Abstract
AIM
To evaluate the efficacy and safety of subconjunctival triamcinolone acetonide (TA) injections for treating uveitic macular edema (UME).
METHODS
This retrospective case series study included patients with UME who received subconjunctival TA injections with a minimum follow-up period of 6mo. The main outcome measure was central macular thickness (CMT). The secondary outcome measures included best-corrected visual acuity (BCVA), recurrence rate and intraocular pressure (IOP).
RESULTS
In total, 65 patients (80 eyes), mainly including idiopathic uveitis in 33 patients (50.77%) and Vogt-Koyanagi-Harada (VKH) syndrome in 19 patients (29.23%), were enrolled in this study. The mean CMT decreased from 457.6±173.0 µm at baseline to 325.9±176.8, 302.7±148.2, 332.2±177.3 and 270.6±121.6 µm at 1-, 2-, 3- and 6-months postinjection, respectively (all P<0.001). BCVA increased from logMAR 0.5±0.3 at baseline to logMAR 0.4±0.3, 0.4±0.3, 0.4±0.4 and 0.4±0.3 at the 1-, 2-, 3- and 6-months postinjection visits, respectively (all P<0.001). Twenty-one (21/80, 26.25%) eyes underwent relapse of UME within 6mo. A total of 20/80 (25%) eyes exhibited elevated IOPs, of which 13 eyes were controlled with topical IOP-lowering agents and 7 eyes underwent surgical removal of subconjunctival TA deposit.
CONCLUSION
Subconjunctival TA injections appear to be safe and effective for UME.
Keywords: triamcinolone acetonide, subconjunctival injection, uveitis, macular edema, intraocular pressure
INTRODUCTION
Macular edema (ME) is one of the most common complications of uveitis which may result in visual impairment and even blindness[1]–[4]. The mechanism of ME is believed to result from fluid leakage across the blood-retinal barrier and fluid accumulation in the macular region, sometimes with a characteristic distribution in the outer plexiform layer and subretinal area[1]. While corticosteroids remain the first line treatment for uveitic macular edema (UME), immunosuppressants such as cyclosporine, methotrexate, azathioprine and mycophenolate mofetil are usually required for chronic and intractable UME. Sustained-release corticosteroid implants[5], anti-vascular endothelial growth factor (VEGF) agents and anti-tumor necrosis factor-α (TNF-α) agents have recently emerged as options for UME[1],[6].
Despite the advantages of the corticosteroid implants, triamcinolone acetonide (TA), a long-acting glucocorticoid, is still widely used for its efficacy and affordable cost[7]. However, although the reports on periocular[8] or intraocular injections of TA are numerous[9]–[13], few studies have been conducted on subconjunctival injections of TA to treat UME[14]–[16].
This study aimed to evaluate the efficacy and safety of subconjunctival TA injections in treating UME.
SUBJECTS AND METHODS
Ethical Approval
The study followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Peking Union Medical College Hospital. Written informed consent was obtained from all participants before the subconjunctival TA injection(s) and any other invasive procedures/examinations.
Patient Eligibility and Exclusion Criteria
The clinical data of UME patients who received subconjunctival TA injections from January 2009 to December 2018 in the Department of Ophthalmology, Peking Union Medical College Hospital were collected and analyzed. All patients underwent a complete ophthalmic examination at each visit, which included measures of the best-corrected visual acuity (BCVA), intraocular pressure (IOP), slit-lamp examination, and fundus examination under pupil dilation. A routine work-up, including a complete blood cell count; urinalysis; liver and renal function marker analysis; human immunodeficiency virus antibody, rapid plasma regain (RPR), hepatitis C virus antibody, and hepatitis B virus soluble antigen tests; chest X-ray analysis; purified protein derivative test; erythrocyte sedimentation rate analysis; and antinuclear antibody and human leukocyte antigen-B27 tests, was performed at presentation. The inclusion criteria were as follows: 1) new onset of unilateral UME, or bilateral UME with unilateral aggravation, of any anatomical type (anterior, middle, or posterior uveitis or panuveitis); 2) dose of ≤15 mg prednisone or equivalent if the patient was on systemic corticosteroid; 3) absence of significant ocular inflammation requiring initiation or uptitration of systemic corticosteroids and/or immunosuppressants or a patient refusing or having contraindications for these drugs; 4) no use of corticosteroid eye drops and topical nonsteroidal anti-inflammatory drugs (NSAIDs); and 5) patients with complete clinical data at baseline and the 1-, 2-, 3- and 6-months postinjection visits. The exclusion criteria were as follows: 1) patients with infectious uveitis were excluded from this study; 2) history of any other ocular disease (e.g., diabetic retinopathy or retinal vascular obstruction) that may cause ME; 3) periocular or intraocular injections received within 6mo before the subconjunctival TA injection; and 4) presence or development of posterior synechia or media opacity such as cataract that compromise satisfactory fundus evaluation and the quality of optical coherence tomography (OCT) images.
