Summary
PhenolaTi is an advanced non-toxic anticancer chemotherapy; this inert bis(phenolato)bis(alkoxo) Ti(IV) complex demonstrates the intriguing combination of high and wide efficacy with no detected toxicity in animals. Here we unravel the cellular pathways involved in its mechanism of action by a first genome study on Ti(IV)-treated cells, using an attuned RNA sequencing-based available technology. First, phenolaTi induced apoptosis and cell-cycle arrest at the G2/M phase in MCF7 cells. Second, the transcriptome of the treated cells was analyzed, identifying alterations in pathways relating to protein translation, DNA damage, and mitochondrial eruption. Unlike for common metallodrugs, electrophoresis assay showed no inhibition of DNA polymerase activity. Reduced in vitro cytotoxicity with added endoplasmic reticulum (ER) stress inhibitor supported the ER as a putative cellular target. Altogether, this paper reveals a distinct ER-related mechanism by the Ti(IV) anticancer coordination complex, paving the way for wider applicability of related techniques in mechanistic analyses of metallodrugs.
Subject Areas: Organometallic Chemistry, Biochemistry, Genomics, Cancer
Graphical Abstract
Highlights
-
•
First in-depth mechanistic analysis of a non-toxic Ti-based anticancer metallodrug
-
•
A comprehensive RNA-seq analysis to map the transcriptomic netowrk initiated by phenolaTi.
-
•
Unraveling a distinct mechanism through ER stress, not through direct DNA binding
-
•
Toward understanding and applicability of diverse better-tolerable chemotherapies
Organometallic Chemistry; Biochemistry; Genomics; Cancer
Introduction
Chemotherapeutic drugs are essential in the treatment of a variety of cancers with mechanisms ranging from DNA alkylation to antimetabolites. Specifically, metallodrugs can serve as effective anti-neoplastic agents, as first discovered with the pioneering Pt-based drug cisplatin (CDDP). Nearly 50% of patients with cancer worldwide receive platinum-based drugs to cope with testicular, ovarian, head and neck, and other cancer types (Brabec et al., 2017; Komeda and Casini, 2012; Mjos and Orvig, 2014; Riddell and Lippard, 2018). The mode of action of cisplatin was widely explored and is presumed to involve interaction of the Pt center with the N atoms of the purine bases of adjacent DNA nucleotides, eventually leading to apoptosis (Riddell and Lippard, 2018). A limiting drawback of cisplatin and its derivatives, similarly to other chemotherapies, is the severe side effects accompanying their administration, resulting from toxicity to, among other things, the kidneys, liver, and brain. Other metals studied as alternatives include essential (Fe, Cu, Ni, Zn, etc.) and non-essential (Pt, Ru, Ti, etc.) elements, with Ti being the first non-platinum metal reaching clinical trials as an appealing candidate for anticancer chemotherapy.
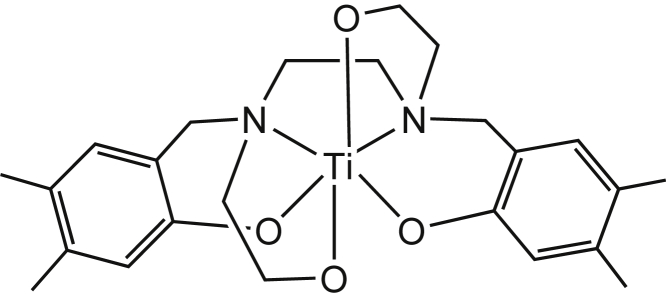
The first generation of anticancer Ti(IV) compounds with diketonato- and cyclopentadenyl-based ligands showed activity both in vitro and in vivo, with reduced toxicity and side effects (Köpf and Köpf-Maier, 1979). Nevertheless, their utility in the clinic was hampered by rapid decomposition in biological environments (Caruso and Rossi, 2004; Caruso et al., 2001; Christodoulou et al., 1998; Cini et al., 2017; Ellahioui et al., 2017; Keppler et al., 1991; Koepf-Maier and Koepf, 1987; Loza-Rosas et al., 2017; Manohari Abeysinghe and Harding, 2007; Meléndez, 2002; Ott and Gust, 2007; Peri et al., 2009; Toney and Marks, 1985; Tshuva and Ashenhurst, 2009; Tshuva and Miller, 2018), as occurs often with coordination complexes of the labile and oxophilic Ti(IV) metal. Insoluble and undefined O-bridged aggregates of different sizes and nuclearities are formed instantaneously upon interaction with water, leading to uncontrolled solution chemistry, difficult to monitor and analyze. Therefore, despite some mechanistic clues gained throughout the years, the cellular mode of action of these compounds is yet unknown (Cini et al., 2017). Still, a major benefit in using Ti(IV)-based coordination compounds is the biofriendly nature of the metal: its lability, although responsible for the rapid hydrolysis in biological environments, turns into an advantage with the ultimate hydrolysis product being the safe titanium dioxide, often found in a variety of daily products (e.g., sunscreen, food coloring, drugs). Thus, advanced Ti(IV) coordination compounds with enhanced hydrolytic stability were developed to prolong the anticancer activity window before titanium dioxide is formed and is safely excreted. Specifically, the phenolato-based Ti(IV) complexes that we introduced showed a wide range of in vitro cytotoxic effects with no signs of in vivo toxicity; importantly, their high hydrolytic stability facilitates investigation of their molecular mechanisms of action (Barroso et al., 2015; Glasner and Tshuva, 2011, 2014; Immel et al., 2010, 2011, 2012; Manna et al., 2012; Meker et al., 2012, 2014, 2015; Miller et al., 2016; Peri et al., 2011a, 2011b; Shavit et al., 2007; Tinoco et al., 2012; Tshuva and Tzubery, 2017; Tzubery and Tshuva, 2012). In particular, we recently showed that a bis(phenolato)bis(alkoxo)Ti(IV) compound (phenolaTi, Scheme 1), conveniently synthesized from available starting materials, demonstrates high activity toward all cancer cell lines in the NCI-60 panel of the NIH with an average growth inhibition value (GI 50) of 4.6 ± 2 μM (slightly better activity than cisplatin: 5.6 μM; Figure S1) (Meker et al., 2016). No correlations in the cytotoxicity pattern to known drugs in the NIH database, as deduced from COMPARE analysis, implies a distinct mechanism of action. PhenolaTi also demonstrates the following: (1) high cytotoxicity toward cisplatin-resistant and multi-drug-resistant cell lines (Ganot and Tshuva, 2018; Meker et al., 2016); (2) in vivo efficacy with no indication of toxicity (Ganot et al., 2018); (3) high water stability for weeks in biological medium (Meker et al., 2016); (4) cellular accumulation and induction of apoptosis within 24–48 h following administration to human colon HT-29 cancer cells (Meker et al., 2016; Miller et al., 2016).
Scheme 1.
PhenolaTi
Herein we aimed to elucidate the mechanism of action of phenolaTi, as a lead anticancer complex, representative of Ti(IV)-based metallodrugs. To that effect, we applied RNA sequencing (RNA-seq) using CEL-Seq2 methodology (Hashimshony et al., 2016) on cell populations at different time points (up to 72 h) following exposure to the drug. Despite the significance of RNA-seq as a research tool, to our knowledge, only a single work used RNA-seq and a few more used microarray analyses to study the effect of metallodrugs (Bergamo et al., 2015; Grozav et al., 2015; Jovanović et al., 2016; Velma et al., 2016), whereby no studies were reported on Ti(IV) cytotoxicity pathways. Genes related to cell-cycle checkpoints, protein translation, and the endoplasmic reticulum (ER) pathway were significantly altered, thus introducing the first indication of ER involvement in the distinct action of anticancer Ti(IV) phenolato compounds.
