Abstract
The contribution of both common and rare risk variants to the genetic architecture of schizophrenia (SZ) has been documented in genome-wide association studies, whole exome and whole genome sequencing approaches. As SZ is highly heritable and segregates in families, highly penetrant rare variants are more likely to be identified through analyses of multiply affected families. Further, much of the gene mapping studies in SZ have utilized individuals of Caucasian ancestry. Analysis of other ethnic groups may be informative. In this study, we aimed at identification of rare, penetrant risk variants utilizing whole exome sequencing (WES) in a three-generation Indian family with multiple members affected. Filtered data from WES, combined with in silico analyses revealed a novel heterozygous missense variant (NM_080841:c.1730C>G:p.T577R; exon18) in Protein tyrosine phosphatase, receptor type A (PTPRA 20p13). The variant was located in an evolutionary conserved position and predicted to be damaging. Screening for variants in this gene in the WES data of an independent SZ cohort (n = 350) of matched ethnicity, identified five additional rare missense variants with MAF < 0.003, which were also predicted to be damaging. In conclusion, the rare missense variants in PTPRA identified in this study could confer risk for SZ. This has also derived support from concordant data from prior linkage and association, as well as animal studies which indicated a role for PTPRA in glutamate function.
1. Introduction
Schizophrenia (SZ) is a debilitating disorder, with a life time prevalence of ~1%. It is characterised by positive and negative symptoms and cognitive impairments which create emotional distress and lifelong disability in the affected individuals (Lewis and Lieberman, 2000). SZ is considered as a manifestation of an aberrant neurodevelopmental phenomenon (Lewis and Levitt, 2002) and abnormalities in neurotransmitter systems including dopaminergic, glutamatergic and gamma amino butyric acid (GABA) have been reported (Howes et al., 2015; Wassef et al., 2003). Genetic risk factors for SZ have been demonstrated through family based and twin and adoption studies, which also indicate the contribution of environmental perturbations (Gottesman and Gottesman, 1991; Tsuang, 2000). SZ is typically considered as an outcome of cumulative contribution of large number of genes with minor effects and transmitted in a non-Mendelian fashion. Based on this assumption, the last decade witnessed a large number of genome wide association studies (GWASs) carried out across ethnic groups, which identified a large number of common variants in several genes/loci. A recent meta-analysis using 36,989 cases and 113,075 controls identified 108 loci associated with disease with genome wide significance (Ripke et al., 2014). Of note, several of the genes encoded proteins involved in dopaminergic and glutamatergic functions. However, a recent study has shown that the associated SNPs only explain a small fraction (hg2 = 0.27) of genetic liability to the disease (Loh et al., 2015), warranting newer paradigms to uncover the components to explain total heritability.
The rapidly decreasing cost of next generation sequencing (NGS) technology facilitated one such approach, leading to rapid identification of rare variants in SZ etiology. Several studies using whole exome sequencing (WES) of case-parent trios have identified over 1000 rare de novo variants predicted to be disturbing protein function (Ambalavanan et al., 2016; Fromer et al., 2014; Girard et al., 2011; Guipponi et al., 2014; Gulsuner et al., 2013; Gulsuner and McClellan, 2014; Kranz et al., 2015; McCarthy et al., 2014; Singh et al., 2016; Takata et al., 2014; Xu et al., 2012). These de novo mutations are rare, nevertheless they were enriched in genes involved in synaptic transmission, glutamatergic post synaptic proteins and N-methyl-D-aspartate receptor (NMDAR) complexes the systems previously implicated in SZ pathogenesis. However, neither the common nor the de novo rare variants sufficiently explain the large fraction of genetic liability and/or the high heritability of SZ suggesting the presence of few rare high risk conferring variants transmitted across generations. At this juncture identifying such highly penetrant rare variants in functionally relevant gene(s) segregating with the disease phenotype in familial forms of SZ is appealing. We and others in the recent past have used small to medium size families with SZ and reported moderately to highly penetrant rare protein coding variants segregating with disease phenotype (Egawa et al., 2016; Homann et al., 2016; Hornig et al., 2017; John et al., 2018, 2017; Kos et al., 2016; Shirzad et al., 2017; Steinberg et al., 2017; Timms et al., 2013; Zhou et al., 2016) Several genes identified in these studies were broadly connected with glutamatergic pathway and supported the commonly accepted glutamatergic dysfunction hypothesis. These studies reaffirm the highly complex and heterogeneous nature of SZ and at the same time provide evidence for a major/predominant role of glutamatergic pathway genes in SZ etiology based on common and rare variants identified in GWASs and WES respectively. Yet, a substantial fraction of genetic determinants remains to be elucidated, encouraging additional studies. In the present study we analysed a small multigenerational SZ family using WES approach and identified a novel missense variant in PTPRA segregating with the phenotype. Furthermore, five additional rare coding variants in this gene were also observed on screening an independent SZ cohort.
2. Methods
2.1. Sample recruitment
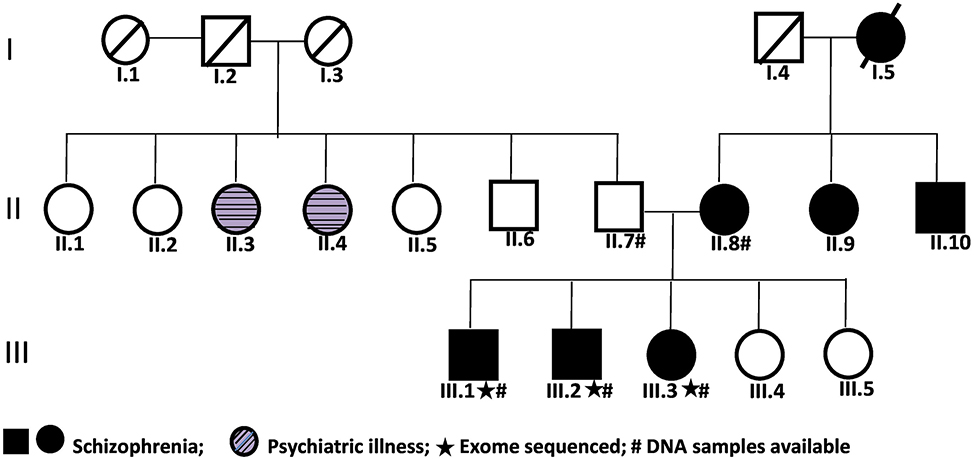
The family in this study was recruited from Department of Psychiatry, Dr. Ram Manohar Lohia Hospital, New Delhi and was of north Indian origin. Consensus diagnosis of SZ was made by psychiatrists and well trained psychologists using DSMIV criteria. For getting additional information for the genetic studies, the Hindi version of Family Interview for Genetic Studies (FIGS) and Diagnostic interview for genetic studies (DIGS) were used (Deshpandeetal., 1998; John etal., 2016). The family comprised of seven affected, 11 unaffected and two other members with other psychiatric illness (Fig. 1). Of these, DNA from four affected and one unaffected individual was available for study. Any other psychiatric/behavioural phenotype in the unaffected individual was ruled out by same clinicians. The study was approved by institutional ethical committee of both participating institutions.
