Significance
In this report we identify genetic susceptibility variants for periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome, the most common periodic fever syndrome in children. PFAPA shares risk loci at IL12A, STAT4, IL10, and CCR1-CCR3 with Behçet’s disease and recurrent aphthous stomatitis, defining a family of Behçet’s spectrum disorders. Differential HLA associations along this spectrum may determine where individual phenotypes fall among the Behçet’s spectrum disorders.
Keywords: PFAPA, periodic fever, Behçet’s disease, aphthous ulcers, tonsillitis
Abstract
Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome is the most common periodic fever syndrome in children. The disease appears to cluster in families, but the pathogenesis is unknown. We queried two European–American cohorts and one Turkish cohort (total n = 231) of individuals with PFAPA for common variants previously associated with two other oropharyngeal ulcerative disorders, Behçet’s disease and recurrent aphthous stomatitis. In a metaanalysis, we found that a variant upstream of IL12A (rs17753641) is strongly associated with PFAPA (OR 2.13, P = 6 × 10−9). We demonstrated that monocytes from individuals who are heterozygous or homozygous for this risk allele produce significantly higher levels of IL-12p70 upon IFN-γ and LPS stimulation than those from individuals without the risk allele. We also found that variants near STAT4, IL10, and CCR1-CCR3 were significant susceptibility loci for PFAPA, suggesting that the pathogenesis of PFAPA involves abnormal antigen-presenting cell function and T cell activity and polarization, thereby implicating both innate and adaptive immune responses at the oropharyngeal mucosa. Our results illustrate genetic similarities among recurrent aphthous stomatitis, PFAPA, and Behçet’s disease, placing these disorders on a common spectrum, with recurrent aphthous stomatitis on the mild end, Behçet’s disease on the severe end, and PFAPA intermediate. We propose naming these disorders Behçet’s spectrum disorders to highlight their relationship. HLA alleles may be factors that influence phenotypes along this spectrum as we found new class I and II HLA associations for PFAPA distinct from Behçet’s disease and recurrent aphthous stomatitis.
Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome is considered to be the most common periodic fever syndrome in children and is characterized by recurrent, regular attacks of high fever associated with pharyngeal inflammation, aphthous stomatitis, and/or cervical lymphadenopathy (1, 2). Affected children typically begin having fever episodes between 1 and 5 y of age and continue to have these inflammatory attacks for 3 to 7 y until episodes spontaneously resolve (3, 4). Studies of family history suggest that PFAPA clusters within families, with nearly a quarter of patients with PFAPA having a family member with the disease; moreover, families of patients with PFAPA have a high prevalence of family members with recurrent aphthous stomatitis (5, 6). However, queries for rare variants associated with the disease have not yielded promising candidates, suggesting that PFAPA is a complex genetic disorder (6).
Aphthous ulcers and inflammation at the oropharyngeal mucosa are hallmarks not only of PFAPA but also of other disorders, including Behçet’s disease and recurrent aphthous stomatitis. Behçet’s disease is a systemic inflammatory disease primarily characterized by mucosal ulcers in the mouth, genital area, and gastrointestinal tract, as well as inflammatory attacks affecting the eyes, skin, joints, blood vessels, and brain. Several genome-wide association studies (GWAS) of Behçet’s disease in Turkish and Japanese individuals implicate variants near or within the IL10, IL23R-IL12RB2, CCR1-CCR3, STAT4, KLRC4, IL12A, IL1A-IL1B, IRF8, CEBPB-PTPN1, ADO-EGR2, RIPK2, LACC1, and FUT2 loci as risk factors for the disease (7–10). In addition, the major histocompatibility complex (MHC) allele HLA-B*51 consistently shows a strong association with Behçet’s disease (11). Among individuals with HLA-B*51, missense variants in ERAP1, which encodes a protein that trims peptides loaded onto class I MHC molecules, also confer risk for Behçet’s disease (8). In aggregate, these risk loci suggest that the pathogenesis of Behçet’s disease involves a complex interplay of aberrant innate immune and T cell responses to microbial antigens and disrupted barrier function at mucosal surfaces. Interestingly, a recent GWAS of recurrent aphthous stomatitis in the United States and United Kingdom also revealed variants near genes previously associated with Behçet’s disease, including those near IL12A, STAT4, IL10, and CCR1 (12). Although several statistically significant class I and class II HLA allele associations were identified, the most significantly associated allele, HLA-DRB1*01:03, is of low prevalence among individuals of European ancestry.
In light of the phenotypic similarities among Behçet’s disease, recurrent aphthous stomatitis, and PFAPA, we hypothesized that these diseases may also have genotypic overlap and screened three cohorts of patients with PFAPA for variants previously associated with Behçet’s disease or recurrent aphthous stomatitis. We identified common variants associated with PFAPA and tested their functional significance with in vitro cellular assays. In addition, we discovered class I and class II classical HLA alleles that are risk factors for PFAPA.
Results
Common Variants Associated with PFAPA Syndrome.
