of Staphylococcus aureus by polymorphonuclear leukocytes, Eur. J. Clin. Microbiol 3 (1984) 108–112. [DOI] [PubMed] [Google Scholar]
- [3].Mezzenga R, Mitsi M, The molecular dance of fibronectin: conformational flexibility leads to functional versatility, Biomacromolecules 20 (2019) 55–72, 10.1021/acs.biomac.8b01258. [DOI] [PubMed] [Google Scholar]
- [4].Czop JK, Phagocytosis of particulate activators of the alternative complement pathway: effects of fibronectin, Adv. Immunol 38 (1986) 361–398. [DOI] [PubMed] [Google Scholar]
- [5].Astrof S, Crowley D, Hynes RO, Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin, Dev. Biol 311 (2007) 11–24, 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO, Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin, Development 119 (1993) 1079–1091. [DOI] [PubMed] [Google Scholar]
- [7].George EL, Baldwin HS, Hynes RO, Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells, Blood 90 (1997) 3073–3081. [PubMed] [Google Scholar]
- [8].To WS, Midwood KS, Plasma and cellular fibronectin: distinct and independent functions during tissue repair, Fibrogenesis Tissue Repair 4 (2011) 21, 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwarzbauer JE, Alternative splicing of fibronectin: three variants, three functions, Bioessays 13 (1991) 527–533, 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- [10].Paul JI, Schwarzbauer JE, Tamkun JW, Hynes RO, Cell-type-specific fibronectin subunits generated by alternative splicing, J. Biol. Chem 261 (1986) 12258–12265. [PubMed] [Google Scholar]
- [11].Lenselink EA, Role of fibronectin in normal wound healing, Int. Wound J 12 (2015) 313–316, 10.1111/iwj.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grinnell F, Fibronectin and wound healing, J. Cell. Biochem 26 (1984) 107–116, 10.1002/jcb.240260206. [DOI] [PubMed] [Google Scholar]
- [13].Yamada KM, Clark RA, Provisional matrix, in: The Molecular and Cellular Biology of Wound Repair, Kluwer Academic Publishers, New York, 1996, pp. 51–93. [Google Scholar]
- [14].Clark RAF, Fibronectin in the skin, J. Invest. Dermatol 81 (1983) 475–479, 10.1111/1523-1747.ep12522718. [DOI] [PubMed] [Google Scholar]
- [15].Clark RA, Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin, J. Invest. Dermatol 94 (1990) 128S–134S. [DOI] [PubMed] [Google Scholar]
- [16].Walraven M, Hinz B, Therapeutic approaches to control tissue repair and fibrosis: extracellular matrix as a game changer, Matrix Biol. 71–72 (2018) 205–224, 10.1016/j.matbio.2018.02.020. [DOI] [PubMed] [Google Scholar]
- [17].Stoppacciaro A, Pietrucci A, Fofi C, Raffa S, Torrisi MR, Menè P, Fibronectin glomerulopathy: an uncommon cause of nephrotic syndrome in systemic lupus erythematosus, NDT Plus 1 (2008) 225–227, 10.1093/ndtplus/sfn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Altrock E, Sens C, Wuerfel C, Vasel M, Kawelke N, Dooley S, Sottile J, Nakchbandi IA, Inhibition of fibronectin deposition improves experimental liver fibrosis, J. Hepatol 62 (2015) 625–633, 10.1016/j.jhep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- [19].Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK, The roles of α(V) integrins in lens EMT and posterior capsular opacification, J. Cell Mol. Med 18 (2014) 656–670, 10.1111/jcmm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barker TH, Engler AJ, The provisional matrix: setting the stage for tissue repair outcomes, Matrix Biol. 60–61 (2017) 1–4, 10.1016/j.matbio.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Lin Z, Foolen J, Schoen I, Santoro A, Zenobi-Wong M, Vogel V, Disentangling the multifactorial contributions of fibronectin, collagen and cyclic strain on MMP expression and extracellular matrix remodeling by fibroblasts, Matrix Biol. 40 (2014) 62–72, 10.1016/j.matbio.2014.09.001. [DOI] [PubMed] [Google Scholar]
- [22].Fontana L, Chen Y, Prijatelj P, Sakai T, Fassler R, Sakai LY, Rifkin DB, Fibronectin is required for integrin alphavbeta6-mediated activation of latent, Faseb. J 19 (2005) 1798–1808, 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- [23].Zollinger AJ, Smith ML, Fibronectin, the extracellular glue, Matrix Biol. 60–61 (2017) 27–37, 10.1016/j.matbio.2016.07.011. [DOI] [PubMed] [Google Scholar]
