Highlights
-
•
Biomass production of Justicia gendarussa Burm.f. for supporting drug material provided by callus culture method.
-
•
Metabolite profiles of Justicia gendarussa Burm.f callus for supporting data base in drug developed from this plant.
-
•
Callus of Justicia gendarussa Burm.f. production for supporting cell culture system of this plant.
-
•
Repetitive subculture and the best sucrose concentration will be supporting to scale up the biomass production from Justicia gendarussa Burm.f.
Abbreviations: BAP, Benzyl amino purine; MS, Murashige and Skoog; 2,4-D, 2,4- Dichlorophenoxyacetic Acid; GC/MS, Gas chromatography/mass spectrometry; KOH, Kalium hydroxide /Sodium hydroxide
Keywords: Callus, Gas chromatography/mass spectrometry, Justicia gandarussa Burm.f., Light, Repetitive subculture, Sucrose
Abstract
Objective
This research aimed at the discovering the effects of light, sucrose concentration and repetitive subculture on the growth of Justicia gendarussa Burm.f. calluses based on biomass, morphological characters and metabolic profiles.
Methods
The second and third leaves of young J. gendarussa Burm.f. were isolated and cultured in solid Murashige and Skoog (MS) medium with the addition of 1 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.5 mg/L benzyl amino purine (BAP). Different sucrose concentrations, varying from 0 to 5%, were included in the medium. The cultures were incubated under light and dark conditions for 6 weeks. Repetitive subculture was carried out every 2 weeks for a total of four times, and 3% sucrose gave the best callus growth. Dry calluses were extracted with methanol, and their metabolic profiles were analyzed using gas chromatography/mass spectrometry (GC/MS). Compound identification was performed by comparing the mass spectra to references from the WILEY version 7n.1 library.
Results
Among the 12 conditions tested for the prolonged 6-week culture, the 2–5 % sucrose treatments under light and 3–5 % sucrose treatments under dark exhibited the highest dry weight. For repetitive subculture, the highest wet and dry weights were identically detected under both light and dark conditions after the second repetitive subculture. A total of 19 metabolites was identified by GC/MS, with major compounds being taraxasterol, monoplex D, 2-methoxy-4-vinylphenol, and palmitic acid.
Conclusion
Light, sucrose concentration, and repetitive subculture all significantly impacted the growth and metabolic profiles of J. gendarussa Burm.f. calluses. A concentration of 3% sucrose demonstrated the best growth of callus and could be further applied for mass production.
1. Introduction
Gandarusa (Justicia gendarussa Burm.f.) is an Indonesian medicinal plant belonging to Acanthaceae. It is a small, erect shrub growing 0.6–1.2 m tall and is widely distributed over South and Southeast Asia, from lowlands up to 1500 m above sea level [1,2]. Gandarusa has been reported to contain saponins, flavonoids, polyphenols, essential oils, flavanol-3-glycosides, flavones, luteolins, iso-orientin, coumarin, iridoids, sterols, tannins, and calcium oxalate [3]. Traditionally, it has been used to treat muscle spasms, rheumatism, common cold, headache, pharyngitis, bronchitis, dyspepsia, eye diseases [3], antifertility [4], analgesic [5] and antihyperuricemic effects [6].
Nowadays, tissue culture approaches have been developed to produce specific plants in large quantities in order to support biotechnological researches [7,8]. Currently, gandarusa is in the public spotlight in Indonesia and is the subject of extensive research due to its medicinal potential. As a result of the increasing demand, its availability in nature has been visibly impacted. Therefore, tissue culture can be developed into a whole plant or cell suspensions to obtain only the desired secondary metabolites [9,10]. Nevertheless, the success of callus culture relies on several key factors, including the plant genotype and variety, culture medium [11], growth-controlling substances [11,12], growth environment [13], carbon source [[12], [13], [14]] and light [15,16]. Changes in light condition and sucrose concentration could affect the production of such molecules [17,18]. Moreover, repetitive subculture is a critical step in plant tissue culture in order for culture strategies to take into account how many times subculture is performed.
GC/MS has been successfully employed to identify metabolite patterns in a variety of medicinal plant cultures [19]. Secondary metabolite analysis has been conducted using GC/MS on both the leaf [20] and callus of gandarusa in the presence of various growth-regulating substances [21]. Some natural compound has been identified by GC/MS, such as taraxasterol which has many important pharmacological actions including anticancer activity. Palmitic acid, monoplex D and 2-methoxy-4-vinylphenol were also used as antioxidant and antimicrobial [20,21].
In this study, we measured the biomass of gandarusa callus through fresh and dry weights and used GC/MS method to characterize small metabolites produced from the callus; we then compared them among different culture conditions. Three major factors–light, sucrose concentration and number of repetitive subcultures–were varied in this study to determine which conditions were best for gandarusa callus growth. This research provided useful information about the effects of light, sucrose concentration, and repetitive subculture on the growth and metabolic profiles of gandarusa callus. In addition, it could inform the mass production of gandarusa in both agricultural and industrial settings in the future.
2. Materials and methods
2.1. Explant preparation
Gandarusa plant (Justicia gandarussa Burm.f.) was acquired from Taman Husada Graha Famili, Surabaya, Indonesia, and identified by botanists from the Biosystematic Laboratory, Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya, Indonesia. The second and third leaves were collected from young shoots and used as the initial explants. They were cut and washed carefully with running tap water. To sterilize the explants, the cut leaves were soaked in 2 g/L dithane fungicide for 5 min and rinsed with distilled water, three times each. They were then immersed in clorox solution (50 %, v/v) and shaken for 7 min. Lastly, the explants were rinsed three times with sterile distilled water inside a laminar airflow cabinet.
2.2. Medium preparation
Murashige and Skoog (MS) medium was used for callus culture [31]. Sucrose (0%, 1%, 2%, 3%, 4% and 5%; w/v), 8 g/L agar, 1 mg/L 2,4-D and 0,5 mg/L BAP (the best growth regulator substance data for initiation that already published) were subsequently added to the medium. The pH of the medium was adjusted to 5.6–5.8 using 1 N of KOH or HCl. Prepared stock medium was divided into a small bottles (100 mL bottle, 30 mL medium), covered with aluminium foil and sterilized by autoclaving at 121 °C and 1 atm of pressure for 15 min. The medium with sucrose (3%, w/v) gave the best callus growth and was identified as the best medium used in the repetitive subculture experiments.
