Significance
The basic mechanism by which vertebrate collagenous tissues are mineralized remains unclear, despite the importance of mineralization for skeletal formation and regeneration. The present study of normally mineralizing avian leg tendon, that presents structural, molecular biological, and biochemical features common to other collagenous vertebrate tissues, reveals previously unknown three-dimensional interrelationships between an extensive tendon lacuno-canalicular network, the extracellular matrix, and mineral deposits. These interrelationships suggest a mechanism where the lacuno-canalicular system facilitates the transport of mineral ions and possibly mineralization precursors. These ions and putative precursors are initially present in interfibrillar collagen spaces and subsequently translocated to neighboring collagen fibrils. Mineral particles then nucleate in association with collagen to form the well-known collagen–mineral composite material of the skeleton.
Keywords: biomineralization, lacuno-canalicular network, inter- and intrafibrillar mineralization, FIB-SEM
Abstract
The spatial-temporal relationship between cells, extracellular matrices, and mineral deposits is fundamental for an improved understanding of mineralization mechanisms in vertebrate tissues. By utilizing focused ion beam-scanning electron microscopy with serial surface imaging, normally mineralizing avian tendons have been studied with nanometer resolution in three dimensions with volumes exceeding tens of micrometers in range. These parameters are necessary to yield sufficiently fine ultrastructural details while providing a comprehensive overview of the interrelationships between the tissue structural constituents. Investigation reveals a complex lacuno-canalicular network in highly mineralized tendon regions, where ∼100 nm diameter canaliculi emanating from cell (tenocyte) lacunae surround extracellular collagen fibril bundles. Canaliculi are linked to smaller channels of ∼40 nm diameter, occupying spaces between fibrils. Close to the tendon mineralization front, calcium-rich deposits appear between the fibrils and, with time, mineral propagates along and within them. These close associations between tenocytes, tenocyte lacunae, canaliculi, small channels, collagen, and mineral suggest a concept for the mineralization process, where ions and/or mineral precursors may be transported through spaces between fibrils before they crystallize along the surface of and within the fibrils.
Normally mineralized tendon, bone, and other collagen-based mineralized tissues such as dentin or cementum are materials remarkable for their hierarchical structure based on a unique combination of protein, mineral, and water that provides relatively high fracture resistance (1, 2). Such mechanical competence implies not only a well-balanced ratio between its content of mineral and collagen in extracellular matrices (ECMs) but also appropriate spatial relations between all its components (3–5). Development of mineralized collagenous tissue is orchestrated in part by tissue-forming cells: Tenocytes in tendon, osteoblasts in bone, odontoblasts in dentin, cementocytes in cementum, and chondrocytes in cartilage synthesize and secrete collagen as a principal product together with noncollagenous proteins and other molecules that comprise ECMs where mineral deposition predominantly occurs (6–9). Tenocytes and osteocytes, and presumably certain of the other cell types above, generate and extend a series of processes that interconnect cells, conduct fluid, and provide essential means for cell–cell and cell–matrix crosstalk and communication (10, 11).
While the structure of mineralized collagen fibrils has long been studied by transmission electron microscopy (TEM) and more recently by electron tomography (12–14), surprisingly little is known about the three-dimensional (3D) arrangement of collagen fibrils and mineral with respect to the cell network within a mineralizing matrix. Such information is difficult to obtain because it requires nanometer resolution and a large field of view in the range of tens of micrometers, in order to visualize several cells with their processes (15). In this study, focused ion beam-scanning electron microscopy (FIB-SEM) utilized in serial surface view mode (16) was used to examine simultaneously cells, ECM, and mineral deposits in turkey leg tendon (TLT) as a model system for collagen mineralization. Normally mineralizing tendons are representative of other vertebrate mineralizing tissues and are structurally more advantageous for study and analysis (17, 18). TLT is comprised of collagen fibrils elaborated in essentially parallel arrays rather than in more complex overlapping or twisted assemblages found in bone and other vertebrate systems (19). Furthermore, the sequence of events of mineral deposition may be followed and described spatially and temporally along the tendon length because the mineralization begins in the distal aspect of the tissue and continues uninterrupted proximally from that origin. In the context of location and time of tendon mineralization, events in mineral formation and development are independent within individual collagen fibrils and between neighboring fibrils comprising a particular fibril bundle. They are also independent within different bundles comprising a particular tendon and, as just noted, within the gross anatomical regions (distal–to–proximal) of a single tendon (17). Thus, the avian tendon represents timelines in the progressive formation of mineral at various spatial sites of different length scales and levels of hierarchical structure. These tissue characteristics have provided insight into certain aspects of the basic mechanism(s) of vertebrate mineralization and avian tendon adaptation in material properties (17, 18, 20).
In fully mineralized tendon, a previously unknown extensive 3D network of tenocytes–canaliculi resembling the network of osteocytes–canaliculi in bone was found. This network branches into finer channels that appear to occupy spaces between collagen fibrils. At the tendon mineralization front, arrays of calcium-rich deposits were observed in such channels between fibrils which subsequently propagated into neighboring fibrils. Collectively, these data begin to define possible temporal and spatial relationships between tendon cells, their elaborated ECM, and both inter- and intrafibrillar collagen mineralization, as well as further understanding of critical events mediating mineralization in vertebrate tissues.
Results and Discussion
Tenocyte Networks in the Mineralized Zone of Turkey Leg Tendon.
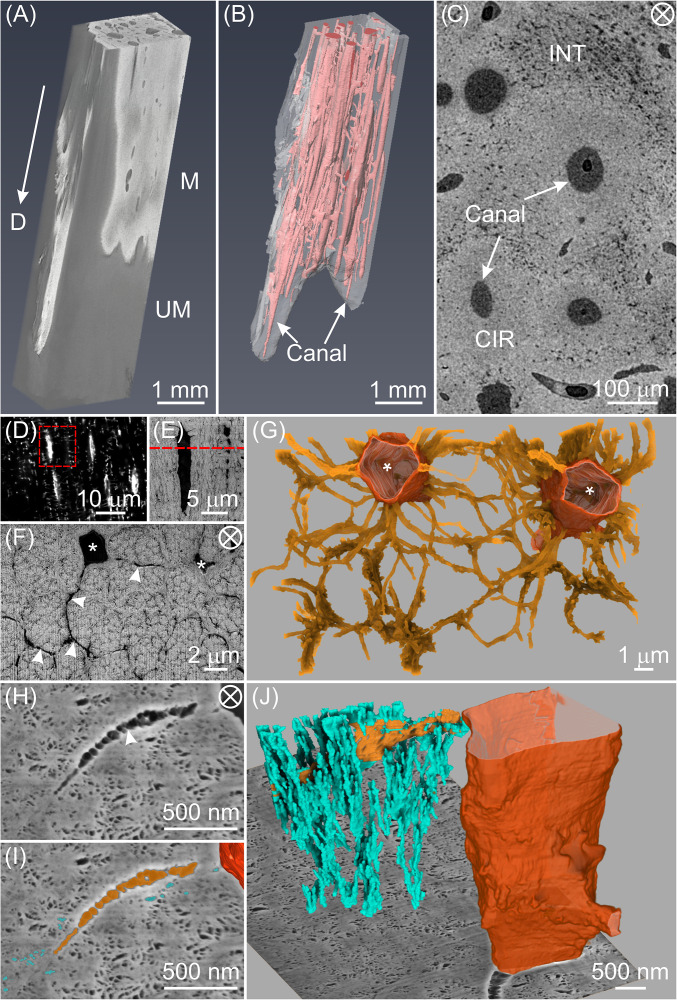
The fully mineralized zone of the tibialis cranialis tendon from the 26-wk-old male domestic turkey, Meleagris gallopavo, was first investigated across multiple length scales. Microcomputed tomography (micro-CT) of the distal tendon (D) showed a clear contrast between its radiopaque mineralized region (M) and dark gray unmineralized zone (UM) (Fig. 1A). Large canals ∼100 µm in diameter were clearly visible in the mineralized zone (Fig. 1B, pink; SI Appendix, Fig. S1 A–D), oriented mainly along the longitudinal direction of the tendon and interconnected through oblique perforating channels. Transverse sections of the fully mineralized zone (Fig. 1C) revealed two types of tissue: Circumferential (CIR) tissue with lower porosity surrounding the canals and interstitial (INT) tissue with higher porosity occupying the spaces between CIR zones. These tissues in avian tendon have been described previously by light microscopy and backscattered electron microscopy (21, 22) where collagen fibrils and pores of different sizes were also noted. In agreement with related published results (21), the volume fraction of CIR tissue qualitatively decreased from the more highly mineralized (mature) tendon region to the region closer to the mineralization front (Movie S1). Interestingly, the CIR tissue together with the canals resembles the morphology of Haversian systems in bone (23) and the central canals may contain blood vessels to provide ions, nutrients, and other substituents for cell viability, which may play a critical role in the mineralization process (24).