The main outcome measure was central macular thickness (CMT) measured by OCT. The secondary outcome measures included BCVA, recurrence rate and IOP within 6mo after the injection.
Examination and Treatment Procedures
The procedure was performed in the Outpatient Department. Patients received subconjunctival injections of TA in a supine position. To anesthetize the injected eye, a single application of 0.4% oxybuprocaine hydrochloride eye drops (Santen Pharmaceutical Co., Ltd. Japan) was applied. A 1-mL syringe containing 20 mg TA (Kunming Jida Pharmaceutical Co., Ltd., China, concentration: 40 mg/mL) was injected into the inferior fornix, and the drug deposit could be seen under the conjunctiva. Patients were asked to monitor their eye pressure every 2wk after the intervention. Systemic corticosteroids or immunosuppressants were not initiated or uptitrated. Corticosteroid eye drops and topical NSAIDs were not used in any of the cases. Meanwhile, topical IOP-lowering agents such as beta-blockers, carbonic anhydrase inhibitors, and alpha-agonists were applied as first-line treatment for IOP elevation. For patients with IOP over 30 mm Hg that could not be controlled with topical eye drops, surgery to remove the TA deposit was recommended.
Optical Coherence Tomography Acquisition
The CMT was measured using an Optovue OCT (Optovue, Fremont, CA) or 3D-OCT 2000 (Topcon Corporation, Japan) devices. For the follow-up, the same device was applied for each patient. AutoRescan features were used to ensure that the follow-up scans matched the baseline.
Statistical Analysis
Statistical analysis was performed using IBM SPSS software, version 25.0 (IBM SPSS, USA). Visual acuity was obtained from each patient's medical records and converted to a logarithm of the minimal angle of resolution (logMAR) for statistical analysis. Paired t-tests were performed to analyze logMAR visual acuity and CMT. A P-value <0.05 was considered significant difference.
RESULTS
In this retrospective, observational case series study, 65 patients (16 males and 49 females, 80 eyes) were enrolled. The age of the included patients ranged from 11 to 78 (49.2±14.1)y; 35/65 patients (53.85%) received only one injection, while other patients received several injections in one eye or in both eyes. Of the 15 patients with both eyes included, none received bilateral subconjunctival TA injection simultaneously. The demographic features of patients at baseline were shown in Table 1.
Table 1. Demographic features of the patients.
| Subjects | No. of patients/eyes |
| Uveitis diagnosis | |
| Idiopathic | 33/65 (50.77) |
| Vogt-Koyanagi-Harada disease | 19/65 (29.23) |
| JIA-associated | 3/65 (4.62) |
| Sarcoidosis | 2/65 (3.08) |
| Bechςet's disease | 2/65 (3.08) |
| HLA-B27 associated | 1/65 (1.54) |
| Other | 5/65 (7.69) |
| Lens condition | |
| No cataract | 41/80 (51.25) |
| Cataract | 21/80 (26.25) |
| IOL eyes | 18/80 (22.50) |
| Periocular steroid injection times | |
| Unilateral | 50/65 (76.92) |
| Bilateral | 15/65 (23.07) |
| 7 times | 1/65 (1.54) |
| 6 times | 1/65 (1.54) |
| 5 times | 1/65 (1.54) |
| 4 times | 5/65 (7.69) |
| 3 times | 7/65 (10.77) |
| 2 times | 15/65 (23.08) |
| 1 time | 35/65 (53.85) |
| Systemic therapy | 42/65 (64.62) |
| Prednisolone alone | 5/65 (7.69) |
| Prednisolone+1 immunosuppressant | 24/65 (36.80) |
| Prednisolone+2 immunosuppressant | 8/65 (12.31) |
| 1 immunosuppressant | 3/65 (4.62) |
| 2 immunosuppressant | 2/65 (3.08) |
JIA: Juvenile idiopathic arthritis; HLA: Human leukocyte antigen. Immunosuppressant including cyclosporine, methotrexate, azathioprine.
