Highlights
-
•
Hippocampal stiffness in MTLE is measured with magnetic resonance elastography.
-
•
The epileptogenic hippocampus is stiffer than non-epileptogenic hippocampus in MTLE.
-
•
Hippocampal stiffness ratio is higher in MTLE patients than in healthy participants.
-
•
Stiffness ratio provides additional diagnostic information to hippocampal volume.
Keywords: Temporal lobe epilepsy, Magnetic resonance elastography, Hippocampus, Stiffness, Volume
Abstract
Mesial temporal lobe epilepsy (MTLE) is the most common form of refractory epilepsy. Common imaging biomarkers are often not sensitive enough to identify MTLE sufficiently early to facilitate the greatest benefit from surgical or pharmacological intervention. The objective of this work is to establish hippocampal stiffness measured with magnetic resonance elastography (MRE) as a biomarker for MTLE; we hypothesized that the epileptogenic hippocampus in MTLE is stiffer than the non-epileptogenic hippocampus. MRE was used to measure hippocampal stiffness in a group of patients with unilateral MTLE (n = 12) and a group of healthy comparison participants (n = 13). We calculated the ratio of hippocampal stiffness ipsilateral to epileptogenesis to the contralateral side for both groups. We found a higher hippocampal stiffness ratio in patients with MTLE compared with healthy participants (1.14 v. 0.99; p = 0.004), and that stiffness ratio differentiated MTLE from control groups effectively (AUC = 0.85). Hippocampal stiffness ratio, when added to volume ratio, an established MTLE biomarker, significantly improved the ability to differentiate the two groups (p = 0.038). Stiffness measured with MRE is sensitive to hippocampal pathology in MTLE and the addition of MRE to neuroimaging assessments may improve detection and characterization of the disease.
1. Introduction
Epilepsy of the temporal lobe is common and often leads to persistent, medically-intractable seizures that are not well-controlled by pharmacotherapy with anti-epileptic drugs (Semah et al., 1998). The epileptogenic source in many temporal lobe epilepsy cases is the mesial temporal lobe, particularly the hippocampus, and mesial temporal lobe epilepsy (MTLE) is considered the most common form of refractory epilepsy in humans (Engel, 2001, Semah et al., 1998). Progressive sclerosis and scarring of the hippocampus results in greater resistance to treatment over time (Labate et al., 2016) and the development of mesial temporal sclerosis (MTS). Under-treated MTS eventually leads to contralateral seizure onset and bilateral pathology (Coan and Cendes, 2013), at which point the patient is no longer a candidate for surgery – the most effective treatment option (Wiebe and Jette, 2012). Preventing disease progression is the goal of medication and surgical intervention, though most patients present to epilepsy centers only after unilateral MTLE is clearly measured by current methods (Berg et al., 2006), generally precluding very early treatment (i.e. before onset of sclerosis) that could possibly change the course of the disease.
While early pharmacological interventions could yield positive outcomes, early symptoms (e.g., olfactory aura, acute bouts of anxiety, déjà vu) (Beyenburg et al., 2005, Chen et al., 2003) seem relatively benign and patients rarely present to epilepsy centers in the early stages of the disease (Engel, 2008, Engel et al., 2012). Accordingly, with delayed presentation to the clinic, MTLE is one of the most pharmacologically refractory epilepsies; however, it is also one of the epilepsies most effectively treated through surgery (Engel, 2002, Wiebe et al., 2001). Studies repeatedly find hippocampal resection to result in seizure-free outcomes in 65% of patients (Engel et al., 2003, Wiebe and Jette, 2012) and surgical groups also experience significantly greater quality-of-life than subjects receiving only anti-epileptic drugs (Pauli et al., 2017). Importantly, earlier use of surgical intervention for MTLE improves outcomes (Engel et al., 2012, Wiebe et al., 2001). Despite the positive outcomes of surgery, use of surgical intervention can be limited by the use of traditional imaging biomarkers (based on structural MRI) that often do not confirm disease presence (Duncan, 2010, Koepp and Woermann, 2005) and thus engender reluctance to perform surgery on what appears to be normal tissue. As a result, interest is high in identifying and developing new imaging biomarkers for MTLE that may provide more accurate diagnosis and potentially enable earlier detection of abnormal tissue. Previous studies have employed diffusion MRI (Thivard et al., 2005), quantitative T1 and T2 relaxometry (Bernhardt et al., 2018, Coan et al., 2014, Mueller et al., 2007), and functional MRI (Golby et al., 2002, Haneef et al., 2014), amongst other advanced MRI methods, and have generally found differences in tissue that are asymmetric between the hippocampi. Methods to better identify degraded or degrading tissue as early as possible are critical in order to deliver more effective pharmacological or surgical interventions to MTLE patients.