Fig. 1.
The tenocyte network in a highly mineralized zone of TLT from a tendon prepared by chemical fixation and stained with osmium tetroxide alone. (A) Three-dimensional reconstruction of micro-CT data from the more heavily mineralized distal end of the leg tendon. This region contains both mineralized (radiopaque, M) and unmineralized (darker gray, UM) zones. The arrow points toward the distal end (D) of the tendon. (B) The mineralized zone in A contains multiple unmineralized canals (pink) principally oriented in the tendon longitudinal direction. (C) High-resolution micro-CT image of a representative transverse section from a fully mineralized zone of the tendon reveals circumferential regions (CIRs) with qualitatively lower porosity surrounding the canals and interstitial tissue (INT) with higher porosity between CIRs. (D) Confocal image of a longitudinal section in the mineralized zone of a rhodamine-stained tendon showing tenocyte lacunae and canaliculi (white streaks and small white dots/lines, respectively). An area framed in red is enlarged and shown by energy selective backscattered (EsB) imaging in E where lacunae and canaliculi are more apparent. (F) EsB image of a transverse section (marked by the red dashed line in E) showing two tenocyte lacunae (*) and radiating canaliculi (arrowheads) surrounding mineralized collagen fibril bundles. (G) FIB-SEM 3D reconstruction of a volume of F illustrating the two tenocyte lacunae (tangerine) and their associated canaliculi (gold). (H) High-resolution SE image of a tendon section showing a portion of a cell lacuna, canaliculus (arrowhead) and fine pores, highlighted in dark tangerine, gold, and turquoise, respectively in I. (J) Three-dimensional rendering corresponding to H and I of the tenocyte lacuno-canalicular network intersecting with a FIB-SEM background image plane and demonstrating the numerous secondary channels (turquoise) branching from a canaliculus (gold) and disposed primarily in the longitudinal tendon direction. The tenocyte lacuna is defined by a contoured surface (tangerine). The circled cross in C, F, and H denotes the view along the longitudinal direction of the tendon.
Reconstruction of micro-CT images (SI Appendix, Fig. S1 E–H) revealed elongated/prolate cell lacunae in the CIR regions principally oriented along the tendon long axis. At higher resolution, laser scanning confocal microscopy (LSCM) imaging (Fig. 1D, SI Appendix, Fig. S2, and Movie S2) demonstrated a cellular network that is similar to the lacuno-canalicular system in bone (10), where osteoblasts differentiated into osteocytes and formed a complex 3D network of cells connected through canaliculi. This network is known to be related to the microarchitecture of collagenous tissue, most notably the local fiber orientation (10, 25).
The spindle-shaped cell lacunae in mineralized tendon were oriented along the longitudinal direction of the tendon, a result consistent with that found in a serial block-face SEM study of healthy human tendon (26). LSCM also showed that the canaliculi were radiating from the lacunae, mainly perpendicular to the lacunae long axes. The same area was further investigated by FIB-SEM imaging with a voxel size of 10.6 × 10.6 × 21 nm3 (Fig. 1 E–G and Movie S3), which showed the canaliculi with an average diameter of ∼100 nm, similar to that found in mouse femoral bone (27), surrounding and occasionally traversing collagen fibril bundles (Fig. 1G).
High-resolution FIB-SEM imaging with a voxel size of 6.0 × 6.0 × 8.7 nm3 (Fig. 1 H–J and Movie S4) revealed a significant number of finer pores with a diameter of ∼40 nm connected to the canaliculi. Three-dimensional reconstruction of these pores showed that they formed channels (Fig. 1J) predominantly along the longitudinal direction of collagen fibril bundles. Whether these pores are filled with fluid or may contain organic material such as glycosaminoglycans and/or cell processes is difficult to determine with current FIB-SEM analysis. Nonetheless, these smaller interconnected pores, termed secondary channels, might facilitate the mineralization of collagen fibrils that are far from the tenocytes of the tissue through possible mechanisms of transport or diffusion of ions or mineral precursors, for example.
Tenocytes, Collagen Fibrils, and Mineral Deposits at the Mineralization Front.