We selected six single nucleotide polymorphisms (SNPs) previously associated with Behçet’s disease (near or within the IL12A, IL10, STAT4, CCR1-CCR3, IL23R-IL12RB2, and FUT2 loci) because these are among the strongest susceptibility loci found in Turkish cohorts that also have minor allele frequency greater than 5% (7–9). Three of the variants we selected were also found to be in strong linkage disequilibrium (LD) with those associated with recurrent aphthous stomatitis (IL12A, IL10, and STAT4) (12). The CCR1-CCR3 variant we selected is associated with Behçet’s disease, but several nearby SNPs that are not in LD are reported to be significantly associated with recurrent aphthous stomatitis (8, 12). Four of the variants we selected are in noncoding regions near genes involved in CD4+ T cell activation (rs17753641 near IL12A, rs1518110 near IL10, rs7574070 near STAT4, rs924080 near IL23R-IL12RB2), while the CCR1-CCR3 variant (rs7616215) is associated with diminished monocyte chemotaxis (7–9). Homozygous inheritance of the nonsense mutation (W143X) in FUT2 (rs601338) among Caucasians impairs secretion of ABO antigens at mucosal surfaces (13); this and other nonsecretor alleles modulate risk not only for Behçet’s disease but also for Crohn’s disease and some intestinal infections (14, 15). In addition, we screened our cohorts for a frameshift insertion in CARD8 (rs140826611), which was reported by another group to be significantly associated with PFAPA (16).
Patients who met the diagnostic criteria for PFAPA were included (SI Appendix, Materials and Methods). We initially recruited a cohort of 129 European–American individuals with PFAPA and compared their ancestry to that of individuals of European ancestry in the HapMap database (17) using genotyping data generated from the OmniExpressExome-8 (v1.4 or 1.6) chip from Illumina; 7 individuals with PFAPA who clustered away from the HapMap Caucasian cohort were excluded (SI Appendix, Fig. S1). Therefore, in total, we screened a discovery cohort of 122 European–American patients with PFAPA. We compared the frequencies of the candidate variants to those of 1,927 participants in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, a primarily Caucasian cohort from the United Kingdom. The frequency of the FUT2 variant was not available in the ALSPAC cohort; therefore, the frequency among 503 Europeans in the 1000 Genomes cohort was used as the comparator. Later, we recruited a replication cohort of 64 European–American individuals with PFAPA; 4 of these individuals did not genetically cluster with individuals of European ancestry from the HapMap cohort and were excluded, and another individual was found to be identical to an individual in the discovery cohort and was excluded (SI Appendix, Fig. S1). In total, we screened a replication cohort of 59 European–American patients with PFAPA and compared the frequencies of the candidate noncoding variants to those of ∼7,700 non-Finnish Europeans in the Genome Aggregation Database (gnoMAD) and coding variants to ∼33,000 non-Finnish Europeans in the Exome Aggregation Consortium (ExAC). We also genotyped a replication cohort of 50 patients with PFAPA from Turkey and compared the allele frequencies to that of about 1,880 Turkish controls without Behçet’s disease or recurrent aphthous stomatitis who were previously genotyped (8, 9).
Risk allele frequencies and odds ratios (ORs) in each of the three cohorts and ORs and P values from a metaanalysis of these three cohorts are shown in Table 1 (additional data in SI Appendix, Table S1). A variant upstream of IL12A (rs17753641) was consistently the most significant variant in all three cohorts and in the metaanalysis with an ORmeta of 2.13 (Pmeta = 6 × 10−9). Three additional variants near IL10, STAT4, and CCR1-CCR3 were also significantly associated with PFAPA in the metaanalysis. Variants we queried in or near IL23R-IL12RB2, FUT2, and CARD8 were not significantly associated with PFAPA in the metaanalysis.
Table 1.
Candidate gene allele frequencies in three cohorts of individuals with PFAPA and in metaanalysis of three cohorts
| SNP | Nearest gene | Risk allele | Risk allele frequency among PFAPA cases* | Risk allele frequency among controls† | ORs | ORmeta (95% CI) | Pmeta | Bonferroni corrected Pmeta‡ |
| rs17753641 | IL12A | G | 0.21 | 0.12 | 2.03 | |||
| 0.22 | 0.12 | 2.12 | 2.13 | |||||
| 0.10 | 0.04§ | 2.67 | (1.67–2.72) | 9 × 10−10 | 6 × 10−9 | |||
| rs7574070 | STAT4 | A | 0.43 | 0.35 | 1.42 | |||
| 0.51 | 0.33 | 2.09 | 1.51 | |||||
| 0.46 | 0.42¶ | 1.16 | (1.25–1.82) | 2 × 10−5 | 0.0001 | |||
| rs1518110 | IL10 | A | 0.27 | 0.20 | 1.42 | |||
| 0.30 | 0.21 | 1.62 | 1.45 | |||||
| 0.37 | 0.30§ | 1.34 | (1.18–1.79) | 0.0004 | 0.003 | |||
| rs7616215 | CCR1-CCR3 | T | 0.72 | 0.65 | 1.39 | |||
| 0.69 | 0.62 | 1.32 | 1.38 | |||||
| 0.73 | 0.66¶ | 1.44 | (1.12–1.69) | 0.002 | 0.02 | |||
| rs924080 | IL23R-IL12RB2 | T | 0.58 | 0.53 | 1.25 | |||
| 0.57 | 0.55 | 1.07 | 1.22 | |||||
| 0.68 | 0.61§ | 1.35 | (1.00–1.47) | 0.04 | 0.3 | |||
| rs601338 | FUT2 | Homozygous AA (nonsecretor) | 0.33 | 0.21 (1000G, n = 503) | 1.85 | |||
| 0.27 | 0.22 (ExAC, n = 32934) | 1.35 | 1.37 | |||||
| 0.18 | 0.26§ | 0.61 | (1.00–1.88) | 0.05 | 0.3 | |||
| rs140826611 | CARD8 | TT insertion | 0.061 | 0.057 | 1.09 | |||
| 0.035 | 0.058 (ExAC, n = 33357) | 0.60 | 0.95 | |||||
| 0.050 | N/A | N/A | (0.59–1.52) | 0.83 | 1.0 |
Values are listed in the following order: American discovery cohort (n = 122); American replication cohort (n = 59); Turkish replication cohort (n = 50).