Fig. 1.
Pedigree of the multiplex family with schizophrenia.
Whole Exome Sequencing (WES)
DNA from three affected members (Fig. 1) was used for WES. Agilent SureSelect Human All ExonV5+UTR kit was used for target enrichment and library preparation. Sequencing was performed in 101-bp paired-end mode using Illumina® HiSeq™ 2000. All these processes was carried out at a commercial facility (Medgenome; https://www.medgenome.com/).
2.2. Whole exome data analysis
The raw FastQ data were processed as per the recommendations of Genome Analysis Toolkit (GATK) “Best Practices for Germline SNP & Indel Discovery”. Basic QC checking of the raw data obtained from the service provider was performed using FastQC tool and Adapter and low quality sequences (phred score < 15) were removed using cutadapt (Martin, 2011). The QC passed sequences were aligned to the reference human genome (hg19) using BWA-MEM algorithm (Li, 2013; Li and Durbin, 2009). Aligned data in SAM format were then sorted, converted into BAM file and PCR duplicates were removed using Picard Tools (http://broadinstitute.github.io/picard/). Subsequently, realignment around indels and base recalibration were performed using GATK and cleaned BAM file was generated. Alignment QC and target region coverage in both depth and breadth were calculated from the BAM file generated from the preceding step using Qualimap (García-Alcalde et al., 2012). Variants in VCF format were created from the cleaned BAM file using GATK (McKenna et al., 2010). The variants were annotated using Kggseq (Li et al., 2012).
2.3. Variant prioritisation
For the prioritisation of variants we followed the recommendations from three previous publications (Dashti and Gamieldien, 2017; Kircher et al., 2014; Richards et al., 2015) and used Kggseq (Li et al., 2012). Keeping in mind the small sized study family and therefore, a greater chance of identifying shared variants among affected just by chance alone, and thus to avoid false positive detection, we primarily focused on protein disturbing rare variants in previously reported and/or functionally relevant candidate genes (Purcell et al., 2014) and adopted the following steps for variant prioritisation. To start with, all the protein coding variants were extracted from the annotated file. All common variants (MAF > 0.001) catalogued in different public databases available namely 1000 genome (1000G), Exome Aggregation Consortium (ExAC r0.3.1), dbSNP, Genome Aggregation Database (gnomAD) browser and NHLBI GO Exome Sequencing Project (ESP) and all the synonymous variants were removed. Variants shared among three affected WES individuals were then taken forward to check for segregation in the remaining two individuals (one affected and one unaffected) using target capture sequencing. Only variants shared among all four affected individuals in the family were considered and variants in segmentally duplicated regions or in “polymorphic” genes (Fuentes Fajardo et al., 2012) and genes containing four or more variants each in an individual were removed from this list. Variants which were common (MAF > 0.001) in in-house WES data and shared with the unaffected member in the family were also removed. Finally, the variants that were present only in all affected members were further prioritised based on their predicted deleterious nature (by SIFT or Polyphen2_HDlV or Polyphen2_HVAR and with CADD score > 15) and on their relevance in SZ etiology, based on available reports of association/linkage/exome sequencing/animal studies/presence in pathways implicated in SZ and/or other neuropsychiatric disorders and gene functions. Variant (s) thus shortlisted were confirmed by Sanger sequencing (primer details in Supplementary Table 1; Supplementary Fig. 1).
2.4. In silico analysis of prioritised variants
To check if the variants were predicted to be damaging we used SlFT, Polyphen2_HDlV, Polyphen2_HVAR, LRT, MutationTaster, LR, FATHOM, MutationAssessor, MetaLR, PROVEAN, MetaSVM, RadialSVM, Variant Effect Scoring Tool3 (VEST3) and Combined Annotation Dependent Depletion (CADD) score. Evolutionary conservation score of the variant positions was calculated using phastCons7way_vertebrate, GERP+ +_RS, SiPhy_29way_logOdds and GERP+ +_NR and gene-based pathogenicity estimation were calculated using Residual Variation Intolerance Score (RVIS). All the algorithms were part of dbNSFP2.9 (Liu et al., 2013) and are included in Kggseq (Li et al., 2017, 2012).
2.5. Screening for additional rare variants in the prioritised gene(s) in an independent cohort
For screening of rare variants in the prioritised gene(s), we used WES data from i) unrelated SZ patients (n = 350) of matched ethnicity, recruited previously by the clinician (SND) based on consensus diagnosis using DSMlV criteria and available in the laboratory and ii) individuals without any psychiatric disorder (n = 150, considered as controls), both of which were also used in our previous study (John et al., 2018). All these samples were from the same geographical regions as the family recruited for the study.
3. Results
Three affected members namely III.1, III.2 and III.3 of the study family (Fig. 1) were used for WES. The mean target depth of sequencing observed across these three samples was 58.82×. On an average > 97% of the target regions were with 10× and >89% with 20× coverage. The mean mapping observed across the three samples was 46.99. Detailed information of target region both in depth and breadth aspects are given in Supplementary Table 2.
A scheme for prioritisation of variants detected with WES is provided in supplementary Table 3. After prioritisation, 20 variants were identified to be shared among all the affected but not in the unaffected member of the study family. Of these, 13 variants were predicted to be damaging by SIFT or Polyphen2_HDlV or Polyphen2_HVAR and CADD score > 15 (Supplementary Table 3). In order to identify high risk conferring variant(s) from among these, the 13 genes encompassing the rare variants were further scrutinised for their known/likely involvement in neurobiology. Among these, four genes namely NRROS, L3MBTL1, RTTN and PTPRA seemed to have some neurologically relevant functions. A critical review of all the available literature on genetic/knockout/over expression/pharmacological studies and animal models showing SZ or other neuropsychiatric disorder-related symptoms, suggesting the direct/indirect roles of these genes in disease biology (summarised in supplementary Table 6), revealed significant support for the involvement of Protein Tyrosine Phosphatase, Receptor Type A (PTPRA) but not the other three genes. Therefore, though the variants with complete annotation in these genes are catalogued (Supplementary Table 4), NRROS, L3MBTL1 and RTTN were not considered for further analysis in the present study. However, this does not imply that these three genes may not have a role in SZ etiology, but sufficient support, based on functional and/or animal model studies are currently lacking. On the other hand, PTPRA is involved in various neurodevelopmental processes and also in glutamatergic and dopamine signalling pathways and previously implicated in SZ (as detailed in discussion below). The novel index variant (NM_080841:c.1730C>G: p.T577R; exon18) in PTPRA is in the Tyrosine-protein phosphatase 2 domain. The gene is expressed in different brain regions as evidenced in two public databases BrainSpan (http://www.brainspan.org) and Genotype-Tissue Expression (GTEx) portal (https://www.gtexportal.org/).
