Summary
The microtubule-associated tau protein forms pathological inclusions that accumulate in an age-dependent manner in tauopathies including Alzheimer's disease (AD). Since age is the major risk factor for AD, we examined endogenous tau species that evolve during aging in physiological and diseased conditions. In aged mouse brain, we found tau-immunoreactive clusters embedded within structures that are reminiscent of periodic acid-Schiff (PAS) granules. We showed that PAS granules harbor distinct tau species that are more prominent in 3xTg-AD mice. Epitope profiling revealed hypo-phosphorylated rather than hyper-phosphorylated tau commonly observed in tauopathies. High-resolution imaging and 3D reconstruction suggest a link between tau clusters, reactive astrocytes, and microglia, indicating that early tau accumulation may promote neuroinflammation during aging. Using postmortem human brain, we identified tau as a component of corpora amylacea (CA), age-related structures that are functionally analogous to PAS granules. Overall, our study supports neuroimmune dysfunction as a precipitating event in tau pathogenesis.
Subject Areas: Neuroscience, Molecular Neuroscience, Cellular Neuroscience
Graphical Abstract

Highlights
-
•
Tau is present in mouse hippocampal granules and human corpora amylacea
-
•
Tau accumulates with age in hippocampal granules and is accelerated in 3xTg-AD mice
-
•
Tau immunoreactive corpora amylacea are present in Alzheimer's disease brain
-
•
Age-related tau deposits are associated with reactive astrocytes
Neuroscience; Molecular Neuroscience; Cellular Neuroscience
Introduction
One of the defining features of Alzheimer's disease (AD) is the formation of tau species that accumulate with age, initially as soluble tau, then transitioning to tau oligomers and neurofibrillary tangles (NFTs) in end-stage AD brain (Lasagna-Reeves et al., 2012, Maeda et al., 2006). A major challenge when using rodent models of AD is generating robust tau pathology to the extent observed in human tauopathies. Indeed, the formation of tau inclusion pathology in rodents typically requires tau over-expression paradigms, the incorporation of mutant aggregate-prone versions of tau, or prion-like templating to seed tau into an insoluble, hyper-phosphorylated state that resembles that observed in late-stage human tauopathies (Puzzo et al., 2015). Surprisingly, few studies have analyzed normal endogenous tau conformations that emerge during the aging process, well before the onset of cognitive deficits. A better understanding of tau in this early stage could identify pre-pathological tau forms, allow the early detection of tauopathy prior to over symptoms, and potentially even provide a framework for tau-targeted drug discovery.
Recently, we identified a tau species that accumulated in aged wild-type mice (Tseng et al., 2017). This species was distinct from classic tau pathology since it was soluble and immunoreactive with the tau-1 antibody (e.g., tau-1 immunoreactive, or tau-1IR), which detects hypo-phosphorylated tau in the region spanning residues S192–T205 (Binder et al., 1985). The aged tau species accumulated as punctate clusters in the hippocampus and cortex of middle-aged (∼12 months old) and older mice (>24 months old) but was nearly undetectable in young mice (<6 months old). Using a cell culture model of acute neuroinflammation, we determined that tau-1IR structures contained tau species that were acetylated (Cohen et al., 2011) and phosphorylated (Drewes et al., 1997) within the microtubule-binding domain (MTBR) (e.g., ac-K280 and p-S262-positive) but not the flanking regions. Thus, we proposed that tau-1IR clusters represent a feature of the aged brain that is accelerated under conditions of neuronal stress or damage. Consistent with this possibility, genetic depletion of the deacetylase HDAC6 in wild-type mice, which confers a neuroprotective effect in some models (Selenica et al., 2014, Zhang et al., 2014a), led to reduced tau-1IR cluster formation. Conversely, neuroinflammatory triggers including lipopolysaccharide (LPS) exposure were associated with accumulation of tau-1IR clusters (Tseng et al., 2017). These findings suggested that an age-dependent form of tau might emerge in the brains of vulnerable mice.
Morphologically similar to tau-1IR clusters, carbohydrate-rich deposits known as periodic acid-Schiff (PAS) granules (also referred to as Wirak bodies) are spheroid ∼3-μm-diameter structures detected by PAS staining of hippocampal brain sections of aged mice (Cana et al., 2015, Jucker et al., 1994, Kuo et al., 1996, Manich et al., 2014a, Manich et al., 2014b, Manich et al., 2015, Manich et al., 2016, Wirak et al., 1991). In addition, similarly spherical, age-related structures known as corpora amylacea (CA) have been described in human brain (Auge et al., 2017, Auge et al., 2018, Auge et al., 2019, Manich et al., 2016, Singhrao et al., 1993). Both PAS granules and CA are thought to emerge during aging as a result of abnormal or pathological processes, potentially arising from diverse stressors including oxidative stress (Porquet et al., 2013), neuroinflammation (Tseng et al., 2017), or environmental triggers such as fungal (Pisa et al., 2016) or viral (Libard et al., 2014) infection. Like tau-1IR clusters, PAS granules increase in the aged mouse brain (Manich et al., 2014a). Primarily thought to be composed of glycoproteins and carbohydrate moieties, the protein constituents of PAS granules remain poorly characterized, largely due to false-positive PAS granule detection from non-specific IgM components present in many natural and commercially available antibodies (Manich et al., 2015). In light of this confounding issue, the mechanism of PAS granule biogenesis and its role in neurodegenerative disease has been a highly debated topic.
At the ultrastructural level, PAS granules were shown to contain degenerating cytoplasmic organelles including mitochondria, irregular vacuolar structures, fragmented cytoskeletal components, and breakdown products derived from degenerating neurons and astrocytes (Kuo et al., 1996, Manich et al., 2014a, Mitsuno et al., 1999). A link between tau, PAS granules, and AD is bolstered by the presence of degenerating dendrites immediately surrounding PAS deposits (Manich et al., 2014a), as well as the potential identification of tau and other MAPs within CA by proteomics approaches (Pisa et al., 2018). Furthermore, a significant correlation was observed between PAS granule formation and hippocampal-dependent spatial memory defects (Jucker et al., 1994, Knuesel et al., 2009). Recent findings indicate that CA or PAS granules may be engulfed by macrophages after they are extruded from the brain into the cerebrospinal fluid (CSF) (Riba et al., 2019). Thus, PAS granules may act in a protective capacity during aging to isolate or limit the accumulation of damaged, misfolded, and aggregated proteins including tau. Excessive PAS granule formation, coupled with its failed clearance, could exacerbate disease pathology and promote neurodegeneration. Connecting these structures to the progression of AD and other tauopathies remains an unresolved issue that has major implications for brain aging, AD pathology, and cognitive decline.
Here, we performed a detailed characterization of tau-1IR structures and found striking parallels between tau-1IR clusters and mouse PAS granules. We then validated the specific immunoreactivity of tau-1 and tau5 mouse monoclonal antibodies using genetic tau-knockout mice and immunoadsorption techniques. We show that the formation of tau-immunoreactive clusters, which accumulate predominantly in the CA1 region, are accelerated in the 3xTg-AD model mice and are present in the hippocampus of aged and AD postmortem human brain. High-resolution 3D microscopy indicates that the cluster-associated pool of tau is engaged by astrocytic and microglial processes. Overall, our data highlight mouse PAS granules and human CA as unanticipated hubs for the emergence of tau species that may be linked to the evolution of tau pathology in normal aging, AD, and related tauopathies.
Results
Characterizing Tau-1IR Cluster Formation by Tau Epitope Immunoreactivity
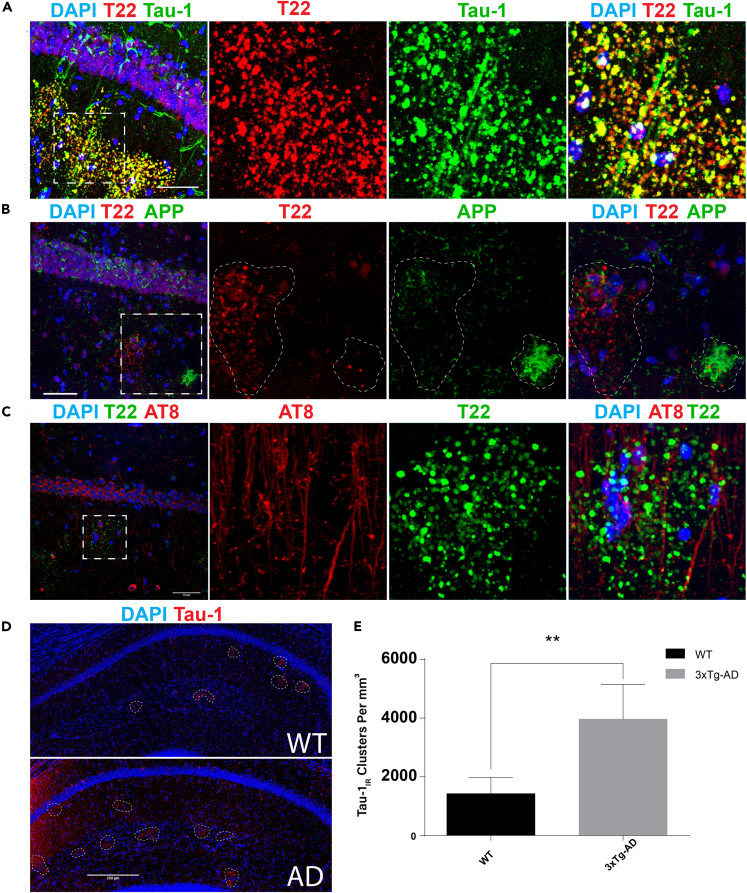
In our previous study, we showed that the non-phosphorylation-specific monoclonal tau-1 antibody and the tau-oligomer-specific polyclonal T22 antibody both partly labeled hippocampal age-related clusters in wild-type mice (Tseng et al., 2017) (Table S1). To evaluate tau-1IR clusters under pathological conditions, we performed double-labeling immunofluorescence on wild-type and transgenic AD mice (3xTg-AD). These mice harbor three mutations associated with familial AD (APP Swedish, MAPT P301L, and PSEN1 M146V), which drive cortical Aβ plaque deposition at 3–4 months of age, whereas transgenic human tau pathology is not observed robustly until around 12 months of age, at which point cognitive deficits become more apparent (Oddo et al., 2003). Tau-1IR/T22IR clusters were detected in the molecular layer (ML), stratum oriens (SO), and prominently in the stratum radiatum (SR) of aged 3xTg-AD mice (Figure 1A). These observations are consistent with the prior detection of PAS granules in the SR and nearby hippocampal regions such as the stratum lacunosum moleculare (SLM) (Manich et al., 2014b), indicating that tau-1IR clusters and PAS granules correspond, at least morphologically, to identical age-related deposits. Importantly, analysis of Aβ plaques in 3xTg-AD mice, detected with the 6E10 monoclonal antibody, showed that tau-1IR clusters were not associated with plaque pathology in these regions (Figure 1B).