Results
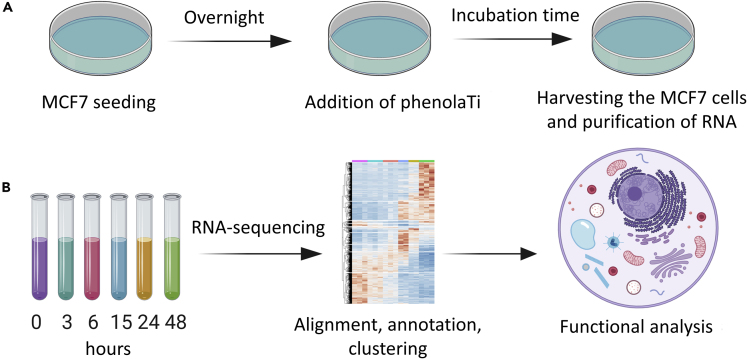
To perform in-depth mechanistic analysis of phenolaTi, we aimed to map its dose- and time-dependent effects on the MCF7 human breast adenocarcinoma cell line and analyze the cellular behavior (Figure 1). Breast cancer is the most frequent malignancy in females; therefore, MCF7 cells are widely employed as an in vitro model in cancer research (Comşa et al., 2015) and were thus selected for this study. PhenolaTi was synthesized as previously described, from Ti(OiPr)4 and the ligand precursor; the latter had been synthesized by a single-step condensation reaction from available starting materials (Meker et al., 2016). Preliminary cytotoxicity studies showed no evident toxicity for the first 6 h of exposure and maximal effect was detected at 72 h (Figure S2). Therefore, we incubated the cells with phenolaTi (54 μM; selected according to the cytotoxicity curves; Figure S2) and analyzed cell-cycle effects, cytotoxicity, apoptosis, and expression profiles, untreated (time 0) and after 3, 6, 15, 24, and 48 h of exposure.
Figure 1.
General Experimental Procedure
(A) Experimental in vitro workflow; MCF7 cells were seeded overnight, phenolaTi was added at 54 μM at different time points, harvesting was generated at the same time point to obtain 3, 6, 15, 24, and 48 h of incubation (with untreated control samples, 0). (B) Generated samples were sequenced, aligned, annotated, clustered, and functionally analyzed. MCF7 cells undergo changes in response to phenolaTi treatment.
PhenolaTi Induces Apoptosis and Cell-Cycle Arrest
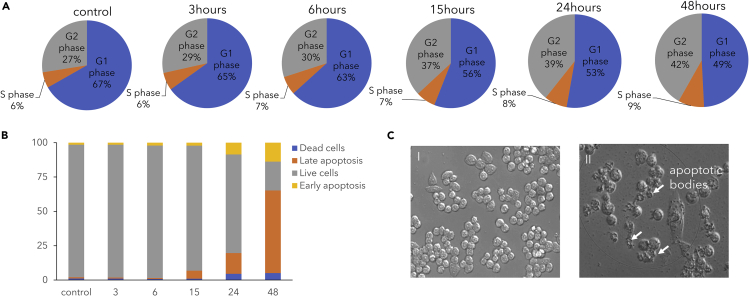
The effect of phenolaTi on MCF7 cells was first measured using flow cytometry to determine cell kinetics, proliferation, and apoptosis. Cells accumulated at the G2/M phase (from 27% to 42%; whereby some accumulation also at S phase can't be ruled out) with major reduction in the percentage of cells in the G1 phase (from 67% to 49%) (Figures 2A and S3A), suggesting inhibition of cell-cycle checkpoints. Additionally, apoptosis induction was evident: (1) double staining with Annexin V-FITC and propidium iodide (PI) revealed an increase in the percentage of late and early apoptotic cells within 24 h (Figures 2B and S3B); (2) characteristic morphological changes indicative of regulated cell death were detected (Figure 2C). These observations overall point to regulated, programmed cellular death executed by the cellular machinery in response to treatment with phenolaTi (Manna et al., 2012; Meker et al., 2016; Miller and Tshuva, 2018; Miller et al., 2016).
Figure 2.
MCF7 Cells undergo Changes in Response to PhenolaTi Treatment
(A) Cell-cycle distribution following incubation with phenolaTi at 54 μM at different time points. (B) Time-dependent effect of phenolaTi at 54 μM on apoptosis in MCF7 cancer cells, as recorded using flow cytometry. (C) Microscopic images of MCF7 cells (I) untreated cells, control (II) treated with phenolaTi at 54 μM for 36 h of incubation. Gene expression alteration in response to phenolaTi in MCF7 cells.
Cellular Pathways Identified by Transcriptomic Characterization
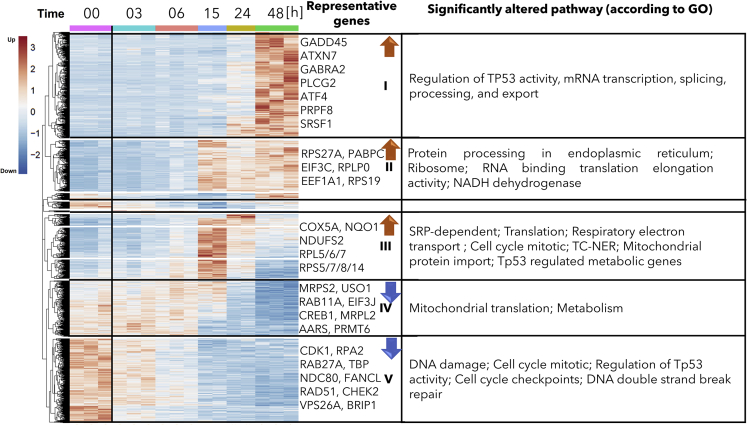
After establishing the effect of phenolaTi on cell viability and proliferation, we aimed to offer a plausible mechanism of action by mapping transcriptomic changes following phenolaTi treatment, namely, changes in transcribed mRNA molecules. To that effect, MCF7 cells were treated with phenolaTi (54 μM) and incubated for 0 (untreated), 3, 6, 15, 24, and 48 h in triplicates. Then, the mRNA was extracted and sequenced. Both upregulation and downregulation compared with time zero (untreated cells) were considered for analysis, as shown in the heatmap. The 5,000 most variably expressed genes were further analyzed. These genes were divided into five main clusters, applying hierarchical clustering methodology to group similarly behaving genes (Figure 3).
Figure 3.
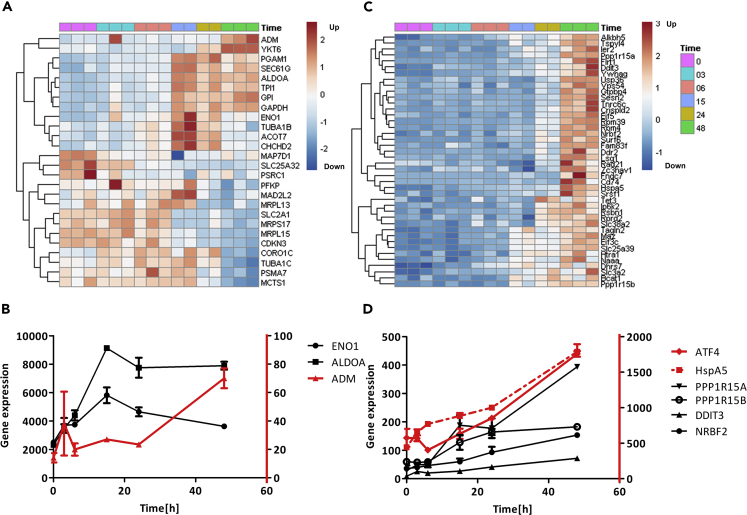
Gene Expression (RPM, Reads per Million) Alteration in Response to PhenolaTi in MCF7 Cells at 54 μM
MCF7 were treated with 54 μM phenolaTi and sequenced in triplicates or duplicates after 0 (untreated), 3, 6, 15, 24, and 48 h. Expression analysis included CEL-seq2, Z-scoring, and hierarchical clustering. Roughly, the genes can be divided into five main clusters with distinct expression and significant biological functions. The biological functions of the genes were analyzed using GeneAnalytics, an integrative gene set analysis tool (Ben-Ari Fuchs et al., 2016). Blue, increased gene expression; red, decreased gene expression. Changes in expression of ER/hypoxia-related genes in response to phenolaTi in MCF7 cells.