3.1. In silico analysis
The index variant (p.T577R) in PTPRA was located at an evolutionarily conserved residue (Supplementary Fig. 2) and is predicted to be damaging by seven of 13 in-silico tools and with a CADD score of 32. This indicates that the variant is among the top 0.1% of deleterious variants in the human genome (Supplementary Table 4).
3.2. Gene level pathogenic analysis
Residual Variation lntolerance Score (RVIS) of PTPRA showed that the gene is among 7.15% of the most intolerant genes in human.
3.3. Additional variants identified in PTPRA in an independent SZ cohort
Based on all the findings presented above, screening for additional variants, if any, in PTPRA was undertaken in an independent SZ cohort. This would lend additional support to this gene being important in SZ etiology. WES data from a SZ cohort (n = 350) of matched ethnicity available in the laboratory and used in a previous study (John et al., 2018) were utilized for screening of variants in PTPRA. We identified five additional rare (MAF < 0.003) missense heterozygous variants namely; p.T57N, p.E506G, p.V664l and p.R759W in one individual each and p.A129V in two different individuals in the cohort. All these rare variants were also predicted to be damaging with several in silico tools and with CADD score was >15 (Supplementary Table 5). Except for p.T57N in PTPRA which was present in a heterozygous state in one healthy individual, none of the other variants were present in the exome data of 150 non-SZ individuals (non-disease controls) of lndian origin also available in the laboratory. All the five variants thus identified have been reported with MAF <0.003 in South Asian and few other populations in ExAC and gnomAD browsers (supplementary Table 5) and therefore, catalogued as rare in this study. lt may be relevant to mention here that p.T57N was reported in two SZ patients but was absent in 912 healthy controls in a Japanese population (Xing et al., 2014).
3.4. Analysis of PGC dataset
On screening the Psychiatric Genomics Consortium (PGC) data (https://www.med.unc.edu/pgc/), we found two common (MAF > 0.05) intronic SNPs namely rs6037443 (p = 0.0006) and rs1178029 (p = 0.01) in PTPRA nominally associated with SZ.
4. Discussion
We analysed a multi-member affected SZ family by WES and identified 13 rare variants that were predicted to be damaging and were present in all affected but not in the unaffected member in the study family. Based on multiple levels of contextual support from pre-existing genetic and animal studies (detailed below), the novel heterozygous missense variant ( p.T577R) in PTPRA emerged as the most compelling contributor to the disease in study family (Fig. 1; Supplementary Fig. 1). Five additional rare missense variants in this gene were also identified among 350 unrelated SZ patients and four of these were not found in non-SZ exomes (n = 150) screened in the laboratory (Supplementary Table 5). A large number of in silico tools predicted all these variants to be deleterious and they are located in evolutionarily conserved positions (Supplementary Table 5). Furthermore, likely involvement of PTPRA is extensively supported by available literature. PTPRA is a member of protein tyrosine phosphatase (PTP) family. By regulating the phosphorylation of potassium channels (Kv1.1 and Kv1.2), PTPRA modulates acetylcholine and serotonin mediated activity response (Imbrici et al., 2000; Tsai, 1999). Kv1.2 is involved in D2 dopamine autoreceptor mediated dopamine release (Fulton et al., 2011; Martel et al., 2011). The gene is also known to regulate the kinase activity of Src and Fyn (Ponniah et al., 1999) and the role of Src in N-methyl-D-aspartate (NMDA) receptors hypofunction in SZ is evident from a previous report (Li et al., 2012). Fyn has also been shown to be involved in the phosphorylation and trafficking of NMDA receptors (Trepanier et al., 2012). Further as evident from literature, through the interactions with neural recognition molecules namely NB-3 and CHL1, PTPRA has been shown to be involved in apical dendrite development in the deep layer pyramidal neurons of the caudal neocortex (Ye et al., 2008). PTPRA is also involved in NCAM mediated neurite elongation (Bodrikov et al., 2008, 2005).
Interestingly PTPRA (20p13) has been previously reported to be linked to SZ in a large Arab Israeli pedigree (Teltsh et al., 2008) and with SZ and various psychotic illness in high-density Irish families with psychotic illness (Fanous et al., 2008). Two common intronic variants (rs6037443; p = 0.0006 and rs1178029; p = 0.01)} were shown to be nominally associated with SZ in Psychiatric Genomics Consortium (PGC) study (https://purces04.u.hpc.mssm.edu/ldookup/ldookup.cgi). A common intronic SNP (rs1016753) from this gene has been reported to be significantly associated (p = 0.0008) with SZ in a Japanese population (1420 cases, 1377 controls). Expression studies showing reduced expression of this gene in SZ brain samples compared to controls substantiated these findings and the same study also showed a trend of reduced expression in bipolar patients (Takahashi et al., 2011). In another study on Japanese population, where protein coding regions of the gene were re-sequenced in 382 SZ patients, eight rare variants were identified, which were further tested for association using 944 SZ patients, 336 autism spectrum disorders patients, and 912 healthy controls but no association was reported (possibly due to insufficient power). However, as already mentioned in the results section, in two individuals with SZ they observed PT57N (Xing et al., 2014), a rare variant that we also identified in one individual (Supplementary Table 5). Besides PTPRA variants in SZ, one de novo missense variant (NM_002836.3: c.2116G>C p.Gly706Arg) in this gene was also reported in an individual with autism spectrum disorder (Yuen et al., 2017). Two other intronic SNPs namely rs12151888 (p = 0.02) and rs77646362 (p = 0.04) from this gene have been reported to be nominally associated with autism (https://purces04.u.hpc.mssm.edu/ldookup/ldookup.cgi).
PTPRA knock out studies in mice further substantiate the relevance of this gene in brain functions. Association of this phosphatase with neurodevelopmental abnormalities include defects in pyramidal neuronal migration, synaptic plasticity, long-term potentiation (LTP) and hippocampal development (Petrone et al., 2003), oriented growth of apical dendrites of deep layer pyramidal neurons in caudal cortex (Ye et al., 2008), central nervous system myelination and oligodendrocyte differentiation (Wang et al., 2009). Knock out mice showed impaired src family kinases mediated NMDAR tyrosine phosphorylation and subsequent aberrant NMDAR-associated functions (Le et al., 2006; Lei et al., 2002). In another study on knock out mice, enhanced methamphetamine induced hyperactivity has been observed suggesting an augmented dopaminergic system and defect in prepulse inhibition (PPI) of the startle response and the defect in PPI is considered as an endophenotype of SZ (Takahashi et al., 2011). Thus, substantial evidence for involvement of PTPRA in SZ etiology is available.