- [24].Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE, Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan, J. Cell Biol 162 (2003) 149–160, 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stenzel D, Lundkvist A, Sauvaget D, Busse M, Graupera M, van der Flier A, Wijelath ES, Murray J, Sobel M, Costell M, Takahashi S, Fässler R, Yamaguchi Y, Gutmann DH, Hynes RO, Gerhardt H, Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis, Development 138 (2011) 4451, 10.1242/dev.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iwasaki A, Sakai K, Moriya K, Sasaki T, Keene DR, Akhtar R, Miyazono T, Yasumura S, Watanabe M, Morishita S, Sakai T, Molecular mechanism responsible for fibronectin-controlled alterations in matrix stiffness in advanced chronic liver fibrogenesis, J. Biol. Chem 291 (2016) 72–88, 10.1074/jbc.M115.691519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhu Y, Soderblom C, Trojanowsky M, Lee D-H, Lee JK, Fibronectin matrix assembly after spinal cord injury, J. Neurotrauma 32 (2015) 1158–1167, 10.1089/neu.2014.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moriya K, Sakai K, Yan MH, Sakai T, Fibronectin is essential for survival but is dispensable for proliferation of hepatocytes in acute liver injury in mice, Hepatology 56 (2012) 311–321, 10.1002/hep.25624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vega ME, Schwarzbauer JE, Collaboration of fibronectin matrix with other extracellular signals in morphogenesis and differentiation, Curr. Opin. Cell Biol 42 (2016) 1–6, 10.1016/j.ceb.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E, A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1), J. Biol. Chem 265 (1990) 5934–5937. [PubMed] [Google Scholar]
- [31].Kumra H, Reinhardt DP, Fibronectin-targeted drug delivery in cancer, Adv. Drug Deliv. Rev 97 (2016) 101–110, 10.1016/j.addr.2015.11.014. [DOI] [PubMed] [Google Scholar]
- [32].Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010, Invest. Ophthalmol. Vis. Sci 56 (2015) 6762–6769, 10.1167/iovs.15-17201. [DOI] [PubMed] [Google Scholar]
- [33].Liu Y-C, Wilkins M, Kim T, Malyugin B, Mehta JS, Cataracts, Lancet 390 (2017) 600–612, 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- [34].Lee CM, Afshari NA, The global state of cataract blindness, Curr. Opin. Ophthalmol 28 (2017) 98–103, 10.1097/ICU.0000000000000340. [DOI] [PubMed] [Google Scholar]
- [35].Wormstone IM, Wang L, Liu CSC, Posterior capsule opacification, Exp. Eye Res 88 (2009) 257–269, 10.1016/j.exer.2008.10.016. [DOI] [PubMed] [Google Scholar]
- [36].Awasthi N, Guo S, Wagner BJ, Posterior capsular opacification: a problem reduced but not yet eradicated, Arch. Ophthalmol 127 (2009) 555–562, 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- [37].Vasavada AR, Praveen MR, Posterior capsule opacification after phacoemulsification: annual review, Asia Pac. J. Ophthalmol. (Phila). 3 (2014) 235–240, 10.1097/APO.0000000000000080. [DOI] [PubMed] [Google Scholar]
- [38].Marcantonio JM, Vrensen GF, Cell biology of posterior capsular opacification, Eye 13 (Pt 3b) (1999) 484–488, 10.1038/eye.1999.126. [DOI] [PubMed] [Google Scholar]
- [39].Dawes LJ, Eldred JA, Anderson IK, Sleeman M, Reddan JR, Duncan G, Wormstone IM, TGF beta-induced contraction is not promoted by fibronectin-fibronectin receptor interaction, or alpha SMA expression, Invest. Ophthalmol. Vis. Sci 49 (2008) 650–661, 10.1167/iovs.07-0586. [DOI] [PubMed] [Google Scholar]
- [40].Gyorfi AH, Matei A-E, Distler JHW, Targeting TGF-beta signaling for the treatment of fibrosis, Matrix Biol. 68–69 (2018) 8–27, 10.1016/j.matbio.2017.12.016. [DOI] [PubMed] [Google Scholar]
- [41].Tiwari A, Kumar R, Ram J, Sharma M, Luthra-Guptasarma M, Control of fibrotic changes through the synergistic effects of anti-fibronectin antibody and an RGDS-tagged form of the same antibody, Sci. Rep 6 (2016) 30872, 10.1038/srep30872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].VanSlyke JK, Boswell BA, Musil LS, Fibronectin regulates growth factor signaling and cell differentiation in primary lens cells, J. Cell Sci 131 (2018), 10.1242/jcs.217240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shirai K, Saika S, Okada Y, Oda S, Ohnishi Y, Histology and immunohistochemistry of fibrous posterior capsule opacification in an infant, J. Cataract Refract. Surg 30 (2004) 523–526, 10.1016/S0886-3350(03)00616-3. [DOI] [PubMed] [Google Scholar]
- [44].Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, McAvoy JW, TGFβ induces morphological and molecular changes similar to human anterior subcapsular cataract, Br. J. Ophthalmol 86 (2002) 220–226. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771017/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW, Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation, Cells Tissues Organs 179 (2005) 43–55, 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- [46].Jiang J, Shihan MH, Wang Y, Duncan MK, Lens epithelial cells initiate an inflammatory response following cataract surgery, Invest. Ophthalmol. Vis. Sci 59 (2018) 4986–4997, 10.1167/iovs.18-25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hales AM, Chamberlain CG, McAvoy JW, Cataract induction in lenses cultured with transforming growth factor-beta, Invest. Ophthalmol. Vis. Sci 36 (1995) 1709–1713. [PubMed] [Google Scholar]
- [48].Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP, Fassler R, Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis, Nat. Med 7 (2001) 324–330, 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- [49].Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML, Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice, Invest. Ophthalmol. Vis. Sci 45 (2004) 1930–1939, 10.1167/iovs.03-0856. [DOI] [PubMed] [Google Scholar]