The five clusters, according to their pattern of expression, are the following (Figure 3) (large to small): (1) cluster (I) of 1,426 genes that were upregulated at 48 h; (2) cluster (V) of 1,284 genes that were downregulated within 3 h; (3) cluster (II) of 684 genes that were upregulated at 15 h; (4) cluster (III) of 406 genes that were locally upregulated at 15 h; (5) cluster (IV) of 251 genes that were downregulated at 15 h. GeneAnalytics (Ben-Ari Fuchs et al., 2016) was employed to annotate the genes in each cluster and associate it with the biological function. Figure 3 presents the most relevant results; full data are provided in the Supplemental Information (Table S1).
The first event following exposure to phenolaTi was downregulation within 3 h and then low expression across all time points (Figure 3 cluster (V)). The genes in this cluster are significantly associated with DNA repair (p value < 3.79 × 10−8) and cell cycle (p value < 2.07 × 10−10) (e.g., CHEK2, RAD51, BRIP1, and FANCL). The second transcriptional event was downregulation of mitochondrial translation genes after a 15-h incubation period (Figure 3 cluster (IV)). Simultaneously, temporary upregulation was observed of genes related to different mitochondrial processes (e.g., respiration, electron transport, ATP synthesis), as well as those related to ribosome subunits and other proteins required for translation (Figure 3 cluster (III)). From the 15th h onward upregulation of multiple translational processes included genes associated with ER and the ribosome, which were constantly high (Figure 3 cluster (II)). These processes include cytoplasmic translation, ribosomal small/large subunit assembly and biogenesis and translational elongation. Lastly, at 48 h time, mRNA transcription, transport, and splicing were also upregulated (Figure 3 cluster (I)). Among the most significantly altered genes during the course of the measurement were ATXN7 (p value 6.4 × 10−14), GABRA2 (p value 2.4 × 10−11), and PLCG2 (p value 7.3 × 10−12), which relate to ER processes (Dai et al., 2009; Kurosaki et al., 2000; Nagy et al., 2009).
The transcriptomic analysis correlated with the cell cycle and apoptosis experiments (Figures 2 and 3). As supported by flow cytometry, genes related to cell cycle were affected (Figure 3 cluster (III)/(V)), such as cyclin dependent kinase 1 (CDK1), responsible for progression of cells into the M phase (DiPaola, 2002; Khazaei et al., 2017; Wang et al., 2016), which is downregulated within 3 h (Figure 3 cluster (V)). Moreover, pro-apoptotic genes, such as Growth Arrest and DNA Damage 45 (GADD45) and Activating Transcription Factor 4 (ATF4) were upregulated within 48 h (Figure 3 cluster (I)), in agreement with the observed changes (Figures 2B and 2C). Importantly, ATF4 is a key player in the cellular response to hypoxia evolved in ER stress (Rzymski et al., 2009).
Overall, phenolaTi treatment upregulated processes related to the translation of proteins in the ER and ribosomal biogenesis (Figure 3 cluster (II)/(III)). A variety of ribosomal genes (RPL and RPS) were upregulated within 15 h, among which some were consequently downregulated, and others were continuously highly expressed at 24/48 h. Moreover, significant upregulation of EIF3C gene (p value 6.7 × 10−7) was observed, a key player in translation initiation within cells (Wagner et al., 2014); together with high expression of ATF4, this observation supports involvement of ribosome and ER in the cellular response to phenolaTi.
Short-Term Effect (3–15 h) of PhenolaTi Supports Hypoxia and ER Stress
The analysis points to major changes in gene expression, mainly observed 15 h or more following treatment. To focus on the short-term effect of the drug, gene expression in the first 15 h following exposure (3, 6, and 15 h) was specifically analyzed relative to control (0 h), looking at genes most variable at short time points (Figure S4). The most significantly altered gene already within 3 h of exposure was SLC30A1 (p value 4.7 × 10−35), associated with cation transmembrane activity; interestingly, this gene is involved in zinc efflux through the ER membrane (Barresi et al., 2018). Additional altered genes within 6 h of exposure include EGLN2 (p value 4.66 × 10−6), involved in oxygen sensing related to hypoxia tolerance; PNRC2 (p value 5.6 × 10−24), associated with energy balance/storage; and TMEM177 (p value 2.2 × 10−22), a mitochondrial respiratory chain complex assembly factor. This gene signature further supports the effect on mitochondria previously observed for related Ti phenolato compounds, accumulated in the mitochondria organelle (Schur et al., 2013). Inspecting the genes altered within 15 h of treatment, the downregulated genes relate to cell-cycle checkpoints (e.g., CDC23) and DNA damage (e.g., BRCA1). Overall, examining the most significant genes over the entire short time course of 3/6/15 h, three genes are constantly upregulated: CYP1A1, SLC30A1, and PYGM (Figures S4D and S4E). The first two genes relate to cellular response to metal ions (Chen and Chan, 2016; Zogzas and Mukhopadhyay, 2018), whereby CYP1A1 is a member of cytochrome p450 superfamily enzymes, which are localized mainly in the inner membrane of the mitochondria or the ER and are associated with cancer susceptibility (Agúndez, 2004; Brignac-Huber et al., 2016; Kawajiri et al., 1993; Sharma et al., 2014). The alterations in CYP1A1 together with those of SLC30A1 again focused our attention specifically on the ER as a putative cellular target.
Analysis of Pathway-Related Genes: PhenolaTi Causes Changes in Hypoxia and Endoplasmic Reticulum-Related Genes
Known metallodrugs, particularly cisplatin and its Pt-based derivatives, operate by direct DNA binding (Meier-Menches et al., 2018; Riddell and Lippard, 2018). For any similarly operating metallodrug, upregulation of genes related to DNA damage would be expected, such as the homologous recombination repair (HRR)-related genes. In contrast, our data show downregulation of DNA damage-related genes (Figure 3 cluster (V)). This indication implies that direct DNA damage is not induced by phenolaTi. Nonetheless, previous studies showed connection between downregulation of HRR genes and hypoxia (Bindra et al., 2004, 2005; Chan et al., 2008; Meng et al., 2005), which in turn can correlate with ER stress.
Owing to the aforementioned results, we evaluated specifically the possible induction of hypoxic mimetic conditions by phenolaTi. Interestingly, from a set of 51 genes previously reported to be strongly related to hypoxia (Buffa et al., 2010), 26 genes were significantly altered herein (p value < 0.0005 based on hypergeometric distribution) (Figure 4A). Of these 26 genes, 12 were strongly upregulated within 15 h and the rest were downregulated. The downregulated genes are annotated to mitochondrial translation processes, whereas the upregulated genes relate to the glucose metabolism pathway or hypoxia inducing factor 1 (HIF1) signaling pathway (Figure 4B), further supporting phenolaTi-induced hypoxia. The mechanism by which phenolaTi mimics hypoxia induction remains to be elucidated; but it may also relate to the altered expression of mitochondrial genes and reduction in oxygen levels (Figure 3 clusters (III)/(IV)).