However, it may be reiterated that prioritisation and identification of PTPRA from among the 20 variants that segregated with SZ in the study family has relied on previous knowledge only (as discussed above) and needs to be validated by functional experiments, but which are currently unavailable. Furthermore, contribution of other common/regulatory variants, CNVs etc. to disease in this family cannot also be ruled out considering the commonly accepted polygenic nature of this illness. In addition, a few other limitations in this study warrant mention. Replication cohort used in the study (350 SZ cases and 150 controls) is small. Data on genetic variation among the ethnically distinct Indian population is also limited to 1000 Genomes and ExAC databases bu. These together greatly limit identification of variants with a reliable population frequency to enable rare variant association testing. Nevertheless, the variant data presented in this study may be useful for meta-analysis. In conclusion, based on the genomic data from the present study, in conjunction with findings from previously reported biochemical and animal studies, the rare variants in PTPRA may be implicated for SZ etiology encouraging their functional validation.
Supplementary Material
Acknowledgements
Junior and Senior Research Fellowship (09/045(1166)/2012-EMR-I) to Jibin John from Council for Scientific and Industrial Research (CSIR), New Delhi; Junior and Senior Research Fellowship from the Department of Genetics under UGC-Special Assistance Program Meritorious Award scheme to Aditya Sharma; and DSK-PDF (BL/13-14/0404) to Dr. Prachi Kukshal from UGC, New Delhi are gratefully acknowledged. We are thankful for, the study sample collection by trained and dedicated staff at Dr. RML hospital, DNA isolation by Mrs. Anjali Dabral at the University of Delhi South Campus; help with linkage analysis by Mr. Navneesh Yadav and computational facility provided by Central Instrumentation Facility, University of Delhi South Campus. We gratefully acknowledge infrastructure support provided by the UGC, New Delhi, through Special Assistance Programme and Department of Science and Technology, New Delhi, through FIST and DU-DST PURSE programmes to the Department of Genetics, UDSC.
Role of funding sources
This work was supported in part by grants #BT/MB/Project-Schizophrenia/2012-2013 and #BT/PR2425/Med13/089/2001 to Prof. B.K. Thelma and Prof. S. N. Deshpande from the Department of Biotechnology, Government of India, New Delhi; Grant #MH093246, #MH063480 and #TW009114 to Prof. V. L. Nimgaonkar from NIMH, the Fogarty International Center, USA. All funding sources had no further role in in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest in relation to the subject of this study.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.schres.2018.12.012.
References
- Ambalavanan A, Girard SL, Ahn K, Zhou S, Dionne-Laporte A, Spiegelman D, Bourassa CV, Gauthier J, Hamdan FF, Xiong L, Dion PA, Joober R, Rapoport J, Rouleau GA, 2016. De novo variants in sporadic cases of childhood onset schizophrenia. Eur. J. Hum. Genet 24, 944–948. 10.1038/ejhg.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrikov V, Leshchyns’ka I, Sytnyk V, Overvoorde J, Den Hertog J, Schachner M, 2005. RPTPα is essential for NCAM-mediated p59 fyn activation and neurite elongation. J. Cell Biol 168, 127–139. 10.1083/jcb.200405073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrikov V, Sytnyk V, Leshchyns’ka I, Den Hertog J, Schachner M, 2008. NCAM induces CaMKIIα-mediated RPTPα phosphorylation to enhance its catalytic activity and neurite outgrowth. J. Cell Biol 182, 1185–1200. 10.1083/jcb.200803045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti MJS, Gamieldien J, 2017. A practical guide to filtering and prioritizing genetic variants. BioTechniques 62, 18–30. 10.2144/000114492. [DOI] [PubMed] [Google Scholar]
- Deshpande SN, Mathur MNL, Das SK, Bhatia T, Sharma S, Nimgaonkar VL, 1998. A Hindi version of the diagnostic interview for genetic studies. Schizophr. Bull 24, 489–493. 10.1093/oxfordjournals.schbul.a033343. [DOI] [PubMed] [Google Scholar]
- Egawa J, Hoya S, Watanabe Y, Nunokawa A, Shibuya M, Ikeda M, Inoue E, Okuda S, Kondo K, Saito T, Kaneko N, Muratake T, Igeta H, Iwata N, Someya T, 2016. Rare UNC13B variations and risk of schizophrenia: whole-exome sequencing in a multiplex family and follow-up resequencing and a case–control study. Am. J. Med. Genet. B Neuropsychiatr. Genet 171, 797–805. 10.1002/ajmg.b.32444. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Webb BT, Straub RE, O’Neill FA, Walsh D, Riley BP, Kendler KS, 2008. Novel linkage to chromosome 20p using latent classes of psychotic illness in 270 Irish high-density families. Biol. Psychiatry 64, 121–127. 10.1016/j.biopsych.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC, O’Donovan MC, O’Donovan MC, 2014. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184. 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes Fajardo KV, Adams D, Mason CE, Sincan M, Tifft C, Toro C, Boerkoel CF, Gahl W, Markello T, 2012. Detecting false-positive signals in exome sequencing. Hum. Mutat 33, 609–613. 10.1002/humu.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Thibault D, Mendez JA, Lahaie N, Tirotta E, Borrelli E, Bouvier M, Tempel BL, Trudeau LE, 2011. Contribution of Kv1.2 voltage-gated potassium channel to D2 autoreceptor regulation of axonal dopamine overflow. J. Biol. Chem 286, 9360–9372. 10.1074/jbc.M110.153262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alcalde F, Okonechnikov K, Carbonell J, Cruz LM, Götz S, Tarazona S, Dopazo J, Meyer TF, Conesa A, 2012. Qualimap: evaluating next-generation sequencing alignment data. Bioinformatics 28, 2678–2679. 10.1093/bioinformatics/bts503. [DOI] [PubMed] [Google Scholar]