Figure 1.
Tau-Immunoreactive Clusters in the Hippocampus Are Distinct from Plaque Pathology in 3xTg-AD Mice
(A) Immunofluorescent confocal images of aged (21-month-old [mo]) 3xTg-AD mice indicate that Tau-1 (green) and T22 (red) co-localize in granules in the CA1 and SR.
(B) T22 (red) does not co-localize with plaques (6E10) or neuronal soma (green) within the CA1 and SR.
(C) AT8-immunoreactive neuronal soma and axons (red) associate, but do not co-localize, with T22-positive granules (green) in the CA1 or SR.
(D) Representative immunofluorescent images of 3xTg-AD and WT hippocampal fields captured by confocal microscopy at 40x magnification. Dashed circles indicate tau-1IR clusters (red).
(E) Quantification of tau-1IR clusters showing that they are significantly elevated in the hippocampi of 6-mo 3xTg-AD mice when compared with controls.
Scale bars, 50 μm (A–C), 250 μm (D). Statistical significance was assessed using Student's t test from N = 4 biological replicates (∗∗p < 0.01). Error bars represent SEM. See also Figure S1.
To determine whether tau-1IR clusters in wild-type (WT) and 3xTg-AD mice contain phosphorylated tau, we evaluated their immunoreactivity with AT8, a monoclonal antibody commonly used to mark NFT pathology, which detects tau phosphorylated at S202/T205. Consistent with tau-1 and AT8 targeting complimentary epitopes (Szendrei et al., 1993), we found that tau-1IR/T22IR clusters were AT8-negative in the SR of 3xTg-AD mice (Figure 1C), indicating enrichment for hypo-phosphorylated tau, at least within the region detected by tau-1 (residues S195-T205) and T22 (oligomeric) antibodies. Quantification of tau-1IR cluster density from hippocampal sections of 6-month-old mice showed a significant increase from 1,425 granules/mm3 in WT to 3,965 granules/mm3 in 3xTg-AD mice (Figures 1D and 1E). The difference between genotypes gradually normalized as the mice aged (Figure S1A). These results suggest that tau-1IR cluster formation is accelerated at early stages in an AD mouse model and support the independent spatial accumulation of tau clusters and Aβ in the aging hippocampus.
Evaluating Tau as a Component of PAS Granules
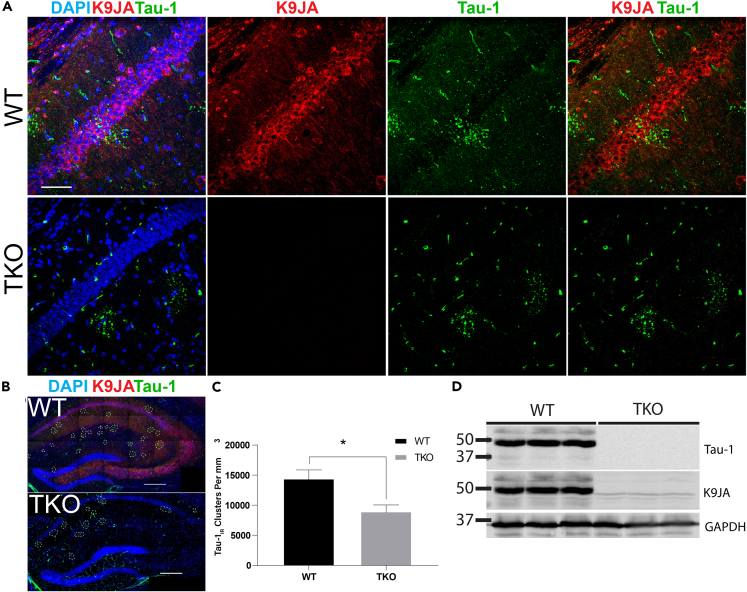
To confirm the incorporation of tau into PAS granules, we performed genetic validation experiments using tau knockout (TKO) mice to evaluate the specificity of tau within PAS granules. Surprisingly, although total tau (K9JA) immunoreactivity was absent, quantification of confocal images revealed that tau-1IR clusters were detectable yet significantly less dense in the hippocampi of 23-month-old TKO mice (∼9,000 granules/mm3) compared with age-matched WT mice (∼14,400 granules/mm3) (Figures 2A–2C), indicating that tau depletion reduced but did not eliminate tau-1 immunoreactivity. Immunoblotting of hippocampal lysates showed the expected tau depletion (Figure 2D), excluding the potential for any residual tau expression in the TKO mice.
Figure 2.
Tau-Immunoreactive Clusters Are Reduced, but Not Eliminated, in Tau KO Mice When Detected with Pan-Reactive Secondary Antibodies
(A) Immunofluorescent confocal images of aged (23-mo) wild-type (WT) and tau knockout (TKO) mice show immunoreactivity with K9JA (red) and tau-1 (green) in the CA1 and SR of WT mice, with only tau-1IR cluster immunoreactivity in TKO mice.
(B) Representative images of full hippocampal fields in aged WT and TKO mice captured by confocal microscopy at 40x magnification.
(C) Quantification of tau-1IR cluster density showing a reduction in granules in 23-mo TKO mice when compared with age-matched WT mice.
(D) Immunoblots of tau (tau-1, K9JA) and GAPDH in 23-mo WT and TKO mouse hippocampal lysates confirm the absence of tau expression in TKO mice.
Scale bars, 50 μm (A), 250 μm (B). Statistical significance was assessed using a Student's t test from N = 4 biological replicates (∗p < 0.05). Error bars represent SEM. See also Figure S2.
To further address the presence of tau clusters in TKO mice, we investigated their molecular and histological properties. Tau-1IR clusters are highly reminiscent of PAS granules, also known as Wirak bodies, which form in aged mouse brain (Wirak et al., 1991). Mass spectrometry-based proteomics of related CA deposits from human brain hinted that tau and related MAPs may be present within these structures (Pisa et al., 2018), but this has not been confirmed or thoroughly investigated. Intriguingly, PAS granules and CA comprise neo-epitopes containing carbohydrates that are recognized by natural and commercially available antibodies containing a non-specific IgM fraction (Manich et al., 2015). Therefore, to clarify whether the tau-1IR clusters contain tau or rather result from non-specific immunohistochemical detection due to anti-IgM immunoreactivity, we performed a series of experiments using more highly specific immunohistological approaches. We used isotype-specific anti-mouse IgG and anti-mouse IgM secondary antibodies to distinguish among the components found within the primary tau antibodies (Table S1).