2.3. Callus initiation and repetitive subculture
Leaf explants, 1 cm2 in size, were transferred to petri dishes lined with sterile filter paper. They were gently embedded on the surface of the varied sterile medium formulations inside the culture bottles. The bottles were tightly tied with a rubber band. Calluses were harvested every 2 weeks to examine their growth over a total of 6 weeks. A half gram of callus obtained from the initiation step was transferred into a subculture bottle. The first repetitive subculture was conducted on a 4-week-old callus and performed every 2 weeks afterward for total four times. Culture bottles were placed in a preservation room at 25 ± 2 °C, equipped with continuous (24 h) light of neon lamp (general electric cool white fluorescent tube) to provide 650 ± 45 lx light intensity (the lamp was off for the dark condition). The distance between the light source and culture bottles was approximately 60 cm. Every treatment was done in four times.
2.4. Callus collection and extraction
Calluses were harvested at every 2 weeks (repetitive subculture period). They were freshly weighed and measured again after complete drying in room temperature (25 ± 2 °C) with continuous light. After drying (measured until the constant weight), they were ground in preparation for extraction. Ten millilitres of absolute methanol were added to 1 g dried samples and macerated for 24 h in a closed falcon tube (15 mL, shaken every 6 h). This step was repeated three times, and the fractions were pooled together for evaporating. This methanol extract was kept at 4 °C until further analysis.
2.5. GC/MS analysis
The metabolite profiles of methanol callus extracts were determined using GC/MS. Extract of 50 mg was dissolved in 0.5 mL n-hexane and then filtrated by 0.45 μm of filter paper. GC/MS sample analysis was performed using an Agilent 6980 N Network GC System with autosampler and detector (Agilent 5973 inert MSD), fitted with HP-5 5% phenylmethylsiloxane capillary column (30 m ×0.32 mm, with 0.2-μm film thickness; J&W Scientific). The oven temperature was programmed from 50 °C to 280 °C at 10 °C/min and held for 10 min. Helium was used as the carrier gas at flow rate of 1.3 mL/min. The injector temperature was 280 °C and the injection volume 1 μL, with a split ratio of 1:10. The interface and MS ion source were maintained at 230 °C and 150 °C, respectively, the mass spectra were taken at 70 eV with a mass scan range of 20–700 amu. Data handling was done using GC/MS solution software. The identification of compounds was based on comparisons of their mass spectra with those of the WILEY version 7n.1 library. The relative percentage of each component was calculated by the relative percentage of the total peak area in the chromatogram.
2.6. Data analysis
Two types of data were collected, they were qualitative data which included callus morphology, including colour and texture callus, while quantitative data which included fresh and dry callus weights. The quantitative data were analyzed statistically using SPSS 20 software. They were also tested for normality using one-sample Kolmogorov-Smirnov test, followed by the Kruskal-Wallis test. The Mann-Whitney test was used to test significant differences among treatments.
3. Results
3.1. The effects of light, sucrose concentration and repetitive subculture on Justicia gendarussa Burm.f. callus morphology
One indicator of growth during in vitro culture is the appearance of calluses in explants. The emergence of callus begins with swelling on the side of the wound in explants. Callus formation in wound tissue was stimulated by explant growth medium, which includes growth regulators and sucrose. In this study, leaf explants were placed on MS medium with the addition of 1 mg/L 2,4-D hormone, 0.5 mg/L BAP and sucrose at various concentrations and subsequently incubated in the dark or the light condition. In culture medium without sucrose, explants incubated in the dark did not form calluses and became brown after the first week of observation, whereas in light incubation, the calluses formed in the third week. Using culture medium with 1%–5% sucrose, callus induction started growing in the second week. The effects of different sucrose concentrations and light condition were recorded. The callus texture was friable at first, and then, when incubated in both dark and light conditions, the texture became compact in all treatments, while the callus colour varied among the treatments (Table 1 and sure 1). Overall, the dark-incubated calluses were brighter than the light-incubated calluses. The first callus appeared translucent. The calluses grew until they became enlarged and dark brown in colour. The colour of the calluses became darker with age and increase of sucrose concentration.
Table 1.
Formation time, morphology, and intensity of callus from Justicia gendarussa Burm. f., with variation in sucrose concentration for 6 weeks in dark and light conditions.
| Week | Sucrose concetration (%) | Dark |
Light |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eficiency of callus initiation (%) | Callus formation time (week) | Callus morphology |
Callus intensity | Eficiency of callus initiation (%) | Callus formation time (week) | Callus morphology |
Callus intensity | ||||
| Colour | Texture | Colour | Texture | ||||||||
| 1 | 0 | – | – | – | – | – | – | – | – | – | – |
| 10 | – | – | – | – | – | – | – | – | – | – | |
| 20 | – | – | – | – | – | – | – | – | – | – | |
| 30 | – | – | – | – | – | – | – | – | – | – | |
| 40 | – | – | – | – | – | – | – | – | – | – | |
| 50 | – | – | – | – | – | – | – | – | – | – | |
| 2 | 0 | – | – | – | – | – | – | – | – | – | – |
| 10 | 100 | 2 | White | Friable | + | 100 | 2 | White | Friable | + | |
| 20 | 100 | 2 | White | Friable | + | 100 | 2 | White | Friable | + | |
| 30 | 100 | 2 | White | Friable | + | 100 | 2 | White | Friable | + | |
| 40 | 100 | 2 | White | Friable | + | 100 | 2 | White | Friable | + | |
| 50 | 100 | 2 | White | Friable | + | 100 | 2 | White | Friable | + | |
| 3 | 0 | – | – | – | – | – | 100 | 3 | White | Friable | + |
| 10 | 100 | 2 | White | Friable | + | 100 | 2 | White | Friable | + | |
| 20 | 100 | 2 | Yellowish | Compact | ++ | 100 | 2 | Yellowish | Compact | ++ | |
| 30 | 100 | 2 | Yellowish | Compact | ++ | 100 | 2 | Yellowish | Compact | ++ | |
| 40 | 100 | 2 | Yellowish | Compact | ++ | 100 | 2 | Yellowish | Compact | ++ | |
| 50 | 100 | 2 | Brown | Compact | ++ | 100 | 2 | Brownish | Compact | ++ | |
| 4 | 0 | – | – | – | – | – | 100 | 3 | White | Friable | + |
| 10 | 100 | 2 | White | Friable | + | 100 | 2 | White | Friable | + | |
| 20 | 100 | 2 | Yellowish | Compact | +++ | 100 | 2 | Yellowish | Compact | +++ | |
| 30 | 100 | 2 | Yellowish | Compact | ++++ | 100 | 2 | Yellowish | Compact | ++++ | |
| 40 | 100 | 2 | Muddy white | Compact | ++++ | 100 | 2 | Muddy white | Compact | ++++ | |
| 50 | 100 | 2 | Brown | Compact | ++++ | 100 | 2 | Brownish | Compact | ++++ | |
| 5 | 0 | – | – | – | – | – | 100 | 3 | Yellowish | Compact | ++ |
| 10 | 100 | 2 | Yellowish | Compact | ++ | 100 | 2 | Yellowish | Compact | ++ | |
| 20 | 100 | 2 | Brownish | Compact | +++ | 100 | 2 | Muddy white | Compact | ++++ | |
| 30 | 100 | 2 | Brownish | Compact | ++++ | 100 | 2 | Brownish | Compact | ++++ | |
| 40 | 100 | 2 | Brownish | Compact | ++++ | 100 | 2 | Brown | Compact | ++++ | |
| 50 | 100 | 2 | Dark brown | Compact | ++++ | 100 | 2 | Brown | Compact | ++++ | |
| 6 | 0 | – | – | – | – | – | 100 | 2 | Brown | Compact | ++ |
| 10 | 100 | 2 | Dark brown | Compact | +++ | 100 | 2 | Dark brown | Compact | ++++ | |
| 20 | 100 | 2 | Dark brown | Compact | ++++ | 100 | 2 | Dark brown | Compact | ++++ | |
| 30 | 100 | 2 | Dark brown | Compact | ++++ | 100 | 2 | Dark brown | Compact | ++++ | |
| 40 | 100 | 2 | Dark brown | Compact | ++++ | 100 | 2 | Dark brown | Compact | ++++ | |
| 50 | 100 | 2 | Dark brown | Compact | ++++ | 100 | 2 | Dark brown | Compact | ++++ | |
Note: -, no callus is formed; +, 25 % callus of explants; ++, 50 % callus of explants; +++, 75 % callus of explants; ++++, 100 % callus of explant.
