Abstract
AIM
To investigate the changes in levels of the lactate dehydrogenase (LDH) enzyme in corneal edema after cataract surgery with trans-corneal oxygenation therapy.
METHODS
This pre-post design study design conducted on 15 patients with corneal edema after cataract surgery and receiving trans-corneal oxygenation therapy. Tear sample (using Schirmer paper, from the inferior fornix of the conjunctiva) was carried out prior to trans-corneal oxygenation therapy, on the day 2 (D2) and day 5 (D5) postoperatively before and after trans-corneal oxygenation therapy. Visual acuity [VA (LogMAR)], corneal endothelial density, central corneal thickness (CCT), and coefficient of variation corneal endothelial (CoV) were recorded. The value of LDH was measured using ELISA. The difference in mean LDH value before and after trans-corneal oxygenation therapy, between two groups were analyzed using Wilcoxon signed rank test.
RESULTS
There was a decrease in LDH tear concentration at D2 (pre vs post: 1127.54±497.09 vs 696.91±489.49; P=0.002) and D5 (pre vs post: 1064.17±677.77 vs 780.28±428.95; P=0.027) after trans-corneal oxygenation therapy as well as decrease in LDH concentration on the D2 compared to D5 (P=0.041). The mean CCT was decreased significantly after the administration of trans-corneal oxygenation (pre vs post: 632.10±25.66 vs 563.90±51.54; P=0.005). The mean VA and CoV increased significantly after the administration of trans-corneal oxygenation (P=0.001 and P=0.028, respectively). However, there was no difference in mean of corneal endothelial density (P=0.814).
CONCLUSION
Trans-corneal oxygenation therapy is associated with significant decrease of tears LDH levels in post cataract surgery with corneal edema. It is accompanied by clinical improvement such as significant reduction of CCT.
Keywords: corneal edema after cataract surgery, central corneal thickness, lactate dehydrogenase, trans-corneal oxygenation
INTRODUCTION
Cataracts are the main cause of blindness and vision problems throughout the world, therefore cataract surgery is the most common surgical procedure performed by ophthalmologists. As medical science and technology develop, evolutionary and revolutionary changes occur in cataract surgery. This is in line with the change in the ophthalmology paradigm from rehabilitation of blindness to optimization of vision function[1]. Cataract surgery aims to produce an optimal function of vision characterized by rapid recovery, measured with minimal side effects, long-term stability, and provide satisfaction to patients. With the development of cataract surgical technology today, intraoperative, and postoperative complications of cataracts are increasingly reduced[2].
Corneal edema is one of the complications following cataract surgery with an incidence of 0.03% to 5.18% of cases[3]. Previous randomized control trial by Sharipfour et al[4] has revealed that administration of trans-corneal oxygenation as an adjuvant therapy in patients with post cataract surgery corneal edema was effective in reducing edema. The exact mechanism is remained unclear, however this might due to the function of endothelial pump (regulated by Na+/K+-ATPase) required oxygen from trans-corneal diffusion.
Therefore, in attempt to explain the early hypoxia process in corneal edema after cataract surgery, it is necessary to investigate the biomolecular markers such as lactate dehydrogenase (LDH). Study by Fullard and Carney[5] has revealed that in the condition of corneal hypoxia there is increase of LDH. The present study was to investigate the changes of LDH enzyme in cataract surgery corneal edema patients following trans-corneal oxygenation therapy.
SUBJECTS AND METHODS
Ethical Approval
The study followed the tenets of the Declaration of Helsinki and was approved by Ethical Review Board of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada (No.KE/FK/0528/EC/2019). Written informed consent was obtained from all study participants.