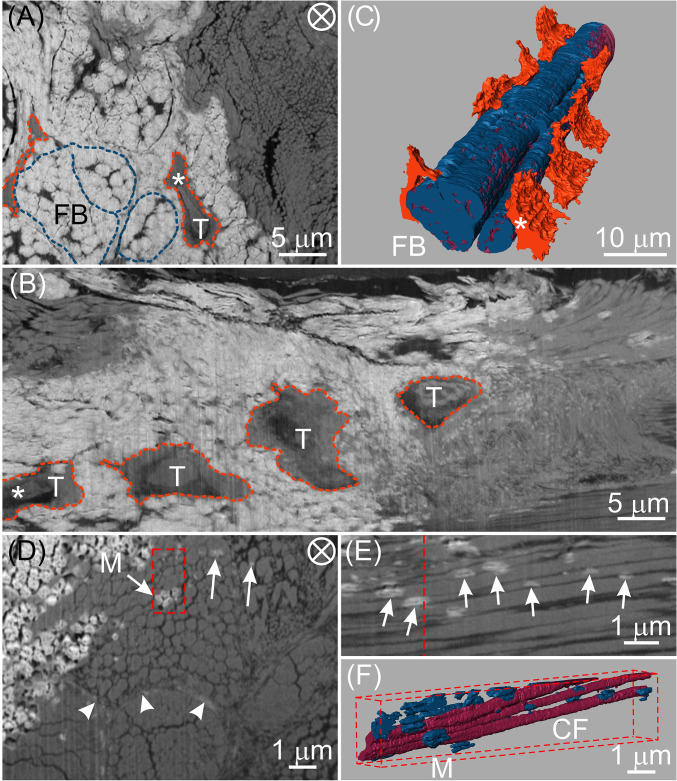
To gain insight into the process of vertebrate mineralization, thin longitudinal sections (∼150 µm) of leg tendons from freshly killed 23-wk-old turkeys were investigated by FIB-SEM. The tissues contained the transitional region between unmineralized and mineralized portions of the tendon and were prepared by high-pressure freezing (HPF) followed by automated freeze substitution (AFS) with uranyl acetate staining. Tenocytes, ECM components, particularly collagen fibril bundles, and mineral deposits could be clearly identified in SEM images (Fig. 2A). From longitudinal views with respect to the tendon long axis (Fig. 2B), for example, four tenocytes were seen aligned along collagen fibril bundles. Such alignment could be more clearly visualized by 3D rendering where tenocyte lacunae were in close contact with mineralized collagen fibril bundles (Fig. 2C and Movie S5). The bundles were circumferentially enclosed by a thin sheath, known as the endotendon (9), and themselves contained individual mineralized collagen fibrils (SI Appendix, Fig. S3). Such sheaths enclosing collagen bundles were also observed in unmineralized tendon regions, where cells and their extensions were situated in the spaces between several collagen fibril bundles (SI Appendix, Fig. S4). A recent mouse tendon study by Kalson et al. (28) demonstrated similar results where cells had elongated and interconnected cell processes, which delimited collagen fibril bundles. These data suggest that cell processes may overlap and/or may be embedded in the sheath surrounding collagen fibril bundles. While collagen becomes mineralized at later stages of tendon development, the canaliculi associated with sheath regions remain unmineralized and may serve to accommodate cell processes when the tissue becomes more completely mineralized (SI Appendix, Fig. S4).
Fig. 2.
FIB-SEM images and corresponding 3D reconstructions of tenocyte lacunae, collagen fibrils, and mineral deposits at an interface between mineralized and unmineralized avian tendon zones. Images are from the same specimen, prepared by cryomicrotomy and HPF. AFS followed these procedures and the sample was stained with uranyl acetate alone. (A) EsB image of a transverse section of a typical tendon volume showing mineralized (white) and unmineralized (gray) zones. Two collagen fibril bundles (FB) and tenocytes (T) may be identified in the mineralized zone and are denoted by dashed blue and tangerine lines, respectively. There are thin, dark structures of unknown nature that appear to separate individual mineralized units within bundles. Such structures may possibly be related to canaliculi surrounding fibril bundles. (B) Longitudinal view obtained by digital processing the full stack of FIB-SEM slices through the same tendon volume represented by the single transverse EsB image in A. Tenocytes (T) within their lacunae are outlined in tangerine. (C) Three-dimensional reconstruction of the FIB-SEM data of the volume of A and B showing tenocyte lacunae (tangerine) adjacent to and aligned along the longitudinal direction of mineralized collagen fibril bundles. Bundles may be distinguished by their component collagen fibrils (wine) and mineral (blue). The same tenocyte (T) in its lacuna has been followed and identified (*) in each of the images, A and B, and their reconstruction, C. (D) High-resolution EsB image showing mineral (arrows, M) within collagen fibril bundles and sheath regions (arrowheads). (E) Longitudinal view obtained by digital processing the full stack of FIB-SEM slices through the same tendon volume represented by the transverse EsB image in the red-framed area of D. The red dashed line in E represents the location of the framed area of D. Numerous mineral deposits (arrows) are associated with collagen fibrils and appear predominantly as prolate ellipsoids in shape and elongated in the tendon longitudinal direction. (F) Three-dimensional reconstruction of the volume marked by the red-framed area in D showing several collagen fibrils (CF, wine) and their associated discrete mineral deposits (M, blue) that vary in length and thickness. As in E, the longitudinal view reveals numerous mineral deposits interrelated with several collagen fibrils, many of which are separated by dark, narrow spaces. The circled cross in A and D denotes the view along the longitudinal direction of the tendon.
High-resolution FIB-SEM data with a voxel size of 12.0 × 12.0 × 24.0 nm3 revealed isolated mineral deposits far from tenocytes and within both collagen fibril bundles (arrows, Fig. 2D) and sheath regions (arrowheads, Fig. 2D). Mineral deposits within collagen fibril bundles framed in Fig. 2D were digitally processed in a longitudinal view (Fig. 2E) and rendered three-dimensionally in a slightly oblique view (Fig. 2F). Such longitudinal perspectives clearly demonstrate that the mineral deposits (arrows, Fig. 2E; blue, Fig. 2F) have a prolate ellipsoidal shape elongated along the tendon longitudinal direction. This shape suggests that the mineral grows faster along the longitudinal rather than the radial direction of collagen fibrils. This observation is consistent with reports that mineral crystals grow preferentially along their crystallographic c-axes and in the direction of the collagen long axis (29). Fig. 2E also shows spaces averaging ∼40 nm in width between adjacent collagen fibrils viewed in longitudinal profile. Such spaces may correspond to the secondary channels identified and described in Fig. 1H.
To investigate the spatial relationships between collagen fibrils and mineral deposits and to understand the progression of mineralization events, tendons from 23-wk-old turkeys were cryofixed utilizing HPF, stained with osmium tetroxide and uranyl acetate and examined ultrastructurally with FIB-SEM with a voxel size of 6.0 × 6.0 × 8.7 nm3. Both transverse (Fig. 3 A and B) and longitudinal (Fig. 4 D and F) sections of tendons showed relatively thinner collagen fibrils (∼30 to 50 nm in diameter) joining or fusing with thicker fibrils (∼200 to 300 nm in diameter). Thinner fibrils could represent more recently synthesized and secreted type I collagen compared to thicker fibrils (30) or they may be the characteristically very fine type XII collagen fibrils that are also known to comprise tendon (31). Both thinner and thicker fibrils maintain the characteristic collagen periodicity (Fig. 4F). In many instances, such thinner fibrils, as well as thicker fibrils, were found in the vicinity of the earliest detectable mineral deposits in tendon regions (Figs. 3A and 4 D and F). Deposits may come into close contact with both thin and thick fibrils (Fig. 4 D and F) and their disposition between fibrils of any size constitutes interfibrillar mineral. Deposits located within fibrils define intrafibrillar mineral.
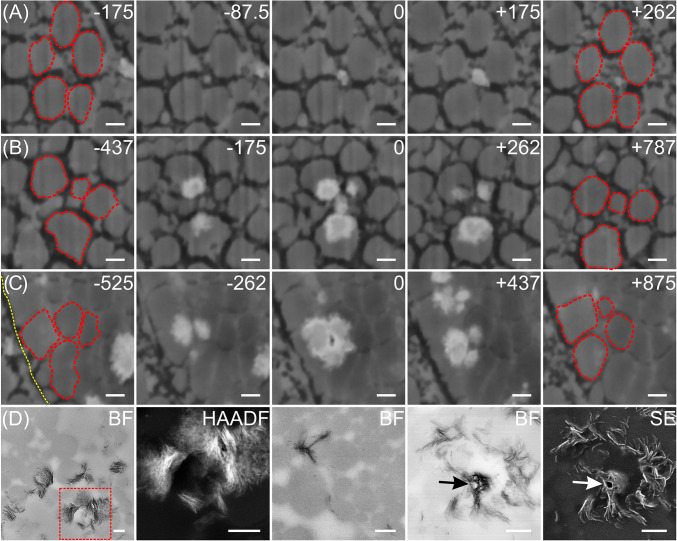
Fig. 3.