Values are listed in the following order (unless otherwise indicated): ALSPAC (n = 1,927); gnoMAD (n ∼ 7,690); Turkish controls (n ∼ 1,880).
Metaanalysis P values (Pmeta) were corrected for seven comparisons using the Bonferroni correction.
Data from Takeuchi et al. (9).
Data from Kirino et al. (8).
Three patients in our cohorts who met the diagnostic criteria for PFAPA also developed additional clinical features of Behçet’s disease but did not fulfill the International Study Group Criteria for Behçet’s disease (18). Two patients (one in the discovery cohort and one in the European–American replication cohort) initially had regular febrile episodes with oral ulcers, cervical adenopathy, and/or tonsillar inflammation that met the diagnostic criteria for PFAPA. However, both later reported genital ulcers during some of their flares. Another Caucasian patient reported pustular and papular lesions around the anal and genital areas during febrile episodes. Thus, some patients have phenotypic features of both PFAPA and Behçet’s disease, supporting the genetic similarities we find in these disease entities.
Greater Portion of Monocytes Express Both p35 and p40 during PFAPA Flares.
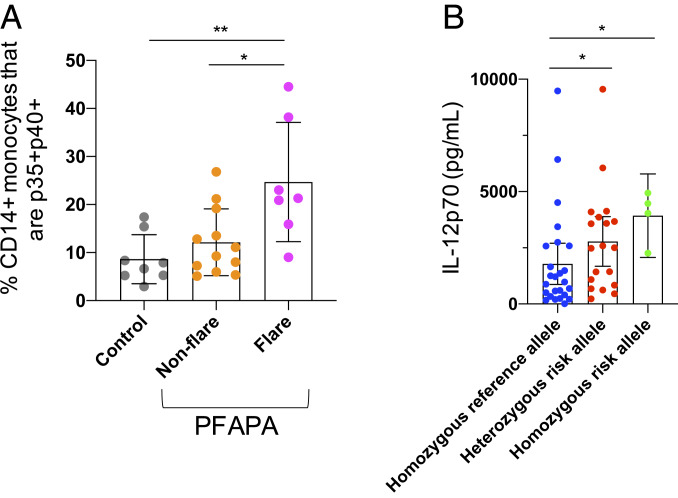
IL12A encodes the p35 subunit of both the proinflammatory cytokine IL-12 and the antiinflammatory cytokine IL-35. The p35 subunit associates with p40 to form IL-12 (IL-12p70), while the p35 subunit interacts with EBI3 to form IL-35. We measured the percentage of CD14+ monocytes from peripheral blood mononuclear cells (PBMCs) expressing both p35 and p40 directly ex vivo from patients with PFAPA during a febrile episode (n = 7) and during an asymptomatic period (n = 12) and from healthy pediatric controls (n = 8). We found that during a PFAPA febrile episode, a significantly higher proportion of CD14+ monocytes expressed both subunits of IL-12 (p35+p40+) than those from asymptomatic periods (P = 0.02) and from healthy controls (P = 0.004) (Fig. 1A and SI Appendix, Fig. S2).
Fig. 1.
Expression of IL-12p70 subunits during PFAPA flares and comparison of IL-12p70 production according to IL12A risk allele. During PFAPA flares, a higher percentage of patient CD14+ monocytes express both subunits of IL-12p70, p35 and p40, compared with nonflare periods and with healthy controls; ex vivo FACS analysis of PBMCs (A). Peripheral blood CD14+ monocytes from adults homozygous and heterozygous for the risk allele near IL12A (rs17753641) secrete more IL-12p70 following priming with IFN-γ and stimulation with LPS than monocytes from individuals without the risk allele; ELISA on supernatant of in vitro-stimulated cells (B). Each dot represents an individual. Mean and SD are plotted. *P < 0.05, **P < 0.005.
Monocytes from Patients Heterozygous and Homozygous for the rs17753641 Risk Allele near IL12A Produce More IL-12p70.
In order to determine whether the risk allele rs17753641 affects IL-12p70 production, we obtained PBMCs from adults from the National Institutes of Health Blood Bank and The Genomic Ascertainment Cohort (T.G.A.C.) who were homozygous for the reference allele (n = 25), heterozygous for the risk allele (n = 19), and homozygous for the risk allele (n = 4). We purified CD14+ monocytes by magnetic-activated cell sorting and primed the CD14+ monocytes with IFN-γ overnight and stimulated with LPS for 8 h. We found that compared to those without the risk allele, monocytes from individuals who were heterozygous or homozygous for the risk allele secreted significantly more IL-12p70 into the supernatant (P = 0.045 and P = 0.027, respectively) (Fig. 1B). However, no significant differences in the geometric mean fluorescence intensity of the individual intracellular subunits (p35, p40, and EBI3) were found by flow cytometry according to genotype at 8 h of stimulation (SI Appendix, Fig. S3).