In this paper, we propose the mechanical stiffness of the hippocampus, measured noninvasively with magnetic resonance elastography (MRE) (Hiscox et al., 2016, Muthupillai et al., 1995), as a biomarker for MTLE complementary to existing imaging methods. MRE offers a unique neuroimaging contrast potentially useful for characterizing MTLE as viscoelastic mechanical properties reflect how tissue components act and interact when forced, and thus reflect the composition and organization of tissue at the microscale (Sack et al., 2013). Previous studies using MRE to examine neurological disorders have shown that brain mechanical properties are affected by aging and dementia (Arani et al., 2015, Hiscox et al., 2020a, Hiscox et al., 2018, Huston et al., 2016, Murphy et al., 2016), and that these parameters are related to the microstructural health of brain tissue (Millward et al., 2015, Munder et al., 2018, Schregel et al., 2012). MRE has also been used in the characterization of intracranial tumors to detect if they are stiff or soft for pre-surgical planning (Hughes et al., 2015, Murphy et al., 2013), which may be relevant for sensing the sclerosis expected to stiffen the hippocampus. We have recently developed high-resolution methodology for reliable, localized MRE measurements of the hippocampus (Johnson et al., 2016), and have used it to examine hippocampal-specific memory performance in young and older adults (Hiscox et al., 2020b, Schwarb et al., 2016).
Using our high-resolution MRE protocol, we examined hippocampal stiffness in patients with MTLE. Our overarching hypothesis was that as sclerosis occurs, the affected hippocampus will become stiff and detectable by MRE, potentially due to reactive gliosis. This hypothesis is further motivated by surgical observations of epileptogenic lesions being stiffer than healthy tissue, which has been previously reported through the use of intraoperative ultrasound elastography (Chan et al., 2014, Mathon et al., 2019). We tested this hypothesis by comparing stiffness of the epileptogenic hippocampus in patients with moderate or severe unilateral MTLE to stiffness of the contralateral hippocampus, and to stiffness of normal hippocampal tissue from a group of healthy comparison participants. We expected that the epileptogenic hippocampus would be stiffer in patients and result in a lateral stiffness asymmetry, which would not be observed in healthy control participants. We further quantified the expected asymmetry via a stiffness ratio and examined how this metric differentiated patient and healthy groups. To our knowledge, this is the first study of MRE characterization of MTLE or any form of human epilepsy.
2. Methods
2.1. Participants
Fifteen participants with moderate or severe unilateral MTLE were enrolled in the study and completed an MRI imaging session that included MRE and a high-resolution T1-weighted acquisition. Each MRI session was completed on a Siemens 3T Trio scanner (Siemens Healthineers; Erlangen, Germany). Clinical inclusion criteria were seizure history consistent with MTLE and positive biomarker findings on one or multiple of EEG (frontotemporal slowing and sharp waves), FLAIR (hyperintensity of mesial structures), T2-weighted MRI (loss of internal hippocampal architecture), and/or PET (hypometabolism). Only unilateral MTLE patients were included, with biomarkers clearly present on one side and absent contralaterally. Severity was determined by number of positive biomarker findings: moderate MTLE exhibited one positive biomarker while severe MTLE exhibited multiple positive biomarkers. Seventeen healthy participants with no history of neurological disorder were enrolled as matched comparison participants and completed an identical MRI exam session. Control participants were matched to patients in terms of age (+/- 5 years), education (+/- 2 years), handedness, and sex. The Institutional Review Boards of Carle Foundation Hospital and the University of Illinois at Urbana-Champaign approved the study and all participants provided written, informed consent.
Two patients and three healthy participants were excluded due to MRE scans with signal-to-noise ratio too low for acceptance (McGarry et al., 2011). One patient and one healthy participant were excluded as outliers due to ratio of hippocampal stiffness (see below). The final patient group included twelve participants (2/10 M/F; 26-61 years; mean age = 45.8 years) and the final healthy group included thirteen participants (1/12 M/F; 20-60 years; mean age = 34.0 years). Of the twelve patients, ten were identified as having left MTLE and two had right MTLE. Table 1 lists age, sex, and severity and lateralization of all MTLE patients included in this study.
Table 1.