Figure 4.
Changes in Expression of ER/Hypoxia-Related Genes in Response to PhenolaTi in MCF7 Cells at 54 μM
(A) A 26-gene cluster of the total 51 genes previously reported to be overexpressed in hypoxia (Buffa et al., 2010); these were significantly altered over time in our experiment (p value < 0.05). (B) Expression of genes ENO1, ALDOA, and ADM, relating to hypoxic conditions. (C) A 52-gene cluster of the total 575 genes previously reported to be related to ER stress (Han et al., 2013); these were upregulated over time in our experiment (p value < 0.05). (D) Expression of genes ATF4, HspA5, PPP1R15A/B, DDIT3. and NRBF2, relating to ER stress. In vitro validation assays support ER-related mechanism of phenolaTi with no direct DNA binding.
As mentioned above, a variety of genes related to ribosome biogenesis (RPL/RPS/CYP1A1) and translational process were significantly changed (Figure 3 cluster (II)/(III)), pointing to ribosome translation processes and the ER as vital participants in the mechanism of phenolaTi. Therefore, 575 genes previously reported to be related to ER stress (Han et al., 2013) were closely examined. A total of 232 genes were significantly altered (p value < 0.05), 95 were clustered (p value < 0.001) (Figure S11), whereby 52 of the most variable genes were upregulated within 48 h (Figure 4C), and the rest were downregulated within the first 3 h. Specifically, upregulation within 48 h occurred for HSPA5, DDIT3, PPP1R15A, and PPP1R15B, namely, of proteins that are usually localized in the ER and elevate in response to ER stress (Figure 4D). Interestingly, Ppp1r15a/b are phosphatase regulators that promote dephosphorylation of eIF2α enabling control of translation processes during cellular stress. Also, it has been reported that, among the 575 genes previously analyzed as ER-related, the majority (472) correlated with expression of ATF4 (Han et al., 2013). Therefore, further analysis was employed herein as well, using HOMER search motif (Heinz et al., 2010), to independently look for a possible common transcription factor (TF) for each cluster (Figure 3). Interestingly, ATF4 was identified here as well as a TF regulating the transcription of the genes upregulated within 48 h (Figure 3 cluster (I)) with a p value of 1 × 10−12. This observation joins the overall data in pointing to involvement of ER in the cellular mechanism of phenolaTi.
ER Stress Is Putative Mechanism of Action of PhenolaTi, rather Than Direct DNA Interaction
Owing to the overall negative charge of the DNA, it is often suspected as the primary direct target of metallodrugs, as occurs for platinum compounds (Riddell and Lippard, 2018). Our transcriptomics demonstrates the opposite. Thus, to directly evaluate possible interactions between DNA and phenolaTi, the effect on DNA polymerase activity was measured using polymerase chain reaction (PCR). The reaction was carried out in the presence of phenolaTi or other drugs with known DNA-related or -unrelated mechanisms. The interaction of cisplatin and doxorubicin with DNA fragments is well established, and therefore, these drugs served as positive controls (Jamieson and Lippard, 1999; Wang and Lippard, 2005; Yang and Wang, 1999). In contrast, 5-fluorouracil is a thymidylate synthase inhibitor (Rustum et al., 1997) and hence served as a negative control. DNA was isolated from MCF7 cancer cells at a concentration of 0.4 ng/μL, amplifying the fragments of the gene that codes for actin. The tested compounds at concentrations of 27 and 54 μM were separately added to PCR tubes for evaluation of possible interaction with DNA, by inspecting inhibition of the DNA polymerase activity, as manifested by inhibition of gene amplification. The results are depicted in Figure 5A. As expected, the actin bands were absent for the drugs operating on DNA: cisplatin and doxorubicin, indicative of inhibition to the DNA polymerase activity due to DNA-drug interaction. The well-detected bands for 5-fluorouracil and phenolaTi imply that, as for fluorouracil, DNA may not be the major target of Ti(IV) phenolato compounds in their cytotoxic effect, supporting the transcriptomics data.
Figure 5.
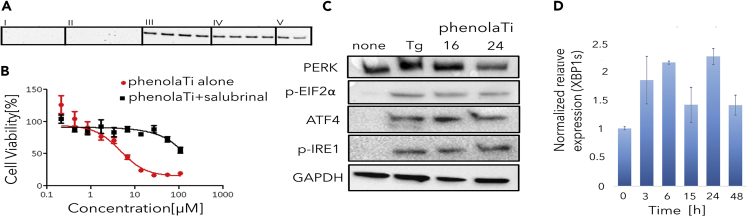
In Vitro Validation Assays Support ER-Related Mechanism of PhenolaTi with No Direct DNA Binding
(A) Agarose gel electrophoresis of the PCR products for detection of actin after co-incubation with (I) cisplatin, (II) doxorubicin, (III) 5-fluorouracil, (IV) phenolaTi, (V) control, at 27 (left 2 bands) or 54 (right 2 bands) μM of each tested compound in duplicates (showing one of three repeats); phenolaTi, as 5-fluorouracil, does not interact directly with DNA.
(B–D) (B) Cytotoxicity curves of phenolaTi toward human MCF7 cancer cells, with and without the addition of salubrinal, using the MTT assay following 72 h of incubation; activity of phenolaTi is abolished with the ER-stress inhibitor. (C) Expression of PERK, p-EIF2α, ATF4, and p-IRE1 levels (evaluated using immunoblotting) in MCF7 cells following incubation with phenolaTi; positive control Thapsigargin (Tg, a known ER Ca2+ ATPase inhibitor; 4 nM for 16 h); GAPDH as a loading control (inactivated PERK is observed at a lower molecular weight at time point 0, whereas activated PERK is observed upon treatment). (D) qPCR levels of XBP1s form over time in MCF7 cells exposed to phenolaTi at 54 μM concentration.
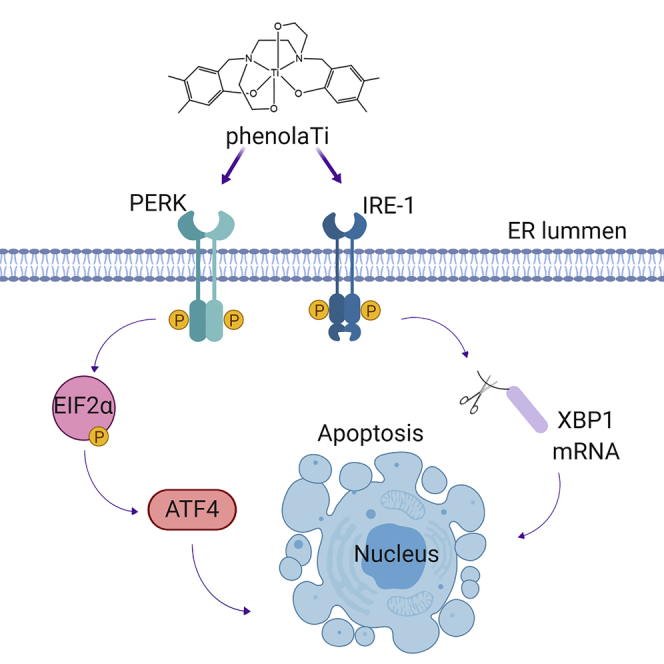
The gene expression analysis pointed to ER as a target involved in the mechanism of action of phenolaTi. ER stress is characterized by disruption of ER homeostasis, which is responsible mainly for production and folding of cellular proteins, storage and regulation of calcium, and glucose metabolism. In response to ER stress, the cell activates a signaling pathway called the unfolded protein response (UPR), which aims to help the cell cope with the induced stress. There are three major sensors (proteins) controlling the UPR: inositol requiring enzyme 1 (IRE1), protein kinase RNA-activated (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6). IRE1 activation induces X-box binding protein 1 (XBP1) splicing. The downstream proteins of PERK are eukaryotic initiation factor 2 (EIF2) and activating transcription factor 4 (ATF4) proteins.