HLA-B*15:01, HLA-B*35:02, HLA-DPA1*02:02, and the Haplotype Comprised of HLA-DRB1*13:01, HLA-DQA1*01:03, and HLA-DQB1*06:03 Are Risk Alleles for PFAPA Syndrome.
In total, 198 European–American cases and 50 Turkish cases with PFAPA were genotyped on the OmniExpressExome-8 (v1.4 or v1.6) chip from Illumina. Genotyping data were obtained from 5,437 controls of European ancestry from six dbGaP studies (study IDs are phs000652.v1.p1, phs000138.v2.p1, phs000346.v2.p2, phs000812.v1.p1, phs000331.v1.p1, and phs000168.v2.p2) and from 1,779 Turkish controls previously genotyped on the Immunochip (9, 19). One European–American case and 3 Turkish cases were excluded due to low genotype call rate, 1 European–American case was excluded for relatedness (identical patient), and another 11 European–American cases were excluded due to poor ancestry matching with individuals of European ancestry from the HapMap database (SI Appendix, Fig. S4). No controls of either ethnicity or Turkish PFAPA cases were excluded. In total, 185 European–American cases and 47 Turkish cases of PFAPA were analyzed. Classical HLA types were imputed from genotyping data from the MHC region of chromosome 6 with SNP2HLA. HLA alleles were converted to numeric values for regression analysis with a dominant model (1 if the allele was present, 0 if absent), and regression analyses were corrected for the top 10 principal components comparing genotypes of cases and controls in each population (SI Appendix, Fig. S5).
Among European–Americans, the classical HLA types with dominant allele frequency greater than 5% that were most significantly associated with PFAPA were HLA-B*15:01 (OR 2.00, P = 0.001), HLA-C*08:02 (OR 0.29, P = 0.002), HLA-B*35:02 (OR = 3.61, P = 0.003) (SI Appendix, Table S2). The following three class II alleles were also among the top 10 most significant alleles associated with PFAPA: HLA-DRB1*13:01, HLA-DQA1*01:03, and HLA-DQB1*06:03. Among Turkish individuals, the most significant risk alleles were HLA-DQB1*06:03 (OR 3.32, P = 0.002) and HLA-DRB1*13:01 (OR 2.97, P = 0.005) (SI Appendix, Table S3)
The 10 most significant HLA allele associations with dominant allele frequency greater than 5% among the European–American cohort were assessed in a metaanalysis of both the Turkish and European–American cohorts. In the metaanalysis, HLA-DQB1*06:03, HLA-DRB1*13:01, HLA-DQA1*01:03, HLA-B*15:01, HLA-B*35:02, and HLA-DPA1*02:02 were significant risk alleles for PFAPA (Table 2). Conditional analyses and linkage analysis on Haploview showed that HLA-DRB1*13:01, HLA-DQA1*01:03, and HLA-DQB1*06:03 are in LD and comprise a haplotype (SI Appendix, Fig. S6 and Tables S2 and S3).
Table 2.
Classical HLA allele associations in cohorts of European and Turkish ancestry and metaanalyses of these two cohorts
| Classical HLA type | Frequency of dominant genotype among PFAPA cases* | Frequency of dominant genotype among controls† | OR | P value | ORmeta (95% CI) | Pmeta | Bonferroni corrected Pmeta‡ |
| HLA-DQB1*06:03§ | 0.18 | 0.12 | 1.83 | 0.006 | 2.13 (1.50–3.03) | 2 × 10−5 | 0.0002 |
| 0.28 | 0.11 | 3.32 | 0.002 | ||||
| HLA-DRB1*13:01§ | 0.18 | 0.12 | 1.79 | 0.008 | 2.04 (1.43–2.91) | 9 × 10−5 | 0.0009 |
| 0.26 | 0.11 | 2.97 | 0.005 | ||||
| HLA-DQA1*01:03§ | 0.20 | 0.13 | 1.83 | 0.004 | 1.97 (1.40–2.77) | 0.0001 | 0.001 |
| 0.32 | 0.17 | 2.44 | 0.015 | ||||
| HLA-B*15:01 | 0.19 | 0.13 | 2.00 | 0.001 | 1.96 (1.33–2.89) | 0.0007 | 0.007 |
| 0.043 | 0.035 | 1.48 | 0.62 | ||||
| HLA-B*35:02 | 0.05 | 0.01 | 3.61 | 0.003 | 2.86 (1.56–5.26) | 0.0007 | 0.007 |
| 0.13 | 0.07 | 1.88 | 0.24 | ||||
| HLA-DPA1*02:02 | 0.12 | 0.06 | 2.03 | 0.01 | 1.93 (1.23–3.04) | 0.004 | 0.04 |
| 0.11 | 0.08 | 1.61 | 0.36 |
Values are listed in the following order: Caucasian cases (n = 185); Turkish cases (n = 47).
Values are listed in the following order: Caucasian controls (n = 5,437); Turkish controls (n = 1,779). HLA types of the 5437 Caucasian controls were previously reported in Gourh et al. (19).
Metaanalysis P values (Pmeta) were corrected for 10 comparisons with Bonferroni correction.