- Girard SL, Gauthier J, Noreau A, Xiong L, Zhou S, Jouan L, Dionne-Laporte A, Spiegelman D, Henrion E, Diallo O, Thibodeau P, Bachand I, Bao JYJ, Tong AHY, Lin C-H, Millet B, Jaafari N, Joober R, Dion PA, Lok S, Krebs M-O, Rouleau GA, 2011. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat. Genet 43, 860–863. 10.1038/ng.886. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gottesman II, 1991. Schizophrenia genesis: the origins of madness. A Ser. Books Psychol 10.1136/jnnp.54.5.480-b [DOI] [Google Scholar]
- Guipponi M, Santoni FA, Setola V, Gehrig C, Rotharmel M, Cuenca M, Guillin O, Dikeos D, Georgantopoulos G, Papadimitriou G, Curtis L, Méary A, Schürhoff F, Jamain S, Avramopoulos D, Leboyer M, Rujescu D, Pulver A, Campion D, Siderovski DP, Antonarakis SE, 2014. Exome sequencing in 53 sporadic cases of schizophrenia identifies 18 putative candidate genes. PLoS One 9, e112745 10.1371/journal.pone.0112745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsuner S, McClellan JM, 2014. De novo mutations in schizophrenia disrupt genes coexpressed in fetal prefrontal cortex. Neuropsychopharmacology 39, 238–239. 10.1038/npp.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, Rippey C, Shahin H, Consortium on the Genetics of Schizophrenia (COGS), D, PAARTNERS Study Group, K.S, Nimgaonkar VL, Go RCP, Savage RM, Swerdlow NR, Gur RE, Braff DL, King M-C, McClellan JM, Light G, Nuechterlein K, Olincy A, Radant A, Ray A, Schork N, Seidman LJ, Siever L, Silverman J, Stone WS, Sugar C, Swerdlow N, Tsuang D, Tsuang M, Turetsky B, Aduroja T, Allen T, Bradford LD, Calkins ME, Devlin B, Edwards NB, Ganguli R, Go RCP, Gur RE, Gur RC, Kwentus J, Lahti AC, Lyons P, Mathos K, May R, McLeod-Bryant S, McEvoy JP, Montgomery-Barefield L, Nimgaonkar VL, O’Jile J, Santos A, Savage RM, Swanson CL, Wilson W, Nimgaonkar VL, Go RCP, Savage RM, Swerdlow NR, Gur RE, Braff DL, King M-C, McClellan JM, Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR, Akil H, Brenner S, Kandel E, Kendler KS, King MC, Scolnick E, Watson JD, Zoghbi HY, Anton ES, Kreidberg JA, Rakic P, Barabási AL, Oltvai ZN, Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, Al E, Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P, Choi KH, Rhim H, Cleveland WS, Grosse E, Shyu MJ, Costa E, Dong E, Grayson DR, Guidotti A, Ruzicka W, Veldic M, Duchrow M, Schlüter C, Key G, Kubbutat MH, Wohlenberg C, Flad HD, Gerdes J, Eisenberg DP, Berman KF, Fitzsimmons J, Kubicki M, Shenton ME, Gaisler-Salomon I, Wang Y, Chuhma N, Zhang H, Golumbic YN, Mihali A, Arancio O, Sibille E, Rayport S, Gilman SR, Chang J, Xu B, Bawa TS, Gogos JA, Karayiorgou M, Vitkup D, Girard SL, Gauthier J, Noreau A, Xiong L, Zhou S, Jouan L, Dionne- Laporte A, Spiegelman D, Henrion E, Diallo O, Al E, Girirajan S, Rosenfeld JA, Coe BP, Parikh S, Friedman N, Goldstein A, Filipink RA, McConnell JS, Angle B, Meschino WS, Al E, Glàscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D, Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Al E, Grantham R, Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, Lagemaat LN, van de Smith KA, Ebbert A, Riley ZL, Al E, Insel TR, Consortium IS, Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Al E, Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, Hermanson O, Rosenfeld MG, Kalkstein S, Hurford I, Gur RC, Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Al E, Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Al E, Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R, Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Jonasdottir A, Al E, Konopka G, Friedrich T, Davis- Turak J, Winden K, Oldham MC, Gao F, Chen L, Wang GZ, Luo R, Preuss TM, Geschwind DH, Krumm N, Sudmant PH, Ko A, O’Roak BJ, Malig M, Coe BP, Quinlan AR, Nickerson DA, Eichler EE, Project NES, Kumar P, Henikoff S, Ng PC, Marín O, Martin C, Jacobi JS, Nava G, Jeziorski MC, Clapp C, Escalera GM, de la Masana M, Santana N, Artigas F, Bortolozzi A, McClellan J, King MC, Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q, Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Al E, O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, Al E, O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Al E, Pacary E, Tixier E, Coulet F, Roussel S, Petit E, Bernaudin M, Patten BA, Sardi SP, Koirala S, Nakafuku M, Corfas G, Press W, Rakic P, Ramocki MB, Zoghbi HY, Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H, Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Al E, Schlesselman JJ, Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Al E, Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ, Shima Y, Kawaguchi SY, Kosaka K, Nakayama M, Hoshino M, Nabeshima Y, Hirano T, Uemura T, Simons CJ, Winkel R, van Group, Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T, Stefansson H, Rujescu D, Cichon S, Pietiläinen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, GROUP, Al E, Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA, Uhlhaas PJ, Singer W, Uslaner JM, Smith SM, Huszar SL, Pachmerhiwala R, Hinchliffe RM, Vardigan JD, Nguyen SJ, Surles NO, Yao L, Barrow JC, Al E, Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandari A, Corominas R, Al E, Vidal M, Cusick ME, Barabási AL, Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH, Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Al E, Walsh T, Shahin H, Elkan-Miller T, Lee MK, Thornton AM, Roeb W, Rayyan AA, Loulus S, Avraham KB, King MC, Kanaan M, Watts DJ, Strogatz SH, Weinberger DR, Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M, Xu B, Roos JL, Dexheimer P, Boone B, Plummer B, Levy S, Gogos JA, Karayiorgou M, Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, Levy S, Gogos JA, Karayiorgou M, Yunker AM, Sharp AH, Sundarraj S, Ranganathan V, Copeland TD, McEnery MW, Zhang C, Gao J, Zhang H, Sun L, Peng G, Aliyu MH, Calkins ME, Swanson CL, Lyons PD, Savage RM, May R, Wiener H, McLeod-Bryant S, Nimgaonkar VL, Ragland JD, Group, P.S., Al E, Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Al E, Cooper GM, Goode DL, Ng SB, Sidow A, Bamshad