To further analyze the effect of phenolaTi on ER stress as suggested by the transcriptomics, the cytotoxicity of phenolaTi was tested on MCF7 breast adenocarcinoma cells, with and without 70 μM of salubrinal, a known ER-stress inhibitor (Boyce et al., 2005; Suntharalingam et al., 2013, 2014) that inhibits eIF2α dephosphorylatoin, using the MTT assay (Ganot et al., 2013). The results are presented in Figure 5B.
The activity of phenolaTi was abolished in the presence of salubrinal. The protective effect of salubrinal on cell viability suggests that ER stress has a role in inducing cell death. We then aimed to characterize the alterations in the different components of the phenolaTi-induced ER-stress response pathway. As mentioned above, there are three main ER-stress regulating proteins: IRE1, PERK, and ATF6. Their activation has both pro-apoptotic and pro-survival effects, depending on stress duration and intensity. At ∼16 and 24 h post phenolaTi treatment, activated IRE1 and XBP1s expression was observed (Figures 5C and 5D). Additionally, activated PERK leads to phosphorylation of the protein Eif2α and, in turn, to ATF4 translation. Again, activated PERK and Eif2α phosphorylation were detected along with ATF4 expression at the same time points (Figure 5C). These results strongly support the hypothesis pointing to ER as at least one of the main molecular targets associated with the apoptotic cell death induced by phenolaTi.
Discussion
In this study, we present the first in-depth mechanistic analysis of a Ti(IV)-based anticancer drug. The scarce genomic analyses reported previously for Pt- and Ru-based metallodrugs involved microarray or conventional RNA-seq methodologies (Bergamo et al., 2015; Grozav et al., 2015; Jovanović et al., 2016; Velma et al., 2016). Herein, the advanced CEL-Seq2 methodology (Hashimshony et al., 2016), which has optimized primers, reagents, clean-up, and library preparation steps, was employed on cell populations rather than applied as a single-cell sequencing technique, enabling conveniently gaining new insights on the pathways involved within the mode of action of the Ti(IV) drug.
A variety of essential signaling pathways were significantly changed over the tested time course. Cell cycle was erupted at G2/M phase, and apoptosis initiation via a mitochondrial pathway is proposed based on microscopically detected changes, buildup of apoptotic cells, and alterations in mitochondria-related genes, all in agreement with some reports in the literature on mechanistic aspects of Ti-based metallodrugs (Manna et al., 2012; Meker et al., 2016; Miller et al., 2016). All of the above support a well-programmed cell death initiated by phenolaTi.
Inspecting correlations to the genomic analyses of other anticancer metallodrugs (Bergamo et al., 2015; Grozav et al., 2015; Jovanović et al., 2016; Velma et al., 2016), it is evident that, despite some similarities relating to apoptosis induction, cell-cycle arrest, and p53-based mediated pathways, phenolaTi operates distinctively as implied previously by the NCI-60 reactivity pattern (Meker et al., 2016). The ancestor drug cisplatin operates directly on DNA (Riddell and Lippard, 2018; Wang and Lippard, 2005), whereas the downregulation of DNA double-strand break pathway within the first hours of exposure to phenolaTi suggests fundamentally different cellular behaviors. The lack of interference with DNA polymerase activity also supports an indirect interaction between phenolaTi and DNA under the tested conditions. Interestingly, downregulation of DNA repair genes is associated with hypoxic conditions in cells (Bindra et al., 2004, 2005; Chan et al., 2008; Meng et al., 2005), which was corroborated through direct analysis of hypoxia-related genes (Buffa et al., 2010). Also, when comparing the results with those previously reported for Ru-based compounds, phenolaTi does not activate similar metastasis-related TFs (Bergamo et al., 2015), thus further confirming its distinct cellular impact.
The main pathways that stand out as particularly influenced by phenolaTi are those related to the ER, as proposed previously for osmium-based anticancer drugs (Boyce et al., 2005; Suntharalingam et al., 2013, 2014). A variety of proteins and metabolic processes were altered, ATF4 was identified in one cluster (upregulation at 48 h), and genes associated with ER function based on previous work were upregulated following phenolaTi treatment. Notably, in vitro cytotoxicity toward MCF7 cells was abolished upon addition of salubrinal, a known ER-stress inhibitor (Boyce et al., 2005), which further supports ER involvement in the mechanism of action of phenolaTi. Upregulation of both IRE1 and PERK pathways further validate ER-stress activation. Interestingly, previous cell imaging studies with fluorescent salen-type Ti(IV) complexes have suggested possible accumulation of the fluorescent species near the ER region in the cell (Tzubery et al., 2018). Since the ER membrane mainly comprises phospholipids (Brignac-Huber et al., 2016), direct interaction of the oxophilic Ti(IV) metal with the ER to give strong Ti(IV)-phosphate bonds is plausible, although such interaction would not be ER specific.
To conclude, unlike cisplatin and related known drugs, ER stress and hypoxia appear to govern Ti(IV)-based cytotoxic reactivity, conditions that could be related (Corazzari et al., 2017; Pereira et al., 2014). Nevertheless, additional pathways activated in parallel or on different lines/by different derivatives cannot be ruled out. The specific interactions between the drug and its direct target are yet to be elucidated, as well as the source for cancer selectivity as manifested by the unique combination of wide activity and no toxic effects for phenolaTi (Ganot et al., 2018). ATF4 was previously reported to contribute to tumor progression (Fels and Koumenis, 2006), as well as malfunction or overexpression of phosphorylation process (Ardito et al., 2017), all of which may serve a plausible explanation for cancer selectivity observed in vivo for phenolaTi. Overall, being a highly promising new-generation anticancer chemotherapeutic drug, the mechanistic insights provided herein for phenolaTi promote the understanding and advancing of modern non-toxic chemotherapy. Lastly, the wide applicability and availability of the RNA-seq methodology applied on cell populations as described herein should progress more rapid, economical, and in-depth mechanistic analyses of various metallodrugs operating by various mechanisms, for a marked leap in cancer research and better accessibility of various tolerable chemotherapies.
Limitations of the Study
The study was performed on breast adenocarcinoma MCF7 cell line and therefore is limited by the mutational status of the current tested cells.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contacts, Edit Y. Tshuva (edit.tshuva@mail.huji.ac.il) and Yuval Tabach (yuvaltab@ekmd.huji.ac.il).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The raw and the processed data files of the current study can be accessed through Gene Expression Omnibus (GEO) with the GSE148239 accession number.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Mohamed Mahameed for his fruitful discussion regarding the endoplasmic reticulum response. Funding was received from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Program (grant agreement 681243), The U.S.- Israel Binational Science Foundation (BSF) (grant agreement 0375055), and the Israel Science Foundation (ISF) (grant agreement 1591/19), Melanoma Research Association (grant agreement 402792).
Author Contributions
Conceptualization, M.M., E.Y.T., and Y.T.; Investigation, M.M., A.M., M.B., E.C., Z.S., E.Y.T., and Y.T.; Data curation and software, E.C. and M.M.; Writing - Original Draft, M.M. and E.Y.T.; Writing - Review and Editing, M.M., D.S.-R., I.U., O.B., J.H., E.Y.T., and Y.T.; Funding acquisition, E.Y.T.; Resources, Y.T. and E.Y.T.