These HLA alleles are in LD.
Discussion
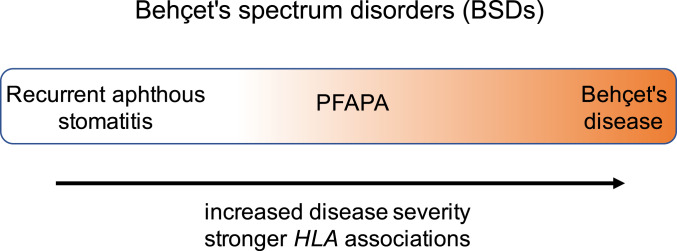
In this study, we found that common variants previously associated with two other disorders characterized by aphthous ulcers (Behçet’s disease and recurrent aphthous stomatitis) are also strongly associated with PFAPA (Table 3). The genetic similarities among these three diseases suggest that they share features of their pathogenesis and could be placed on a continuum. We propose grouping these diseases as a family called Behçet’s spectrum disorders (BSDs). The BSDs span recurrent aphthous ulcers as the mildest, PFAPA as the intermediary, and Behçet’s disease as the most severe (Fig. 2).
Table 3.
Genetic similarities among PFAPA, recurrent aphthous stomatitis, and Behçet’s disease
| Recurrent aphthous stomatitis (RAS) | PFAPA | Behçet’s disease | |||||
| Gene | Associated SNP/allele | OR/P value | Associated SNP/allele | OR/P value | Associated SNP/allele | OR/P value | Notes |
| IL12A | rs76830965* | 0.72 | rs17753641 | 2.13 | rs17753641† | 1.90 | ORs for rs17753641 and rs76830965 are reported for opposite alleles. Risk alleles for these two SNPs are in high LD with each other (D′=1, r2 = 1). |
| 4 × 10−483 | 6 × 10−9 | 1 × 10−9 | |||||
| STAT4 | rs11684030* | 1.06 | rs7574070 | 1.51 | rs7574070‡ | 1.27 | rs7574070 and rs11684030 are in high LD with each other (D′=0.99, r2 = 0.93). |
| 1 × 10−42 | 1 × 10−4 | 1 × 10−9 | |||||
| IL10 | rs1800871* | 1.18 | rs1518110 | 1.45 | rs1518110† | 1.34 | rs1518110 and rs1800871 are in high LD with each other (D′=0.98, r2 = 0.89). |
| 6 × 10−236 | 0.003 | 5 × 10−9 | |||||
| CCR1-CCR3 | rs4493469* | 1.10 | rs7616215 | 1.38 | rs7616215† | 1.40 | Several variants near the CCR3 and/or CCR1 loci are associated with RAS. |
| 2 × 10−43 | 0.02 | 1 × 10−10 | |||||
| IL23R-IL12RB2 | Not reported as a top association* | Not significant | rs924080† | 1.28 | |||
| 3 × 10−7 | |||||||
| FUT2 | Not reported as a top association* | Not significant | rs601338† | 1.52 | |||
| 7 × 10−9 | |||||||
| HLA | HLA-DRB1*01:03* | 1.33 | HLA-DQB1*06:03 (in LD with HLA-DRB1*13:01 & HLA-DQA1*01:03) | 2.13 | HLA-B*51:01§ | 3.3 | In Behçet’s disease, HLA-B*15:01 was nearly significant in conditional analyses. HLA-B*15 was statistically significant among Behçet’s disease patients without HLA-B*51 (OR 2.0, P = 3 × 10−5).§ |
| 2.0 × 10−24 | HLA-B*15:01 | 0.0002 | HLA-B*15:01§ | 5 × 10−58 | |||
| HLA-B*15:01* | 1.10 | 1.96 | 1.24 | ||||
| 6.4 × 10−9 | 0.007 | 0.2 | |||||
Fig. 2.
The BSDs include recurrent aphthous stomatitis, PFAPA, and Behçet’s disease. Recurrent aphthous ulcers are on the mild end of the spectrum with relatively weak HLA associations, while Behçet's disease has the most severe disease manifestations and strongest HLA associations, and PFAPA is between the two.
Clinically, patients who exhibit features of more than one of these disease entities have been described. Many adults with Behçet’s disease have reported symptoms earlier in childhood that fulfill the diagnostic criteria for PFAPA (3, 20). Two patients in our cohort reported genital ulcers during fever flares, and similar patients have been reported in the literature (21, 22). In light of these findings, it is possible that patients with bipolar or complex aphthosis also fall on this spectrum. On the milder end of the spectrum, patients with PFAPA are significantly more likely to have first-degree family members with recurrent aphthous ulcers (5). Patients who resist classification with existing disease entities may better be referred to as having a BSD.
Although these aphthous ulcer disorders have overlapping genetic risk factors and clinical features, differences in the severity and tissue involvement of these diseases necessitate investigation of additional factors that influence the ultimate phenotype of an individual. Our results suggest that MHC may be one of these factors. Although HLA-B*15 has been identified as a significant risk allele for all three diseases (11, 12), other significant HLA associations are distinct for each disease (Table 3). In Behçet’s disease, HLA-B*51 is the most significant risk factor for the disease, but other class I HLA alleles also contribute relatively weakly. For recurrent aphthous ulcers, the most significant MHC association among Caucasians is a rare class II allele, HLA-DRB1*01:03, but other more common class I and class II alleles also contribute to risk, many of which are distinct from those associated with Behçet’s disease. In PFAPA, we found both class I and class II HLA associations. Aside from HLA-B*15, the other risk alleles we found appear to be uniquely associated with PFAPA. This suggests that the presence of particular HLA types, which affects antigen presentation to and thereby activation of T cells, may play a role in determining the phenotype of the patient.