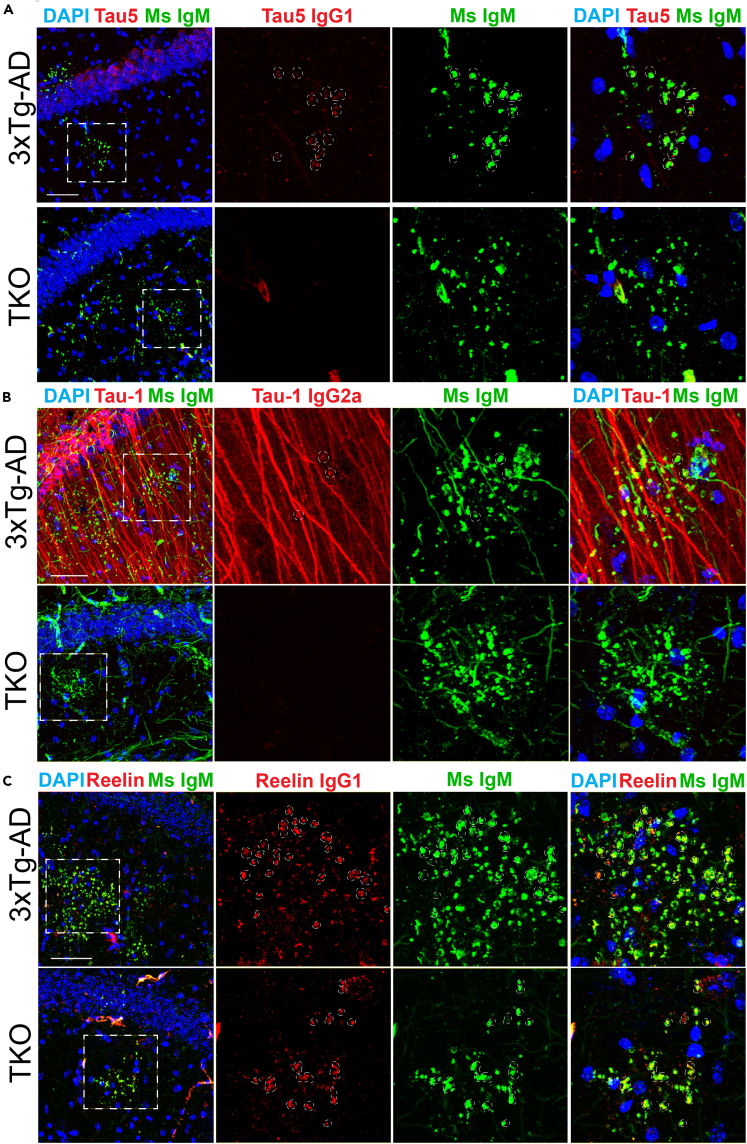
Tau-1IR and tau5IR clusters were indeed detected with anti-mouse IgM-specific secondary antibodies in aged mice (Figures 3A and 3B, green) but not in young 2-month-old WT or AD mice (Figure S1B), suggesting a non-specific IgM component is present in these monoclonal antibodies. This result is not unexpected, as many commercial antibodies contain trace amounts of IgM antibodies directed against poorly defined neo-epitopes that can lead to false-positive detection of PAS granules or analogous structures (Auge et al., 2018). We therefore subclassified tauIR clusters into anti-mouse IgG-specific and anti-mouse IgM-specific (anti-IgM, μ-chain specific) granules using secondary antibodies against tau-1 (anti-IgG2a, γ-chain specific) or tau5 (anti-IgG1, γ-chain specific) (Table S2). Although the tau-1 and tau5 monoclonal antibodies harbor an IgM component, we observed anti-mouse IgG-specific tauIR granules within the anti-mouse IgM-specific granules, indicating that a pool of tau does indeed localize to PAS granules in 3xTg-AD mice (Figures 3A and 3B, red). Moreover, we excluded any non-specific IgG immunoreactivity using an isotype control antibody (Figure S2). Further confirming the presence of tau within these structures, tauIR granules were not detectable using anti-mouse IgG-specific secondary antibodies in TKO mice that lack the tau protein, despite the fact that anti-mouse IgM-specific immunoreactivity remained (Figures 3A and 3B, see TKO panel).
Figure 3.
Analysis of Hippocampal Tau Clusters Using Isotype-Specific Immunodetection
(A) Immunofluorescent confocal images of aged (23-mo) 3xTg-AD and TKO mice using isotype-specific secondary antibodies. Tau5 detects tau granules when labeled with an anti-mouse IgM μ-chain-specific secondary antibody (Ms IgM, green) in both genotypes and partially detects tau granules when labeled with an anti-mouse IgG1 γ-chain-specific secondary antibody (Tau5 IgG1, red) in 3xTg-AD but not TKO mice.
(B) Tau-1 detects tau granules when labeled with an anti-mouse IgM μ-chain-specific secondary antibody (Ms IgM, green) in both genotypes and partially detects tau granules when labeled with an anti-mouse IgG2a γ-chain-specific secondary antibody (Tau-1 IgG2a, red) in 3xTg-AD mice but not TKO mice.
(C) Reelin partially detects granules when labeled with both anti-mouse IgM μ-chain-specific (Ms IgM, green) and anti-mouse IgG1 γ-chain-specific (Reelin IgG1, red) secondary antibodies in both 3xTg-AD and TKO mice.
Scale bars, 50 μm; dashed white circles indicate regions of co-localization. See also Figure S3.
As an IgM and IgG1-containing positive control primary antibody, we used a monoclonal anti-Reelin antibody (clone G10) and confirmed the expected non-specific IgM component that was previously shown to label PAS granules, referred to as “Reelin plaques” (Doehner et al., 2010, Knuesel et al., 2009, Manich et al., 2016). Both anti-mouse IgG1-specific and anti-mouse IgM-specific secondary antibodies labeled Reelin granules in the 3xTg-AD mouse, and neither signal was effectively depleted in TKO mice (Figure 3C).
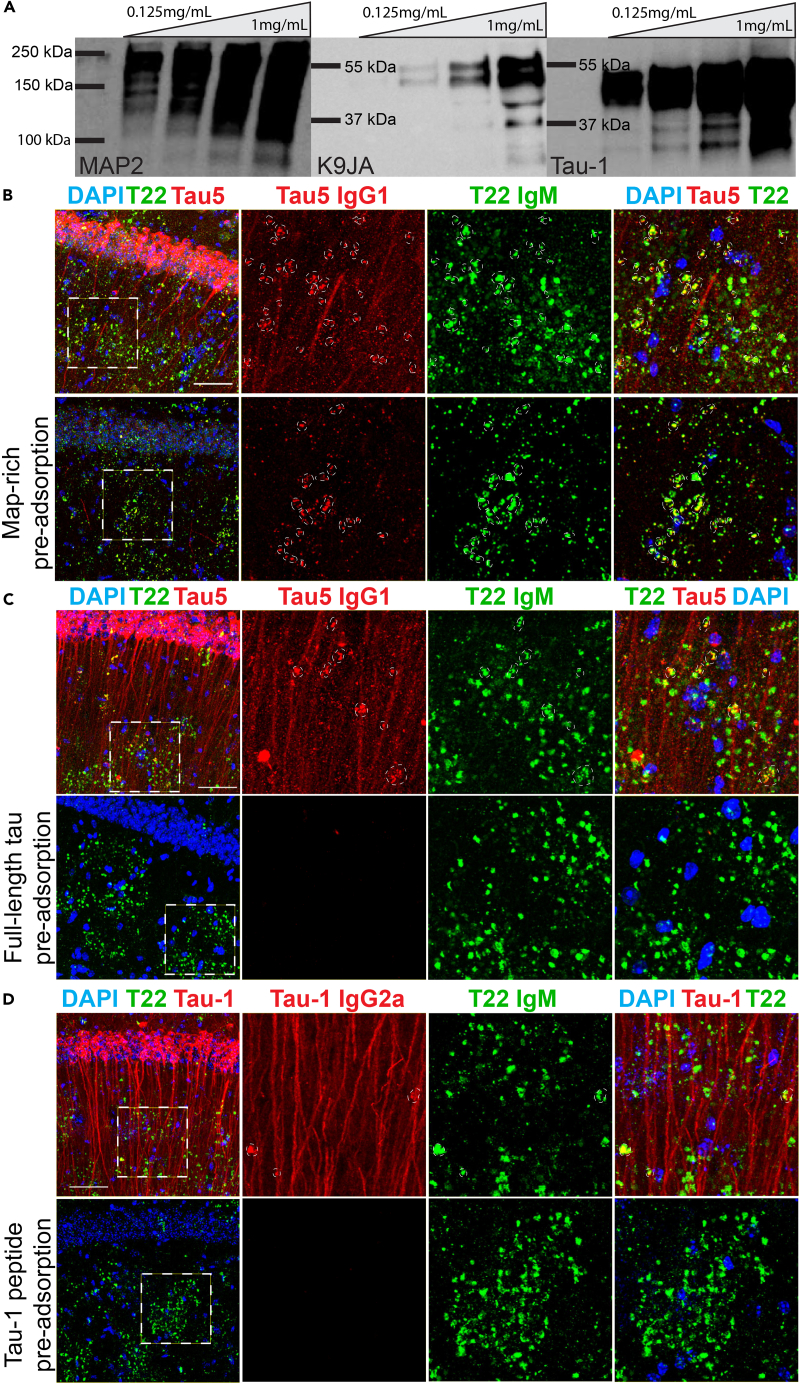
To further validate the specificity of tau-1 and tau5 antibodies, we performed immunoadsorption experiments to block the monoclonal antibody epitopes and hence neutralize tau immunoreactivity prior to immunofluorescence analysis. To deplete the tau signal with native tau isoforms, we used either recombinant human tau protein (2N4R full-length tau) or a brain-derived MAP-rich fraction (containing tau) isolated by temperature-induced tubulin polymerization, ionic exchange chromatography, and high salt elution. The MAP mixture contains multiple neuronal tau and MAP isoforms that we confirmed by immunoblotting of serially diluted MAP-rich fractions (Figure 4A). Pre-incubating the tau5 or tau-1 antibodies with the MAP-rich fraction or with recombinant full-length tau led to antibody neutralization and a reduction in the anti-mouse IgG-specific detection of tau in dendrites and granules of 3xTg-AD mice (Figures 4B and 4C) as well as aged WT mice (Figures S3A and S3B). Pre-incubation of the tau-1 primary antibody with a purified peptide containing only the tau-1 epitope sequence nearly eliminated the anti-mouse IgG-specific detection of tau-1IR granules (Figure 4D), whereas anti-mouse IgM-specific granules remained. Therefore, combining genetic and histological validation controls, we conclude that tau is a bona fide constituent of PAS granules that accumulates in the aged mouse brain.