Series of FIB-SEM (A–C) and TEM images (D) showing spatial relationships between collagen fibrils (A–C, red outlines) and mineral deposits (white) in a distal region of an avian tendon. The FIB-SEM panels show different tissue regions in transverse profile in a single tendon specimen, prepared by HPF followed by AFS and staining with osmium tetroxide-uranyl acetate. Each FIB-SEM panel of five images represents mineralization occurring at different degrees at the specific locations imaged. A–C illustrate three sequences of mineralization events from sites in two different fibril bundles in the same tendon (A and B from the same bundle; C from a bundle different from that of A and B). Because all three sites are distinct within the same or different fibril bundles, A–C illustrate sequences of mineralization events that are spatially and temporally independent of each other. The panels represent various stages of mineral deposition based on increasing mineral content apparent in the images: An early stage (A), an intermediate stage (B), and a relatively more mature stage (C). Numbers at the Top Right corners of A–C represent the distance (in nanometers) of each image to the reference image (marked by 0) along the longitudinal direction of the collagen fibrils (the tendon long axis) in the specimen volume investigated. (A) Early stage mineral formation beginning and developing among five unmineralized collagen fibrils. The mineral in this sequence appears (image −87.5) in close association with a fibril(s) of small diameter located between five other fibrils of larger diameter, the mineral deposit(s) increases in size (image 0 and 175), and it gradually disappears (image 262) as the distance increases along the fibrils from the reference image (0). Because the association between the fibril(s) and mineral is not explicitly clear, it is difficult to determine whether these images illustrate intra- or interfibrillar mineralization or both processes. (B) Several mineral deposits appearing principally as intrafibrillar collagen mineralization. The intrafibrillar deposits occur at certain sites along the periphery of fibrils, only partially mineralizing them, and they do not form a shell of mineral about the fibril perimeter. As it grows and propagates from such sites, mineral changes in size and expanse with distance radially and along the fibrils (images −175, 0, and 262). The central deposit (image 0) would appear to define both intra- and interfibrillar mineralization. (C) A region of densely packed collagen fibrils illustrating a degree of intrafibrillar mineralization that reveals a small dark area, possibly a narrow channel, in the central interfibrillar space (image 0). The mineral deposits are predominantly intrafibrillar and they appear and disappear along the length of the several fibrils as at other sites (A and B). The yellow dotted line (image −525) represents the boundary of the collagen fibril bundle containing the fibrils of interest. (D) Brightfield (BF, far Left) and high angle annular darkfield (HAADF) images of a tendon sample prepared by chemical fixation. The images show mineral deposits principally surrounding collagen fibrils as viewed in transverse profiles. The HAADF image was obtained from the red-framed region of BF (far Left). The Middle BF image demonstrates intrafibrillar mineral deposits (black) spanning several collagen fibrils (gray). BF and SE images (panels 4 and 5, respectively, from the Left) show a possible channel (arrows) encircled by mineral in interfibrillar space. (Scale bar: 200 nm for all panels.)
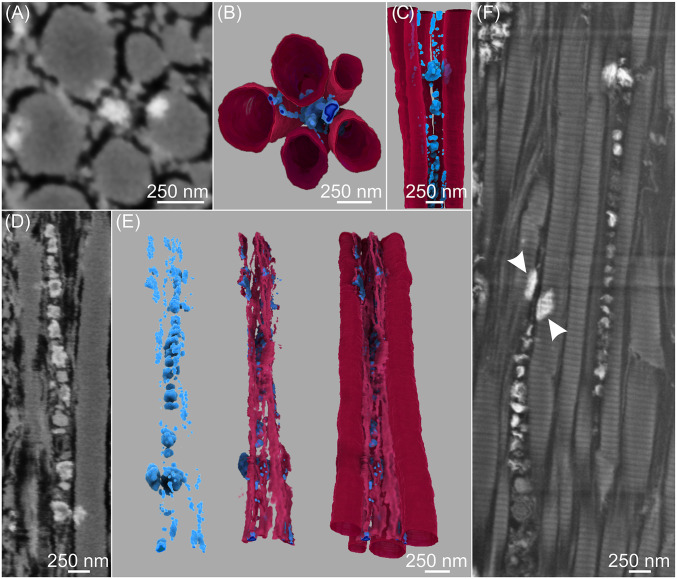
Fig. 4.
FIB-SEM images (A, D, and F) and 3D reconstructions (B, C, and E) of early stages of mineralization showing structural and spatial relationships between mineral deposits, possible secondary channels, and collagen fibrils in avian tendon. The tendon specimen was prepared by HPF followed by AFS and staining with osmium tetroxide-uranyl acetate. A–C, D and E, and F represent three different regions of the same tendon: distal (A–C and D and E) and proximal (F). (A) SE image of a transverse section of five collagen fibrils, the intervening spaces between them (black) which may be secondary channels, and several small mineral deposits (white). (B) Transverse, top-down, and (C) longitudinal view of the 3D reconstruction of the volume represented by A, showing the mineral deposits (blue) both within and outside the collagen fibrils (wine). (D) SE image of the longitudinal section illustrates linearly arranged mineral deposits (white) of different sizes predominantly between thin and thick collagen fibrils in possible secondary channels (black). (E) Three-dimensional reconstructions of D showing mineral deposits only (blue; Left image), mineral associated with thin collagen fibrils (light wine; Middle image), and mineral associated with both thin and thick fibrils (wine; Right image) with mineral deposits aligned along the longitudinal collagen axes. (F) High-resolution SE image of a region of tendon ECM different from that in D but showing similar collagen mineralization characteristics; periodic banding of both thin and thick collagen fibrils is clear, thin, and thick fibrils fuse together, and mineral (white) is found in linear disposition between fibrils as well as on the surfaces of and within fibrils (arrowheads). The mineral may be associated with matrix vesicles as noted previously although such a relationship has not been established definitively. Spaces (black) between fibrils may denote putative secondary channels in the tendon matrix.
To examine possible spatial relationships between inter- and intrafibrillar mineralization, both transverse (Fig. 3) and longitudinal tissue sections (Fig. 4) were imaged and analyzed in 3D. Along the tendon long axis, successive FIB slices represent progressive changes in tissue location, time, and degree of tendon mineralization, and corresponding SEM images showed that mineral deposits grew along and within collagen fibrils (inter- and intrafibrillar collagen mineralization, respectively) (Fig. 3 A–C and Movie S6). Certain early-stage mineral deposits were found to associate with thin collagen fibrils between larger ones (Fig. 3A) and all deposits appeared and disappeared along the fibril lengths (Fig. 3 A–C). In some aspects of mineralization, a possible channel ∼40 nm in diameter was observed in the central space within a group of relatively more heavily mineralized fibrils (Fig. 3C). Such spaces may correspond to the dark, narrow separations found in longitudinal sections of tendon (Fig. 2E). TEM analysis of similar mineralizing tendon regions (Fig. 3D) also indicated the presence of plate-like mineral crystals within and outside collagen fibrils. Further, these mineral crystals were found to encircle channel-like structures (arrows) observed in brightfield and secondary electron (SE) images (panels 4 and 5 from the Left of Fig. 3D), results consistent with FIB-SEM findings (Figs. 2E and 3C).