n (%)
The mean CMTs of the subconjunctival TA-injected eyes were significantly reduced. The mean CMT decreased from 457.6±173.0 µm before the injection to 325.9±176.8 µm (P<0.001), 302.7±148.2 µm (P<0.001), 332.2±177.3 µm (P<0.001) and 270.6±121.6 µm (P<0.001) at 1-, 2-, 3- and 6-months postinjection, respectively (Figure 1).
Figure 1. CMT changes after the treatment with subconjunctival injection of TA.
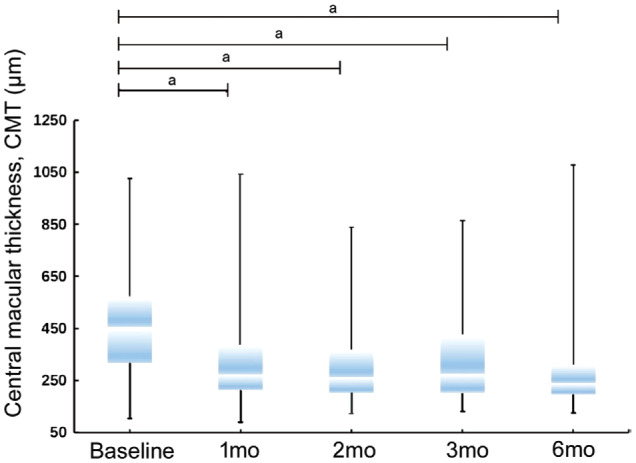
aP<0.001.
BCVA increased from logMAR 0.5±0.3 at baseline to logMAR 0.4±0.3 (P<0.001), logMAR 0.4±0.3 (P<0.001), logMAR 0.4±0.4 (P<0.001) and logMAR 0.4±0.3 (P<0.001) at the 1-, 2-, 3- and 6-months post-injection visits, respectively (Figure 2).
Figure 2. Mean visual acuity at baseline and the changes over time.
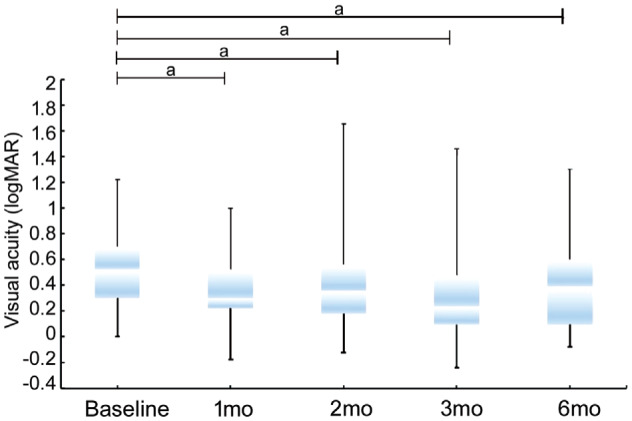
aP<0.001.
We observed twenty-one (21/80, 26.25%) eyes underwent relapse of UME within 6mo. Among these eyes, 5 (23.81%), 7 (33.33%) and 9 (42.86%) eyes relapsed less than 2mo, 2 to 3mo, and 3 to 6mo after the injection, respectively. Ten of 21 (47.62%) eyes received a second injection and were still responsive.
Elevation of IOP (≥21 mm Hg) was observed in 20/80 (25.0%) eyes. Among them, 8/20 (40.0%), 5/20 (25.0%) and 3/20 (15.0%) eyes had peak IOPs between 21 to 25 mm Hg, 25 to 30 mm Hg and 30 to 35 mm Hg, respectively, and 4/20 (20.0%) eyes had peak IOPs over 35 mm Hg. Furthermore, 13/20 (65.0%) eyes were well controlled by 1 or 2 types of topical IOP-lowering agents, while 7 eyes (35.0%) underwent surgical removal of the subconjunctival TA deposit. Eight eyes had IOP elevation during the first month after the injection, 7 eyes had IOP elevation during the second month after the injection, 4 eyes had IOP elevation during the 3rd month after the injection, and only 1 case had an IOP elevation in the 4th month (15wk). The time frame for IOP rise was in the first 2mo (15/20, 75%) after the injections.