Figure 4.
Analysis of Tau Clusters Following Epitope Neutralization by Immunoadsorption
(A) Immunoblots of MAP-rich fractions (MRFs) from porcine brain that were used to pre-adsorb tau-1, tau5, and MAP2 primary antibodies showing MAP2 and tau (K9JA, tau-1) immunoreactivity.
(B) The tau5 antibody detects granules when labeled with an anti-mouse IgM μ-chain-specific secondary antibody (Ms IgM, green), labels both dendrites and granules when labeled with an anti-mouse IgG1-specific secondary antibody (Tau5 IgG1, red), and is partially pre-adsorbed when neutralized with MRF, whereas anti-mouse IgM-specific granules (Ms IgM, green) remain in 21-mo 3xTg-AD mice.
(C) Tau5 anti-mouse IgG1-specific (Tau5 IgG1, red) immunoreactivity is neutralized with recombinant full-length (2N4R isoform) tau protein, whereas anti-mouse IgM-specific granules (green) remain in 21-mo 3xTg-AD mice.
(D) The tau-1 antibody detects granules when labeled with anti-mouse IgM μ-chain-specific secondary antibody (Ms IgM, green), detects dendrites and some, but not all, granules when labeled with anti-IgG2a secondary antibody (Tau-1 IgG2a, red) in 21-mo 3xTg-AD mice. Tau-1 anti-mouse IgG2a-specific immunoreactivity is neutralized by the purified tau-1 peptide, whereas anti-mouse IgM-specific (Ms IgM, green) immunoreactivity remains. Scale bars, 50 μm, dashed white circles indicate regions of co-localization.
See also Figure S4.
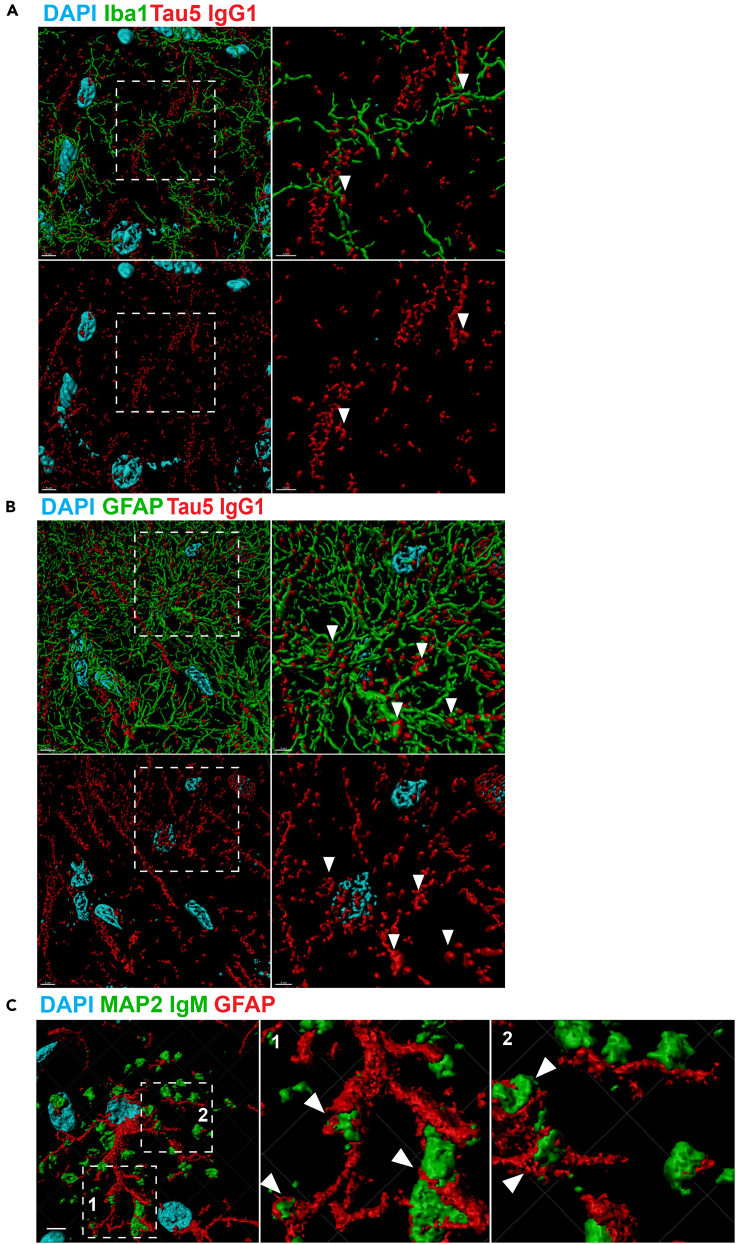
We also investigated the possibility of MAP2 accumulation within tauIR clusters. Multiple MAPs were found by mass spectrometry analysis of CA isolated from human brain tissue, with MAP2 being the most commonly observed peptide (Pisa et al., 2018). We used a mouse monoclonal MAP2 antibody (IgG1) that contains an IgM component in conjunction with isotype-specific secondary antibodies and observed that MAP2 labeled with anti-mouse IgG1-specific secondary antibodies reliably marked dendrites but was not immunoreactive within anti-mouse IgM-specific clusters in aged mice (Figure S4). We did, however, observe PAS granules closely associated with MAP2-positive dendrites, suggesting that PAS granules may partly originate from neurons.
Tau-1IR Cluster Formation Is Associated with Reactive Astrocytes
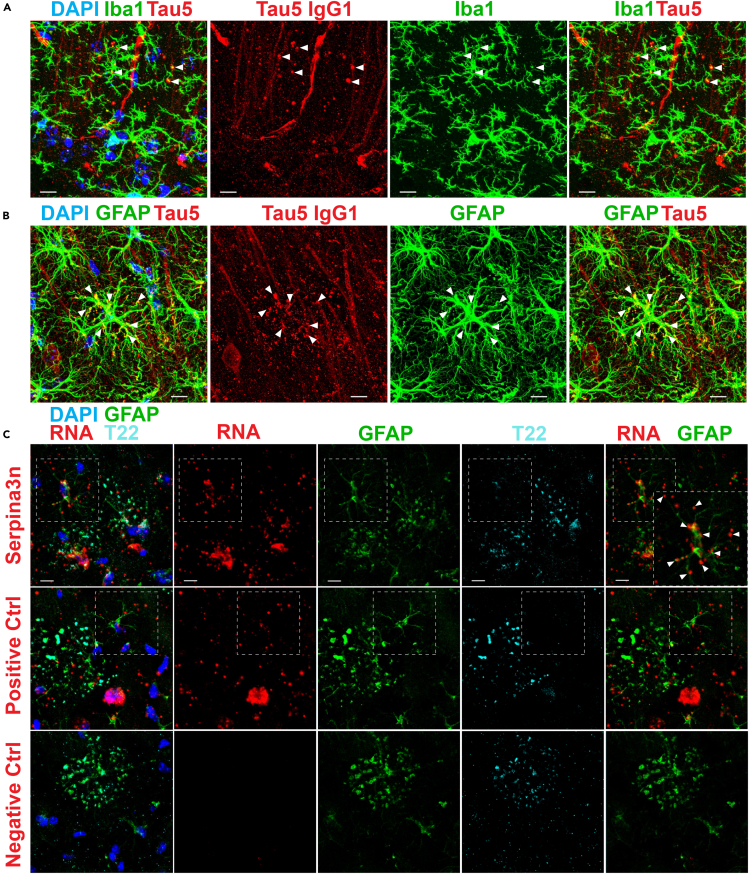
Previous studies support a glial origin of PAS granules, as > 60% of these structures are reported to associate with glial fibrillary acidic protein (GFAP)-immunoreactive astrocytic processes (Akiyama et al., 1986, Jucker et al., 1994, Kuo et al., 1996, Madhusudan et al., 2009, Manich et al., 2014a, Nakamura et al., 1995, Robertson et al., 1998). Moreover, we showed that tau-1IR clusters correlate with inflammatory microglia in the hippocampus (Tseng et al., 2017). We therefore explored the interactions of anti-mouse IgG-specific tauIR granules with microglia and astrocytes. Iba-1-positive microglial processes were in close proximity with anti-mouse IgG-specific tauIR granules and were observed surrounding these structures (see arrowheads marking these interactions) (Figure 5A). More prominently, GFAP-positive astrocytic processes were strongly co-localized with anti-mouse IgG-specific tauIR granules, and the astrocytic somas were frequently at the center of individual tauIR cluster patches (Figure 5B). Isotype-specific staining using a mouse monoclonal GFAP antibody detected a substantial IgM component, whereas the IgG component of the GFAP antibody labeled with anti-mouse IgG1-specific secondary showed very close juxtaposition of astrocytic processes that terminated with PAS granules (Figure S5).
Figure 5.
Tau-Immunoreactive Granules Are Associated with Reactive Astrocytes
(A) Immunofluorescent confocal images of aged (21-mo) 3xTg-AD mice show distal processes of microglia detected with anti-rabbit Iba1 (Iba1, green) that associate with and envelop anti-mouse IgG1-specific tau5IR granules (Tau5 IgG1, red) in the CA1 and SR of aged 3xTg-AD mice.