MJ, Shendure J, Nickerson DA, Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN, First MB, Gibbon M, Spitzer RL, Williams J, Horsfield JA, Anagnostou SH, Hu JK, Cho KH, Geisler R, Lieschke G, Crosier KE, Crosier PS, Imamura Y, Oda A, Katahira T, Bundo K, Pike KA, Ratcliffe MJ, Kitamura D, Jastak S, Wilkinson G, Kähler AK, Djurovic S, Kulle B, Jönsson EG, Agartz I, Hall H, Opjordsmoen S, Jakobsen KD, Hansen T, Melle I, Al E, Kikuchi M, Yamada K, Toyota T, Yoshikawa T, Li H, Durbin R, Li Y, Lalwani AK, Mhatre AN, Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Subgroup, 1000 Genome Project Data Processing, Liu JS, Maxwell ME, Miró X, Meier S, Dreisow ML, Frank J, Strohmaier J, Breuer R, Schmäl C, Albayram O, Pardo-Olmedilla MT, Mühleisen TW, Al E, Moreno-De-Luca D, Mulle JG, Kaminsky EB, Sanders SJ, Myers SM, Adam MP, Pakula AT, Eisenhauer NJ, Uhas K, Weik L, Consortium, S., Consortium, S.S.C.G., GeneSTAR, Al E, Mühleisen TW, Mattheisen M, Strohmaier J, Degenhardt F, Priebe L, Schultz CC, Breuer R, Meier S, Hoffmann P, Rivandeneira F, Investigators G, Al E, Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T, Rajanikanth V, Srivastava SS, Singh AK, Rajyalakshmi M, Chandra K, Aravind P, Sankaranarayanan R, Sharma Y, Rozen S, Skaletsky HJ, Sato M, Nagano T, Stano JF, Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E, Vawter MP, Weickert CS, Ferran E, Matsumoto M, Overman K, Hyde TM, Weinberger DR, Bunney WE, Kleinman JE, Verstreken P, Ohyama T, Haueter C, Habets RL, Lin YQ, Swan LE, Ly CV, Venken KJ, De Camilli P, Bellen HJ, 2013. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154, 518–529. 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann OR, Misura K, Lamas E, Sandrock RW, Nelson P, McDonough SI, DeLisi LE, 2016. Whole-genome sequencing in multiplex families with psychoses reveals mutations in the SHANK2 and SMARCA1 genes segregating with illness. Mol. Psychiatry, 1–6 10.1038/mp.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig T, Grüning B, Kundu K, Houwaart T, Backofen R, Biber K, Normann C, 2017. GRIN3B missense mutation as an inherited risk factor for schizophrenia: whole-exome sequencing in a family with a familiar history of psychotic disorders. Genet. Res. (Camb) 99, e1 10.1017/S0016672316000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O, McCutcheon R, Stone J, 2015. Glutamate and dopamine in schizophrenia: an update for the 21st century. J. Psychopharmacol 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbrici P, Tucker SJ, D’Adamo MC, Pessia M, 2000. Role of receptor protein tyrosine phosphatase α (RPTPα) and tyrosine phosphorylation in the serotonergic inhibition of voltage-dependent potassium channels. Pflugers Arch. - Eur. J. Physiol 441, 257–262. 10.1007/s004240000406. [DOI] [PubMed] [Google Scholar]
- John J, Bhatia T, Kukshal P, Chandna P, Nimgaonkar VL, Deshpande SN, Thelma BK, 2016. Association study of MiRSNPs with schizophrenia, tardive dyskinesia and cognition. Schizophr. Res. 174, 29–34. 10.1016/j.schres.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Kukshal P, Bhatia T, Chowdari KV, Nimgaonkar VL, Deshpande SN, Thelma BK, 2017. Possible role of rare variants in trace amine associated receptor 1 in schizophrenia. Schizophr. Res. 189, 190–195. 10.1016/j.schres.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Sharma A, Kukshal P, Bhatia T, Nimgaonkar VL, Deshpande SN, Thelma BK, 2018. Rare variants in tissue inhibitor of metalloproteinase 2 as a risk factor for schizophrenia: evidence from familial and cohort analysis. Schizophr. Bull 10.1093/schbul/sbx196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J, 2014. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet 46, 310–315. 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos MZ, Carless MA, Peralta J, Blackburn A, Almeida M, Roalf D, Pogue-Geile MF, Prasad K, Gur RC, Nimgaonkar V, Curran JE, Duggirala R, Glahn DC, Blangero J, Gur RE, Almasy L, 2016. Exome sequence data from multigenerational families implicate AMPA receptor trafficking in neurocognitive impairment and schizophrenia risk. Schizophr. Bull 42, 288–300. 10.1093/schbul/sbv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz TM, Harroch S, Manor O, Lichtenberg P, Friedlander Y, Seandel M, Harkavy-Friedman J, Walsh-Messinger J, Dolgalev I, Heguy A, Chao MV, Malaspina D, 2015. De novo mutations from sporadic schizophrenia cases highlight important signaling genes in an independent sample. Schizophr. Res 166, 119–124. 10.1016/j.schres.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HT, Maksumova L, Wang J, Pallen CJ, 2006. Reduced NMDA receptor tyrosine phosphorylation in PTP??-deficient mouse synaptosomes is accompanied by inhibition of four src family kinases and Pyk2: an upstream role for PTP? In NMDA receptor regulation. J. Neurochem 98, 1798–1809. 10.1111/j.1471-4159.2006.04075.x. [DOI] [PubMed] [Google Scholar]
- Lei G, Xue S, Chery N, Liu Q, Xu JD, Kwan CL, Fu YP, Lu YM, Lu MY, Harder KW, Yu XM, 2002. Gain control of N-methyl-D-aspartate receptor activity by receptor-like protein tyrosine phosphatase alpha. EMBO J. 21, 2977–2989. 10.1093/emboj/cdf292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P, 2002. Schizophrenia as a disorder of neurodevelopment. Annu. Rev. Neurosci 25, 409–432. 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA, 2000. Catching up on schizophrenia: natural history and neurobiology. Neuron 28, 325–334. [DOI] [PubMed] [Google Scholar]
- Li H, 2013. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. https://doi.org/arXiv:1303.3997 [q-bio.GN]. [Google Scholar]