- [50].Manthey AL, Terrell AM, Lachke SA, Polson SW, Duncan MK, Development of novel filtering criteria to analyze RNA-sequencing data obtained from the murine ocular lens during embryogenesis, Genom Data 2 (2014) 369–374, 10.1016/j.gdata.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Scholzen T, Gerdes J, The Ki-67 protein: from the known and the unknown, J. Cell. Physiol 182 (2000) 311–322, . [DOI] [PubMed] [Google Scholar]
- [52].Maruno KA, Lovicu FJ, Chamberlain CG, McAvoy JW, Apoptosis is a feature of TGF beta-induced cataract, Clin. Exp. Optom 85 (2002) 76–82, 10.1111/j.1444-0938.2002.tb03012.x. [DOI] [PubMed] [Google Scholar]
- [53].Cvekl A, Zhang X, Signaling and gene regulatory networks in mammalian lens development, Trends Genet. 33 (2017) 677–702, 10.1016/j.tig.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sindhu Kumari S, Gupta N, Shiels A, FitzGerald PG, Menon AG, Mathias RT, Varadaraj K, Role of Aquaporin 0 in lens biomechanics, Biochem. Biophys. Res. Commun 462 (2015) 339–345, 10.1016/j.bbrc.2015.04.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lovicu FJ, Ang S, Chorazyczewska M, McAvoy JW, Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFβeta-induced anterior subcapsular cataract, Dev. Neurosci 26 (2004) 446–455, 10.1159/000082286. [DOI] [PubMed] [Google Scholar]
- [56].Wells A, Yates C, Shepard CR, E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas, Clin. Exp. Metastasis 25 (2008) 621–628, 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hosper NA, van den Berg PP, de Rond S, Popa ER, Wilmer MJ, Masereeuw R, Bank RA, Epithelial-tomesenchymal transition in fibrosis: collagen type I expression is highly upregulated after EMT, but does not contribute to collagen deposition, Exp. Cell Res 319 (2013) 3000–3009, 10.1016/j.yexcr.2013.07.014. [DOI] [PubMed] [Google Scholar]
- [58].Trackman PC, Lysyl oxidase isoforms and potential therapeutic opportunities for fibrosis and cancer, Expert Opin. Ther. Targets 20 (2016) 935–945, 10.1517/14728222.2016.1151003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ricard-Blum S, Baffet G, Theret N, Molecular and tissue alterations of collagens in fibrosis, Matrix Biol. 68–69 (2018) 122–149, 10.1016/j.matbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- [60].Jones PL, Jones FS, Tenascin-C in development and disease: gene regulation and cell function, Matrix Biol. 19 (2000) 581–596, 10.1016/s0945-053x(00)00106-2. [DOI] [PubMed] [Google Scholar]
- [61].Singh P, Carraher C, Schwarzbauer JE, Assembly of fibronectin extracellular matrix, Annu. Rev. Cell Dev. Biol 26 (2010) 397–419, 10.1146/annurev-cell-bio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Murphy-Ullrich JE, Suto MJ, Thrombospondin-1 regulation of latent TGF-beta activation: a therapeutic target for fibrotic disease, Matrix Biol. 68–69 (2018) 28–43, 10.1016/j.matbio.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen H, Jia W, Li J, ECM1 promotes migration and invasion of hepatocellular carcinoma by inducing epithelialmesenchymal transition, World J. Surg. Oncol 14 (2016) 195, 10.1186/s12957-016-0952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tan K, Lawler J, The interaction of Thrombospondins with extracellular matrix proteins, J. Cell Commun. Signal 3 (2009) 177–187, 10.1007/s12079-009-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sercu S, Zhang M, Oyama N, Hansen U, Ghalbzouri AEL, Jun G, Geentjens K, Zhang L, Merregaert JH, Interaction of extracellular matrix protein 1 with extracellular matrix components: ECM1 is a basement membrane protein of the skin, J. Invest. Dermatol 128 (2008) 1397–1408, 10.1038/sj.jid.5701231. [DOI] [PubMed] [Google Scholar]
- [66].Walker J, Menko AS, Integrins in lens development and disease, Exp. Eye Res 88 (2009) 216–225, 10.1016/j.exer.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kokkinos MI, Brown HJ, de Iongh RU, Focal adhesion kinase (FAK) expression and activation during lens development, Mol. Vis 13 (2007) 418–430. https://www.ncbi.nlm.nih.gov/pubmed/17417603. [PMC free article] [PubMed] [Google Scholar]