Callus morphology also varied during subculture. In both light and dark conditions, the first and second subcultures showed compact-textured calluses, whereas the third and fourth showed friable-textured calluses. Callus colour also changed during repetitive subculture treatments. In both light and dark conditions, the first subculture showed a white callus and the second showed a muddy white callus. In light condition, the third subculture showed a muddy white callus, but in dark condition, it showed a brown callus. In light condition, the fourth subculture remained muddy white, while in the dark they were dark brown (Fig. 2).
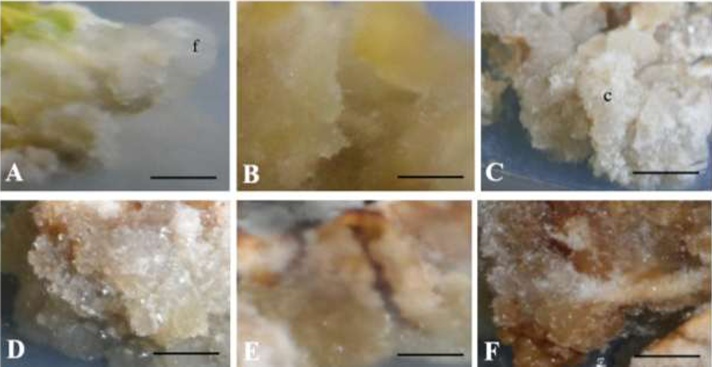
Fig. 2.
Representative morphological traits of Justicia gendarussa Burm.f. calluses treated with different sucrose concentrations (%) with dark or light incubation. (A) Translucent white callus, (B) yellowish callus, (C) muddy white callus, (D) brownish callus, (E) brown callus, (F) dark brown callus, (f) friable callus, (c) compact callus; (bar =2 mm).
3.2. The effects of light, sucrose concentration and repetitive subculture on Justicia gendarussa Burm.f. callus induction and growth
The present study showed that calluses protruded from the leaves and were clearly visible after fourteen days of culture. Compact and light yellowish calluses were obtained after 21 days (Fig. 1C). Based on Fig. 1 and Table 1, all treatments produced callus except those without sucrose in dark condition.
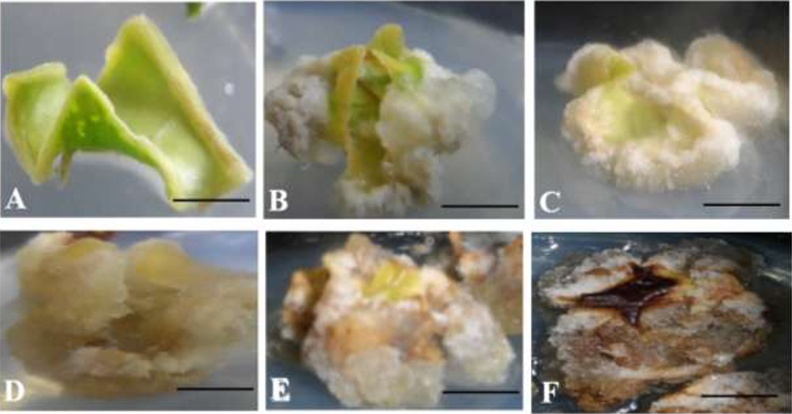
Fig. 1.
Representative morphological traits of Justicia gandarussa Burm.f. calluses treated with 3% sucrose every week. (A) First week culture, (B) second week culture, (C) third week culture, (D) fourth week culture, (E) fifth week culture, (F) sixth week culture; (bar =1 cm).
Callus biomass accumulation was recorded for 6 weeks, at 2-week intervals, under various concentrations of sucrose, and incubated either in the dark or light. The different sucrose concentrations affected both the fresh weight and dry weights of the calluses. The highest fresh weight of callus was with 2% sucrose (light) (1.19 ± 0.18 g), 3% (light) (1.19 ± 0.18 g) and 3% (dark) (1.08 ± 0.03 g), followed by 5% (dark) (0.89 ± 0.13 g), 1% (light) (0.78 ± 0.11 g), 2% (light) (0.72 ± 0.06 g), 4 % (light) (0.72 ± 0.06 g), 4% (dark) (0.70 ± 0.12 g), 2% (dark) (0.70 ± 0.09 g), 5% (light) (0.68 ± 0.41 g), 1% (dark) (0.64 ± 0.10 g) and 0% (dark and light) (0.00 ± 0.00 g) after 6 weeks of callus culture (Table 2).
Table 2.