Research Design and Subjects
The present study was a pre-posttest study design. Fifteen subjects who diagnosed with moderate-to-severe corneal edema at day 2 (D2) post cataract surgery and underwent trans-corneal oxygenation therapy were recruited. Moderate edema was defined as increased stromal thickness, less than ten folds in the Descemet's membrane and hazy iris details. The edema was labeled as being severe if the iris details were not visible; there were more than 10 folds in the Descemet's membrane and a marked increase in the corneal thickness. The inclusion criteria in this study were post cataract surgery patients with phacoemulsification technique with less than 1h surgical time and corneal edema at time of follow up, endothelial corneal counts ≥2000 cells/mm2 and central corneal thickness (CCT) ≥450 µm, do not have other causes of corneal edema, such as: uveitis, glaucoma, congenital eye disorders, other corneal disorders such as corneal dystrophy, do not suffer from systemic disorders such as diabetes mellitus and immune disorders, do not have eye trauma, willing to do trans-corneal oxygenation therapy, and willing to participate in research and approve research sheets. Exclusion criteria in this study were patients who received other intervention therapies during follow-up and whose tear specimens were damaged so that LDH levels cannot be measured.
Data Collection and Study Protocol
Tear sampling was performed before trans-corneal oxygenation therapy, using Schimer paper sterilely, from the inferior fornix of the conjunctiva. Data recording and tear sampling were carried out at D2 and D5 postoperatively before and after trans-corneal oxygenation therapy. The primary outcome was LDH concentration in tears, obtained from ELISA results done at the Laboratory of Biomolecular and Cell Biology, Faculty of Medicine, Universitas Gadjah Mada, Indonesia. Clinical outcomes were visual acuity (VA) and specular microscopy parameters (SP-1000, Topcon Corp., Tokyo, Japan) such as: corneal endothelial density, CCT, and coefficient of variation corneal endothelial (CoV).
All patients received sodium hyaluronate eye drops (Cendo Siloxan®, Cendo Pharmaceutical industries, Indonesia) for corneal edema despite their routines post-cataract eye drops. Transcorneal oxygenation therapy was done using 100% oxygen at a flow rate of 5 L/min for 30min with an eyeshield at postoperative D2 and D5. The shield was firmly taped over the eye with all holes were closed except 1 for inserting the oxygen cannula and another for the exit of oxygen to keep the condition normobaric. Oxygen for transcorneal delivery was humidified using a bottle humidifier filled with distilled water. Oxygen was bubbled through the water, and a relative humidity of 80% was achieved. Patients blinked normally under the shield while receiving transcorneal oxygen.
Statistical Analysis
Statistical analysis was performed using SPSS 24.0 for Windows software. Data were expressed as mean±standard deviation (SD) and range, normality of all data samples was first confirmed by the Saphiro-Wilk test. Difference in subject research characteristics were analyzed using paired t-test if the distribution was normal and Mann-Whitney U test if the distribution was not normal. Changes in LDH concentration were analyzed using Wilcoxon signed rank test. Clinical outcome including VA, corneal endothelial density, CCT, and CoV pre- and post-oxygenation were analyzed using Wilcoxon signed rank test. The P<0.05 was considered significant.
RESULTS
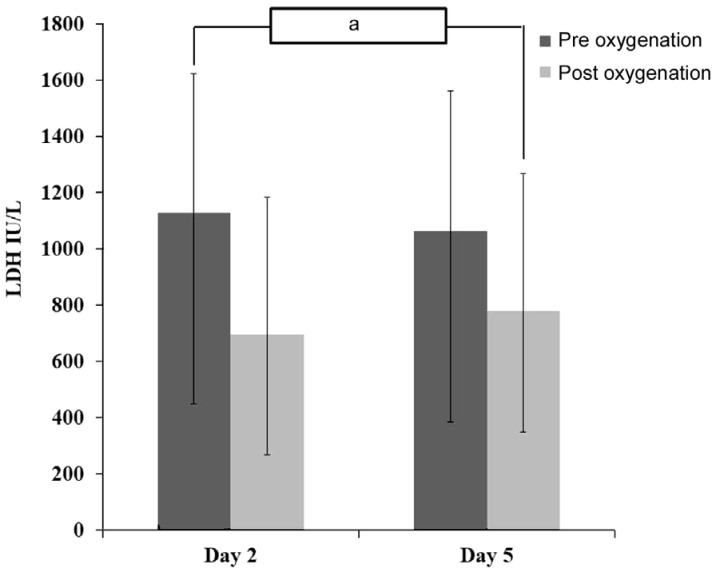
Characteristics of the subjects are shown in Table 1. There was a significant decrease in tear LDH concentration pre vs post oxygenation therapy at D2 with difference 430.62±7.59 IU/L (pre vs post: 1127.54±497.09 vs 696.91±489.49; P=0.002) and D5 with difference 283.88±248.81 IU/L (pre vs post: 1064.17±677.77 vs 780.28±428.95; P=0.027; Table 2, Figure 1). A significant mean difference (pre vs post) in VA, CCT and CoV were found (P=0.001, P=0.005 and P=0.028, respectively; Table 3).