DISCUSSION
ME is frequently encountered in patients with uveitis[17]–[18], and it can cause permanent vision loss. The management varies significantly among different centers. The options for local corticosteroids included periocular or intraocular injections of TA and intraocular sustained-release glucocorticoid implants[11],[19]. Of interest is the POINT trial which compared the effectiveness of 3 treatment modalities of local corticosteroids in UME, in particular periocular injections of 40 mg TA (periorbital floor or posterior sub-Tenon's approach), intraocular injections of 4 mg TA and a 0.7 mg dexamethasone intravitreal implant[15]. The results showed that all treatment groups had clinically meaningful reductions in central subretinal thickness compared with baseline[15]. However, subconjunctival injections of TA have rarely been reported[14]–[16].
Regarding Central Macular Thickness
In the first month after injection of 20 mg TA, 62/71 eyes (87.32%) showed a reduction in CMT with 59/71 eyes (83.09%) by at least 20%, which is very close to the overall response rate (88%) observed in a previous study[20] aiming to compare subconjunctival TA, intravitreal TA and intravitreal dexamethasone implants. Other studies, however, revealed lower levels of effectiveness of subtenon TA injections. Bae and colleagues[21] reported that 53.1% of the eyes treated with peribulbar injections of 40 mg TA showed reduction in CMT after 1mo. Leder et al[22] observed that UME was clinically resolved in 53% and 57% of treated eyes 1 and 3mo respectively after a single posterior-subtenon TA (40 mg) injection. Furthermore, CMT reduction was observed only in 23% eyes 2mo after a periocular injections of 40 mg TA[11].
Regarding Relapse
As presented previously, 21 (21/80, 26.25%) eyes underwent relapse of UME within 6mo. Among these eyes, 5 (23.81%), 7 (33.33%) and 9 (42.86%) eyes relapsed less than 2mo, 2 to 3mo, and 3 to 6mo after the injection, respectively. In addition, the majority of uveitis types enrolled in our study were idiopathic and VKH. We found that 6/21 (28.57%) eyes got relapse in VKH group. while 12/46 (26.09%) eyes in idiopathic group, with no statistical difference between the two subgroups (P=0.526, P>0.05). Some cases are worth noting. In one patient, the first injection resulted in resolution of UME for 6mo, but the therapeutic effect of the second injection given 1.5y later lasted only 2mo. Another patient received 7 injections with good responsiveness observed every time in a 10-year follow up period, and the longest resolution lasted for more than 6mo.
Regarding Intraocular Pressure
An elevated IOP was observed in 20/80 eyes (25.0%) in our study. However, Byun and Park[23] reported that 18 eyes (11.3%) required glaucoma medications after a posterior-subtenon injection. Another study reported that 34.9% of the patients after a posterior-subtenon injection had elevated IOPs, and 4.7% of the patients needed trabeculectomy ultimately[24].
Anterior subtenon injection of TA was found to be 2.4 times more likely (95%CI, 1.02-5.9) to cause elevated IOPs than posterior subtenon injection[25], which could be explained by the notion that a higher aqueous level of TA is associated with a higher incidence of IOP elevation. However, our data showed a similar rate of IOP elevation as compared to posterior subtenon injection. In addition, elevated IOP was observed mainly (15/20 eyes, 75%) within the first 2mo, which indicated that the patients should be close monitoring of IOP during the first 2mo after the intervention. While IOP-lowering eye drops were sufficient for the majority of the patients, 7 eyes (7 patients) underwent surgical removal of the subconjunctival TA deposit, and the IOP returned to normal within 1mo after the surgery. Subconjunctival hemorrhage is also a well-known but trivial side effect. Other reported side effects[16] of subconjunctival TA such as conjunctival ulceration[26], ischemia, necrosis[27] and infectious scleritis were not observed in our patients.