Average fresh and dry weights of Justicia gandarussa Burm.f. calluses after 2, 4, and 6 weeks of incubation.
| Time incubation (week) | Sucrose concentration (%) | Dark |
Light |
||
|---|---|---|---|---|---|
| Fresh weight (g) | Dry weight (g) | Fresh weight (g) | Dry weight (g) | ||
| 2 | 0 | 0.00 ± 0.00a | 0.00 ± 0.00q | 0.00 ± 0.00a | 0.00 ± 0.00q |
| 1 | 0.09 ± 0.00abcd | 0.01 ± 0.00qrs | 0.06 ± 0.01abc | 0.01 ± 0.00qrs | |
| 2 | 0.01 ± 0.01abcd | 0.01 ± 0.00qrst | 0.12 ± 0.02abcde | 0.01 ± 0.0qrst | |
| 3 | 0.19 ± 0.06bcdef | 0.02 ± 0.01stuv | 0.11 ± 0.00abcde | 0.02 ± 0.00stuv | |
| 4 | 0.12 ± 0.01abcde | 0.01 ± 0.00qrs | 0.09 ± 0.02abcd | 0.01 ± 0.00qrstu | |
| 5 | 0.23 ± 0.06def | 0.03 ± 0.01wx | 0.18 ± 0.02abcdef | 0.03 ± 0.00uvw | |
| 4 | 0 | 0.00 ± 0.00a | 0.00 ± 0.00q | 0.04 ± 0.00abc | 0.00 ± 0.00q |
| 1 | 0.07 ± 000abcd | 0.01 ± 0.00qrs | 0.16 ± 0.02abcdef | 0.01 ± 0.00rst | |
| 2 | 0.29 ± 0.06fg | 0.02 ± 0.00stuv | 0.09 ± 0.02abcd | 0.01 ± 0.00qrst | |
| 3 | 0.28 ± 0.10fg | 0.03 ± 0.00tuv | 0.13 ± 0.01abcde | 0.02 ± 0.00stu | |
| 4 | 0.05 ± 0.01ab | 0.01 ± 0.00qrs | 0.21 ± 0.03cdef | 0.02 ± 0.00tuv | |
| 5 | 0.42 ± 0.09g | 0.04 ± 0.00wx | 0.26 ± 0.03ef | 0.03 ± 0.00uvw | |
| 6 | 0 | 0.00 ± 0.00a | 0.00 ± 0.00q | 0.06 ± 0.01abc | 0.01 ± 0.00q |
| 1 | 0.64 ± 0.10h | 0.04 ± 0.00wx | 0.78 ± 0.11hi | 0.05 ± 0.00xy | |
| 2 | 0.70 ± 0.09h | 0.05 ± 0.00xy | 1.19 ± 0.18j | 0.08 ± 0.01z | |
| 3 | 1.08 ± 0.03j | 0.09 ± 0.00z | 1.19 ± 0.12j | 0.09 ± 0.01z | |
| 4 | 0.70 ± 0.12h | 0.09 ± 0.03z | 0.72 ± 0.06h | 0.09 ± 0.00z | |
| 5 | 0.89 ± 0.13i | 0.09 ± 0.01z | 0.68 ± 0.41h | 0.09 ± 0.01z | |
Note: Number followed by the same letter indicates no significant differences according to Mann-Whitney Test (α = 0.05).
The highest dry weight of callus was with the addition of 3% sucrose (dark and light) (0.09 ± 0.00 g) and (0.09 ± 0.01 g), respectively, 4% (dark) (0.09 ± 0.03 g) and 5% (dark and light) (0.09 ± 0.01 g) and (0.09 ± 0.01 g), respectively, which did not differ significantly from 2% (light) (0.08 ± 0.01 g), followed by 4% (light) (0.06 ± 0.00 g), 1% (light) (0.05 ± 0.00 g), 2% (dark) (0.05 ± 0.00 g), 1% (dark) (0.04 ± 0.00 g) and 0% (dark and light) (0.00 ± 0.00 g) after 6 weeks of callus culture (Table 2).
The 3% sucrose media was used for subcultures, because it was the lowest sucrose level that gave the highest biomass. Callus biomass was tracked for 2 months, recorded every 2 weeks (subculture period). Calluses showed growth after the second subculture. After repeating subculture, callus growth in term of gross weight was changed (Table 3). Fresh and dry weights of the calluses increased after the first and second subculture and decreased in the third subculture. Calluses grown in both light and dark conditions demonstrated the same pattern. The highest callus weight was observed in the second subculture under dark conditions (1.72 ± 0.02 g), but this was not significantly higher than the light treatment (1.68 ± 0.01 g).
Table 3.
Average fresh and dry weights of Justicia gandarussa Burm.f. calluses after 1, 2, 3 and 4 repetitive subcultures.
| Treatment | First repetitive |
Second repetitive |
Third Repetitive |
Forth repetitive |
||||
|---|---|---|---|---|---|---|---|---|
| Fresh weight | Dry weight | Fresh weight | Dry weight | Fresh weight | Dry weight | Fresh weight | Dry weight | |
| Light | 0.90 ± 0.04b | 0.07 ± 0.01y | 1.68 ± 0.01d | 0.11 ± 0.00z | 1.30 ± 0.01c | 0.07 ± 0.01x | 0.87 ± 0.02a | 0.07 ± 0.00x |
| Dark | 0.88 ± 0.03b | 0.07 ± 0.00y | 1.72 ± 0.02d | 0.08 ± 0.00z | 1.27 ± 0.01c | 0.06 ± 0.00x | 0.61 ± 0.01a | 0.06 ± 0.01x |
Note: Number followed by the same letter shows no significant differences according to the Duncan test (α = 0.05).
3.3. Phytochemical profile
Phytochemical profiling of Justicia gendarussa Burm.f. (gandarusa) calluses was carried out using GC/MS. Representative chromatograms from each condition are shown in Fig. 3. Lists of chemical identifications are reported in Table 4. A total of 19 compounds was detected from the eight different conditions. Under light conditions, the highest number of compounds was identified from the first subculture (12 compounds), followed by the third subculture (10 compounds), second subculture (8 compounds) and fourth subculture (4 compounds). Under dark conditions, the fourth subculture had the highest number of detected components (11 compounds), followed by the first and third subculture (9 compounds), and second subculture (8 compounds).