Declaration of Interests
The authors declare no competing interests. Relating patent: Tshuva EY and Hochman J; Cytotoxic titanium and vanadium complexes PCT/IL2013/05,069 filled 15/08/2013.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101262.
Contributor Information
Edit Y. Tshuva, Email: edit.tshuva@mail.huji.ac.il.
Yuval Tabach, Email: yuvaltab@ekmd.huji.ac.il.
Supplemental Information
References
- Agúndez J.A.G. Cytochrome P450 gene polymorphism and cancer. Curr. Drug Metab. 2004;5:211–224. doi: 10.2174/1389200043335621. [DOI] [PubMed] [Google Scholar]
- Ardito F., Giuliani M., Perrone D., Troiano G., Lo Muzio L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int. J. Mol. Med. 2017;40:271–280. doi: 10.3892/ijmm.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi V., Valenti G., Spampinato G., Musso N., Castorina S., Rizzarelli E., Condorelli D.F. Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J. Cell Biochem. 2018;119:9707–9719. doi: 10.1002/jcb.27285. [DOI] [PubMed] [Google Scholar]
- Barroso S., Coelho A.M., Gomez-Ruiz S., Calhorda M.J., Zizak Z., Kaluderovic G.N., Martins A.M. Correction: synthesis, cytotoxic and hydrolytic studies of titanium complexes anchored by a tripodal diamine bis(phenolate) ligand. Dalt. Trans. 2015;44:2497. doi: 10.1039/c4dt90194k. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Fuchs S., Lieder I., Stelzer G., Mazor Y., Buzhor E., Kaplan S., Bogoch Y., Plaschkes I., Shitrit A., Rappaport N. GeneAnalytics: an integrative gene set analysis tool for next generation sequencing, RNA-seq and microarray data. Omi. A J. Integr. Biol. 2016;20:139–151. doi: 10.1089/omi.2015.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamo A., Gerdol M., Lucafo M., Pelillo C., Battaglia M., Pallavicini A., Sava G. RNA-seq analysis of the whole transcriptome of MDA-MB-231 mammary carcinoma cells exposed to the antimetastatic drug NAMI-A. Metallomics. 2015;7:1439–1450. doi: 10.1039/c5mt00081e. [DOI] [PubMed] [Google Scholar]
- Bindra R.S., Schaffer P.J., Meng A., Woo J., Måseide K., Roth M.E., Lizardi P., Hedley D.W., Bristow R.G., Glazer P.M. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol. Cell. Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra R.S., Gibson S.L., Meng A., Westermark U., Jasin M., Pierce A.J., Bristow R.G., Classon M.K., Glazer P.M. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- Boyce M., Bryant K.F., Jousse C., Long K., Harding H.P., Scheuner D., Kaufman R.J., Ma D., Coen D.M., Ron D. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Brabec V., Hrabina O., Kasparkova J. Cytotoxic platinum coordination compounds. DNA binding agents. Coord. Chem. Rev. 2017;351:2–31. [Google Scholar]
- Brignac-Huber L.M., Park J.W., Reed J.R., Backes W.L. Cytochrome P450 organization and function are modulated by endoplasmic reticulum phospholipid heterogeneity. Drug Metab. Dispos. 2016;44:1859–1866. doi: 10.1124/dmd.115.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa F.M., Harris A.L., West C.M., Miller C.J. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br. J. Cancer. 2010;102:428. doi: 10.1038/sj.bjc.6605450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso F., Rossi M. Antitumor titanium compounds and related metallocenes. Met. Ions Biol. Syst. 2004;42:353–384. [PubMed] [Google Scholar]
- Caruso F., Rossi M., Pettinari C. Anticancer titanium agents. Expert Opin. Ther. Patents. 2001;11:969–979. [Google Scholar]
- Chan N., Koritzinsky M., Zhao H., Bindra R., Glazer P.M., Powell S., Belmaaza A., Wouters B., Bristow R.G. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008;68:605–614. doi: 10.1158/0008-5472.CAN-07-5472. [DOI] [PubMed] [Google Scholar]
- Chen Y.Y., Chan K.M. Differential effects of metal ions on TCDD-induced cytotoxicity and cytochrome P4501A1 gene expression in a zebrafish liver (ZFL) cell-line. Metallomics. 2016;8:236–251. doi: 10.1039/c5mt00219b. [DOI] [PubMed] [Google Scholar]
- Christodoulou C., Eliopoulos A., Young L., Hodgkins L., Ferry D., Kerr D. Antimproliferative activity and mechanism of action of titanocene dichloride. Br. Joumal Cancer. 1998;77:2088–2097. doi: 10.1038/bjc.1998.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cini M., Bradshaw T.D., Woodward S. Using titanium complexes to defeat cancer: the view from the shoulders of titans. Chem. Soc. Rev. 2017;46:1040–1051. doi: 10.1039/c6cs00860g. [DOI] [PubMed] [Google Scholar]
- Comşa Ş., Cimpean A.M., Raica M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 2015;35:3147–3154. [PubMed] [Google Scholar]
- Corazzari M., Gagliardi M., Fimia G.M., Piacentini M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front. Oncol. 2017;7:78. doi: 10.3389/fonc.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Wang X., Chen Y., Wang X., Zhu J., Lu L. Expression quantitative trait loci and genetic regulatory network analysis reveals that Gabra2 is involved in stress responses in the mouse. Stress. 2009;12:499–506. doi: 10.3109/10253890802666112. [DOI] [PubMed] [Google Scholar]
- DiPaola R.S. To arrest or not to G2-M Cell-cycle arrest. Clin. Cancer Res. 2002;8:3512–3519. [PubMed] [Google Scholar]
- Ellahioui Y., Prashar S., Gomez-Ruiz S. Anticancer applications and recent investigations of metallodrugs based on gallium, tin and titanium. Inorganics. 2017;5:4. [Google Scholar]
- Fels D.R., Koumenis C. The PERK/eIF2α/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol. Ther. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- Ganot N., Tshuva E.Y. In vitro combinations of inert phenolato Ti (iv) complexes with clinically employed anticancer chemotherapy: synergy with oxaliplatin on colon cells. RSC Adv. 2018;8:5822–5827. doi: 10.1039/c8ra00229k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganot N., Meker S., Reytman L., Tzubery A., Tshuva E.Y. Anticancer metal complexes: synthesis and cytotoxicity evaluation by the MTT assay. J. Vis. Exp. 2013;10:e50767. doi: 10.3791/50767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganot N., Briaitbard O., Gammal A., Tam J., Hochman J., Tshuva E.Y. In vivo anticancer activity of a nontoxic inert phenolato titanium complex: high efficacy on solid tumors alone and combined with platinum drugs. ChemMedChem. 2018;13:2290–2296. doi: 10.1002/cmdc.201800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner H., Tshuva E.Y. A marked synergistic effect in antitumor activity of salan titanium(IV) complexes bearing two differently substituted aromatic rings. J. Am. Chem. Soc. 2011;133:16812–16814. doi: 10.1021/ja208219f. [DOI] [PubMed] [Google Scholar]
- Glasner H., Tshuva E.Y. C-1-Symmetrical titanium(IV) complexes of salan ligands with differently substituted aromatic rings: enhanced cytotoxic activity. Inorg. Chem. 2014;53:3170–3176. doi: 10.1021/ic500001j. [DOI] [PubMed] [Google Scholar]