In addition, the strength of HLA associations is different in each of these diseases. In Behçet’s disease, class I HLA associations are the major risk alleles for the disease with considerably stronger and more significant disease associations than the non-MHC genomic variants; the strongest risk allele, HLA-B*51, increases the odds of having Behçet’s disease by three- to sevenfold in most studies (7, 11, 23). On the other hand, in recurrent aphthous stomatitis, non-MHC genomic variants appear to play a stronger role. Although the HLA-DRB1*01:03 allele had an OR of 1.33, other more common class I and class II alleles had weaker contributions to the disease, with ORs between 1.05 and 1.12 (12). In the European–American PFAPA cohort we assessed, we found that the class I and class II HLA associations increase the odds of having PFAPA by about twofold, which is stronger than in recurrent aphthous stomatitis and weaker than in Behçet’s disease, with the caveat that these association studies were performed in different populations.
The BSDs exhibit differences in organ involvement. For example, in contrast to individuals with PFAPA and recurrent aphthous stomatitis, those with Behçet’s disease may present with vascular and ocular disease. A GWAS of PFAPA would facilitate a deeper characterization of the genetic architecture of the disease, allowing us to compare the attributable risk of HLA and non-HLA susceptibility loci and identify differences in genetic risk loci that modulate phenotype among the BSDs. In addition to genetic risk loci, other factors—such as the microbiome, nutritional deficits, and age of the individual—may also influence disease manifestations.
The strongest susceptibility loci for PFAPA that we found (rs17753641) is located in a regulatory region 50,000 bases upstream of the IL12A gene, which encodes the p35 subunit of the cytokines IL-12 and IL-35. IL-12 is produced primarily by antigen-presenting cells and is a major promoter of CD4+ T helper 1 (Th1) cell polarization and induction of IFN-γ production by Th1 cells, CD8+ T cells, and NK cells (24). We found that rs17753641 is associated with increased IL-12p70 production from monocytes upon stimulation, suggesting that individuals with this variant are prone to excess Th1 cell activation. IL-35 is an antiinflammatory cytokine produced by induced T regulatory cells, B cells, and tolerogenic dendritic cells (25–29); the role of this susceptibility variant in regulating IL-35 production remains unknown.
Some of the other risk alleles that we found for PFAPA also affect antigen-presenting cell function and CD4+ T cell activation. The risk SNP near IL10 has previously been associated with reduced IL-10 production from monocytes, which may augment the effects of proinflammatory mediators like IL-12 and IL-23 (7). The susceptibility allele near STAT4 is associated with elevated STAT4 mRNA expression (8), which presumably would enhance signaling from IL-12, IL-23, and type 1 IFN stimulation in CD4+ T cells (30). On the other hand, the susceptibility SNP near CCR1-CCR3 is correlated with a reduction in CCR1 expression and reduced monocyte migration in response to MIP-1α, which may diminish mucosal barrier function (8). Other studies indicate that CCR1 expression is higher among M2 macrophages, which have regulatory roles, and so the risk variant may disproportionately decrease M2 macrophage migration to sites of inflammation (31). Another study suggested that this variant more prominently increases CCR2 expression, thereby increasing monocyte migration to sites of inflammation (32). In aggregate, these risk alleles likely render individuals more susceptible to abnormal innate-immune cell function and heightened T cell activation. Further studies are necessary to understand the role of these risk variants and HLA alleles in the periodicity of PFAPA.
In concordance with the predicted effects of the genomic variants we identified and in line with our finding of more p35+p40+ monocytes during flares, immunologic profiling of peripheral blood during febrile PFAPA episodes indicates heightened monocyte and Th1 activation with up-regulation of IFN-induced transcripts and increased levels of IL-12, IFN-γ, and Th1-associated chemokines during flares (33–36). Elevated expression of Th1-associated chemokines was also noted in the tonsils of patients with PFAPA (37). Similarly, in both Behçet’s disease and recurrent aphthous stomatitis, heightened Th1 and Th17 activation have been noted in both the peripheral blood and around ulcerative lesions (38–43).
Our comparisons of genetic risk alleles for PFAPA, Behçet’s disease, and recurrent aphthous ulcers begin to build a framework for understanding the genetic architecture of ulcerative diseases of the oropharynx. The overlap in genetic susceptibility loci may also extrapolate to treatment strategies. Apremilast, a phosphodiesterase 4 inhibitor, has been shown to be effective in reducing the number of aphthous ulcers in patients with Behçet’s disease and a small number of individuals with treatment-refractory recurrent aphthous stomatitis (44–46). Because apremilast reduces production of proinflammatory cytokines, including IL-12p70 and IFN-γ from human PBMCs, it may have efficacy in treating patients with PFAPA (47, 48). In addition, because IL-12 has significance in the pathogenesis of PFAPA, ustekinumab, a monoclonal antibody targeting the p40 subunit of IL-12 and IL-23, may be a potential therapy for treatment-refractory cases. Clinical trials of these medications for PFAPA would provide more information.