- [68].Robertson IB, Rifkin DB, Regulation of the bioavailability of TGF-beta and TGF-beta-related proteins, Cold Spring Harb. Perspect. Biol 8 (2016), 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Griggs LA, Hassan NT, Malik RS, Griffin BP, Martinez BA, Elmore LW, Lemmon CA, Fibronectin fibrils regulate TGF-beta1-induced epithelial-mesenchymal transition, Matrix Biol. 60–61 (2017) 157–175, 10.1016/j.matbio.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hayashi H, Sakai T, Biological significance of local TGF-beta activation in liver diseases, Front. Physiol 3 (2012) 12, 10.3389/fphys.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Courousse T, Sakai LY, Rifkin DB, Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin, J. Cell. Physiol 227 (2012) 3828–3836, 10.1002/jcp.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.[] Todorovic V, Rifkin DB, LTBPs, more than just an escort service, J. Cell. Biochem 113 (2012) 410–418, 10.1002/jcb.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rifkin DB, Rifkin WJ, Zilberberg L, LTBPs in biology and medicine: LTBP diseases, Matrix Biol. 71–72 (2018) 90–99, 10.1016/j.matbio.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Faber SC, Robinson ML, Makarenkova HP, Lang RA, Bmp signaling is required for development of primary lens fiber cells, Development 129 (2002) 3727–3737. [DOI] [PubMed] [Google Scholar]
- [75].Huang J, Liu Y, Filas B, Gunhaga L, Beebe DC, Negative and positive auto-regulation of BMP expression in early eye development, Dev. Biol 407 (2015) 256–264, 10.1016/j.ydbio.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Boswell BA, Musil LS, Synergistic interaction between the fibroblast growth factor and bone morphogenetic protein signaling pathways in lens cells, Mol. Biol. Cell 26 (2015) 2561–2572, 10.1091/mbc.E15-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Brazil DP, Church RH, Surae S, Godson C, Martin F, BMP signalling: agony and antagony in the family, Trends Cell Biol. 25 (2015) 249–264, 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- [78].Staloch D, Gao X, Liu K, Xu M, Feng X, Aronson JF, Falzon M, Greeley GH, Rastellini C, Chao C, Hellmich MR, Cao Y, Ko TC, Gremlin is a key profibrogenic factor in chronic pancreatitis, J. Mol. Med. (Berl.) 93 (2015) 1085–1093, 10.1007/s00109-015-1308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].McDowell CM, Hernandez H, Mao W, Clark AF, Gremlin induces ocular hypertension in mice through smad3-dependent signaling, Invest. Ophthalmol. Vis. Sci 56 (2015) 5485–5492, 10.1167/iovs.15-16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li G, Li Y, Liu S, Shi Y, Chi Y, Liu G, Shan T, Gremlin aggravates hyperglycemia-induced podocyte injury by a TGFβeta/smad dependent signaling pathway, J. Cell. Biochem 114 (2013) 2101–2113, 10.1002/jcb.24559. [DOI] [PubMed] [Google Scholar]
- [81].Ma B, Jing R, Liu J, Qi T, Pei C, Gremlin is a potential target for posterior capsular opacification, Cell Cycle 18 (2019) 1714–1726, 10.1080/15384101.2019.1632125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.[] Church RH, Ali I, Tate M, Lavin D, Krishnakumar A, Kok HM, Hombrebueno JR, Dunne PD, Bingham V, Goldschmeding R, Martin F, Brazil DP, Gremlin1 plays a key role in kidney development and renal fibrosis, Am. J. Physiol. Ren. Physiol 312 (2017) F1141–F1157, 10.1152/ajprenal.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Margadant C, Sonnenberg A, Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing, EMBO Rep. 11 (2010) 97–105, 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vesaluoma M, Mertaniemi P, Mannonen S, Lehto I, Uusitalo R, Sarna S, Tarkkanen A, Tervo T, Cellular and plasma fibronectin in the aqueous humour of primary open-angle glaucoma, exfoliative glaucoma and cataract patients, Eye 12 (Pt 5) (1998) 886–890, 10.1038/eye.1998.224. [DOI] [PubMed] [Google Scholar]
- [85].Kii I, Periostin functions as a scaffold for assembly of extracellular proteins, in: Kudo A (Ed.), Periostin, Springer; Singapore, Singapore, 2019, pp. 23–32, 10.1007/978-981-13-6657-4_3. [DOI] [PubMed] [Google Scholar]
- [86].Kii I, Ito H, Periostin and its interacting proteins in the construction of extracellular architectures, Cell. Mol. Life Sci 74 (2017) 4269–4277, 10.1007/s00018-017-2644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Liu AY, Zheng H, Ouyang G, Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments, Matrix Biol. 37 (2014) 150–156, 10.1016/j.matbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- [88].Chen D, Wang X, Liang D, Gordon J, Mittal A, Manley N, Degenhardt K, Astrof S, Fibronectin signals through integrin alpha5beta1 to regulate cardiovascular development in a cell type-specific manner, Dev. Biol 407 (2015) 195–210, 10.1016/j.ydbio.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Parmigiani CM, McAvoy JW, The roles of laminin and fibronectin in the development of the lens capsule, Curr. Eye Res 10 (1991) 501–511. [DOI] [PubMed] [Google Scholar]
- [90].Huang J, Rajagopal R, Liu Y, Dattilo LK, Shaham O, Ashery-Padan R, Beebe DC, The mechanism of lens placode formation: a case of matrix-mediated morphogenesis, Dev. Biol 355 (2011) 32–42, 10.1016/j.ydbio.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Boyd W, Peiffer RL, Siegal G, Eifrig DE, Fibronectin as a component of pseudophakic acellular membranes, J. Cataract Refract. Surg 18 (1992) 180–183, 10.1016/S0886-3350(13)80928-5. [DOI] [PubMed] [Google Scholar]