- Grozav A., Balacescu O., Balacescu L., Cheminel T., Berindan-Neagoe I., Therrien B. Synthesis, anticancer activity, and genome profiling of thiazolo arene ruthenium complexes. J. Med. Chem. 2015;58:8475–8490. doi: 10.1021/acs.jmedchem.5b00855. [DOI] [PubMed] [Google Scholar]
- Han J., Back S.H., Hur J., Lin Y.-H., Gildersleeve R., Shan J., Yuan C.L., Krokowski D., Wang S., Hatzoglou M. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013;15:481. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimshony T., Senderovich N., Avital G., Klochendler A., de Leeuw Y., Anavy L., Gennert D., Li S., Livak K.J., Rozenblatt-Rosen O. CEL-Seq2: sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 2016;17:77. doi: 10.1186/s13059-016-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S., Benner C., Spann N., Bertolino E., Lin Y.C., Laslo P., Cheng J.X., Murre C., Singh H., Glass C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immel T.a., Groth U., Huhn T. Cytotoxic titanium salan complexes: surprising interaction of salan and alkoxy ligands. Chem. A. Eur. J. 2010;16:2775–2789. doi: 10.1002/chem.200902312. [DOI] [PubMed] [Google Scholar]
- Immel T.A., Groth U., Huhn T., Öhlschläger P. Titanium salan complexes displays strong antitumor properties in Vitro and in Vivo in mice. PLoS One. 2011;6:e17869. doi: 10.1371/journal.pone.0017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immel T.A., Gruzke M., Spae A.-K., Groth U., Hlschlaer P., Huhn T. Synthesis and X-ray structure analysis of a heptacoordinate titanium(IV)-bis-chelate with enhanced in vivo antitumor efficacy. Chem. Commun. 2012;48:5790–5792. doi: 10.1039/c2cc31624b. [DOI] [PubMed] [Google Scholar]
- Jamieson E.R., Lippard S.J. Structure, recognition, and processing of cisplatin−DNA adducts. Chem. Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- Jovanović K.K., Tanić M., Ivanović I., Gligorijević N., Dojčinović B.P., Radulović S. Cell cycle, apoptosis, cellular uptake and whole-transcriptome microarray gene expression analysis of HeLa cells treated with a ruthenium (II)-arene complex with an isoquinoline-3-carboxylic acid ligand. J. Inorg. Biochem. 2016;163:362–373. doi: 10.1016/j.jinorgbio.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Nakachi K.I., zue Imai K., Watanabe J., Hayashi S. The CYP1A1 gene and cancer susceptibility. Crit. Rev. Oncol. Hematol. 1993;14:77–87. doi: 10.1016/1040-8428(93)90007-q. [DOI] [PubMed] [Google Scholar]
- Keppler B.K., Friesen C., Moritz H.G., Vongerichten H., Vogel E. Bioinorganic Chemistry. Springer Berlin Heidelberg; 1991. Tumor-inhibiting bis(β-Diketonato) metal complexes. Budotitane, cis-diethoxybis(1-phenylbutane-1,3-dionato)titanium(IV) pp. 97–127. [Google Scholar]
- Khazaei S., Esa N.M., Ramachandran V., Hamid R.A., Pandurangan A.K., Etemad A., Ismail P. In vitro antiproliferative and apoptosis inducing effect of Allium atroviolaceum bulb extract on breast, cervical, and liver cancer cells. Front. Pharmacol. 2017;8:5. doi: 10.3389/fphar.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepf-Maier P., Koepf H. Non-platinum group metal antitumor agents. History, current status, and perspectives. Chem. Rev. 1987;87:1137–1152. [Google Scholar]
- Komeda S., Casini A. Next-generation anticancer metallodrugs. Curr. Top. Med. Chem. 2012;12:219–235. doi: 10.2174/156802612799078964. [DOI] [PubMed] [Google Scholar]
- Köpf H., Köpf-Maier P. Titanocene dichloride—the first metallocene with cancerostatic activity. Angew. Chem. Int. Ed. 1979;18:477–478. doi: 10.1002/anie.197904771. [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Maeda A., Ishiai M., Hashimoto A., Inabe K., Takata M. Regulation of the phospholipase C-gamma2 pathway in B cells. Immunol. Rev. 2000;176:19–29. doi: 10.1034/j.1600-065x.2000.00605.x. [DOI] [PubMed] [Google Scholar]
- Loza-Rosas S.A., Saxena M., Delgado Y., Gaur K., Pandrala M., Tinoco A.D. A ubiquitous metal, difficult to track: towards an understanding of the regulation of titanium(iv) in humans. Metallomics. 2017;9:346–356. doi: 10.1039/c6mt00223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna C.M., Braitbard O., Weiss E., Hochman J., Tshuva E.Y. Cytotoxic salan-titanium(IV) complexes: high activity toward a range of sensitive and drug-resistant cell lines, and mechanistic insights. ChemMedChem. 2012;7:703–708. doi: 10.1002/cmdc.201100593. [DOI] [PubMed] [Google Scholar]
- Manohari Abeysinghe P., Harding M.M. Antitumour bis(cyclopentadienyl) metal complexes: titanocene and molybdocene dichloride and derivatives. Dalt. Trans. 2007;32:3474–3482. doi: 10.1039/b707440a. [DOI] [PubMed] [Google Scholar]
- Meier-Menches S.M., Gerner C., Berger W., Hartinger C.G., Keppler B.K. Structure–activity relationships for ruthenium and osmium anticancer agents–towards clinical development. Chem. Soc. Rev. 2018;47:909–928. doi: 10.1039/c7cs00332c. [DOI] [PubMed] [Google Scholar]
- Meker S., Margulis-Goshen K., Weiss E., Magdassi S., Tshuva E.Y. High antitumor activity of highly resistant salan-titanium(IV) complexes in nanoparticles: an identified active species. Angew. Chem. Int. Ed. 2012;51:10515–10517. doi: 10.1002/anie.201205973. [DOI] [PubMed] [Google Scholar]
- Meker S., Margulis-Goshen K., Weiss E., Braitbard O., Hochman J., Magdassi S., Tshuva E.Y. Anti-proliferative activity of nano-formulated phenolato titanium(IV) complexes against cancer cells. ChemMedChem. 2014;9:1294–1298. doi: 10.1002/cmdc.201400038. [DOI] [PubMed] [Google Scholar]
- Meker S., Braitbard O., Margulis-Goshen K., Magdassi S., Hochman J., Tshuva E.Y. Highly stable tetra-phenolato titanium(IV) agent formulated into nanoparticles demonstrates anti-tumoral activity and selectivity. Molecules. 2015;20:18526–18538. doi: 10.3390/molecules201018526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meker S., Braitbard O., Hall M.D., Hochman J., Tshuva E.Y. Specific design of titanium(IV) phenolato chelates yields stable and accessible, effective and selective anticancer agents. Chem. Eur. J. 2016;22:9986–9995. doi: 10.1002/chem.201601389. [DOI] [PubMed] [Google Scholar]
- Meléndez E. Titanium complexes in cancer treatment. Crit. Rev. Oncol. 2002;42:309–315. doi: 10.1016/s1040-8428(01)00224-4. [DOI] [PubMed] [Google Scholar]
- Meng A.X., Jalali F., Cuddihy A., Chan N., Bindra R.S., Glazer P.M., Bristow R.G. Hypoxia down-regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother. Oncol. 2005;76:168–176. doi: 10.1016/j.radonc.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Miller M., Tshuva E.Y. Synthesis of pure enantiomers of titanium (IV) complexes with chiral diaminobis (phenolato) ligands and their biological reactivity. Sci. Rep. 2018;8:9705. doi: 10.1038/s41598-018-27735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Braitbard O., Hochman J., Tshuva E.Y. Insights into molecular mechanism of action of salan titanium(IV) complex with in vitro and in vivo anticancer activity. J. Inorg. Biochem. 2016;163:250–257. doi: 10.1016/j.jinorgbio.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Mjos K.D., Orvig C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014;114:4540–4563. doi: 10.1021/cr400460s. [DOI] [PubMed] [Google Scholar]