(B) Distal processes of astrocytes detected with anti-rabbit GFAP (GFAP, green) contain and envelop many anti-mouse IgG1-specific tau5IR granules (Tau5 IgG1, red) in the CA1 and SR of aged 3xTg-AD mice.
(C) Confocal images of dual in situ RNA labeling and immunofluorescence shows Serpina3n expression (red) in processes of reactive astrocytes (GFAP, green) entangled with T22-positive hippocampal clusters (T22, cyan). Robust expression of Ppib (positive control, red) is also detected in affected astrocytes (green) and other cells (DAPI, blue), whereas the negative control probe (red) is not detected in the hippocampus. Scale bars, 10 μm; arrowheads indicate sites of co-localization. See also Figure S5.
Both astrocytes and microglia are known to alter synapses, and synaptic dysfunction is observed under neuroinflammatory conditions including LPS exposure, which increases PAS granule burden and astrocyte reactivity throughout aging (Chen et al., 2014). Reactive astrocytes may therefore represent a major source of neuronal toxicity via the release of factors that induce microglial activation throughout normal aging and in many neurodegenerative disorders (Liddelow et al., 2017). Astrocytic reactivity is known to increase owing to inflammation and injury, and astrocytes increasingly display transcript profiles indicative of a neurotoxic A1 phenotype that arises during normal aging in rodents (Clarke et al., 2018). Among the most prominent up-regulated genes throughout aging was Serpina3n, a serine-threonine protease inhibitor (Baker et al., 2007), the expression of which increases sharply during normal aging in the rodent hippocampus, but not the cortex, mirroring the pattern of PAS granule formation (Vanni et al., 2017). Given their close proximity, we hypothesized that the formation of PAS granules during aging could coincide with an increase in astrocyte reactivity.
To investigate this possibility, we used in situ RNA probes and performed double labeling using a combination of RNA probes and antibodies marking astrocytes (GFAP) and PAS granules (T22) in aged mouse brain. As expected, the negative control RNA probe (DapB) showed limited reactivity (Figure 5C, bottom row), whereas abundant signal was detected with the positive control probe (Mm-PpiB) (Figure 5C, middle row), a factor involved in collagen formation. Using the Serpina3n probe (Mm-Serpina3n), we found that Serpina3n expression was enriched in the soma and processes of the same astrocytes that also harbored tauIR clusters (Figure 5C, top row, see co-localized regions marked by arrowheads). These results suggest that tau granule formation is linked to increased astrocytic reactivity during normal rodent aging.
To further visualize the association of microglia and astrocytes interacting with tau granules, we used 3D reconstruction imaging techniques (Figure 6). The analysis revealed extensive interactions between anti-IgG-specific tau5IR granules and either microglia (Figure 6A) or astrocytes (Figure 6B) (see arrowheads marking regions of co-localization). The prominent association of astrocytes, in particular, with PAS granules was even more apparent with GFAP labeling and high-resolution 3D reconstruction of the astrocytic processes wrapping around PAS granules (labeled with anti-IgM-specific secondary antibodies to label the entire PAS granule pool) (Figure 6C). Our findings strongly support the notion that microglia and reactive astrocytes are intimately associated with anti-mouse IgG-specific tauIR granules.
Figure 6.
3D Reconstruction of Tau-Immunoreactive Granules Reveals Extensive Association with Microglia and Astrocytes
(A) 3D reconstruction of confocal images obtained at 60x magnification (with 2× zoom) show that Iba1-positive microglial processes (green) associate with anti-mouse IgG-specific tau5IR dendrites and granules (Tau5 IgG1, red) in the SR of 3xTg-AD mice.
(B) 3D reconstruction shows that GFAP-positive astrocytic processes (green) associated with anti-mouse IgG-specific tau5IR dendrites and granules (Tau5 IgG1, red) in the SR of 3xTg-AD mice.
(C) 3D reconstruction shows that anti-mouse IgM-specific granules from the mouse monoclonal MAP2 primary antibody (MAP2 IgM, green) are closely juxtaposed along processes of GFAP-positive astrocytes (red). Scale bar, 5 μm (A, B, left); 3 μm (A, B, right); 5 μm (C, left). Arrowheads indicate regions of association.
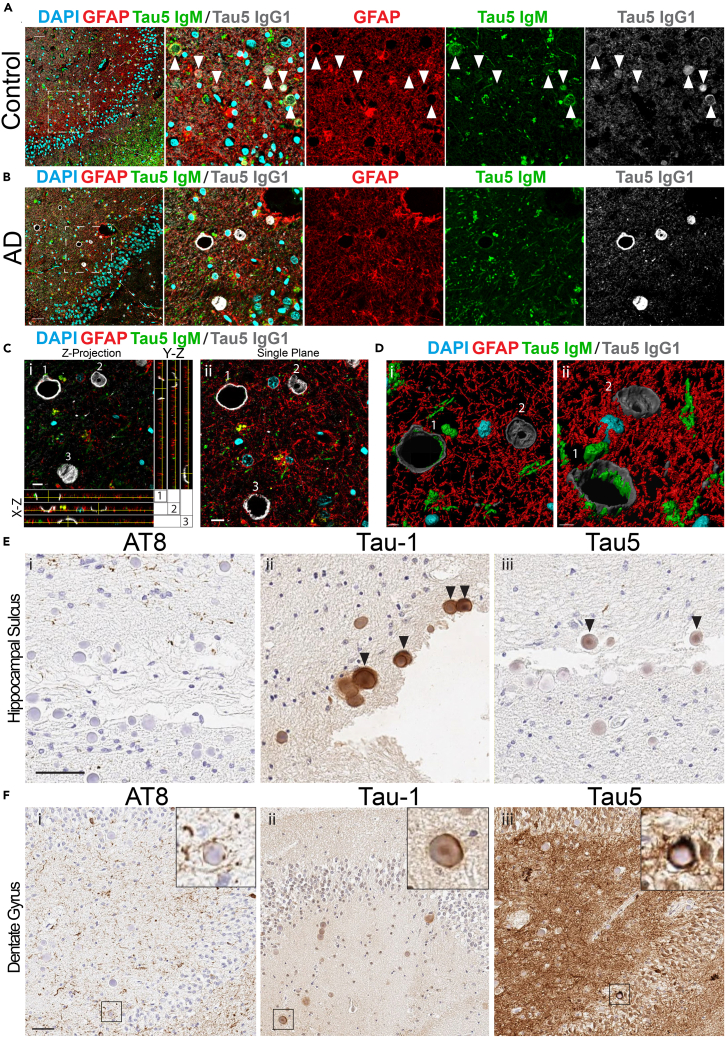
Next, we sought to corroborate our observations in mice with human postmortem brain tissue. Corpora amylacea (CA) in humans are thought to represent structural and functional homologues of mouse hippocampal PAS granules (Auge et al., 2019). Therefore, we analyzed hippocampal tissue sections from human control subjects and patients with AD with polyclonal GFAP and monoclonal tau5 antibodies, followed by labeling with isotype-specific secondary antibodies (Figures 7A–7C). We closely monitored anti-mouse IgG-specific tau5 immunoreactivity within CA structures, which are routinely observed in the subpial regions of the hippocampus. Consistent with our findings in mice, tau-positive CA were marked with both anti-mouse IgM-specific and anti-mouse IgG-specific secondary antibodies and were often in close proximity to GFAP-positive astrocytes (Figures 7A and S6, arrowheads indicate CA). Immunoadsorption of the tau5 primary antibody with full-length recombinant tau protein eliminated anti-mouse IgG-specific tau5 immunoreactivity in human CA, whereas anti-mouse IgM-specific immunoreactivity remained (Figures S7A and S7B). In many instances, we observed striking hollowed-out or dome-like CAs that were prominently tau5 immunoreactive using anti-mouse IgG-specific secondaries in AD brain (Figure 7B), which was further analyzed by (1) average-Z projection and orthogonal views (Figure 7C-i), (2) single confocal planes (Figure 7C-ii), and (3) 3D reconstruction analysis using IMARIS software (Figures 7D-i and 7D-ii, alternate views). The hollowed appearance of tau-positive CA could potentially arise from the harsh antigen retrieval process that may remove the internal CA contents but leave membrane-associated tau intact. In AD brain, we observed typical neuritic tau pathology that was largely enveloped by GFAP-positive processes and was in close proximity to CA membrane-associated tau (Figure S6).
Figure 7.
Tau-Immunoreactive CA in Human Control and AD Brain
(A) Confocal images of human control hippocampus immunostained with tau5 and GFAP primary antibodies and anti-mouse IgM μ-chain (Tau5 IgM, green), anti-rabbit (GFAP, red), and anti-mouse IgG1 γ-chain-specific (Tau5 IgG1, gray) secondary antibodies. Tau5 detects tau within CA when labeled with an anti-mouse IgG1 secondary antibody (gray). CA were labeled with an anti-mouse IgM μ-chain-specific secondary antibody (green). GFAP surrounds CA when labeled with anti-rabbit secondary antibody (red). Scale bar, 50 μm (arrowheads indicate immunoreactive CA).