- [92].Duncan MK, Kozmik Z, Cveklova K, Piatigorsky J, Cvekl A, Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of (alpha)5(beta)1 integrin expression, J. Cell Sci 113 (Pt 18) (2000) 3173–3185. [DOI] [PubMed] [Google Scholar]
- [93].Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA, iSyTE 2.0: a database for expression-based gene discovery in the eye, Nucleic Acids Res. 46 (2018) D875–D885, 10.1093/nar/gkx837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li H, Yuan X, Li J, Tang X, Implication of Smad2 and Smad3 in transforming growth factor-beta-induced posterior capsular opacification of human lens epithelial cells, Curr. Eye Res 40 (2015) 386–397, 10.3109/02713683.2014.925932. [DOI] [PubMed] [Google Scholar]
- [95].Das S, Wikstrom P, Walum E, Lovicu FJ, A novel NADPH oxidase inhibitor targeting Nox4 in TGFβeta-induced lens epithelial to mesenchymal transition, Exp. Eye Res 185 (2019) 107692, 10.1016/j.exer.2019.107692. [DOI] [PubMed] [Google Scholar]
- [96].Wernecke L, Keckeis S, Reichhart N, Strauss O, Salchow DJ, Epithelial-mesenchymal transdifferentiation in pediatric lens epithelial cells, Invest. Ophthalmol. Vis. Sci 59 (2018) 5785–5794, 10.1167/iovs.18-23789. [DOI] [PubMed] [Google Scholar]
- [97].Wormstone IM, Tamiya S, Anderson I, Duncan G, TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag, Invest. Ophthalmol. Vis. Sci 43 (2002) 2301–2308. [PubMed] [Google Scholar]
- [98].Marcantonio JM, Reddan JR, TGFβeta2 influences alpha5-beta1 integrin distribution in human lens cells, Exp. Eye Res 79 (2004) 437–442, 10.1016/j.exer.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [99].Desai VD, Wang Y, Simirskii VN, Duncan MK, CD44 expression is developmentally regulated in the mouse lens and increases in the lens epithelium after injury, Differentiation 79 (2010) 111–119, 10.1016/j.diff.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tait SWG, Ichim G, Green DR, Die another way–nonapoptotic mechanisms of cell death, J. Cell Sci 127 (2014) 2135–2144, 10.1242/jcs.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rousselle P, Montmasson M, Garnier C, Extracellular matrix contribution to skin wound re-epithelialization, Matrix Biol. 75–76 (2019) 12–26, 10.1016/j.matbio.2018.01.002. [DOI] [PubMed] [Google Scholar]
- [102].Karamanos NK, Theocharis AD, Neill T, Iozzo RV, Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases, Matrix Biol. 75–76 (2019) 1–11, 10.1016/j.matbio.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Schwarzbauer JE, DeSimone DW, Fibronectins, their fibrillogenesis, and in vivo functions, Cold Spring Harb. Perspect. Biol 3 (2011), 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sottile J, Hocking DC, Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions, Mol. Biol. Cell 13 (2002) 3546–3559, 10.1091/mbc.e02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sercu S, Zhang L, Merregaert J, The extracellular matrix protein 1: its molecular interaction and implication in tumor progression, Canc. Invest 26 (2008) 375–384, 10.1080/07357900701788148. [DOI] [PubMed] [Google Scholar]
- [106].Chen S, Birk DE, The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly, FEBS J. 280 (2013) 2120–2137, 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Dallas SL, Sivakumar P, Jones CJP, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM, Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1, J. Biol. Chem 280 (2005) 18871–18880, 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- [108].Rodrigues-Diez R, Lavoz C, Carvajal G, Rayego-Mateos S, Rodrigues Diez RR, Ortiz A, Egido J, Mezzano S, Ruiz-Ortega M, Gremlin is a downstream profibrotic mediator of transforming growth factor-beta in cultured renal cells, Nephron Exp. Nephrol 122 (2012) 62–74, 10.1159/000346575. [DOI] [PubMed] [Google Scholar]
- [109].Li Y, Wang Z, Wang S, Zhao J, Zhang J, Huang Y, Gremlin-mediated decrease in bone morphogenetic protein signaling promotes aristolochic acid-induced epithelial-tomesenchymal transition (EMT) in HK-2 cells, Toxicology 297 (2012) 68–75, 10.1016/j.tox.2012.04.004. [DOI] [PubMed] [Google Scholar]
- [110].Shu DY, Wojciechowski MC, Lovicu FJ, Bone morphogenetic protein-7 suppresses TGFβeta2-induced epithelial-mesenchymal transition in the lens: implications for cataract prevention, Invest. Ophthalmol. Vis. Sci 58 (2017) 781–796, 10.1167/iovs.16-20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kim J-T, Lee DH, Chung K-H, Kang I-C, Kim D-S, Joo CK, Inhibitory effects of salmosin, a disintegrin, on posterior capsular opacification in vitro and in vivo, Exp. Eye Res 74 (2002) 585–594, 10.1006/exer.2001.1150. [DOI] [PubMed] [Google Scholar]