Whether collagen fibril mineralization occurs in an inter- or intrafibrillar manner is critical to understanding mineralization mechanisms in vertebrates in general. Recently, by using FIB-SEM and TEM imaging, Reznikov et al. (14) suggested that mineral deposits in bone formed an interlinked network through cross-fibrillar mineralization, that is, by both inter- and intrafibrillar mineralization. This concept was implicit and proposed in earlier work with avian tendon examined by TEM (17, 30, 32). Here, segmentation and 3D reconstruction of tendon collagen fibrils and mineral deposits provided insight into collagen-mineral interactions regarding inter- and intrafibrillar mineralization of this tissue (Fig. 4). Numerous mineral deposits of variable sizes clearly appeared both between and within collagen fibrils. From the images presented here, the sizes of intrafibrillar mineral deposits may appear larger in general than those found in interfibrillar tissue regions. Such a result is only apparent, however, as the size of the mineral comprising either type of deposition varies greatly at each nucleation site as a function of several factors, including time (the stage of mineral growth and development).
Fibrils, themselves, were of different and variable diameters, as noted above. The individual mineral deposits (for example, Fig. 4 A, D, and F), rich in calcium and phosphate as detected by energy dispersive X-ray spectroscopy (EDS) (SI Appendix, Fig. S5), appeared to be located between collagen fibrils, whether thin or thick (interfibrillar mineral), as well as within thick fibrils (intrafibrillar mineral) (Fig. 4F). Deposits furthermore were frequently found in linear arrangements in interfibrillar spaces (Fig. 4 C–F) and, as such, were consistent with those reported in previous TEM studies (17, 30) of normally mineralizing avian tendon. The deposits were suggested to be associated with extracellular vesicles disposed in spaces between collagen fibrils (17, 30), and the vesicles appeared to maintain a close spatial relation with the extensive network of tenocyte cell processes in the tissue (30).
In the present investigation, extracellular vesicles could not be directly visualized since their investing membranes were not entirely clear. Previous studies reported that vesicles underwent changes resulting, for example, in the degradation of their membranes and the subsequent loss or release of their mineral contents to the ECM milieu (33, 34). Further, staining methods utilized in this investigation may not have enhanced the membranes sufficiently. Because their membranes were also uncertain, cell processes have not been directly identified in the current work, but it may be assumed that they occupy the spaces defined by canaliculi and secondary channels, as mentioned above. Thus, the tendon establishes and maintains an ultrastructural organization of collagen and spatially interrelated cell processes, ECM vesicles, and mineral deposits. In this regard, the processes, vesicles, and deposits provide a pathway by which mineral ions and/or mineral precursors may be actively transported, become accessible to collagen, and lead to collagen mineralization. This concept is supported by results shown in both Figs. 3 and 4. Further, the image data of the present work (Fig. 3 and 4, for example) also support the additional concept that interfibrillar mineral deposition precedes intrafibrillar mineralization, a result that is consistent with previous reports (32, 35).
The work here has shown mineral deposition associated with the surfaces and intrafibrillar domains of collagen (Fig. 4F). Such mineral formation within collagen commonly occurred at sites near every or nearly every putative mineralizing vesicle noted above in the interfibrillar collagen spaces (Fig. 4F and Movie S7). On reconstruction of mineral deposits in a representative volume of the ECM, the interfibrillar mineralized sites were isolated from each other and much more numerous than could be revealed in individual two-dimensional (2D) SEM slices (Movie S7). It also appeared as if individual interfibrillar mineral deposits were separated by a similar distance as they formed in linear arrangements (Movie S7). No direct interconnections could be clearly detected between inter- and intrafibrillar mineralization sites, so an immediate structural relationship, if any, between them cannot be determined. In interfibrillar spaces, it may be reasonable to assume that local changes occur in the concentrations of mineral ions or possible mineral precursors resulting from their loss or release from vesicles as noted above. Such fluctuations in mineral ion or precursor concentrations may then alter the physicochemical metastability of the fluid ion concentration surrounding collagen. The subsequent ion concentration changes may serve as a driving force for crystal nucleation, mediating formation and propagation of mineral over collagen surfaces and/or within collagen intrafibrillar domains. In addition, the establishment of simultaneous electrostatic neutrality and osmotic equilibrium between inter- and intrafibrillar spaces of collagen, as described by the Gibbs–Donnan model (36), has been proposed recently to explain the migration of mineral ions or mineral precursors into intrafibrillar collagen spaces (37). Thus, the normally mineralizing tendon model illustrates both inter- and intrafibrillar mineral deposition and yields results consistent with those of bone and tendon as described previously (14, 17, 30, 32).
The presence of a canalicular network throughout the ECM of avian tendon is compatible with the idea that mineral ions may be passively transported in its fluid-filled cavities. In this regard, the possibility exists that such mineral ions move about the interfibrillar collagen spaces and may mediate mineral deposition and its progression. This mechanism of passive transport may be independent or complementary to that provided by the putative active transport of ions and/or mineral precursors through vesicles described previously. Whatever the situation, the space between collagen fibrils appears to be critical for ion transport leading to mineral deposition of those fibrils comprising tendon structure.
Nucleation of mineral in association with collagen surfaces or within collagen fibrils has been conceptualized through different mechanisms. One such proposal involves calcium ion transport to collagen by binding to a negatively charged polyelectrolyte, such as phosphorylated osteopontin (38). Alternatively, ions may diffuse or be actively or passively transported as described above. In either case, the mineral ions are thought to associate and bind to specific sites defined stereochemically by charged amino acid residues comprising collagen α-chains located at the surfaces or within collagen fibrils (35, 39). Other potential sites of mineral ion binding at the collagen surfaces may be provided by charged, noncollagenous proteins such as fetuin, bone sialoprotein, osteocalcin, and osteopontin, which themselves are surface bound to collagen (35, 40–42). Additionally, osteocalcin is known to reside within collagen and in its so-called hole zones where it might bind calcium to facilitate nucleation (40).