Table 1. Characteristics of the study subjects.
| Characteristics | Case (n=15) |
| Age (y) | |
| Mean±SD | 70±6 |
| Range (min-max) | 60-82 |
| Sex, n (%) | |
| Male | 7 (46.7) |
| Female | 8 (53.3) |
| Grading of edema, n (%) | |
| Moderate | 11 (73.3) |
| Severe | 4 (26.7) |
| Visual acuity (logMAR) | |
| Mean±SD | 1.67±0.55 |
| Range (min-max) | 1.18-2.48 |
| Corneal endothelial density (cell/mm2) | |
| Mean±SD | 2305±160.92 |
| Range (min-max) | 2105-2465 |
| Central corneal thickness (µm) | |
| Mean±SD | 632±25.66 |
| Range (min-max) | 600-678 |
| Coefficient of variation corneal endothelial (%) | |
| Mean±SD | 58.00±3.89 |
| Range (min-max) | 53-63 |
Table 2. Changes in LDH concentration pre and post trans-corneal oxygenation therapy.
| LDH (UI/L) | Pre-oxygenation | Post-oxygenation | P |
| Day 2 | |||
| Mean±SD | 1127.54±497.09 | 696.91±489.49 | 0.002a |
| Range (min-max) | 537.08-1989.53 | 133.46-1764.22 | |
| Day 5 | |||
| Mean±SD | 1064.17±677.77 | 780.28±428.95 | 0.027a |
| Range (min-max) | 266.91-2499.85 | 171.61-1633.31 |
LDH: Lactate dehydrogenase; aP<0.05 (Wilcoxon signed rank test).
Figure 1. Changes in LDH concentration day 2 and day 5.
aP=0.041.
Table 3. Changes in visual acuity, corneal endothelial density, central corneal thickness, and coefficient of variation corneal endothelial.
| Clinical outcome | Pre-oxygenation | Post-oxygenation | P |
| Visual acuity (logMAR) | 1.67±0.55 | 1.06±0.27 | 0.001a |
| Corneal endothelial density (cell/mm2) | 2105.33±160.19 | 2007±503.35 | 0.814 |
| Central corneal thickness (µm) | 632.10±25.66 | 563.90±51.54 | 0.005a |
| Coefficient of variation corneal endothelial (%) | 58.00±3.89 | 38.50±4.18 | 0.028a |
LDH: Lactate dehydrogenase; aP<0.05 (Wilcoxon signed rank test).
DISCUSSION
To our best knowledge, no previous studies have investigated tear LDH concentration differences in pre and post trans-corneal oxygenation therapy. The present study revealed that trans-corneal oxygenation therapy was accompanied by a significant reduction in LDH levels. A study by Guo and Zhang[6] showed LDH concentration in normal people was 126.86±60.84 UI/L. Elevated concentration of LDH in tears are associated with its important role in the mechanism of corneal metabolism[5],[7].