- [112].Qin Y, Zhu Y, Luo F, Chen C, Chen X, Wu M, Killing two birds with one stone: dual blockade of integrin and FGF signaling through targeting syndecan-4 in postoperative capsular opacification, Cell Death Dis. 8 (2017), e2920, 10.1038/cddis.2017.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Liu J, Xu D, Li J, Gao N, Liao C, Jing R, Wu B, Ma B, Shao Y, Pei C, The role of focal adhesion kinase in transforming growth factor-beta2 induced migration of human lens epithelial cells, Int. J. Mol. Med 42 (2018) 3591–3601, 10.3892/ijmm.2018.3912. [DOI] [PubMed] [Google Scholar]
- [114].Mamuya FA, Duncan MK, aV integrins and TGF-b-induced EMT: a circle of regulation, J. Cell Mol. Med 16 (2012) 445–455, 10.1111/j.1582-4934.2011.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Xue M, Jackson CJ, Extracellular matrix reorganization during wound healing and its impact on abnormal scarring, Adv. Wound Care 4 (2015) 119–136, 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Shihan MH, Novo SG, Duncan MK, Cataract surgeon viewpoints on the need for novel preventative anti-inflammatory and anti-posterior capsular opacification therapies, Curr. Med. Res. Opin (2019), 10.1080/03007995.2019.1647012, 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rockey DC, Bell PD, Hill JA, Fibrosis–A common pathway to organ injury and failure, N. Engl. J. Med 373 (2015) 96, 10.1056/NEJMc1504848. [DOI] [PubMed] [Google Scholar]
- [118].Wynn TA, Ramalingam TR, Mechanisms of fibrosis: therapeutic translation for fibrotic disease, Nat. Med 18 (2012) 1028–1040, 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ghosh AK, Quaggin SE, Vaughan DE, Molecular basis of organ fibrosis: potential therapeutic approaches, Exp. Biol. Med 238 (2013) 461–481, 10.1177/1535370213489441. [DOI] [PubMed] [Google Scholar]
- [120].Wang Y, Mahesh P, Wang Y, Novo SG, Shihan MH, Hayward-Piatkovskyi B, Duncan MK, Spatiotemporal dynamics of canonical Wnt signaling during embryonic eye development and posterior capsular opacification (PCO), Exp. Eye Res 175 (2018) 148–158, 10.1016/j.exer.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Leavitt T, Hu MS, Marshall CD, Barnes LA, Lorenz HP, Longaker MT, Scarless wound healing: finding the right cells and signals, Cell Tissue Res. 365 (2016) 483–493, 10.1007/s00441-016-2424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shirai K, Tanaka S-I, Lovicu FJ, Saika S, The murine lens: a model to investigate in vivo epithelial-mesenchymal transition, Dev. Dynam 247 (2018) 340–345, 10.1002/dvdy.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Allinovi M, De Chiara L, Angelotti ML, Becherucci F, Romagnani P, Anti-fibrotic treatments: a review of clinical evidence, Matrix Biol. 68–69 (2018) 333–354, 10.1016/j.matbio.2018.02.017. [DOI] [PubMed] [Google Scholar]
- [124].Iozzo RV, Gubbiotti MA, Extracellular matrix: the driving force of mammalian diseases, Matrix Biol. 71–72 (2018) 1–9, 10.1016/j.matbio.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hewlett JC, Kropski JA, Blackwell TS, Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets, Matrix Biol. 71–72 (2018) 112–127, 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Manthey AL, Lachke SA, FitzGerald PG, Mason RW, Scheiblin DA, McDonald JH, Duncan MK, Loss of Sip1 leads to migration defects and retention of ectodermal markers during lens development, Mech. Dev 131 (2014) 86–110, 10.1016/j.mod.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA, Lens regeneration in mice: implications in cataracts, Exp. Eye Res 78 (2004) 297–299, 10.1016/j.exer.2003.10.021. [DOI] [PubMed] [Google Scholar]
- [128].Shiels A, King JM, Mackay DS, Bassnett S, Refractive defects and cataracts in mice lacking lens intrinsic membrane protein-2, Invest. Ophthalmol. Vis. Sci 48 (2007) 500–508, 10.1167/iovs.06-0947. [DOI] [PubMed] [Google Scholar]
- [129].Scheiblin DA, Gao J, Caplan JL, Simirskii VN, Czymmek KJ, Mathias RT, Duncan MK, Beta-1 integrin is important for the structural maintenance and homeostasis of differentiating fiber cells, Int. J. Biochem. Cell Biol 50 (2014) 132–145, 10.1016/j.biocel.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L, Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks, Nat. Protoc 7 (2012) 562–578, 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kalari KR, Nair AA, Bhavsar JD, O’Brien DR, Davila JI, Bockol MA, Nie J, Tang X, Baheti S, Doughty JB, Middha S, Sicotte H, Thompson AE, Asmann YW, Kocher J-PA, MAP-RSeq: mayo analysis pipeline for RNA sequencing, BMC Bioinf. 15 (2014) 224, 10.1186/1471-2105-15-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Audette DS, Anand D, So T, Rubenstein TB, Lachke SA, Lovicu FJ, Duncan MK, Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression, Development 143 (2016) 318–328, 10.1242/dev.127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K, KEGG: new perspectives on genomes, pathways, diseases and drugs, Nucleic Acids Res. 45 (2017) D353–D361, 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tarca AL, Draghici S, Khatri P, Hassan SS, Mittal P, Kim JS, Kim CJ, Kusanovic JP, Romero R, A novel signaling pathway impact analysis, Bioinformatics 25 (2009) 75–82, 10.1093/bioinformatics/btn577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Reed NA, Oh DJ, Czymmek KJ, Duncan MK, An immunohistochemical method for the detection of proteins in the vertebrate lens, J. Immunol. Methods 253 (2001) 243–252, 10.1016/s0022-1759(01)00374-x. [DOI] [PubMed] [Google Scholar]