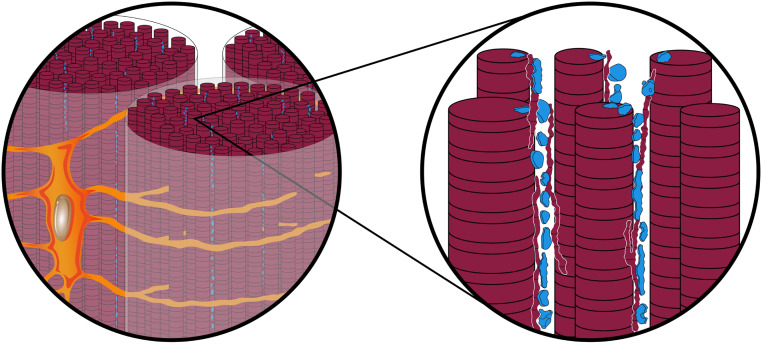
Based on the FIB-SEM observations from tendon mineralization fronts and fully mineralized zones, the spatial interrelations between tenocytes, their lacunae, ECM, and mineral deposits are proposed and schematically shown in Fig. 5. Tenocyte lacunae are interconnected predominantly through canaliculi, which surround each collagen fibril bundle circumferentially and occasionally traverse through them (SI Appendix, Fig. S4). Numerous finer channels branch predominantly in orthogonal directions from the canaliculi and pervade collagen fibril bundles approximately parallel to the fibril bundle longitudinal axes. The extent of such secondary channels intersecting with collagen fibril bundles has not yet been fully documented here by FIB-SEM and 3D reconstruction but, from initial data as represented by Fig. 1J and earlier 2D TEM images (17, 30), it is likely that secondary channels branch at multiple points and provide fluid and mineral ion access through cell processes and matrix vesicles to not only fibril bundle surfaces but also individual collagen fibrils within bundles. The nature of this suggested multiple branching and the direction of secondary channels with respect to bundles and fibrils can only be speculative at this point. Fibril bundles consist of numerous collagen fibrils that are separated by the secondary channels, and mineral deposition occurs in the channel spaces as well as on the surfaces of and within fibrils. Potentially, these interfibrillar secondary channels could allow the infiltration of the collagen matrix by many different ions, for example, calcium ions electrostatically bound to phosphorylated proteins that serve at the same time as calcium transporters and crystallization inhibitors, as proposed from mineralization studies in vitro, while mineral nucleation occurs on the surfaces of and within collagen fibrils (43–45).
Fig. 5.
Schematic illustration showing proposed ultrastructural interrelationships between tenocytes, their lacunae, secreted ECM, and mineral in avian leg tendon. Sections of tissue viewed along the long axis of collagen fibril bundles (Left) show a single tenocyte (tangerine) with its nucleus (gray-brown) and a number of cell processes (amber). Processes enter canaliculi (gold) that envelope fibril bundles about their surfaces and may branch into the bundle sheath (pale white). On enlargement (Right), numerous collagen fibrils (wine) comprising bundles consist of both thinner and thicker structures separated by secondary channels (white spaces) which originate in orthogonal directions from canaliculi and along collagen long axes. Mineral deposits (blue) appear outside and along the surfaces (interfibrillar locations) as well as inside (intrafibrillar locations) collagen fibrils. Banding about collagen represents its characteristic periodicity observed by electron microscopy.
Potential Concerns and Limitations of This Study.
While the data and concepts revealed and discussed above generate information regarding mineralization of avian tendons, there are several areas of the study that remain uncertain in nature. A principal concern in general is the possible creation of a microscopic artifact, potentially the result of dehydration of tendon tissue during specimen processing. In this regard, there may be shrinkage of tissue that leads to spaces between structural components such as thin and thick collagen fibrils and the misleading formation of canaliculi and secondary channels in tendon, for example. Further, dehydration may effectively concentrate mineral ions to produce the small, vesicle-like structures reported between, on the surface of, and within collagen. On the other hand, HPF and AFS mitigate shrinkage and dehydration and optimize tissue preparation for microscopic analyses and, moreover, collagen of various diameters and mineralizing matrix vesicles have been repeatedly documented in numerous previous studies in which tissues have been prepared by a variety of techniques, including conventional, anhydrous, as well as cryofixed methods. The presence of such tissue components observed regardless of preparative techniques would tend to diminish concerns that they are artifactual. This study has revealed both thicker and thinner collagen fibrils, the former representing type I collagen with its characteristic periodicity. The thin fibril type has not been specified although type XII collagen with its fine diameter is a known constituent of tendon (31). Immunocytochemical techniques will be applied in later work to determine thinner fibril type. Definitive identification of matrix vesicles has also not been made in this investigation, possibly because vesicle membranes are inadequately stained or they have been disrupted by their mineral contents as noted previously. Such vesicles are known repositories for alkaline phosphatase (33) and staining for this enzyme will be undertaken in other work to define these organelles unequivocally. Additionally, the present study does not address the possible presence of cell processes that are suggested to reside within canaliculi and secondary channels of the tendon. Detection of such processes will be the subject of further investigation in mineralizing avian tendon. The means by which mineral is propagated from inter- to intrafibrillar tissue spaces remain conjectural and elusive. This subject, too, will be investigated more completely in future work. Finally, there is some caution to this study in terms of its sample pool; here, the number of animals (three) and the type of tendon (tibialis cranialis from among many mineralizing leg tendons) examined in detail were limited and FIB-SEM data were relevant to only a selected few regions of interest. Nonetheless, structural information obtained regarding collagen, mineralizing matrix vesicles, and the presence of mineral deposits associated with these tissue constituents was again largely in accordance with that of previous reports. Considering the highly consistent nature of earlier and present data, the current work has yielded observations of canaliculi and secondary channels and their apparent interconnections in 3D. This study has led further to insight into possible mechanisms by which mineral ions in the supersaturated fluid bathing the tissue as well as potential mineral precursors may be directed and transported throughout the tendon ECM. In particular, the work suggests that tenocytes in mineralizing tendon play a role similar to that of osteocytes in establishing networks which have been associated with mineral homeostasis in the body (46, 47) and with the potential contribution to the transport of mineral precursors to the front of mineralizing osteoid (48–50).
Summary and Outlook.
In summary, FIB-SEM tomographic imaging has revealed 3D ultrastructural interrelations between tenocytes, tenocyte networks, ECM, and mineral deposits in normally mineralizing avian leg tendon. A complex and extensive cellular network was found consisting of canaliculi and much finer secondary channels that enveloped collagen fibril bundles and penetrated their sheaths. Further, secondary channels separated collagen fibrils within bundles and putatively provided the space for cell processes and the fluid environment passageway for mineral ion and/or mineral precursor transport. Possible matrix vesicles, commonly in linear arrangements, may originate from cell processes, and their mineralization outside collagen fibrils appeared to serve as a source of interfibrillar mineralization of the matrices. At subsequent stages of mineral formation, mineral deposits were observed on and along surfaces of collagen fibrils (interfibrillar mineralization) as well as within fibrils (intrafibrillar mineralization). Overall, this report advances knowledge and understanding of cellular and ECM architecture not previously appreciated from 2D image analyses and yields insight into the mechanism(s) of mineralization in avian tendon. The data also have implications for the means of mineralization in bone, dentin, cementum, and other vertebrate tissues. Additional studies of normally mineralizing avian tendon and its counterpart vertebrate tissues to elaborate secondary channel ultrastructure, the nature of both passive and active transport, and the precise means of mineral propagation outside and within collagen will address aspects of mineral deposition that still remain uncertain.
Materials and Methods
Specimen Preparation.
Three 23-wk-old and two 26-wk-old male domestic turkeys, species M. gallopavo, were obtained from local farms (Ullrichs Putenhof and Hartmanns Putenhof) and freshly killed. The tibialis cranialis tendons, containing the transitional region between noncalcified and calcified portions of the tissue, were carefully dissected from turkey legs, and the muscles attached to the tendons were removed using a scalpel. Dissection was conducted on an ice bath of phosphate-buffered saline (PBS) solution (Sigma-Aldrich). The tendons were then either sectioned with a cryomicrotome (HM 560 CryoStar Cryostat, Thermo Scientific) or cut with a scalpel to obtain thin sections 150 to 200 μm thick, followed by HPF. More specifically, samples were sandwiched between two carriers (200 μm brass planchet, type B freezer hats; Ted Pella, Inc.), filled with 1-hexadecane (Sigma-Aldrich) as cryoprotectant and then cryofixed in a model Leica EM HPM100 high-pressure freezing machine (Leica Microsystems).