From our perspective, subconjunctival injection of TA (20 mg) has several advantages over other periocular injections. It is technically an easier procedure and could be safely performed in the outpatient clinics; although it may be more likely to cause IOP elevation, topical IOP-lowering agents are usually sufficient to control the IOP, and surgical removal of subconjunctival TA deposits is easy and effective when intractable IOP elevation occurs.
There are some limitations for our study, including inhomogeneity of the included patients, inevitable biases, missing data and the different follow-up intervals among different patients due to the retrospective nature of the study.
In conclusion, subconjunctival TA injections appear to be safe and effective for UME. Increased IOP is a concern, but it can be well controlled by IOP-lowering eye drops and surgical removal of TA deposits when necessary.
Acknowledgments
Conflicts of Interest: Qu Y, None; Liu XS, None; Liang AY, None; Xiao JY, None; Zhao C, None; Gao F, None; Zhang MF, None.
REFERENCES
- 1.Accorinti M, Okada AA, Smith JR, Gilardi M. Epidemiology of macular edema in uveitis. Ocul Immunol Inflamm. 2019;27(2):169–180. doi: 10.1080/09273948.2019.1576910. [DOI] [PubMed] [Google Scholar]
- 2.Okhravi N, Lightman S. Cystoid macular edema in uveitis. Ocul Immunol Inflamm. 2003;11(1):29–38. doi: 10.1076/ocii.11.1.29.15582. [DOI] [PubMed] [Google Scholar]
- 3.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113(8):1446–1449. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Multicenter Uveitis Steroid Treatment Trial Research Group. Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149(4):550–561.e10. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Symes RJ, Forooghian F. Topical difluprednate monotherapy for uveitic macular edema. Can J Ophthalmol. 2016;51(1):47–49. doi: 10.1016/j.jcjo.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Tallouzi MO, Moore DJ, Calvert M, Murray PI, Bucknall N, Denniston AK. The effectiveness of pharmacological agents for the treatment of uveitic macular oedema (UMO): a systematic review protocol. Syst Rev. 2016;5:29. doi: 10.1186/s13643-016-0203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindholm JM, Taipale C, Ylinen P, Tuuminen R. Perioperative subconjunctival triamcinolone acetonide injection for prevention of inflammation and macular oedema after cataract surgery. Acta Ophthalmol. 2020;98(1):36–42. doi: 10.1111/aos.14175. [DOI] [PubMed] [Google Scholar]
- 8.Sreekantam S, MacDonald T, Keane PA, Sim DA, Murray PI, Denniston AK. Quantitative analysis of vitreous inflammation using optical coherence tomography in patients receiving sub-Tenon's triamcinolone acetonide for uveitic cystoid macular oedema. Br J Ophthalmol. 2017;101(2):175–179. doi: 10.1136/bjophthalmol-2015-308008. [DOI] [PubMed] [Google Scholar]
- 9.Levin MH, Pistilli M, Daniel E, Gangaputra SS, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Jabs DA, Levy-Clarke GA, Kempen JH, Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology. 2014;121(2):588–595.e1. doi: 10.1016/j.ophtha.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tranos PG, Tsaousis KT, Vakalis AN, Asteriades S, Pavesio CE. Long-term follow-up of inflammatory cystoid macular edema. Retina. 2012;32(8):1624–1628. doi: 10.1097/IAE.0b013e3182483348. [DOI] [PubMed] [Google Scholar]
- 11.Choudhry S, Ghosh S. Intravitreal and posterior subtenon triamcinolone acetonide in idiopathic bilateral uveitic macular oedema. Clin Exp Ophthalmol. 2007;35(8):713–718. doi: 10.1111/j.1442-9071.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- 12.Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Jabs DA, Kempen JH. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 2014;121(11):2275–2286. doi: 10.1016/j.ophtha.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steeples LR, Anand N, Moraji J, Jones NP. Clinical outcomes of intravitreal preservative-free triamcinolone preparation (triesence®) for cystoid macular oedema and inflammation in patients with uveitis. Ocul Immunol Inflamm. 2018;26(7):997–1004. doi: 10.1080/09273948.2017.1294185. [DOI] [PubMed] [Google Scholar]