(B) Confocal images of human AD hippocampus immunostained with tau5 and GFAP primary antibodies and isotype-specific secondaries detailed above. Tau5 detects tau within CA when labeled with an anti-mouse IgG1 secondary antibody (gray). CA were labeled with an anti-mouse IgM-specific secondary antibody (green). GFAP outlines but does not fill CA when labeled with an anti-rabbit secondary antibody (red). Scale bar, 50 μm.
(C) Average-intensity Z projection from selected region in (B), surrounded by orthogonal views targeted to individual CA labeled as 1, 2, and 3 (i). A single plane confocal image of AD patient hippocampus is also shown (ii). Scale bar, 10 μm.
(D) 3D reconstruction of inset showing hollowed structure of CA from (i) top-down and (ii) oblique angular view.
(E) Wide-field IHC images of the hippocampal sulcus in serial sections from an AD patient brain stained with (i) AT8, (ii) Tau-1, and (iii) Tau5 primary antibodies and anti-mouse IgG1-specific or anti-mouse IgG2a-specific HRP-conjugated secondary antibodies. Scale bar, 50 μm. Arrowheads indicate tau-immunoreactive CA.
(F) Wide-field IHC images of the dentate gyrus in serial sections from AD patient brain stained with (i) AT8, (ii) tau-1, and (iii) tau5 primary antibodies and detected with anti-mouse IgG1-specific or anti-mouse IgG2a-specific HRP-conjugated secondary antibodies. Scale bar, 50 μm.
See also Figures S6 and S7 and Table S3.
We complemented the human confocal imaging with immunohistochemical (IHC) analysis of a panel of cases using isotype-specific horseradish peroxidase (HRP)-conjugated secondary antibodies to label tau-positive CA (anti-mouse IgG2a or anti-mouse IgG1). Our data confirm that total tau, as detected with anti-mouse IgG-specific secondary antibodies, labels human CA, whereas hyper-phosphorylated tau, detected with AT8 is largely excluded (Figure 7E). However, in some instances, we observed AT8 and tau5 immunoreactivity surrounding and encircling CA (Figure 7F). Based on tau immunoreactivity within CA using AT8, tau5, and tau-1 antibodies, we scored tau-positive CA intensity using a semi-quantitative grading scheme from 0 to 3 in increments of 0.5 (Table S3). We observed a negative correlation for both tau5 (p = 0.0325) and tau-1 (p = 0.0285) immunoreactive CA as a function of increasing Braak stage (Figures S7C and S7D), suggesting tau-immunoreactive CA decrease with increasing burden of insoluble tau pathology in AD. We note that, although this is significant, given the variance in the data, additional studies with larger cohorts are warranted to further correlate tau immunoreactive CA with neuropathological, genetic, and/or cognitive measures of disease progression.
To the best of our knowledge, CA-enriched tau species have not been previously characterized in human brain. Thus, we refer to this tau species as CA-associated tau, or CA-tau. Our IHC analysis indicates that CA-tau is often tau-1 and tau5 immunoreactive within the CA core, whereas phosphorylated tau (AT8-positive), when present, is largely restricted to the CA membrane. Overall, our study highlights mouse PAS granules and human CA as novel hubs involved in tau processing and potential determinants of tau pathogenesis during the aging process and in AD.
Discussion
In this study, we characterized tau-1IR clusters, which are highly reminiscent and overlapping with previously characterized PAS granules that accumulate predominantly in the aged mouse hippocampus. Using histology and molecular approaches, our results indicate that tau is a bona fide constituent of these granules in both mice and humans and that tauIR granules represent a subset of an IgM-reactive pool that has not been defined in the literature. In contrast to more mature tau pathology in AD brain that is typically marked by phospho-tau immunoreactivity, our epitope profiling shows that hypo-phosphorylated, rather than hyper-phosphorylated tau, may be enriched within PAS granules and CA. Since hypo-phosphorylated tau species have been reported to precede subsequent tau hyper-phosphorylation (Liang et al., 2009, Lopresti and Konat, 2001), we propose that PAS granules may harbor a specific age-related conformational tau variant, which, given enough time, may evolve into more mature tau pathology. In light of our study showing that tau-1IR cluster formation occurs in response to neuroinflammatory triggers (e.g., LPS exposure) (Tseng et al., 2017), CA and PAS granules could initially be generated in an attempt to clear tau and other aggregate-prone proteins but may then become stabilized in the aged brain, which could accelerate tau pathogenesis. The biochemical isolation of these structures, followed by mass spectrometry and other proteomics approaches, could identify PTM signatures that more fully characterize this particular tau strain.
We show that tau is present within some, but not all, human CA in control subjects and patients with AD. Our semi-quantitative CA rating has identified a potential negative correlation between tau-positive CA and severity of Braak staging (Figure S7). However, the precise role of CA throughout normal and pathological aging remains unclear, as additional studies are needed to determine whether CA correlates with one or more neuropathological, genetic, or cognitive variables that are linked to clinical disease onset or progression. We speculate that a particular tau strain may originate from CA, an exciting area for further research. Consistent with this possibility, our 3D reconstruction analysis indicates that anti-mouse IgG-specific neuritic tau may coalesce or merge with CA in human brain. The dome-like structures observed indicate that CA are “hollowed out” during antigen retrieval, which is supported by the observation that CA recovered from patient CSF lacks an outer membrane thought to be shed during the extrusion process into ventricular space (Riba et al., 2019). The same group analyzed CA in human tissue using a semithin sectioning approach and was able to capture the interior contents of CA, presumably by stabilizing the CA contents via epoxy resin per standard semithin sectioning procedures (Auge et al., 2019). Although our IHC results demonstrate that a total tau pool localizes within PAS granules and CA, we note that hyper-phosphorylated tau appeared to be restricted from these structures. The close proximity of hyper-phosphorylated tau around CA in AD brain may indicate that, upon exit from CA, tau could become subjected to pathological PTMs, including acetylation and phosphorylation, which may promote its aggregation and/or toxicity.
Previous studies have indicated that tau depletion is beneficial and protective against neuronal damage induced by Aβ pathology (Roberson et al., 2007), epileptic seizures (Devos et al., 2013, Gheyara et al., 2014, Holth et al., 2013, Li et al., 2014), and traumatic brain injury (TBI) (Cheng et al., 2014). Whether such neuroprotection is related to altered PAS granule accumulation is unclear. The reduction of PAS granules in TKO mice and increase in 6-month-old 3xTg-AD mice compared with age-matched controls suggests that tau could contribute to PAS granule stability; however, this possibility requires additional studies. Factors that regulate tau triage including HDAC6 and other autophagy regulators could also be linked to PAS granule formation or dissolution. In support of this possibility, we observed that loss of HDAC6 was sufficient to reduce PAS granules in aged mice (Tseng et al., 2017).
PAS granules and CA deposits increase with aging and are closely associated with astrocytes and microglia. Thus, we performed a more detailed evaluation of glia localization to PAS granules. Indeed, our data indicate that both microglia and, more prominently, astrocytes directly engage PAS granules. Given the steady decline of proteostasis during aging, it is plausible that aberrant glial activity in the aging brain coupled with declining autophagy and proteasome activity could exacerbate tau accumulation and drive chronic PAS granule formation. In fact, complete removal of tau (tau KO mice) was sufficient to eliminate anti-mouse IgG-specific tau antibody immunoreactivity and reduce the anti-mouse IgM-specific PAS granule pool by ∼ 30% (Figures 2B and 2C), indicating that tau depletion could represent a strategy to suppress PAS granule or CA accumulation.
We used Serpina3n to mark reactive astrocytes since Serpina3n expression is sharply elevated after inflammatory stimuli (e.g., LPS injection) (Clarke et al., 2018). The transcriptional pattern of Serpina3n specifically in the hippocampus (Zhang et al., 2014b) is consistent with PAS granule accumulation and may be linked to an age-related increase in A1 neurotoxic astrocytes, which is common in neurodegenerative disorders such as AD or amyotrophic lateral sclerosis (ALS) characterized by complement-mediated synapse loss (Liddelow et al., 2017). Despite the strong correlation between toxic astrocytes and age-related neurodegeneration, little is known about the role of Serpina3n in aging or the pathophysiology of AD. Serpina3n, also commonly referred to as α-1-antichymotrypsin (ACT), is primarily expressed in mature astrocytes in humans (Zhang et al., 2016), is increased in AD patient brain, and also has been shown to promote abnormally modified tau in primary cortical neurons via the c-Jun N-terminal kinase pathway (Padmanabhan et al., 2006). Serpina3n/ACT is an irreversible inhibitor of chymotrypsin, a protease that cleaves tau between the N-terminal projection domain and the microtubule repeat domain (Steiner et al., 1990). Serpina3n-expressing reactive astrocytes may therefore exert neurotoxicity, in part, by impairing the degradation of tau and the clearance of PAS granules.
Our findings strengthen the notion that CA and PAS granules represent a marker of brain vulnerability during aging. CA1-localized astrocytes were reportedly more sensitive to oxidative stress compared with other brain regions (Ouyang et al., 2007), suggesting the CA1 region is particularly vulnerable to proteotoxic stress during aging. The marked association of PAS granules with reactive astrocytes implies that inflammation may trigger further downstream dysfunction during aging. For example, astrocytic endfeet associate with the vasculature in an aquaporin-dependent manner and the localization of aquaporins is increasingly dysregulated in response to brain injury and throughout the aging process (Mccaslin et al., 2011, Nielsen et al., 1997, Rash et al., 1998, Verbavatz et al., 1997). Therefore, chronically reactive astrocytes could impair the clearance of PAS granules or CA, disrupt glymphatic function, and potentially stabilize tau to promote its toxicity (Navarro et al., 2018).