It was also revealed that there was a decrease on CCT after cataract surgery. It was similar with Karekla et al's[8] and Perone et al's[9] study where there is decrease in corneal endothelial density after phacoemulsification by 18.58% in the first week after surgery. Simova et al[10] also stated that after cataract surgery with phacoemulsification there was decrease in corneal endothelial density by 89 cells/mm2 (3.91% compared to baseline). Simova et al[10] stated that the thickness of the central cornea will return into preoperative status after 3 and 12mo postoperatively. It has been shown that a healthy cornea might compensate quickly when there is an increase in the thickness of the central cornea after cataract surgery[11]–[12].
Moderate corneal edema is characterized by an increase in stromal thickness, <10 folds of the Descemet's membrane is found, and faint iris details are seen, however severe edema when iris details are not seen, >10 folds of the Descemet's membrane, and an increase in the thickness of the central cornea[13]. In cataract surgery corneal edema might occur due to manipulation or prolonged or excessive exposure of the cornea that causes endothelial damage, and results in disruption of endothelial pump function[3].
Corneal thickness and clarity are maintained by active pumping mechanism of corneal endothelial cells[14]. This active pump is controlled by the Na+/K+-ATPase mechanism and involves bicarbonate ions gradients and glucose in corneal endothelial cells[8]. The cellular glucose threshold starts from the presence of glucose transporters in the apical and basolateral endothelial cell membranes. Most (85%) of glucose used by the cornea is converted to lactic acid[15] which produced in the cornea when epithelial hypoxia occurs. Epithelial hypoxia might increase osmolarity in the space between the stroma or endothelium, that decrease pressure difference; consequently, less water is released from the stroma. If stromal hydration increases, corneal edema occurs[16].
A study by Sharifipour et al[4] showed that the initial hyperbaric oxygen therapy has been shown to be effective in reducing corneal edema compared to the control group given only medical therapy. The study suggested that oxygen supplements can improve endothelial pump function and restore corneal clarity. Therefore, trans corneal oxygenation is able to increase oxygen levels that improve the condition of corneal hypoxia[17]. We assumed that in the present study, there was an improvement in corneal metabolism in patients who received trans-corneal oxygenation therapy as indicated by a decrease in LDH levels.
The levels of LDH might be used as the indicator of hypoxia and mechanical damage to the corneal epithelium[11],[18]. Lactate production can trigger corneal edema as a result of the cessation of aerobic metabolic pathways[19]. According to Moezzi et al[20] in the condition of lack of oxygen for 3h, the cornea has increased in thickness by 8%. Corneal edema followed by an increase in CCT increases the distance from oxygen to reach corneal endothelial cells, thereby aggravating the hypoxic status of corneal endothelial cells[4]. In conclusion, Trans-corneal oxygenation therapy was associated with significant decrease of tears LDH levels in post cataract surgery with corneal edema which might indicate the improvement of hypoxia condition in the cornea. However, further study is needed to clarify the exact bio-mechanism of LDH in the cornea.
Acknowledgments
Conflicts of Interest: Lahagu EA, None; Fachiroh J, None; Anugrah AS, None; Gunawan W, None; Mahayana IT, None; Suhardjo, None.