- [136].Lewis Carl SA, Gillete-Ferguson I, Ferguson DG, An indirect immunofluorescence procedure for staining the same cryosection with two mouse monoclonal primary antibodies, J. Histochem. Cytochem 41 (1993) 1273–1278, 10.1177/41.8.7687266. [DOI] [PubMed] [Google Scholar]
- [137].Maeda A, Moriguchi T, Hamada M, Kusakabe M, Fujioka Y, Nakano T, Yoh K, Lim K-C, Engel JD, Takahashi S, Transcription factor GATA-3 is essential for lens development, Dev. Dynam 238 (2009) 2280–2291, 10.1002/dvdy.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Grishagin IV, Automatic cell counting with ImageJ, Anal. Biochem 473 (2015) 63–65, 10.1016/j.ab.2014.12.007. [DOI] [PubMed] [Google Scholar]
- [139].Eldred JA, Dawes LJ, Wormstone IM, The lens as a model for fibrotic disease, Phil. Trans. Biol. Sci 366 (2011) 1301–1319, 10.1098/rstb.2010.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Greenburg G, Hay ED, Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells, J. Cell Biol 95 (1982) 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Taiyab A, Korol A, Deschamps PA, West-Mays JA, beta-Catenin/CBP-Dependent signaling regulates TGF-beta-induced epithelial to mesenchymal transition of lens epithelial cells, Invest. Ophthalmol. Vis. Sci 57 (2016) 5736–5747, 10.1167/iovs.16-20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Jeon ES, Kim JH, Ryu H, Kim EK, Lysophosphatidic acid activates TGFBIp expression in human corneal fibroblasts through a TGF-beta1-dependent pathway, Cell. Signal 24 (2012) 1241–1250, 10.1016/j.cellsig.2012.02.009. [DOI] [PubMed] [Google Scholar]
- [143].Meacock WR, Spalton DJ, Stanford MR, Role of cytokines in the pathogenesis of posterior capsule opacification, Br. J. Ophthalmol 84 (2000) 332, 10.1136/bjo.84.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Zhang Y, Huang W, Transforming growth factor beta1 (TGF-beta1)-Stimulated integrin-linked kinase (ILK) regulates migration and epithelial-mesenchymal transition (EMT) of human lens epithelial cells via nuclear factor kappaB (NF-kappaB), Med. Sci. Mon. Int. Med. J. Exp. Clin. Res 24 (2018) 7424–7430, 10.12659/MSM.910601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Rodansky ES, Johnson LA, Huang S, Spence JR, Higgins PDR, Intestinal organoids: a model of intestinal fibrosis for evaluating anti-fibrotic drugs, Exp. Mol. Pathol 98 (2015) 346–351, 10.1016/j.yexmp.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sun P, Li L, Zhao C, Pan M, Qian Z, Su X, Deficiency of a7 nicotinic acetylcholine receptor attenuates bleomycin-induced lung fibrosis in mice, Mol. Med 23 (2017) 34–49, 10.2119/molmed.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wu M, Schneider DJ, Mayes MD, Assassi S, Arnett FC, Tan FK, Blackburn MR, Agarwal SK, Osteopontin in systemic sclerosis and its role in dermal fibrosis, J. Invest. Dermatol 132 (2012) 1605–1614, 10.1038/jid.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wu J, Lubman DM, Kugathasan S, Denson LA, Hyams JS, Dubinsky MC, Griffiths AM, Baldassano RN, Noe JD, Rabizadeh S, Gulati AS, Rosh JR, Crandall WV, Higgins PDR, Stidham RW, Serum protein biomarkers of fibrosis aid in risk stratification of future stricturing complications in pediatric crohn’s disease, Am. J. Gastroenterol 114 (2019) 777–785, 10.14309/ajg.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kumari Suchitra, Panda Tarun Kumar, Pradhan Tapaswini, Oxidase Lysyl, Its diversity in Health and diseases, Indian J. Clin. Biochem 32 (2017) 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Maleszewska M, Gjaltema RAF, Krenning G, Harmsen MC, Enhancer of zeste homolog-2 (EZH2) methyltransferase regulates transgelin/smooth muscle-22alpha expression in endothelial cells in response to interleukin-1beta and transforming growth factor-beta2, Cell. Signal 27 (2015) 1589–1596, 10.1016/j.cellsig.2015.04.008. [DOI] [PubMed] [Google Scholar]
- [151].Idolazzi L, Ridolo E, Fassio A, Gatti D, Montagni M, Caminati M, Martignago I, Incorvaia C, Senna G, Periostin: the bone and beyond, Eur. J. Intern. Med 38 (2017) 12–16, 10.1016/j.ejim.2016.11.015. [DOI] [PubMed] [Google Scholar]
- [152].Stawski L, Trojanowska M, Oncostatin M and its role in fibrosis, Connect. Tissue Res 60 (2019) 40–49, 10.1080/03008207.2018.1500558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Saidenberg Kermanac’h N, Bessis N, Cohen-Solal M, De Vernejoul MC, Boissier M-C, Osteoprotegerin and inflammation, Eur. Cytokine Netw 13 (2002) 144–153. [PubMed] [Google Scholar]