Materials and Methods
Detailed descriptions of patient recruitment, genotyping, ancestry analysis, HLA typing, and assessment of IL-12p70 production from human peripheral blood cells are provided in SI Appendix. This study was approved by the institutional review boards at the National Institutes of Health (Bethesda, MD), Children’s Hospital Boston (Boston, MA), Vanderbilt University School of Medicine (Nashville, TN), and Hacettepe University Faculty of Medicine (Ankara, Turkey). All subjects with PFAPA and/or their parents gave consent for participation in this study.
Data Availability.
The candidate SNP genotyping data for discovery and replication cohorts are in Dataset S1. Genotyping data for the classical HLA alleles for European–American and Turkish cases and controls are in Dataset S2; data are coded according to the dominant model (1 if allele is present and 0 if allele is not present).
Supplementary Material
Acknowledgments
We thank the patients and families who participated in these studies; Kacie Hoyt and Edwin Anderson (Boston Children’s Hospital), Kathryn Garguilo (Vanderbilt University School of Medicine), Silvia Stojanov and Puja Chitkara (National Human Genome Research Institute), Les Biesecker, Priscilla Chan, Jennifer Johnston, Katie Lewis, Justin Paschall, and Tyra Wolfsberg (T.G.A.C.), and Tom Lewis (NIH Blood Bank) for their help in recruiting patients. This work was supported by the Intramural Research Program of the National Human Genome Research Institute and National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Recruitment at Boston Children’s Hospital was funded by the Department of Rheumatology, the Childhood Arthritis and Rheumatology Research Alliance, and a private donation from a family of a child with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis. This work utilized the computational resources of the NIH High Performance Computing Biowulf cluster. T.G.A.C. is supported by the National Institutes of Health Director Challenge Fund. Turkish healthy controls were collected with the support of the Istanbul University Research Fund.
Footnotes
Competing interest statement: F.D. is a consultant to Novartis and receives royalties from UpToDate. K.M.E. is on the Data Safety and Monitoring Board for Seqirus, Pfizer, Sanofi, Moderna, and X4 Pharma, and serves as an advisor to Bio-Net and Merck. P.F.W. is on the scientific advisory boards for GlaxoSmithKline, Sanofi-Pasteur, and Meissa Vaccines.
3A complete list of The Genomic Ascertainment Cohort can be found in the SI Appendix.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002051117/-/DCSupplemental.
Contributor Information
Collaborators: David P. Ascher, Leslie G. Biesecker, Priscilla Chan, Thomas P. Conrads, Jennifer J. Johnston, Alexander E. Katz, Katie L. Lewis, G. Larry Maxwell, Justin Paschall, Henoke Shiferaw, Tyra G. Wolfsberg, Wendy S.W. Wong, and Suiyuan Zhang
References
- 1.Marshall G. S., Edwards K. M., Butler J., Lawton A. R., Syndrome of periodic fever, pharyngitis, and aphthous stomatitis. J. Pediatr. 110, 43–46 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Masters S. L., Simon A., Aksentijevich I., Kastner D. L., Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 27, 621–668 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wurster V. M., Carlucci J. G., Feder H. M. Jr, Edwards K. M., Long-term follow-up of children with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome. J. Pediatr. 159, 958–964 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Feder H. M., Salazar J. C., A clinical review of 105 patients with PFAPA (a periodic fever syndrome). Acta Paediatr. 99, 178–184 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Manthiram K., Nesbitt E., Morgan T., Edwards K. M., Family history in periodic fever, aphthous stomatitis, pharyngitis, adenitis (PFAPA) syndrome. Pediatrics 138, e20154572 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Gioia S. A., et al. , Analysis of the genetic basis of periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. Sci. Rep. 5, 10200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remmers E. F., et al. , Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behçet’s disease. Nat. Genet. 42, 698–702 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirino Y., et al. , Genome-wide association analysis identifies new susceptibility loci for Behçet’s disease and epistasis between HLA-B*51 and ERAP1. Nat. Genet. 45, 202–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi M., et al. , Dense genotyping of immune-related loci implicates host responses to microbial exposure in Behçet’s disease susceptibility. Nat. Genet. 49, 438–443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuki N., et al. , Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet’s disease susceptibility loci. Nat. Genet. 42, 703–706 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Ombrello M. J., et al. , Behçet disease-associated MHC class I residues implicate antigen binding and regulation of cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. U.S.A. 111, 8867–8872 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudding T. et al.; 23andMe Research Team , Genome wide analysis for mouth ulcers identifies associations at immune regulatory loci. Nat. Commun. 10, 1052 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly R. J., Rouquier S., Giorgi D., Lennon G. G., Lowe J. B., Sequence and expression of a candidate for the human Secretor blood group α(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 270, 4640–4649 (1995). [DOI] [PubMed] [Google Scholar]
- 14.McGovern D. P. et al.; International IBD Genetics Consortium , Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum. Mol. Genet. 19, 3468–3476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Graaf M., van Beek J., Koopmans M. P., Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 14, 421–433 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Cheung M. S., et al. , Periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis syndrome is associated with a CARD8 variant unable to bind the NLRP3 inflammasome. J. Immunol. 198, 2063–2069 (2017). [DOI] [PubMed] [Google Scholar]
- 17.International HapMap Consortium et al. , A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Study Group for Behçet’s Disease , Criteria for diagnosis of Behçet’s disease. Lancet 335, 1078–1080 (1990). [PubMed] [Google Scholar]