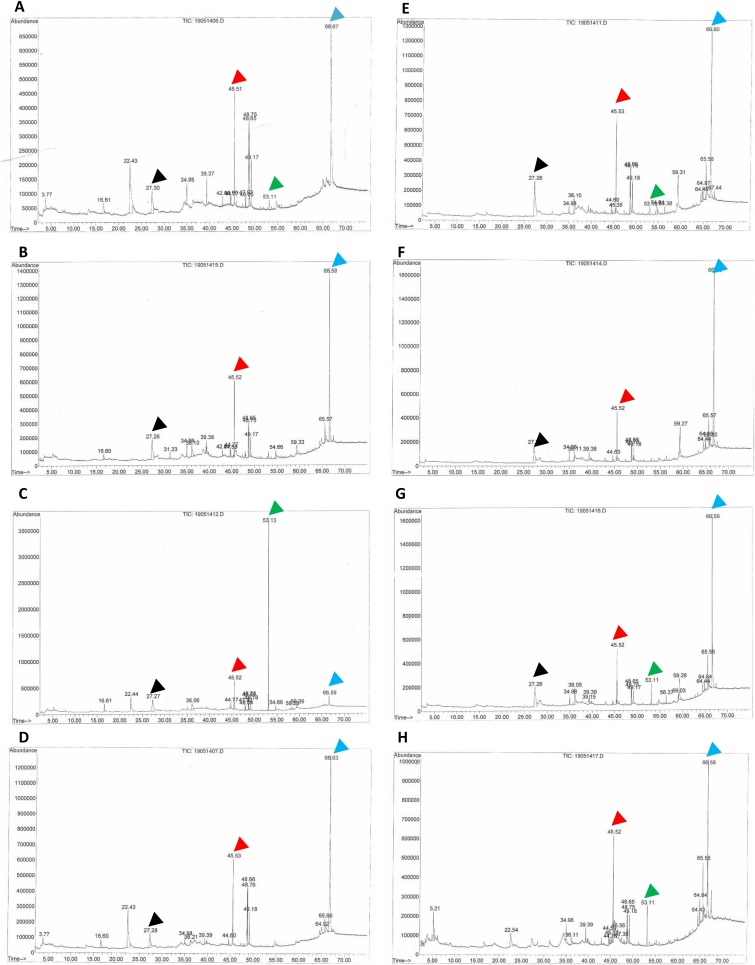
Fig. 3.
Representative chromatogram of methanol extracts of Justicia gendarussa Burm.f. calluses among the different conditions. (A) Light, first subculture; (B) light, second subculture; (C) light, third subculture; (D) light, fourth subculture; (E) dark, first subculture; (F) dark, second subculture; (G) dark, third subculture; (H) dark, fourth subculture. black arrow 2-methoxy-4-vinylphenol; red arrow: palmitic acid; green arrow: monoplex D; blue arrow: traxasterol.
Table 4.
Phytochemical components from GC/MS analysis of Justicia gandarusa Burm.f. calluses in every repetitive subculture.
| No. | Compound | RT (min) | Relative area percentage (peak area relative to the total peak area (%) |
Bioactivity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subculture of Light incubation |
Subculture of Dark incubation |
||||||||||
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | ||||
| Fatty acid | |||||||||||
| 1 | Stearic acid | 49.13 | 2.80 | 2.21 | 2.071 | 6.26 | 2.97 | 1.726 | 1.75 | 3.26 | Antimicrobial, 5α reductase inhibitor, and hypocholesterolemic agent [44] |
| 2 | Palmitic acid | 45.38 | 7.09 | 10.94 | 6.47 | 0.50 | 0.46 | 7.580 | 8.18 | 12.77 | Anti-inflammatory, antidiabetic, antibacterial and antioxidant [44] |
| 3 | Linoleic acid | 48.27 | 5.41 | 4.42 | 2.67 | 7.98 | 3.89 | 2.576 | 2.52 | 3.81 | Anti-inflammatory, acne reductive and moisture-retaining properties [44] |
| 4 | Elaidic acid | 48.74 | 5.01 | – | 2.52 | 5.89 | 5.89 | – | – | 2.21 | Decreases HDL cholesterol[44} |
| 5 | Eudesmane | 44.60 | – | – | – | – | 1.12 | – | – | – | Antioxidant [44] |
| 6 | Hexadecanal | 44.59 | – | – | – | 0.50 | – | 0.891 | – | 1.35 | Antimicrobial and antioxidant [44] |
| 7 | Heptadecanoic acid (CAS) | 47.36 | – | – | – | – | – | – | – | 0.83 | Antioxidant and antimicrobial [44] |
| Phenols | |||||||||||
| 8 | 2-Methoxy-4-vinylphenol | 27.27 | 8.14 | 14.04 | 14.30 | 5.49 | 16.67 | 13.793 | 14.92 | – | Antimicrobial and antioxidant [44] |
| 9 | Homovanillyl alcohol | 36.09 | – | 6.32 | 7.15 | – | 5.55 | 3.874 | 8.05 | 2.90 | Neurotransmitter dopamine, antioxidant [44] |
| Sterol | |||||||||||
| 10 | Taraxasterol | 66.61 | 39.52 | – | – | 45.89 | 28.66 | 42.437 | 35.34 | ` | Antibacterial, antiviral, anti-inflamatory, and anti-apoptosis [44] |
| Silosane | |||||||||||
| 11 | Hexadecamethyl- cyclooctasiloxane | 39.32 | 1.65 | – | – | – | – | 1.072 | 1.36 | 1.80 | Antioxidant, used for treatment and prevention of hypoxia, anemia, and sickle cell disease [44] |
| 12 | Tetradecamethyl-cycloheptasiloxane | 34.97 | 3.43 | 1.73 | – | – | – | – | – | 3.42 | Antimicrobial agent, antifouling immunomodulatory, and has antitumor activities [44] |
| Ester | |||||||||||
| 13 | Methyl palmitate | 44.76 | – | 1.24 | 1.03 | – | – | – | – | 0.59 | Anti-inflammatory, antidiabetic, antibacterial, and antioxidant [44] |
| 14 | Methyl oleate | 48.28 | 0.48 | – | 0.89 | – | – | – | 2.78 | – | Anticancer anti-inflammatory and antioxidant [44] |
| 19 | Monoplex D | 53.11 | 0.54 | – | 39.22 | – | 0.63 | – | 2.32 | – | Antioxidant and antimicrobial [44] |
| 15 | 912-Octadecadienoic acid (Z,Z)-, methyl ester | 47.92 | 0.66 | – | 1.24 | – | – | – | – | – | Anti-inflammatory, anticancer [44] |
| 16 | Pentadecanoic acid, 14-methyl-, methyl ester | 44.77 | 0.52 | – | – | – | – | – | – | – | Antioxidant [44] |
| Others | |||||||||||
| 17 | 6-Aza-571,214-tetrathiapentacene | 39.37 | – | 2.39 | – | – | – | – | – | – | Antibacterial [44] |
| 18 | (+-)-15-Hexadecanolie | 45.11 | – | – | – | – | – | – | – | 0.98 | Antimicrobial [44] |
Among the 19 total compounds detected, stearic acid, palmitic acid, and linoleic acid were found in all eight tested conditions; 2-methoxy-4-vinylphenol and in seven; elaidic acid, taraxasterol, and monoplex D in five; hexadecamethyl-cyclooctasiloxane and tetrade-camethyl-cyclooctasiloxane in four; methyl oleat, methyl palmitate, and hexadecanal in three; 9,12-octadecadienoic acid (Z,Z)-, methyl ester in two of the eight tested conditions. There were 5 compounds identified from just a single condition.