Freeze substitution was carried out in a Leica EM AFS2 automated freeze-substitution unit (Leica Microsystems). The samples, still attached to the frozen carriers, were transferred in liquid nitrogen to 1 mL precooled freeze-substitution media (at −90 °C) in cryovials. The following freeze-substitution protocols were used to enhance the imaging contrast of unmineralized tissue for FIB-SEM and TEM:
Protocol 1: The freeze-substitution media consisted of 0.2% (wt/vol) uranyl acetate (SERVA Electrophoresis GmbH) in absolute acetone (diluted from 20% uranyl acetate stock in methanol) (Sigma-Aldrich). The temperature of the AFS processing chamber was first maintained at −85 °C for 16 h, then raised to −50 °C at a rate of 7 °C/h, held at −50 °C for 7 h, raised to 0 °C at a rate of 5 °C/h, and maintained at 0 °C for 72 h. When the temperature was −50 °C (third step as mentioned above), the sample carriers were removed in liquid nitrogen and the samples were stepwise infiltrated by Lowicryl HM20 (Polysciences, Inc.) with a progressively increasing ratio of HM20 to acetone (volume fraction of HM20: 0%, 30%, 50%, 70%, and 100%; 1 h for each step). Finally, the samples were transferred to fresh 100% HM20. Polymerization under ultraviolet (UV) light was begun at the fourth step of infiltration and continued to the end of the procedure.
Protocol 2: A mixture of 0.1% osmium tetroxide (Electron Microscopy Sciences), 0.1% uranyl acetate (SERVA Electrophoresis), 0.5% glutaraldehyde (Electron Microscopy Sciences), 1.5% H2O and 100% acetone was used for freeze substitution. The AFS temperature progression began at −120 °C for 3 h, followed by warming to −85 °C at a rate of 17.5 °C/h, maintaining −85 °C for 103 h, warming to −20 °C at a rate of 7.3 °C/h, maintaining −20 °C for 12 h, and finally warming to 4 °C at a rate of 2.6 °C/h. At this point, the samples were removed from the specimen carriers (at room temperature) and subsequently embedded in EPON resin (Polysciences, Inc.).
Conventional chemical fixation of freshly acquired samples was also used. After dissection of the turkey leg tendon, samples were chemically fixed in 4% paraformaldehyde (Electron Microscopy Sciences) and 2% glutaraldehyde (Electron Microscopy Sciences) in cacodylate buffer solution (Electron Microscopy Sciences) for 8 h, followed by staining with 2% osmium tetroxide (Electron Microscopy Sciences) in PBS solution for 2 h. After washing with PBS solution, the samples were dehydrated in a graded acetone series (30%, 50%, 70%, 90%, 100%, and 100%; 1 h for each step except for 70% overnight) and then stained with 0.004% (wt/v) Rhodamine 6G (Thermo Fisher) in acetone and finally embedded in Spurr resin (Polysciences, Inc.).
All embedded samples were ground with a series of carbide grinding papers to expose longitudinal sections of turkey leg tendons and then polished with a diamond suspension to improve surface smoothness for subsequent FIB-SEM serial surface imaging.
Microcomputed Tomography.
Spurr-embedded tendon sections containing both mineralized and unmineralized regions of the tissues were imaged using a micro-CT imager (RX Solutions EasyTom160/150 tomographic unit) at a resolution of 4.5 µm/voxel for larger volume observations and 1.0 µm/voxel for more refined volume observations at 100 kV and 200 µA. X-Act CT software (RX Solutions) was used to reconstruct the projection images.
Laser Scanning Confocal Microscopy.
A Leica TCS SP8 LSCM (Leica Microsystems) with a 40×/1.3 oil objective lens was used to identify tendon cells, organization, and connections through canaliculi. The 526 nm line of a multiline argon ion laser was used for Rhodamine 6G excitation with emission at 555 nm. Selected sites of mineralized tissue were further characterized in 3D, during which the specimens were typically imaged from the surface to depths of 30 µm with a step size of 300 nm for each image. Each sequence of the 12-bit images (pixel size 280 nm) thus obtained was segmented and reconstructed using Amira (Version 6.5, Thermo Fisher and Zuse Institute).
Focused Ion Beam-Scanning Electron Microscopy.
FIB-SEM in serial surface mode was performed with a Zeiss Crossbeam 540 station (Carl Zeiss). Carbon-coated specimens were oriented inside the FIB-SEM chamber so that the viewing direction was aligned with the tendon longitudinal direction. A coarse cross section was first milled with a 30 nA gallium beam at 30 kV acceleration voltage to provide a viewing channel for SEM observation. The exposed surface of this cross section was fine-polished by lowering the ion beam current to 1.5 nA. Subsequently, the fine-polished block was serially milled by scanning the ion beam parallel to the surface of the cutting plane using an ion beam of 100 pA, 700 pA, or 1.5 nA at 30 kV. After removal of each tissue slice, the freshly exposed surface was imaged at 1.5 to 3 kV acceleration voltage and 700 pA or 1 nA using both SE and energy selective backscattered (EsB) detectors. The slice thickness was roughly equivalent to the lateral resolution of 2D images, ranging from 6 to 24 nm. In a fully automated procedure, the milling was combined with SEM imaging in sequence (imaging, then sectioning and reimaging) to collect thousands of serial images. Serial EsB images were aligned (image registration) with in-house python script in Anaconda. The same transformation matrix applied to each image of the EsB stack was then applied to the corresponding SE image stack. Following alignment, the images were digitally processed to increase contrast and reduce curtaining effects and noise levels. The resulting stacks of images were then segmented based on their structural features and reconstructed using Amira 3D (v 6.5; Thermo Fisher and Zuse Institute). EDS analysis was performed using an Ultim Extreme silicon drift detector (Oxford Instruments).
Transmission Electron Microscopy.
The same samples of tibialis cranialis tendons, prepared by either HPF or chemical methods and utilized for FIB-SEM, were subsequently examined by transmission electron microscopy. Tissues were stained in block with osmium tetroxide as noted previously and embedded in Spurr resin. Blocks were sliced (100 nm thickness) using a Leica Ultracut UCT ultramicrotome (Leica Microsystems) and sections were floated on a bath of anhydrous ethylene glycol (Electron Microscopy Sciences) to maintain the mineral phase of the tissue as optimally as possible (51). Sections were collected on copper grids with a carbon support film. Images were obtained using a JEOL JEM-ARM200F electron microscope (JEOL, Ltd.) operated at 200 kV, combining traditional TEM and scanning TEM.
Data Availability.
Data presented here have been deposited in Edmond, Open Access Data Repository of the Max Planck Society (https://edmond.mpdl.mpg.de/imeji/collection/5DnfVttwhFkVI78A), or are presented within this paper and in SI Appendix.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. Wolfgang Wagermaier and Richard Weinkamer for insightful discussions and to Birgit Schonert for technical support with sample polishing (all at the Max Planck Institute of Colloids and Interfaces).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All data are deposited in the Open Access Data Repository of the Max Planck Society (Edmond) and can be accessed through the following address: https://edmond.mpdl.mpg.de/imeji/collection/5DnfVttwhFkVI78A.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917932117/-/DCSupplemental.