REFERENCES
- 1.Packer M, Fishkind WJ, Fine IH, Seibel BS, Hoffman RS. The physics of phaco: a review. J Cataract Refract Surg. 2005;31(2):424–431. doi: 10.1016/j.jcrs.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Fine IH, Packer M, Hoffman RS. New phacoemulsification technologies. J Cataract Refract Surg. 2002;28(6):1054–1060. doi: 10.1016/s0886-3350(02)01399-8. [DOI] [PubMed] [Google Scholar]
- 3.Huang S. Cell damage and repair after phacoemulsification. Invest Clín. 2019;60(4) [Google Scholar]
- 4.Sharifipour F, Panahi-Bazaz M, Idani E, Hajizadeh M, Saki A. Oxygen therapy for corneal edema after cataract surgery. J Cataract Refract Surg. 2015;41(7):1370–1375. doi: 10.1016/j.jcrs.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Fullard RJ, Carney LG. Human tear enzyme changes as indicators of the corneal response to anterior hypoxia. Acta Ophthalmol (Copenh) 1985;63(6):678–683. doi: 10.1111/j.1755-3768.1985.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Zhang H. Tear malate dehydrogenase, lactate dehydrogenase and their isoenzymes in normal Chinese subjects and patients of ocular surface disorders. Eye Sci. 1995;11(1):61–64. [PubMed] [Google Scholar]
- 7.Ichijima H, Ohashi J, Cavanagh HD. Effect of contact-lens-induced hypoxia on lactate dehydrogenase activity and isozyme in rabbit cornea. Cornea. 1992;11(2):108–113. doi: 10.1097/00003226-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Karekla AI, Linardi C, Morfopoulos A, Lamprinakis IK. A Clinical Prospective Study: corneal alterations after cataract surgery with the technique of phacoemulsification. Hosp Chron. 2019;14(1):7–12. [Google Scholar]
- 9.Perone JM, Boiche M, Lhuillier L, Ameloot F, Premy S, Jeancolas AL, Goetz C, Neiter E. Correlation between postoperative central corneal thickness and endothelial damage after cataract surgery by phacoemulsification. Cornea. 2018;37(5):587–590. doi: 10.1097/ICO.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 10.Simova J, Radeva M, Grupchev D, Mihova T, Grupcheva C. Central corneal thickness and morphological changes in the cornea after uneventful phacoemulsification. Bulg Rev Ophthalmol. 2018;62(4):10. [Google Scholar]
- 11.Kaup S, Shivalli S, Ks D, Arunachalam C, Varghese RC. Central corneal thickness changes in bevel-up versus bevel-down phacoemulsification cataract surgery: study protocol for a randomised, triple-blind, parallel group trial. BMJ Open. 2016;6(9):e012024. doi: 10.1136/bmjopen-2016-012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galgauskas S, Ignataviciute J, Vieversyte Z, Asoklis R. Endothelial parameters in central and peripheral cornea in patients wearing contact lenses. Int J Ophthalmol. 2018;11(11):1768–1773. doi: 10.18240/ijo.2018.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma N, Singhal D, Nair SP, Sahay P, Sreeshankar SS, Maharana PK. Corneal edema after phacoemulsification. Indian J Ophthalmol. 2017;65(12):1381–1389. doi: 10.4103/ijo.IJO_871_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mencucci R, Ponchietti C, Virgili G, Giansanti F, Menchini U. Corneal endothelial damage after cataract surgery: microincision versus standard technique. J Cataract Refract Surg. 2006;32(8):1351–1354. doi: 10.1016/j.jcrs.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 15.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2012;95(1):2–7. doi: 10.1016/j.exer.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung BK, Bonanno JA, Radke CJ. Oxygen-deficient metabolism and corneal edema. Prog Retin Eye Res. 2011;30(6):471–492. doi: 10.1016/j.preteyeres.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharifipour F, Idani E, Zamani M, Helmi T, Cheraghian B. Oxygen tension in the aqueous humor of human eyes under different oxygenation conditions. J Ophthalmic Vis Res. 2013;8(2):119–125. [PMC free article] [PubMed] [Google Scholar]
- 18.Fullard RJ, Carney LG. Diurnal variation in human tear enzymes. Exp Eye Res. 1984;38(1):15–26. doi: 10.1016/0014-4835(84)90134-9. [DOI] [PubMed] [Google Scholar]
- 19.Brennan NA, Efron N, Carney LG. Critical oxygen requirements to avoid oedema of the central and peripheral cornea. Acta Ophthalmol (Copenh) 1987;65(5):556–564. doi: 10.1111/j.1755-3768.1987.tb07040.x. [DOI] [PubMed] [Google Scholar]
- 20.Moezzi AM, Fonn D, Varikooty J, Richter D. Distribution of overnight corneal swelling across subjects with 4 different silicone hydrogel lenses. Eye Contact Lens. 2011;37(2):61–65. doi: 10.1097/ICL.0b013e31820e0bc3. [DOI] [PubMed] [Google Scholar]