A wide range of chemical components was identified under light conditions. Taraxasterol was abundant in the first and fourth subculture, while palmitic acid and 2-methoxy-4-vinylphenol were highly observed in the second subcultures. Moreover, monoplex D was enriched in the third subculture. Under dark conditions, taraxasterol was a major constituent in the first, second and third subcultures, as was 2-methoxy-4-vinylphenol. Furthermore, in the fourth subculture, palmitic acid was redundant.
The GC/MS analyses revealed that the callus extracts were predominantly composed of fatty acids, in addition to small quantities of a phenolic (2-methoxy-4-vinylphenol), an aliphatic alcohol (homovanillyl alcohol), a sterol (taraxasterol), and ester (hexanedioic acid, bis(2-ethylhexyl) ester). Retention times of fatty acids were recorded in a range of 44–48 min. Taraxasterol was eluted last, at approximately 66 min, indicative of its nonpolar property.
4. Discussion
Although Justicia gendarussa Burm.f. (gandarusa) has pharmaceutical benefits, it is not a cultivated species, and the continued use of natural sources is not sustainable. In vitro cultivation of gandarusa is expected to replace conventional cultivation for the production of bioactive compounds. If achieved, there will be no necessity to take plants directly from nature. Furthermore, in vitro culture facilitates the distribution of gandarusa to other institutions around the world, as this form of plant does not require quarantine [22,23]. In this study, we examined the effects of light, sucrose concentration and repetitive subculture on growth and secondary metabolite profile in gandarusa.
The combination of light, sucrose content and repetitive subculture influenced callus morphology, which was indicated by the colour and texture of the callus. Callus colour was brighter at the first growth, and then, it became darker with age and increase of sucrose concentration. Callus colour in dark conditions was brighter than in light conditions. The colour change of calluses indicated cell activity during cell division [16]. In addition, George and Sherintong [24] also stated that the colour of calluses becomes brown because they produced phenolic compounds that could be toxic to the plants and stop the growth. It has also been reported that increasing the sucrose concentration of the culture medium resulted in the increased production of phenolics, leading to cell death [25].
Further discoloration of the callus, from white to dark brown, was an indicator of the low cleavage activity of callus cells [26]. Taranto et al. [23] found that callus browning was caused by polyphenolic compounds, which were present when the explants were wounded, and the enzymatic browning reaction of phenolic compounds, oxidized by polyphenol oxidase, peroxidized or exposure to air. The oxidation of phenolic compounds was enhanced by light. Light affected the performance of plant hormones. Auxin hormone in the form of 2,4-D worked optimally to improve cell division in dark conditions [27]. Furthermore, calluses would change to a yellowish colour and then turn brown because of the secondary metabolite content [28].
Callus texture showed the quality of callus that was in accordance with the purpose of the study. Friable white calluses indicated high cell activity, in-line with active division and embryogenic behaviour. Callus texture could vary from friable to compact depending on explant variant, basic medium, growth regulators and the biotic and abiotic supplements in the culture [29].
Friable calluses were first observed with all treatments. The friable calluses were formed through the growth of cells at a small site and lost cell interactions that were affected by the presence of auxin. Previous studies have reported that 2.4-D stimulated cell elongation by increasing the plasticity of the cell wall to become loosen, allowing water to easily flow to the inner cell by osmosis, causing the cell to become elongated [3°]. Thus, friable calluses contained a lot of water because the cell wall had not yet reached lignification, and the cells could be easily separated from each other. A friable callus from an explant had loosen cell interactions and could be easily detached using tweezers [24].
Compact calluses were produced in this study after 3 weeks of culture and at the third and fourth subcultures. Compact calluses are composed of tight cells that are difficult to separate, a relatively whitish, light yellowish to brownish colour with a smooth surface. Our finding was in agreement with other reports of callus induction for secondary metabolite production [31]. Based on colour and structural morphology, the calluses obtained from the present experiment were mostly non-embryogenic (compact calluses). Thus, the calluses presented here were suitable for metabolite secondary production.
Following the study of light, sucrose concentration and subculture on callus morphology, we also evaluated their effects on callus growth. The effects varied according to the concentrations added (Table 2, Table 3). In the second and fourth week, the addition of 5% sucrose in dark conditions and 3%–5% sucrose in light conditions gave the highest dry weight. The 2%–5% sucrose treatment under light conditions and 3%–5% sucrose treatment under dark conditions exhibited the highest dry weight at the sixth week.
Callus growth increased from the second to sixth week under dark conditions using 5% sucrose in both dark and light conditions since exogenous carbon source was the main energy sources for explants. This was in accordance with previous studies [30,32]. For the first time, the explant was unable to manufacture its own food, which was called as heterotrophism in nature [33]. The addition of sucrose increased the growth of the callus because sucrose would increase cell respiration, causing in an increase of callus biomass. Low-sucrose concentrations decreased the respiration rate and nitrogen absorption. Thus, the energy supply decreased, and protein synthesis was inhibited because the source of nitrogen decrease [34].
Furthermore, comparing callus growth in light and dark conditions, calluses in light conditions grew faster than in dark conditions at the same sucrose concentration. Our results showed that light influenced explant metabolism related to photosynthesis. Light induced callus cells with photosynthetic pigments that made the callus autotrophic. Hence, calluses might synthesize the primary or secondary metabolites. This result agreed with other reports claiming that the light condition might affect photosynthetic pigments that influenced growth and development of calluses [[35], [36], [37], [38], [39]].
Further, subculture of calluses was essential to ensure optimal growth and development. The known effects of light and subculture on callus growth are varied. Narayanaswamy [40] recommended that calluses be subcultured every 4–6 weeks. In this study, subculture was carried out every 2 weeks up to four total subcultures, and this significantly enhanced callus growth with no morphological variations, and the calluses started to brown during the third week. Among the four subcultures, the second subculture had the highest dry weight. Suman et al. [41] reported that, after 6–8 weeks of inoculation and subculture, the calluses became yellowish, compact, hard and nodular when pedicel and inflorescence parts of safed musli were used as explants. Hence, subcultures had to be carried out before the cultures entered the stationary phase in order to maintain the cell lines for prolonged durations and in a healthy condition. For the duration of the exponential phase, acceleration of cell growth and proliferation was evident since the highest number of cells was in metaphase [42]. Exhaustion of nutrients in the culture medium, drying of solid media or concentration by evaporation of liquid media, accumulation of toxic by product, tissue metabolites or dead cells, and oxygen depletion were the growth-limiting factors for in vitro cultures during the stationary phase [42,43].