- Nagy Z., Riss A., Romier C., le Guezennec X., Dongre A.R., Orpinell M., Han J., Stunnenberg H., Tora L. The human SPT20-containing SAGA complex plays a direct role in the regulation of endoplasmic reticulum stress-induced genes. Mol. Cell. Biol. 2009;29:1649–1660. doi: 10.1128/MCB.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott I., Gust R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. (Weinheim) 2007;340:117–126. doi: 10.1002/ardp.200600151. [DOI] [PubMed] [Google Scholar]
- Pereira E.R., Frudd K., Awad W., Hendershot L.M. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF) J. Biol. Chem. 2014;289:3352–3364. doi: 10.1074/jbc.M113.507194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri D., Meker S., Shavit M., Tshuva E.Y. Synthesis, characterization, cytotoxicity, and hydrolytic behavior of C2- and C1-symmetrical TiIV complexes of tetradentate diamine bis(phenolato) ligands: a new class of antitumor agents. Chem. A Eur. J. 2009;15:2403–2415. doi: 10.1002/chem.200801310. [DOI] [PubMed] [Google Scholar]
- Peri D., Manna C.M., Shavit M., Tshuva E.Y. TiIV complexes of branched diamine bis(phenolato) ligands: hydrolysis and cytotoxicity. Eur. J. Inorg. Chem. 2011;31:4896–4900. [Google Scholar]
- Peri D., Meker S., Manna C.M., Tshuva E.Y. Different ortho and para electronic effects on hydrolysis and cytotoxicity of diamino bis(Phenolato) “Salan” Ti(IV) complexes. Inorg. Chem. 2011;50:1030–1038. doi: 10.1021/ic101693v. [DOI] [PubMed] [Google Scholar]
- Riddell I.A., Lippard S.J. Cisplatin and oxaliplatin: our current understanding of their actions. Met. Ions Life Sci. 2018;18:1–42. doi: 10.1515/9783110470734-007. [DOI] [PubMed] [Google Scholar]
- Rustum Y.M., Harstrick A., Cao S., Vanhoefer U., Yin M.-B., Wilke H., Seeber S. Thymidylate synthase inhibitors in cancer therapy: direct and indirect inhibitors. J. Clin. Oncol. 1997;15:389–400. doi: 10.1200/JCO.1997.15.1.389. [DOI] [PubMed] [Google Scholar]
- Rzymski T., Milani M., Singleton D.C., Harris A.L. Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle. 2009;8:3838–3847. doi: 10.4161/cc.8.23.10086. [DOI] [PubMed] [Google Scholar]
- Schur J., Manna C.M., Deally A., Koester R.W., Tacke M., Tshuva E.Y., Ott I. A comparative chemical-biological evaluation of titanium(IV) complexes with a salan or cyclopentadienyl ligand. Chem. Commun. 2013;49:4785–4787. doi: 10.1039/c3cc38604j. [DOI] [PubMed] [Google Scholar]
- Sharma K.L., Agarwal A., Misra S., Kumar A., Kumar V., Mittal B. Association of genetic variants of xenobiotic and estrogen metabolism pathway (CYP1A1 and CYP1B1) with gallbladder cancer susceptibility. Tumor Biol. 2014;35:5431–5439. doi: 10.1007/s13277-014-1708-4. [DOI] [PubMed] [Google Scholar]
- Shavit M., Peri D., Melman A., Tshuva E.Y. Antitumor reactivity of non-metallocene titanium complexes of oxygen-based ligands: is ligand lability essential? J. Biol. Inorg. Chem. 2007;12:825–830. doi: 10.1007/s00775-007-0236-8. [DOI] [PubMed] [Google Scholar]
- Suntharalingam K., Johnstone T.C., Bruno P.M., Lin W., Hemann M.T., Lippard S.J. Bidentate ligands on osmium (VI) nitrido complexes control intracellular targeting and cell death pathways. J. Am. Chem. Soc. 2013;135:14060–14063. doi: 10.1021/ja4075375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam K., Lin W., Johnstone T.C., Bruno P.M., Zheng Y.-R., Hemann M.T., Lippard S.J. A breast cancer stem cell-selective, mammospheres-potent osmium (VI) nitrido complex. J. Am. Chem. Soc. 2014;136:14413–14416. doi: 10.1021/ja508808v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco A.D., Thomas H.R., Incarvito C.D., Saghatelian A., Valentine A.M. Cytotoxicity of a Ti ( IV ) compound is independent of serum proteins. Proc. Natl. Acad. Sci. U S A. 2012;109:5016–5021. doi: 10.1073/pnas.1119303109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney J.H., Marks T.J. Hydrolysis chemistry of the metallocene dichlorides M(η5-C5H5)2Cl2, M = titanium, vanadium, or zirconium. Aqueous kinetics, equilibria, and mechanistic implications for a new class of antitumor agents. J. Am. Chem. Soc. 1985;107:947–953. [Google Scholar]
- Tshuva E.Y., Ashenhurst J.A. Cytotoxic titanium(IV) complexes: renaissance. Eur. J. Inorg. Chem. 2009;2009:2203–2218. [Google Scholar]
- Tshuva E.Y., Miller M. Metallo-Drugs: Development and Action of Anticancer Agents. 2018. Coordination complexes of titanium(IV) for anticancer therapy; pp. 219–249. [DOI] [PubMed] [Google Scholar]
- Tshuva E.Y., Tzubery A. Cytotoxic titanium(IV) complexes of salalene-based ligands. Eur. J. Inorg. Chem. 2017;12:1695–1705. [Google Scholar]
- Tzubery A., Tshuva E.Y. Cytotoxicity and hydrolysis of trans -Ti(IV) complexes of salen ligands: structure-activity relationship studies. Inorg. Chem. 2012;51:1796–1804. doi: 10.1021/ic202092u. [DOI] [PubMed] [Google Scholar]
- Tzubery A., Melamed-Book N., Tshuva E.Y. Fluorescent antitumor titanium(IV) salen complexes for cell imaging. Dalt. Trans. 2018;47:3669–3673. doi: 10.1039/c7dt04828a. [DOI] [PubMed] [Google Scholar]
- Velma V., Dasari S.R., Tchounwou P.B. Low doses of cisplatin induce gene alterations, cell cycle arrest, and apoptosis in human promyelocytic leukemia cells. Biomark. Insights. 2016;11:113–121. doi: 10.4137/BMI.S39445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Herrmannová A., Malík R., Peclinovská L., Valášek L.S. Functional and biochemical characterization of human eukaryotic translation initiation factor 3 in living cells. Mol. Cell. Biol. 2014;34:3041–3052. doi: 10.1128/MCB.00663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang T., Sun W., Wang Z., Zuo D., Zhou Z., Li S., Xu J., Yin F., Hua Y. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016;7:e2247. doi: 10.1038/cddis.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-L., Wang A.H.-J. Structural studies of atom-specific anticancer drugs acting on DNA. Pharmacol. Ther. 1999;83:181–215. doi: 10.1016/s0163-7258(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Zogzas C.E., Mukhopadhyay S. Putative metal binding site in the transmembrane domain of the manganese transporter SLC30A10 is different from that of related zinc transporters. Metallomics. 2018;10:1053–1064. doi: 10.1039/c8mt00115d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw and the processed data files of the current study can be accessed through Gene Expression Omnibus (GEO) with the GSE148239 accession number.