- Li H, Durbin R, 2009. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 25, 1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MX, Gui HS, Kwan JSH, Bao SY, Sham PC, 2012. A comprehensive framework for prioritizing variants in exome sequencing studies of Mendelian diseases. Nucleic Acids Res. 40, e53 10.1093/nar/gkr1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li J, Li MJ, Pan Z, Hsu JS, Liu DJ, Zhan X, Wang J, Song Y, Sham PC, 2017. Robust and rapid algorithms facilitate large-scale whole genome sequencing downstream analysis in an integrative framework. Nucleic Acids Res. 45, gkx019 10.1093/nar/gkx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jian X, Boerwinkle E, 2013. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum. Mutat. 34, E2393–E2402. 10.1002/humu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P-R, Bhatia G, Gusev A, Finucane HK, Bulik-Sullivan BK, Pollack SJ, Schizophrenia Working Group of Psychiatric Genomics Consortium, de Candia TR, Lee SH, Wray NR, Kendler KS, O’Donovan MC, Neale BM, Patterson N, Price AL, 2015. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat. Genet 47, 1385–1392. 10.1038/ng.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel P, Leo D, Fulton S, Brard M, Trudeau LE, 2011. Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PLoS One 6, e20402 10.1371/journal.pone.0020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.Journal 17, 10 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y, Mistry M, Pavlidis P, Solomon R, Ghiban E, Antoniou E, Kelleher E, O’Brien C, Donohoe G, Gill M, Morris DW, McCombie WR, Corvin A, 2014. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol. Psychiatry 19, 652–658. 10.1038/mp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA, 2010. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone A, Battaglia F, Wang C, Dusa A, Su J, Zagzag D, Bianchi R, Casaccia-Bonnefil P, Arancio O, Sap J, 2003. Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long-term potentiation. EMBO J. 22, 4121–4131. 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponniah S, Wang DZM, Kah Leong L, Pallen CJ, 1999. Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol 9, 535–538. 10.1016/S0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Káhler A, Duncan L, Stahl E, Genovese G, Fernández E, Collins MO, Komiyama NH, Choudhary JS, Magnusson PKE, Banks E, Shakir K, Garimella K, Fennell T, Depristo M, Grant SGN, Haggarty SJ, Gabriel S, Scolnick EM, Lander ES, Hultman CM, Sullivan PF, McCarroll SA, Sklar P, 2014. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506, 185–190. 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med 17, 405–424. 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA, Lee P, Bulik-Sullivan B, Collier DA, Huang H, Pers TH, Agartz I, Agerbo E, Albus M, Alexander M, Amin F, Bacanu SA, Begemann M, Belliveau RA Jr., Bene J, Bergen SE, Bevilacqua E, Bigdeli TB, Black DW, Bruggeman R, Buccola NG, Buckner RL, Byerley W, Cahn W, Cai G, Campion D, Cantor RM, Carr VJ, Carrera N, Catts SV, Chambert KD, Chan RCK, Chen RYL, Chen EYH, Cheng W, Cheung EFC, Ann Chong S, Robert Cloninger C, Cohen D, Cohen N, Cormican P, Craddock N, Crowley JJ, Curtis D, Davidson M, Davis KL, Degenhardt F, Del Favero J, Demontis D, Dikeos D, Dinan T, Djurovic S, Donohoe G, Drapeau E, Duan J, Dudbridge F, Durmishi N, Eichhammer P, Eriksson J, Escott-Price V, Essioux L, Fanous AH, Farrell MS, Frank J, Franke L, Freedman R, Freimer NB, Friedl M, Friedman JI, Fromer M, Genovese G, Georgieva L, Giegling I, Giusti-Rodriguez P, Godard S, Goldstein JI, Golimbet V, Gopal S, Gratten J, de Haan L, Hammer C, Hamshere ML, Hansen M, Hansen T, Haroutunian V, Hartmann AM, Henskens FA, Herms S, Hirschhorn JN, Hoffmann P, Hofman A, Hollegaard MV, Hougaard DM, Ikeda M, Joa I, Julià A, Kahn RS, Kalaydjieva L, Karachanak-Yankova S, Karjalainen J, Kavanagh D, Keller MC, Kennedy JL, Khrunin A, Kim Y, Klovins J, Knowles JA, Konte B, Kucinskas V, Ausrele Kucinskiene Z, Kuzelova-Ptackova H, Kähler AK, Laurent C, Lee Chee Keong J, Hong Lee S, Legge SE, Lerer B, Li M, Li T, Liang K-Y, Lieberman J, Limborska S, Loughland CM, Lubinski J, Lönnqvist J, Macek M Jr., Magnusson PKE, Maher BS, Maier W, Mallet J, Marsal S, Mattheisen M, Mattingsdal M, McCarley RW, McDonald C, McIntosh AM, Meier S, Meijer CJ, Melegh B, Melle I, Mesholam-Gately RI, Metspalu A, Michie PT, Milani L, Milanova V, Mokrab Y, Morris DW, Mors O, Murphy KC, Murray RM, Myin-Germeys I, Müller-Myhsok B, Nelis M, Nenadic I, Nertney DA, Nestadt G, Nicodemus KK, Nikitina-Zake L, Nisenbaum L, Nordin A, O’Callaghan E, O’Dushlaine C, O’Neill FA, Oh S-Y, Olincy A, Olsen L, Van Os J, Endophenotypes International Consortium P, Pantelis C, Papadimitriou GN, Papiol S, Parkhomenko E, Pato MT, Paunio T, Pejovic-Milovancevic M, Perkins DO, Pietiläinen O, Pimm J, Pocklington AJ, Powell J, Price A, Pulver AE, Purcell SM, Quested D, Rasmussen HB, Reichenberg A, Reimers MA, Richards AL, Roffman JL, Roussos P, Ruderfer DM, Salomaa V, Sanders AR, Schall U, Schubert CR, Schulze TG, Schwab SG, Scolnick EM, Scott RJ, Seidman LJ, Shi J, Sigurdsson E, Silagadze T, Silverman JM, Sim K, Slominsky P, Smoller JW, So H-C, Spencer CA, Stahl EA, Stefansson H, Steinberg S, Stogmann E, Straub RE, Strengman E, Strohmaier J, Scott Stroup T, Subramaniam M, Suvisaari J, Svrakic DM, Szatkiewicz JP, Söderman E, Thirumalai S, Toncheva D, Tosato S, Veijola J, Waddington J, Walsh D, Wang D, Wang Q, Webb BT, Weiser M, Wildenauer DB, Williams NM, Williams S, Witt SH, Wolen AR, Wong EHM, Wormley BK, Simon Xi H, Zai CC, Zheng X, Zimprich F, Wray NR, Stefansson K, Visscher PM, Trust Case-Control Consortium W, Adolfsson R, Andreassen OA, Blackwood DHR, Bramon E, Buxbaum JD, Børglum AD, Cichon S, Darvasi A, Domenici E, Ehrenreich H, Esko T, Gejman PV, Gill M, Gurling H, Hultman CM, Iwata N, Jablensky AV, Jönsson EG, Kendler KS, Kirov G, Knight J, Lencz T, Levinson DF, Li QS, Liu J, Malhotra AK, McCarroll SA, McQuillin A, Moran JL, Mortensen PB, Mowry BJ, Nöthen MM, Ophoff RA, Owen MJ, Palotie A, Pato CN, Petryshen TL, Posthuma D, Rietschel M, Riley BP, Rujescu D, Sham PC, Sklar P, St Clair D, Weinberger DR, Wendland JR, Werge T, Daly MJ, Sullivan PF, O’Donovan MC, 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirzad H, Beiraghi N, Ataei Kachoui M, Akbari MT, 2017. Family-based whole-exome sequencing for identifying novel variants in consanguineous families with schizophrenia. Iran. Red Crescent Med. J 19 (2). [Google Scholar]