- 14.Liu XS, Wang M, Zhao C, Gao F, Zhang MF. The efficacy and safety of subconjunctival triamcinolone acetonide injections in treatment of uveitic macular edema. Zhonghua Yan Ke Za Zhi. 2015;51(10):734–738. [PubMed] [Google Scholar]
- 15.Thorne JE, Sugar EA, Holbrook JT, Burke AE, Altaweel MM, Vitale AT, Acharya NR, Kempen JH, Jabs DA, Multicenter Uveitis Steroid Treatment Trial Research Group Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283–295. doi: 10.1016/j.ophtha.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Athanasiadis Y, Tsatsos M, Sharma A, Hossain P. Subconjunctival triamcinolone acetonide in the management of ocular inflammatory disease. J Ocul Pharmacol Ther. 2013;29(6):516–522. doi: 10.1089/jop.2012.0208. [DOI] [PubMed] [Google Scholar]
- 17.Jones NP. The Manchester Uveitis Clinic: the first 3000 patients—epidemiology and casemix. Ocul Immunol Inflamm. 2015;23(2):118–126. doi: 10.3109/09273948.2013.855799. [DOI] [PubMed] [Google Scholar]
- 18.Lasave AF, Schlaen A, Zeballos DG, Díaz-Llopis M, Couto C, El-Haig WM, Arevalo JF. Twenty-four months follow-up of intravitreal bevacizumab injection versus intravitreal triamcinolone acetonide injection for the management of persistent non-infectious uveitic cystoid macular edema. Ocul Immunol Inflamm. 2019;27(2):294–302. doi: 10.1080/09273948.2017.1400073. [DOI] [PubMed] [Google Scholar]
- 19.Roesel M, Gutfleisch M, Heinz C, Heimes B, Zurek-Imhoff B, Heiligenhaus A. Intravitreal and orbital floor triamcinolone acetonide injections in noninfectious uveitis: a comparative study. Ophthalmic Res. 2009;42(2):81–86. doi: 10.1159/000220600. [DOI] [PubMed] [Google Scholar]
- 20.Carbonnière C, Couret C, Blériot A, Lebreton O, Massé H, Le Meur G, Lebranchu P, Weber M. Treatment of macular edema: Comparison of efficacy and tolerability of subconjunctival triamcinolone injections, sub-tenon's triamcinolone injections and intravitreal dexamethasone implant. J Fr Ophtalmol. 2017;40(3):177–186. doi: 10.1016/j.jfo.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Bae JH, Lee CS, Lee SC. Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina. 2011;31(1):111–118. doi: 10.1097/IAE.0b013e3181e378af. [DOI] [PubMed] [Google Scholar]
- 22.Leder HA, Jabs DA, Galor A, Dunn JP, Thorne JE. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol. 2011;152(3):441–448.e2. doi: 10.1016/j.ajo.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Byun YS, Park YH. Complications and safety profile of posterior subtenon injection of triamcinolone acetonide. J Ocul Pharmacol Ther. 2009;25(2):159–162. doi: 10.1089/jop.2008.0087. [DOI] [PubMed] [Google Scholar]
- 24.Jea SY, Byon IS, Oum BS. Triamcinolone-induced intraocular pressure elevation: intravitreal injection for macular edema and posterior subtenon injection for uveitis. Korean J Ophthalmol. 2006;20(2):99–103. doi: 10.3341/kjo.2006.20.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XL, Li YZ, Zhang Y, Du WN, Sun SM, Lin B, Chen H, Cheng LY. Comparison of intraocular pressure elevation after anterior versus posterior subtenon triamcinolone acetonide acetate injection: a retrospective study. Retina. 2012;32(9):1838–1843. doi: 10.1097/IAE.0b013e31824fd384. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal S, Agrawal J, Agrawal TP. Conjunctival ulceration following triamcinolone injection. Am J Ophthalmol. 2003;136(3):539–540. doi: 10.1016/s0002-9394(03)00320-9. [DOI] [PubMed] [Google Scholar]
- 27.Ying-Jiun C, Chee-Kuen W, Shatriah I. Conjunctival necrosis following a subconjunctival injection of triamcinolone acetonide in a child. Middle East Afr J Ophthalmol. 2015;22(1):125–128. doi: 10.4103/0974-9233.148364. [DOI] [PMC free article] [PubMed] [Google Scholar]