- 19.Gourh P., et al. , HLA and autoantibodies define scleroderma subtypes and risk in African and European Americans and suggest a role for molecular mimicry. Proc. Natl. Acad. Sci. U.S.A. 117, 552–562 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantarini L., et al. , PFAPA syndrome and Behçet’s disease: A comparison of two medical entities based on the clinical interviews performed by three different specialists. Clin. Rheumatol. 35, 501–505 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Scattoni R., et al. , Genital ulcer as a new clinical clue to PFAPA syndrome. Clin. Exp. Dermatol. 40, 286–288 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Lin C. M., et al. , Genital ulcers as an unusual sign of periodic fever, aphthous stomatitis, pharyngotonsillitis, cervical adenopathy syndrome: A novel symptom? Pediatr. Dermatol. 28, 290–294 (2011). [DOI] [PubMed] [Google Scholar]
- 23.de Menthon M., Lavalley M. P., Maldini C., Guillevin L., Mahr A., HLA-B51/B5 and the risk of Behçet’s disease: A systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 61, 1287–1296 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tait Wojno E. D., Hunter C. A., Stumhofer J. S., The immunobiology of the interleukin-12 family: Room for discovery. Immunity 50, 851–870 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collison L. W., et al. , IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 11, 1093–1101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collison L. W., et al. , The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Wang R. X., et al. , Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20, 633–641 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen P., et al. , IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon K. O., van der Kooij S. W., Vignali D. A., van Kooten C., Human tolerogenic dendritic cells produce IL-35 in the absence of other IL-12 family members. Eur. J. Immunol. 45, 1736–1747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watford W. T., et al. , Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 202, 139–156 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Nakano H., et al. , GWAS-identified CCR1 and IL10 loci contribute to M1 macrophage-predominant inflammation in Behçet’s disease. Arthritis Res. Ther. 20, 124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishigaki K., et al. , Polygenic burdens on cell-specific pathways underlie the risk of rheumatoid arthritis. Nat. Genet. 49, 1120–1125 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Stojanov S., et al. , Cytokine profile in PFAPA syndrome suggests continuous inflammation and reduced anti-inflammatory response. Eur. Cytokine Netw. 17, 90–97 (2006). [PubMed] [Google Scholar]
- 34.Stojanov S., et al. , Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) is a disorder of innate immunity and Th1 activation responsive to IL-1 blockade. Proc. Natl. Acad. Sci. U.S.A. 108, 7148–7153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolly L., et al. , Periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome is linked to dysregulated monocyte IL-1β production. J. Allergy Clin. Immunol. 131, 1635–1643 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki T., et al. , Markedly elevated CD64 expressions on neutrophils and monocytes are useful for diagnosis of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome during flares. Clin. Rheumatol. 33, 677–683 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Dytrych P., et al. , Polyclonal, newly derived T cells with low expression of inhibitory molecule PD-1 in tonsils define the phenotype of lymphocytes in children with Periodic Fever, Aphtous Stomatitis, Pharyngitis and Adenitis (PFAPA) syndrome. Mol. Immunol. 65, 139–147 (2015). Erratum in: Mol. Immunol.66, 428 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Borra R. C., et al. , The Th1/Th2 immune-type response of the recurrent aphthous ulceration analyzed by cDNA microarray. J. Oral Pathol. Med. 33, 140–146 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Shimizu J., et al. , Excessive CD4+ T cells co-expressing interleukin-17 and interferon-γ in patients with Behçet’s disease. Clin. Exp. Immunol. 168, 68–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozyurt K., et al. , Serum Th1, Th2 and Th17 cytokine profiles and alpha-enolase levels in recurrent aphthous stomatitis. J. Oral Pathol. Med. 43, 691–695 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Lewkowicz N., Lewkowicz P., Banasik M., Kurnatowska A., Tchórzewski H., Predominance of Type 1 cytokines and decreased number of CD4(+)CD25(+high) T regulatory cells in peripheral blood of patients with recurrent aphthous ulcerations. Immunol. Lett. 99, 57–62 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Geri G., et al. , Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J. Allergy Clin. Immunol. 128, 655–664 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Buño I. J., Huff J. C., Weston W. L., Cook D. T., Brice S. L., Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch. Dermatol. 134, 827–831 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Hatemi G., et al. , Apremilast for Behçet’s syndrome—A phase 2, placebo-controlled study. N. Engl. J. Med. 372, 1510–1518 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Hatemi G., et al. , Trial of apremilast for oral ulcers in Behçet’s syndrome. N. Engl. J. Med. 381, 1918–1928 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Kolios A. G. A., et al. , Apremilast in treatment-refractory recurrent aphthous stomatitis. N. Engl. J. Med. 381, 1975–1977 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Schafer P. H., et al. , Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell. Signal. 26, 2016–2029 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Schafer P. H., et al. , Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br. J. Pharmacol. 159, 842–855 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The candidate SNP genotyping data for discovery and replication cohorts are in Dataset S1. Genotyping data for the classical HLA alleles for European–American and Turkish cases and controls are in Dataset S2; data are coded according to the dominant model (1 if allele is present and 0 if allele is not present).