The variation of the chemical constituents in the studied subcultures was remarkable. The nature and extent of the chemical constituents varied from subculture to subculture. Factors such as media, temperature, light, growth-regulating substance, and carbon source also affected the secondary metabolite profiles of calluses [40]. Some of the chemicals detected from gandarusa calluses in this study had pharmacological activities, bolstering the evidence for its use as herbal medicine. Taraxasterol, a major compound in the first and fourth subcultures light condition and first-third of dark condition, had been reported to possess strong antibacterial, antiviral, anti-inflammatory and anti-apoptotic functions [44,45]. Palmitic acid had anti-inflammatory, antidiabetic, antibacterial and antioxidant activity [44]) and 2-methoxy-4-vinylphenol which was highly observed in the second subculture was used as antimicrobial and antioxidant [44]). Moreover, monoplex D was enriched in the third subculture was also used as antioxidant and antimicrobial [44]).
Palmitic acid was also the main compound in the leaves and callus [20,46]. Taraxasterol was a major compound in arnica, burdock, chicory and dandelion. The distribution of taraxasterol in plants was not extensive, but the biological activity of this compound was very interesting [45]. This finding was the first to report that taraxasterol was present in calluses gandarusa. Overall, our results indicated that gandarusa calluses could be used as an alternative biotechnological resource for obtaining bioactive compounds.
5. Conclusions
In this paper, we have described the effects of light, sucrose concentration and repetitive subculture on callus growth of gandarusa (Justicia gendarussa Burm.f. Our results strongly suggested that 2% sucrose under light conditions could be used for callus initiation, while 3% sucrose could be used for callus subculture. This finding was a starting point for further investigation and for enhancing the production of secondary metabolites in in vitro callus cultures for future use in pharmaceutical applications. Additionally, the establishment of callus cultures was recommended because they could be a source of higher amounts of bioactive metabolites.
Data availability
Complete GC/MS (spectra) data is as supplementary data.
Funding statement
This study was financially supported by Universitas Airlangga Research Institute, Education Ministry of The Republic of Indonesia, 2020 financial year.
Declaration of competing interest
No conflict of interest exists.
Acknowledgements
Thanks to the Universitas Airlangga Research Institute, Education Ministry the Republic of Indonesia for funding this research project (2020 financial year), Pipob Suwanchaikasem, Wahid Dianbudiyanto and all colleagues who gave assistant for improving this manuscript.
References
- 1.Sarvananda L., Abed S.S.A., Rohini J., Sathyamurthy B. Molecular identification of the medicinal plant Justicia gandarusa using MatK gene. EJPMR. 2016;3(1):259–266. [Google Scholar]
- 2.NCBI Taxonomy Homepage: Justicia gandarusa.http://www.ncbi.nlm.nih.gov/ Taxonomy/Browser/wwwtax.cgi (Accessed 30 September 2013 at 23.45).
- 3.Saha M.R., Debnath P.C., Rahman M.A., Islam M.A. Evaluation of in vitro anthelmintic activities of leaf and stem extracts of Justicia gandarusa. Bangladesh J. Pharmacol. 2012;7(1):50–53. [Google Scholar]
- 4.Prajogo B. Isolation of male antifertility compound in n-butanol fraction of Justicia gendarrusa Burm.f. leaves. Folia Medica Indonesia. 2009;45(1):28–31. [Google Scholar]
- 5.Cothimanivannan J., Kumar R.S., Subramanian N. Anti-inflammatory and analgesic activities of ethanol extract of aerial part of Justicia gendarrusa Burm.f. Int. J. Pharmacol. 2010;6(3):276–283. [Google Scholar]
- 6.Basah K., Elya B., Amin J., Julian M.I. Activity of ethanolic extract from Justicia gendarussa Burm.f. leaves on decreasing the uric acid plasma. Makara Sains. 2011;15(1):67–70. [Google Scholar]
- 7.Osman N.I., Sidik N.J., Awal A. Effects of variations in culture media and hormonal treatments upon callus induction potential in endosperm explant of Barringtonia racemosa L. Asian Pac. J. Trop. Biomed. 2016;6(2):143–147. [Google Scholar]
- 8.Taso R.D., Melandri F. Sustainable sourcing of natural food ingredients by plant cell cultures. Agro Food Ind. Hi. 2011;22:26–28. [Google Scholar]
- 9.Azeez H., Ibrahim K., Pop R., Pamfil D., Hârţa M., Bobiș O. Changes induced by gamma ray irradiation on biomass production and secondary metabolites accumulation in Hypericum triquetrifolium Turra callus cultures. Ind. Crop Prod. 2017;108:183–189. [Google Scholar]
- 10.Khalil S.A., Ahmad N., Zamir R. Gamma radiation induced variation in growth characteristics and production of bioactive compounds during callogenesis in Stevia rebaudiana (Bert.). New Neg. Plant Sci. 2015;1(2):1–5. [Google Scholar]
- 11.Sridhar T.M., Naidu C.V. An efficient callus induction and plant regeneration of Solanum nigrum L.: an important antiulcer medicinal plant. J. Phytol. 2011;3:23–28. [Google Scholar]
- 12.Chanchula N., Jaruwattanaphan T., Jala A. Differential effects of sucrose and plant growth regulator on shoot multiplication and bulbil formation in Oxalis Versicolour in vitro. ITJEMAST. 2014;5(4):227–234. [Google Scholar]
- 13.Payyavula R.S., Tschaplinski T.J., Jawdy S.S., Sykes R.W., Tuskan G.A., Kalluri U.C. Metabolic profiling reveals altered sugar and secondary metabolism in response to UGPase overexpression in Populus. BMC Plant Biol. 2014;14:265–287. doi: 10.1186/s12870-014-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hmood A.K., Salim H.A. Effect of different concentrations of sucrose on alkaloids and steroids production in vitro from Withania somnifera (L) Dunal (Ashwagandha) Emer Life Sci. Res. 2017;3(2):32–36. [Google Scholar]