References
- 1.Weiner S., Wagner H. D., The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 28, 271–298 (1998). [Google Scholar]
- 2.Fratzl P., Gupta H. S., Paschalis E. P., Roschger P., Structure and mechanical quality of the collagen-mineral nano-composite in bone. J. Mater. Chem. 14, 2115–2123 (2004). [Google Scholar]
- 3.Peterlik H., Roschger P., Klaushofer K., Fratzl P., From brittle to ductile fracture of bone. Nat. Mater. 5, 52–55 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Burr D. B. et al., Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J. Bone Miner. Res. 12, 6–15 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Wegst U. G. K., Bai H., Saiz E., Tomsia A. P., Ritchie R. O., Bioinspired structural materials. Nat. Mater. 14, 23–36 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Clarke B., Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 3 (suppl. 3), S131–S139 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler W. T., Ritchie H., The nature and functional significance of dentin extracellular matrix proteins. Int. J. Dev. Biol. 39, 169–179 (1995). [PubMed] [Google Scholar]
- 8.Akkiraju H., Nohe A., Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 3, 177–192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannus P., Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 10, 312–320 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Kerschnitzki M. et al., Architecture of the osteocyte network correlates with bone material quality. J. Bone Miner. Res. 28, 1837–1845 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Ciani C., Doty S. B., Fritton S. P., An effective histological staining process to visualize bone interstitial fluid space using confocal microscopy. Bone 44, 1015–1017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landis W. J., Song M. J., Leith A., McEwen L., McEwen B. F., Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 110, 39–54 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Schwarcz H. P., McNally E. A., Botton G. A., Dark-field transmission electron microscopy of cortical bone reveals details of extrafibrillar crystals. J. Struct. Biol. 188, 240–248 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Reznikov N., Bilton M., Lari L., Stevens M. M., Kröger R., Fractal-like hierarchical organization of bone begins at the nanoscale. Science 360, eaao2189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reznikov N., Steele J. A. M., Fratzl P., Stevens M. M., A materials science vision of extracellular matrix mineralization. Nat. Rev. Mater. 1, 16041 (2016). [Google Scholar]
- 16.Shah F. A., Ruscsák K., Palmquist A., 50 years of scanning electron microscopy of bone-a comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy. Bone Res. 7, 15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis W. J., A study of calcification in the leg tendons from the domestic Turkey. J. Ultrastruct. Mol. Struct. Res. 94, 217–238 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Traub W., Arad T., Weiner S., Three-dimensional ordered distribution of crystals in Turkey tendon collagen fibers. Proc. Natl. Acad. Sci. U.S.A. 86, 9822–9826 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reznikov N., Almany-Magal R., Shahar R., Weiner S., Three-dimensional imaging of collagen fibril organization in rat circumferential lamellar bone using a dual beam electron microscope reveals ordered and disordered sub-lamellar structures. Bone 52, 676–683 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Rossetti L. et al., The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 16, 664–670 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Spiesz E. M., Roschger P., Zysset P. K., Influence of mineralization and microporosity on tissue elasticity: Experimental and numerical investigation on mineralized Turkey leg tendons. Calcif. Tissue Int. 90, 319–329 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Spiesz E. M., Zysset P. K., Structure-mechanics relationships in mineralized tendons. J. Mech. Behav. Biomed. Mater. 52, 72–84 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Gupta H. S. et al., Mechanical modulation at the lamellar level in osteonal bone. J. Mater. Res. 21, 1913–1921 (2006). [Google Scholar]
- 24.Haimov H. et al., Mineralization pathways in the active murine epiphyseal growth plate. Bone 130, 115086 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Weinkamer R., Kollmannsberger P., Fratzl P., Towards a connectomic description of the osteocyte lacunocanalicular network in bone. Curr. Osteoporos. Rep. 17, 186–194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pingel J. et al., 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: Evidence of tenocyte and matrix buckling. J. Anat. 224, 548–555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider P., Meier M., Wepf R., Müller R., Serial FIB/SEM imaging for quantitative 3D assessment of the osteocyte lacuno-canalicular network. Bone 49, 304–311 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Kalson N. S. et al., A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. eLife 4, 1–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glimcher M. J., The nature of the mineral component of bone and the mechanism of calcification. Instr. Course Lect. 36, 49–69 (1987). [PubMed] [Google Scholar]
- 30.Landis W. J., Song M. J., Early mineral deposition in calcifying tendon characterized by high voltage electron microscopy and three-dimensional graphic imaging. J. Struct. Biol. 107, 116–127 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Zhang G., Young B. B., Birk D. E., Differential expression of type XII collagen in developing chicken metatarsal tendons. J. Anat. 202, 411–420 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arsenault A. L., Frankland B. W., Ottensmeyer F. P., Vectorial sequence of mineralization in the Turkey leg tendon determined by electron microscopic imaging. Calcif. Tissue Int. 48, 46–55 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Anderson H. C., Matrix vesicles and calcification. Curr. Rheumatol. Rep. 5, 222–226 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Wuthier R. E., The role of phospholipids in biological calcification: Distribution of phospholipase activity in calcifying epiphyseal cartilage. Clin. Orthop. Relat. Res., 191–200 (1973). [PubMed] [Google Scholar]
- 35.Landis W. J., Jacquet R., Association of calcium and phosphate ions with collagen in the mineralization of vertebrate tissues. Calcif. Tissue Int. 93, 329–337 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Philipse A., Vrij A., The Donnan equilibrium: I. On the thermodynamic foundation of the Donnan equation of state. J. Phys. Condens. Matter 23, 194106 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Niu L. N. et al., Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat. Mater. 16, 370–378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thrivikraman G. et al., Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nat. Commun. 10, 3520 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silver F. H., Landis W. J., Deposition of apatite in mineralizing vertebrate extracellular matrices: A model of possible nucleation sites on type I collagen. Connect. Tissue Res. 52, 242–254 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Chen L., Jacquet R., Lowder E., Landis W. J., Refinement of collagen-mineral interaction: A possible role for osteocalcin in apatite crystal nucleation, growth and development. Bone 71, 7–16 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Brylka L., Jahnen-Dechent W., The role of fetuin-A in physiological and pathological mineralization. Calcif. Tissue Int. 93, 355–364 (2013). [DOI] [PubMed] [Google Scholar]
- 42.McKee M. D., Nanci A., Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc. Res. Tech. 33, 141–164 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez D. E. et al., Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater. 10, 494–507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nudelman F. et al., The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 9, 1004–1009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y. et al., The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 11, 724–733 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Teti A., Zallone A., Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone 44, 11–16 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Nakashima T. et al., Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Dallas S. L., Prideaux M., Bonewald L. F., The osteocyte: An endocrine cell ... and more. Endocr. Rev. 34, 658–690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franz-Odendaal T. A., Hall B. K., Witten P. E., Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 235, 176–190 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Bonewald L. F., The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landis W. J., Paine M. C., Glimcher M. J., Electron microscopic observations of bone tissue prepared anhydrously in organic solvents. J. Ultrastruct. Res. 59, 1–30 (1977). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented here have been deposited in Edmond, Open Access Data Repository of the Max Planck Society (https://edmond.mpdl.mpg.de/imeji/collection/5DnfVttwhFkVI78A), or are presented within this paper and in SI Appendix.