Our observations support that astrocytes are intimately engaged with CA in human patients and PAS granules in aged rodents. The precise role of astrocyte reactivity in AD remains unclear, with some studies suggesting astrocytes are vital for neuronal survival in normal (Cui et al., 2001) and pathological conditions (Faulkner et al., 2004), yet exacerbate memory deficits in AD models (Wu et al., 2014). Astrocytes have also been observed clearing Aβ-positive presynaptic dystrophies in amyloid models (Gomez-Arboledas et al., 2018). Astrocyte reactivity in response to the hallmark pathological aggregates in AD may therefore initially represent a compensatory response that eventually becomes pathological.
Given the close association of neuritic tau and astrocytic processes surrounding CA, it is tempting to speculate that reactive astrocytes may accelerate the conversion of anti-mouse IgG-specific tauIR granules into more mature tau pathology or even facilitate tau release and spread from the hippocampus to neocortical brain regions in a prion-like manner. In such a scenario, astrocytes, PAS granules, and CA could potentially play an unanticipated role in mediating tau stabilization, aggregation, and spreading in tauopathies, which could provide new and unanticipated avenues for therapeutic intervention. Conversely, PAS granules and CA may represent an attempt by phagocytic astrocytes to clear neuronal debris including those induced by age-related stress. Related to this point, our correlation analyses of CA-tau may indicate less tau localization to CA in the hippocampal sulcus during AD progression.
In summary, our findings describe a novel tau species in the aging brain and underscore the need to further investigate neuron-glia interactions throughout normal and pathological aging in both human patients and rodent models
Limitations of the Study
Our study demonstrates that tau within PAS granules and CA is predominantly de-phosphorylated at the tau-1 epitope. Future studies are needed to investigate other phospho-epitopes or post-translational modifications that may alter tau's localization to PAS granules or CA. Our experiments do not address the question of whether PAS granules or CA are protective or rather act as potential pathological features of aging. Although our studies indicate that tau localizes to these structures, they may not be completely synonymous between mouse and human species, and therefore the species-specific consequences of tau localization to PAS granules and CA requires further investigation.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Todd J. Cohen (toddcohen@neurology.unc.edu).
Materials Availability
This study did not generate unique reagents.
Data and Code Availability
Data in this study will be provided upon request from the corresponding author.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Support for this work was provided by National Institutes of Health (NIH) grants R01AG061188 (T.J.C) and R21AG058080 (T.J.C.), the National Center for Advancing Translational Sciences (NCATS) grant UL1TR001111 (T.J.C.), Alzheimer's Association grant NIRG-14-321219 (T.J.C.), an Association for Frontotemporal Degeneration (AFTD) pilot grant (T.J.C.), and the American Federation for Aging Research (AFAR) grant RAG15247 (T.J.C.). Microscopy was performed at the UNC Neuroscience Microscopy Core Facility supported by grant P30NS045892 and the NIH-NICHD Intellectual and Developmental Disabilities Research Center Support grant U54HD079124. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. John Trojanowski from the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania for providing human postmortem control and AD brain tissue along with their patient demographics.
Author Contributions
C.M.W. performed most of the experiments in this study. A.A. assisted with immunoblotting, whereas J.-H.T., R.M., S.S., H.A., and S.T. assisted with immunofluorescence staining, tissue sectioning, and confocal microscopy. J.S., R.M., S.S., and Y.-Y.I.S. assisted with data analysis and data interpretation. D.J.I. and R.L. performed IHC analysis of human postmortem brain tissues and imaging. C.M.W. and T.J.C. wrote the manuscript. This study was directed and supervised by T.J.C.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101255.
Supplemental Information
References
- Akiyama H., Kameyama M., Akiguchi I., Sugiyama H., Kawamata T., Fukuyama H., Kimura H., Matsushita M., Takeda T. Periodic acid-Schiff (PAS)-positive, granular structures increase in the brain of senescence accelerated mouse (SAM) Acta Neuropathol. 1986;72:124–129. doi: 10.1007/BF00685973. [DOI] [PubMed] [Google Scholar]
- Auge E., Bechmann I., Llor N., Vilaplana J., Krueger M., Pelegri C. Corpora amylacea in human hippocampal brain tissue are intracellular bodies that exhibit a homogeneous distribution of neo-epitopes. Sci. Rep. 2019;9:2063. doi: 10.1038/s41598-018-38010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auge E., Cabezon I., Pelegri C., Vilaplana J. New perspectives on corpora amylacea in the human brain. Sci. Rep. 2017;7:41807. doi: 10.1038/srep41807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auge E., Duran J., Guinovart J.J., Pelegri C., Vilaplana J. Exploring the elusive composition of corpora amylacea of human brain. Sci. Rep. 2018;8:13525. doi: 10.1038/s41598-018-31766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C., Belbin O., Kalsheker N., Morgan K. SERPINA3 (aka alpha-1-antichymotrypsin) Front. Biosci. 2007;12:2821–2835. doi: 10.2741/2275. [DOI] [PubMed] [Google Scholar]
- Binder L.I., Frankfurter A., Rebhun L.I. The distribution of tau in the mammalian central nervous system. J. Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cana A., Herder V., Hansmann F., Beineke A., Baumgartner W., Spitzbarth I. Characterization of periodic acid-schiff-positive granular deposits in the Hippocampus of SJL/J mice. Toxicol. Pathol. 2015;43:737–742. doi: 10.1177/0192623314564254. [DOI] [PubMed] [Google Scholar]
- Chen Z., Jalabi W., Hu W., Park H.J., Gale J.T., Kidd G.J., Bernatowicz R., Gossman Z.C., Chen J.T., Dutta R., Trapp B.D. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat. Commun. 2014;5:4486. doi: 10.1038/ncomms5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.S., Craft R., Yu G.Q., Ho K., Wang X., Mohan G., Mangnitsky S., Ponnusamy R., Mucke L. Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice. PLoS One. 2014;9:e115765. doi: 10.1371/journal.pone.0115765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L.E., Liddelow S.A., Chakraborty C., Munch A.E., Heiman M., Barres B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. U S A. 2018;115:E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T.J., Guo J.L., Hurtado D.E., Kwong L.K., Mills I.P., Trojanowski J.Q., Lee V.M. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Allen N.D., Skynner M., Gusterson B., Clark A.J. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia. 2001;34:272–282. doi: 10.1002/glia.1061. [DOI] [PubMed] [Google Scholar]
- Devos S.L., Goncharoff D.K., Chen G., Kebodeaux C.S., Yamada K., Stewart F.R., Schuler D.R., Maloney S.E., Wozniak D.F., Rigo F. Antisense reduction of tau in adult mice protects against seizures. J. Neurosci. 2013;33:12887–12897. doi: 10.1523/JNEUROSCI.2107-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehner J., Madhusudan A., Konietzko U., Fritschy J.M., Knuesel I. Co-localization of Reelin and proteolytic AbetaPP fragments in hippocampal plaques in aged wild-type mice. J. Alzheimers Dis. 2010;19:1339–1357. doi: 10.3233/JAD-2010-1333. [DOI] [PubMed] [Google Scholar]
- Drewes G., Ebneth A., Preuss U., Mandelkow E.M., Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Faulkner J.R., Herrmann J.E., Woo M.J., Tansey K.E., Doan N.B., Sofroniew M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheyara A.L., Ponnusamy R., Djukic B., Craft R.J., Ho K., Guo W., Finucane M.M., Sanchez P.E., Mucke L. Tau reduction prevents disease in a mouse model of Dravet syndrome. Ann. Neurol. 2014;76:443–456. doi: 10.1002/ana.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arboledas A., Davila J.C., Sanchez-Mejias E., Navarro V., Nunez-Diaz C., Sanchez-Varo R., Sanchez-Mico M.V., Trujillo-Estrada L., Fernandez-Valenzuela J.J., Vizuete M. Phagocytic clearance of presynaptic dystrophies by reactive astrocytes in Alzheimer's disease. Glia. 2018;66:637–653. doi: 10.1002/glia.23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holth J.K., Bomben V.C., Reed J.G., Inoue T., Younkin L., Younkin S.G., Pautler R.G., Botas J., Noebels J.L. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J. Neurosci. 2013;33:1651–1659. doi: 10.1523/JNEUROSCI.3191-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M., Walker L.C., Schwarb P., Hengemihle J., Kuo H., Snow A.D., Bamert F., Ingram D.K. Age-related deposition of glia-associated fibrillar material in brains of C57BL/6 mice. Neuroscience. 1994;60:875–889. doi: 10.1016/0306-4522(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Knuesel I., Nyffeler M., Mormede C., Muhia M., Meyer U., Pietropaolo S., Yee B.K., Pryce C.R., Laferla F.M., Marighetto A., Feldon J. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol. Aging. 2009;30:697–716. doi: 10.1016/j.neurobiolaging.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kuo H., Ingram D.K., Walker L.C., Tian M., Hengemihle J.M., Jucker M. Similarities in the age-related hippocampal deposition of periodic acid-schiff-positive granules in the senescence-accelerated mouse P8 and C57BL/6 mouse strains. Neuroscience. 