- 15.Ali M., Abbasi B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B: Biol. 2014;140:223–227. doi: 10.1016/j.jphotobiol.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Adil M., Abbasi B.H., ul Haq I. Red light controlled callus morphogenetic patterns and secondary metabolites production in Withania somnifera L. Biotechnol. Rep. 2019;24 doi: 10.1016/j.btre.2019.e00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogewoning S.W., Trouwborst G., Maljaars H., Poorter H., van Ieperen W., Harbinson J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010;61:3107–3117. doi: 10.1093/jxb/erq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor S., Raghuvanshia R., Bhardwaja P., Sood H., Saxenaa S., Chaurasia O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B: Biol. 2018;183:258–265. doi: 10.1016/j.jphotobiol.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Li X., Jiang Q., Sun H., Jiang J., Chen S., Guan Z., Fang W., Chen F. GC-MS Analysis of the volatile constituents in the leaves of 14 compositae plants. Molecules. 2018;23:166–178. doi: 10.3390/molecules23010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahyuni D.K., Hamidah Analysis of phytocomponents in the methanolic extract of Justicia gandarusa Burm.f. (Indonesian plant medicine) Acta Scientiae et Intellectus. 2016;2(5):20–28. [Google Scholar]
- 21.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962;15(3):437–497. [Google Scholar]
- 22.Kalimuthu K., Prabakaran R., Brindha C. Antimicrobial activity of in vitro and in vivo plant extracts of Ceropegia pusilla. Wight and Arn an endemic medicinal plant. Int. J. Pharm. Biol. Sci. 2013;4(4):187–191. [Google Scholar]
- 23.Taranto F., Pasqualone A., Mangini G., Tripodi P., Miazzi M.M., Pavan S., Montemurro C. Polyphenol oxidases in crops: biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017;18:377. doi: 10.3390/ijms18020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George E.F., Sherrington P.D. Cambridge University Press; London: 1992. Plant Propagation by Tissue Culture: Handbook and Directory of Commercial Laboratories. [Google Scholar]
- 25.Kumar G.P., Subiramani S., Govindarajan S., Sadasivam V., Manickam V., Mogilicherla K., Thiruppathi S.K., Narayanasamy J. Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton (Gossypium hirsutum L.) cv. SVPR-2. Biotechnol. Rep. 2015;7:72–80. doi: 10.1016/j.btre.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osman N.I., Sidik N.J., Awal A. Effects of variations in culture media and hormonal treatments upon callus induction potential in endosperm explant of Barringtonia racemosa L. Asian Pac. J. Trop. Biomed. 2016;6(2):143–147. [Google Scholar]
- 27.Taiz L., Zieger E. 3rd ed. Sinauer Associates, Inc.; 2013. Plant Physiology; pp. 317–323. [Google Scholar]
- 28.Manuhara Y.S.W. Airlangga University Press; 2014. Kapita Selekta Kultur Jaringan Tumbuhan, Surabaya; pp. 67–70. [Google Scholar]
- 29.Sitorus E.N., Hastuti E.D., Setiari N. Induksi kalus binahong (Basella rubra L.) secara in vitro pada media Murashige & Skoog dengan konsentrasi sukrosa yang berbeda. Bioma. 2011;13(1):1–7. [Google Scholar]
- 30.Rodrigues A.A.J., Santos E.O., Takane R.J., Carvalho A.C.P.P. Artificial light and growth regulators on the in vitro etiolation of Cattleya labiate. Rev. Ciênc. Agron. 2017;48(2):296–302. [Google Scholar]
- 31.Ribeiro I.G., Gayer C.R.M., de Castro T.C., Coelho M.C.P., Albarello N. Compact callus cultures and evaluation of the antioxidant activity of Hovenia dulcis Thunb. (Rhamnaceae) under in vivo and in vitro culture conditions. J. Med. Plant Res. 2015;9(1):8–15. [Google Scholar]
- 32.Ahmed A.B.A., Rao A.S., Rao M.V., Taha R.M. Production of gymnemic acid depends on medium, explants, PGRs, color lights, temperature, photoperiod, and sucrose sources in batch culture of Gymnema sylvestre. ScientificWorldJournal. 2012;2012:1–12. doi: 10.1100/2012/897867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Lee S.T., Huang W.L. Cytokinin, auxin, and abscisic acid affects sucrose metabolism conduce to de novo shoot organogenesis in rice (Oryza sativa L.) callus. Bot. Stud. 2013;54:5. doi: 10.1186/1999-3110-54-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaneshwari C.A. Effect of plant growth regulators and sucrose concentration on callus induction and shoot differentiation from ovary culture of marigold (Tagetes spp.) Int. J. Chem. Stud. 2018;6(1):618–623. [Google Scholar]
- 35.Kami C., Lorrain S., Hornitschek P., Fankhauser C. Light-regulated plant growth and development. Current Topics Dev. Biol. 2010;91:29–66. doi: 10.1016/S0070-2153(10)91002-8. [DOI] [PubMed] [Google Scholar]
- 36.Afshari R.T., Angoshtari R., Kalantari S. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (Brassica napus L.) genotypes. Plant Omics J. 2011;4(2):60–67. [Google Scholar]
- 37.Paul K.K., Bari M.A., Islam S.M.S., Debnath S.C. In vitro shoot regeneration in elephant Foot Yam, Amorphophallus campanulatus Blume. Plant Tissue Cult. & Biotech. 2013;20(1):55–61. [Google Scholar]
- 38.Güler S. Effects of nitrogen on yield and chlorophyll of potato (Solanum tuberosum L.) cultivars. Bangladesh J. Bot. 2009;38(2):163–169. [Google Scholar]
- 39.Yang X., Chen G. Stimulation of photosynthetic characteristics of Ginkgo biloba L. during leaf growth. Bangladesh J. Bot. 2014;43(1):73–77. [Google Scholar]
- 40.Narayanaswamy S. Tata McGraw-Hill Education; India: 1994. Plant Cell and Tissue Culture; pp. 314–319. 9th reprint. [Google Scholar]
- 41.Suman K., Anuradha P., Shaifali G., Pavan V.V.S., Shashwat S. Isolation and characterization of secondary metabolites “Saponins” from explants of Indian medicinal plant Chlorophytum borivilianum. Int. J. Pharm. Front. 2011;1:214–222. [Google Scholar]
- 42.Smith R.H. 2nd ed. Academic Press; U.S.A: 2000. Plant Tissue Culture: Techniques and Experiments; pp. 112–115. [Google Scholar]
- 43.Neumann K.H., Imani J., Kumar A. Springer; Berlin Heidelberg: 2009. Callus Cultures, Principles and Practice in: Plant Cell and Tissue Culture- A Tool in Biotechnology; pp. 13–42. [Google Scholar]
- 44.Duke J.A. CRC Press; Boca Raton, FL, USA: 1992. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants. [Google Scholar]
- 45.Ovesna Z., Vachalcova A., Horvatova K. Taraxasterol and β-sitosterol: new naturally compounds with chemoprotective/chemopreventive effect. Neoplasma. 2004;51(6):407–414. [PubMed] [Google Scholar]
- 46.Wahyuni D.K., Ansori A.N.M., Vidiyanti F. GC-MS analysis of phytocomponents in methanolic extracts of leaf-derived callus of Justicia gandarusa Burm.f. Biosci. Res. 2017;14(3):668–677. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Complete GC/MS (spectra) data is as supplementary data.