- Singh T, Kurki MI, Curtis D, Purcell SM, Crooks L, McRae J, Suvisaari J, Chheda H, Blackwood D, Breen G, Pietiläinen O, Gerety SS, Ayub M, Blyth M, Cole T, Collier D, Coomber EL, Craddock N, Daly MJ, Danesh J, DiForti M, Foster A, Freimer NB, Geschwind D, Johnstone M, Joss S, Kirov G, Körkkö J, Kuismin O, Holmans P, Hultman CM, Iyegbe C, Lönnqvist J, Männikkö M, McCarroll SA, McGuffin P, McIntosh AM, McQuillin A, Moilanen JS, Moore C, Murray RM, Newbury-Ecob R, Ouwehand W, Paunio T, Prigmore E, Rees E, Roberts D, Sambrook J, Sklar P, Clair DS, Veijola J, Walters JTR, Williams H, Swedish Schizophrenia Study, INTERVAL Study, DDD Study, UK10 K Consortium, Sullivan PF, Hurles ME, O’Donovan MC, Palotie A, Owen MJ, Barrett JC, 2016. Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat. Neurosci 19, 571–577. 10.1038/nn.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, Gudmundsdottir S, Sveinbjornsson G, Suvisaari J, Paunio T, Torniainen- Holm M, Frigge ML, Jonsdottir GA, Huttenlocher J, Arnarsdottir S, Ingimarsson O, Haraldsson M, Tyrfingsson T, Thorgeirsson TE, Kong A, Norddahl GL, Gudbjartsson DF, Sigurdsson E, Stefansson H, Stefansson K, 2017. Truncating mutations in RBM12 are associated with psychosis. Nat. Genet 49, 1251–1254. 10.1038/ng.3894. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nielsen KS, Aleksic B, Petersen S, Ikeda M, Kushima I, Vacaresse N, Ujike H, Iwata N, Dubreuil V, Mirza N, Sakurai T, Ozaki N, Buxbaum JD, Sap J, 2011. Loss of function studies in mice and genetic association link receptor protein tyrosine phosphatase α to schizophrenia. Biol. Psychiatry 70, 626–635. 10.1016/j.biopsych.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata A, Xu B, Ionita-Laza I, Roos JL, Gogos JA, Karayiorgou M, 2014. Loss-of-function variants in schizophrenia risk and SETD1A as a candidate susceptibility gene. Neuron 10.1016/j.neuron.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teltsh O, Kanyas K, Karni O, Levi A, Korner M, Ben-Asher E, Lancet D, Hamdan A, Lerer B, Kohn Y, 2008. Genome-wide linkage scan, fine mapping, and haplotype analysis in a large, inbred, Arab Israeli pedigree suggest a schizophrenia susceptibility locus on chromosome 20p13. Am. J. Med. Genet. B Neuropsychiatr. Genet 147, 209–215. 10.1002/ajmg.b.30591. [DOI] [PubMed] [Google Scholar]
- Timms AE, Dorschner MO, Wechsler J, Choi KY, Kirkwood R, Girirajan S, Baker C, Eichler EE, Korvatska O, Roche KW, Horwitz MS, Tsuang DW, 2013. Support for the N-methyl-d-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiat. 70, 582–590. 10.1001/jamapsychiatry.2013.1195. [DOI] [PubMed] [Google Scholar]
- Trepanier CH, Jackson MF, MacDonald JF, 2012. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- Tsai W, 1999. Receptor protein tyrosine phosphatase alpha participates in the m1 muscarinic acetylcholine receptor-dependent regulation of Kv1.2 channel activity. EMBO J. 18, 109–118. 10.1093/emboj/18.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang M, 2000. Schizophrenia: Genes and environment. Biol. Psychiatry 10.1016/S0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Wang PS, Wang J, Xiao ZC, Pallen CJ, 2009. Protein-tyrosine phosphatase α acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J. Biol. Chem 284, 33692–33702. 10.1074/jbc.M109.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD, 2003. GABA and schizophrenia: a review of basic science and clinical studies. J. Clin. Psychopharmacol 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Xing J, Wang C, Kimura H, Takasaki Y, Kunimoto S, Yoshimi A, Nakamura Y, Koide T, Banno M, Kushima I, Uno Y, Okada T, Aleksic B, Ikeda M, Iwata N, Ozaki N, 2014. Resequencing and association analysis of PTPRA, a possible susceptibility gene for schizophrenia and autism spectrum disorders. PLoS One 9, e112531 10.1371/journal.pone.0112531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, Levy S, Gogos JA, Karayiorgou M, 2012. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat. Genet 44, 1365–1369. 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Tan YLJ, Ponniah S, Takeda Y, Wang SQ, Schachner M, Watanabe K, Pallen CJ, Xiao ZC, 2008. Neural recognition molecules CHL1 and NB-3 regulate apical dendrite orientation in the neocortex via PTPα. EMBO J. 27, 188–200. 10.1038/sj.emboj.7601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen RKC, Merico D, Bookman M, Howe JL, Thiruvahindrapuram B, Patel RV, Whitney J, Deflaux N, Bingham J, Wang Z, Pellecchia G, Buchanan JA, Walker S, Marshall CR, Uddin M, Zarrei M, Deneault E, D’Abate L, Chan AJS, Koyanagi S, Paton T, Pereira SL, Hoang N, Engchuan W, Higginbotham EJ, Ho K, Lamoureux S, Li W, MacDonald JR, Nalpathamkalam T, Sung WWL, Tsoi FJ, Wei J, Xu L, Tasse AM, Kirby E, Van Etten W, Twigger S, Roberts W, Drmic I, Jilderda S, Modi BM, Kellam B, Szego M, Cytrynbaum C, Weksberg R, Zwaigenbaum L, Woodbury-Smith M, Brian J, Senman L, Iaboni A, Doyle-Thomas K, Thompson A, Chrysler C, Leef J, Savion-Lemieux T, Smith IM, Liu X, Nicolson R, Seifer V, Fedele A, Cook EH, Dager S, Estes A, Gallagher L, Malow BA, Parr JR, Spence SJ, Vorstman J, Frey BJ, Robinson JT, Strug LJ, Fernandez BA, Elsabbagh M, Carter MT, Hallmayer J, Knoppers BM, Anagnostou E, Szatmari P, Ring RH, Glazer D, Pletcher MT, Scherer SW, 2017. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat. Neurosci 20, 602–611. 10.1038/nn.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hu ZZ, Zhang L, Hu ZZ, Liu H, Liu Z, Du J, Zhao J, Zhou L, Xia K, Tang B, Shen L, 2016. Identification of RELN variation p.Thr3192Ser in a Chinese family with schizophrenia. Sci. Rep 6, 24327 10.1038/srep24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.