1996;74:733–740. doi: 10.1016/0306-4522(96)00169-8. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves C.A., Castillo-Carranza D.L., Sengupta U., Sarmiento J., Troncoso J., Jackson G.R., Kayed R. Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. FASEB J. 2012;26:1946–1959. doi: 10.1096/fj.11-199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Hall A.M., Kelinske M., Roberson E.D. Seizure resistance without parkinsonism in aged mice after tau reduction. Neurobiol. Aging. 2014;35:2617–2624. doi: 10.1016/j.neurobiolaging.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Liu F., Iqbal K., Grundke-Iqbal I., Gong C.X. Dysregulation of tau phosphorylation in mouse brain during excitotoxic damage. J. Alzheimers Dis. 2009;17:531–539. doi: 10.3233/JAD-2009-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libard S., Popova S.N., Amini R.M., Karja V., Pietilainen T., Hamalainen K.M., Sundstrom C., Hesselager G., Bergqvist M., Ekman S. Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PLoS One. 2014;9:e108861. doi: 10.1371/journal.pone.0108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Munch A.E., Chung W.S., Peterson T.C. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti P., Konat G.W. Hydrogen peroxide induces transient dephosphorylation of tau protein in cultured rat oligodendrocytes. Neurosci. Lett. 2001;311:142–144. doi: 10.1016/s0304-3940(01)02137-1. [DOI] [PubMed] [Google Scholar]
- Madhusudan A., Sidler C., Knuesel I. Accumulation of reelin-positive plaques is accompanied by a decline in basal forebrain projection neurons during normal aging. Eur. J. Neurosci. 2009;30:1064–1076. doi: 10.1111/j.1460-9568.2009.06884.x. [DOI] [PubMed] [Google Scholar]
- Maeda S., Sahara N., Saito Y., Murayama S., Ikai A., Takashima A. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer's disease. Neurosci. Res. 2006;54:197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Manich G., Auge E., Cabezon I., Pallas M., Vilaplana J., Pelegri C. Neo-epitopes emerging in the degenerative hippocampal granules of aged mice can be recognized by natural IgM auto-antibodies. Immun. Ageing. 2015;12:23. doi: 10.1186/s12979-015-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manich G., Cabezon I., Auge E., Pelegri C., Vilaplana J. Periodic acid-Schiff granules in the brain of aged mice: from amyloid aggregates to degenerative structures containing neo-epitopes. Ageing Res. Rev. 2016;27:42–55. doi: 10.1016/j.arr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Manich G., Cabezon I., Camins A., Pallas M., Liberski P.P., Vilaplana J., Pelegri C. Clustered granules present in the hippocampus of aged mice result from a degenerative process affecting astrocytes and their surrounding neuropil. Age (Dordr) 2014;36:9690. doi: 10.1007/s11357-014-9690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manich G., Del Valle J., Cabezon I., Camins A., Pallas M., Pelegri C., Vilaplana J. Presence of a neo-epitope and absence of amyloid beta and tau protein in degenerative hippocampal granules of aged mice. Age (Dordr) 2014;36:151–165. doi: 10.1007/s11357-013-9560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccaslin A.F., Chen B.R., Radosevich A.J., Cauli B., Hillman E.M. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J. Cereb. Blood Flow Metab. 2011;31:795–806. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuno S., Takahashi M., Gondo T., Hoshii Y., Hanai N., Ishihara T., Yamada M. Immunohistochemical, conventional and immunoelectron microscopical characteristics of periodic acid-Schiff-positive granules in the mouse brain. Acta Neuropathol. 1999;98:31–38. doi: 10.1007/s004010051048. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Akiguchi I., Seriu N., Ohnishi K., Takemura M., Ueno M., Tomimoto H., Kawamata T., Kimura J., Hosokawa M. Monoamine oxidase-B-positive granular structures in the hippocampus of aged senescence-accelerated mouse (SAMP8) Acta Neuropathol. 1995;90:626–632. doi: 10.1007/BF00318576. [DOI] [PubMed] [Google Scholar]
- Navarro P.P., Genoud C., Castano-Diez D., Graff-Meyer A., Lewis A.J., De Gier Y., Lauer M.E., Britschgi M., Bohrmann B., Frank S. Cerebral Corpora amylacea are dense membranous labyrinths containing structurally preserved cell organelles. Sci. Rep. 2018;8:18046. doi: 10.1038/s41598-018-36223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Nagelhus E.A., Amiry-Moghaddam M., Bourque C., Agre P., Ottersen O.P. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., Laferla F.M. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ouyang Y.B., Voloboueva L.A., Xu L.J., Giffard R.G. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J. Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan J., Levy M., Dickson D.W., Potter H. Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer's disease brain, induces tau phosphorylation in neurons. Brain. 2006;129:3020–3034. doi: 10.1093/brain/awl255. [DOI] [PubMed] [Google Scholar]
- Pisa D., Alonso R., Marina A.I., Rabano A., Carrasco L. Human and microbial proteins from corpora amylacea of Alzheimer's disease. Sci. Rep. 2018;8:9880. doi: 10.1038/s41598-018-28231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa D., Alonso R., Rabano A., Carrasco L. Corpora amylacea of brain tissue from neurodegenerative diseases are stained with specific antifungal antibodies. Front Neurosci. 2016;10:86. doi: 10.3389/fnins.2016.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porquet D., Casadesus G., Bayod S., Vicente A., Canudas A.M., Vilaplana J., Pelegri C., Sanfeliu C., Camins A., Pallas M., Del Valle J. Dietary resveratrol prevents Alzheimer's markers and increases life span in SAMP8. Age (Dordr) 2013;35:1851–1865. doi: 10.1007/s11357-012-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D., Gulisano W., Palmeri A., Arancio O. Rodent models for Alzheimer's disease drug discovery. Expert Opin. Drug Discov. 2015;10:703–711. doi: 10.1517/17460441.2015.1041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash J.E., Yasumura T., Hudson C.S., Agre P., Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. U S A. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba M., Auge E., Campo-Sabariz J., Moral-Anter D., Molina-Porcel L., Ximelis T., Ferrer R., Martin-Venegas R., Pelegri C., Vilaplana J. Corpora amylacea act as containers that remove waste products from the brain. Proc. Natl. Acad. Sci. U S A. 2019;116:26038–26048. doi: 10.1073/pnas.1913741116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Robertson T.A., Dutton N.S., Martins R.N., Roses A.D., Kakulas B.A., Papadimitriou J.M. Age-related congophilic inclusions in the brains of apolipoprotein E-deficient mice. Neuroscience. 1998;82:171–180. doi: 10.1016/s0306-4522(97)00284-4. [DOI] [PubMed] [Google Scholar]
- Selenica M.L., Benner L., Housley S.B., Manchec B., Lee D.C., Nash K.R., Kalin J., Bergman J.A., Kozikowski A., Gordon M.N., Morgan D. Histone deacetylase 6 inhibition improves memory and reduces total tau levels in a mouse model of tau deposition. Alzheimers Res. Ther. 2014;6:12. doi: 10.1186/alzrt241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhrao S.K., Neal J.W., Newman G.R. Corpora amylacea could be an indicator of neurodegeneration. Neuropathol. Appl. Neurobiol. 1993;19:269–276. doi: 10.1111/j.1365-2990.1993.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Steiner B., Mandelkow E.M., Biernat J., Gustke N., Meyer H.E., Schmidt B., Mieskes G., Soling H.D., Drechsel D., Kirschner M.W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990;9:3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendrei G.I., Lee V.M., Otvos L., Jr. Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J. Neurosci. Res. 1993;34:243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- Tseng J.H., Xie L., Song S., Xie Y., Allen L., Ajit D., Hong J.S., Chen X., Meeker R.B., Cohen T.J. The deacetylase HDAC6 mediates endogenous neuritic tau pathology. Cell Rep. 2017;20:2169–2183. doi: 10.1016/j.celrep.2017.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni S., Moda F., Zattoni M., Bistaffa E., De Cecco E., Rossi M., Giaccone G., Tagliavini F., Haik S., Deslys J.P. Differential overexpression of SERPINA3 in human prion diseases. Sci. Rep. 2017;7:15637. doi: 10.1038/s41598-017-15778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbavatz J.M., Ma T., Gobin R., Verkman A.S. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J. Cell Sci. 1997;110(Pt 22):2855–2860. doi: 10.1242/jcs.110.22.2855. [DOI] [PubMed] [Google Scholar]
- Wirak D.O., Bayney R., Ramabhadran T.V., Fracasso R.P., Hart J.T., Hauer P.E., Hsiau P., Pekar S.K., Scangos G.A., Trapp B.D. Deposits of amyloid beta protein in the central nervous system of transgenic mice. Science. 1991;253:323–325. doi: 10.1126/science.1857970. [DOI] [PubMed] [Google Scholar]
- Wu Z., Guo Z., Gearing M., Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer's [corrected] disease model. Nat. Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu C., Wu J., Tao J.J., Sui X.L., Yao Z.G., Xu Y.F., Huang L., Zhu H., Sheng S.L., Qin C. Tubastatin A/ACY-1215 improves cognition in Alzheimer's disease transgenic mice. J. Alzheimers Dis. 2014;41:1193–1205. doi: 10.3233/JAD-140066. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O'keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S., Li G. Purification and characterization of progenitor and mature human astrocytes Reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data in this study will be provided upon request from the corresponding author.