- [154].Kobayashi Y, The role of chemokines in neutrophil biology, Front. Biosci 13 (2008) 2400–2407. [DOI] [PubMed] [Google Scholar]
- [155].Austermann J, Zenker S, Roth J, S100-alarmins: potential therapeutic targets for arthritis, Expert Opin. Ther. Targets 21 (2017) 739–751, 10.1080/14728222.2017.1330411. [DOI] [PubMed] [Google Scholar]
- [156].Rainard P, Riollet C, Berthon P, Cunha P, Fromageau A, Rossignol C, Gilbert FB, The chemokine CXCL3 is responsible for the constitutive chemotactic activity of bovine milk for neutrophils, Mol. Immunol 45 (2008) 4020–4027, 10.1016/j.molimm.2008.06.010. [DOI] [PubMed] [Google Scholar]
- [157].Lee H-S, Woo SJ, Koh H-W, Ka S-O, Zhou L, Jang KY, Lim HS, Kim H-O, Lee S-I, Park B-H, Regulation of apoptosis and inflammatory responses by insulin-like growth factor binding protein 3 in fibroblast-like synoviocytes and experimental animal models of rheumatoid arthritis, Arthritis Rheum. 66 (2014) 863–873, 10.1002/art.38303. [DOI] [PubMed] [Google Scholar]
- [158].van Zuylen WJ, Garceau V, Idris A, Schroder K, Irvine KM, Lattin JE, Ovchinnikov DA, Perkins AC, Cook AD, Hamilton JA, Hertzog PJ, Stacey KJ, Kellie S, Hume DA, Sweet MJ, Macrophage activation and differentiation signals regulate schlafen-4 gene expression: evidence for Schlafen-4 as a modulator of myelopoiesis, PLoS One 6 (2011), e15723, 10.1371/journal.pone.0015723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Brunner PM, Glitzner E, Reininger B, Klein I, Stary G, Mildner M, Uhrin P, Sibilia M, Stingl G, CCL7 contributes to the TNF-alpha-dependent inflammation of lesional psoriatic skin, Exp. Dermatol 24 (2015) 522–528, 10.1111/exd.12709. [DOI] [PubMed] [Google Scholar]
- [160].Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J, S100A8/A9 in inflammation, Front. Immunol 9 (2018) 1298, 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Moschen AR, Adolph TE, Gerner RR, Wieser V, Tilg H, Lipocalin-2: a master mediator of intestinal and metabolic inflammation, Trends Endocrinol. Metabol 28 (2017) 388–397, 10.1016/j.tem.2017.01.003. [DOI] [PubMed] [Google Scholar]
- [162].Espinoza JA, Gonzalez PA, Kalergis AM, Modulation of antiviral immunity by heme oxygenase-1, Am. J. Pathol 187 (2017) 487–493, 10.1016/j.aj-path.2016.11.011. [DOI] [PubMed] [Google Scholar]
- [163].Ma AS, Grigg JR, Ho G, Prokudin I, Farnsworth E, Holman K, Cheng A, Billson FA, Martin F, Fraser C, Mowat D, Smith J, Christodoulou J, Flaherty M, Bennetts B, Jamieson RV, Sporadic and familial congenital cataracts: mutational spectrum and new diagnoses using next-generation sequencing, Hum. Mutat 37 (2016) 371–384, 10.1002/humu.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Wistow G, The human crystallin gene families, Hum. Genom 6 (2012), 10.1186/1479-7364-6-26, 26–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].De Maria A, Bassnett S, DNase IIbeta distribution and activity in the mouse lens, Invest. Ophthalmol. Vis. Sci 48 (2007) 5638–5646, 10.1167/iovs.07-0782. [DOI] [PubMed] [Google Scholar]
- [166].Chepelinsky AB, Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts, Handb. Exp. Pharmacol (2009) 265–297, 10.1007/978-3-540-79885-9_14. [DOI] [PubMed] [Google Scholar]
- [167].Wen Y, Ibaraki N, Reddy VN, Sachs G, Functional analysis of the promoter and chromosomal localization for human LEP503, a novel lens epithelium gene, Gene 269 (2001) 61–71. [DOI] [PubMed] [Google Scholar]
- [168].Irum B, Khan SY, Ali M, Kaul H, Kabir F, Rauf B, Fatima F, Nadeem R, Khan AO, Al Obaisi S, Naeem MA, Nasir IA, Khan SN, Husnain T, Riazuddin S, Akram J, Eghrari AO, Riazuddin SA, Mutation in LIM2 is responsible for autosomal recessive congenital cataracts, PLoS One 11 (2016), e0162620, 10.1371/journal.pone.0162620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Baker C, Belbin O, Kalsheker N, Morgan K, SERPINA3 (aka alpha-1-antichymotrypsin), Front. Biosci 12 (2007) 2821–2835. [DOI] [PubMed] [Google Scholar]
- [170].Tobias PS, Mathison J, Mintz D, Lee JD, Kravchenko V, Kato K, Pugin J, Ulevitch RJ, Participation of lipopolysaccharide-binding protein in lipopolysaccharide-dependent macrophage activation, Am. J. Respir. Cell Mol. Biol 7 (1992) 239–245, 10.1165/ajrcmb/7.3.239. [DOI] [PubMed] [Google Scholar]
- [171].Tsuritani K, Takeda J, Sakagami J, Ishii A, Eriksson T, Hara T, Ishibashi H, Koshihara Y, Yamada K, Yoneda Y, Cytokine receptor-like factor 1 is highly expressed in damaged human knee osteoarthritic cartilage and involved in osteoarthritis downstream of TGF-β, Calcif. Tissue Int 86 (2010) 47–57, 10.1007/s00223-009-9311-1. [DOI] [PubMed] [Google Scholar]
- [172].Arslan AD, Sassano A, Saleiro D, Lisowski P, Kosciuczuk EM, Fischietti M, Eckerdt F, Fish EN, Platanias LC, Human SLFN5 is a transcriptional corepressor of STAT1-mediated interferon responses and promotes the malignant phenotype in glioblastoma, Onco-gene 36 (2017) 6006–6019, 10.1038/onc.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