List of patients with MTLE included in this study.
| Age [yrs] | Sex | Severity | Lateralization |
|---|---|---|---|
| 26 | F | Moderate | Right |
| 29 | F | Severe | Left |
| 32 | M | Moderate | Left |
| 40 | F | Severe | Left |
| 41 | F | Severe | Left |
| 46 | F | Moderate | Left |
| 50 | M | Moderate | Left |
| 53 | F | Moderate | Left |
| 56 | F | Moderate | Left |
| 58 | F | Moderate | Left |
| 58 | F | Severe | Left |
| 61 | F | Moderate | Right |
2.2. Hippocampal volume
We acquired high-resolution, T1-weighted anatomical images using an MPRAGE sequence (magnetization prepared, rapidly acquired gradient echo). Imaging parameters included: 1900/900/2.32 ms repetition/inversion/echo times; 0.9x0.9x0.9 mm3 resolution. Left and right hippocampi were segmented automatically from the T1-weighted images with FreeSurfer 6.0 (Fischl et al., 2002). All segmentations were visually inspected for accuracy and manual corrections were made when necessary. Volumes of left and right hippocampi were extracted from the segmented data.
2.3. Hippocampal stiffness
We acquired MRE displacement data using a 3D multislab, multishot spiral sequence (Johnson et al., 2014). Imaging parameters included: 2 in-plane, constant density spiral shots (R = 2) (Glover, 1999); 1800/73 ms repetition/echo times; 240 mm field-of-view; 150 × 150 matrix; 60 slices at 1.6 mm thickness; 1.6 × 1.6 × 1.6 mm3 final imaging resolution. Image reconstruction was performed with an iterative algorithm that incorporated field inhomogeneity correction and motion-induced phase error correction (Johnson et al., 2014, Sutton et al., 2003). The sequence was synchronized to applied 50 Hz vibrations delivered to the head using a pneumatic actuator with a soft pillow driver (Resoundant, Inc.; Rochester, MN). Complex, full vector shear wave motion was captured throughout the brain in approximately 12 min.
A nonlinear inversion (NLI) algorithm estimated mechanical properties in the brain from acquired MRE displacement data (McGarry et al., 2012). NLI returns maps of the complex shear modulus, G = G′+iG″, where G’ is the storage modulus and G’’ is the loss modulus. From these parameters, we compute the shear stiffness, μ = 2|G|2/(|G|+G′) (Manduca et al., 2001), and the damping ratio, ξ = G″/2G′ (McGarry and Van Houten, 2008). Soft prior regularization (SPR) was applied in the NLI formulation (McGarry et al., 2013) to improve stability of the hippocampal property estimation through incorporation of spatial priors. We have previously demonstrated that SPR improves reliability of hippocampal MRE measures and have suggested this is due to reducing effects of neighboring tissues and cerebrospinal fluid, thus minimizing any partial volume effects on the property estimates (Johnson et al., 2016). Subject-specific masks of left and right hippocampi were created by registering hippocampal volumes segmented from T1-weighted images (see above) to the native MRE space using FLIRT in FSL (Jenkinson et al., 2012, Jenkinson et al., 2002) for incorporation into the NLI routine.
2.4. Analysis
We compared stiffness measures between hippocampi ipsilateral and contralateral to epileptogenesis in MTLE patients. We also compared hippocampal stiffness measures between MTLE and healthy groups, including stiffness of the hippocampi both ipsilateral and contralateral to epileptogenesis, bilateral hippocampal stiffness, and the ratio of ipsilateral to contralateral hippocampal stiffness. In healthy participants, left was assigned as ipsilateral and right as contralateral for comparison between groups. We calculated the same outcomes for hippocampal volume. Statistical outliers were determined based on the median absolute deviation (MAD) and excluded if the stiffness ratio was beyond the conservative estimate of three times the MAD (Leys et al., 2013). Data from one MTLE patient and one healthy control were excluded as outliers in this analysis, as described above. We further examined a subset of participants with only left MTLE (n = 10) and excluding right MTLE (n = 2), as our sample was unbalanced and previous neuroimaging studies have indicated different patterns of neurological damage based on lateralization (Ahmadi et al., 2009, Besson et al., 2014, Kemmotsu et al., 2011).
Paired t-tests compared hippocampal measures within patients. Analysis of variance (ANOVA) tested differences in hippocampal measures between groups. Effect size was calculated as Cohen’s d and significance was determined at p < 0.025 (with Bonferroni correction for multiple comparisons). Receiver operating characteristic (ROC) curves were constructed to evaluate performance of stiffness ratio and volume ratio in classifying MTLE patients vs. controls, and performance was quantified by area under the curve (AUC). Stepwise logistic regression evaluated improvement in classifier performance by including stiffness ratio in addition to volume ratio. ROC and AUC were calculated for the combined classifier, with significance determined at p < 0.05. Analyses were performed in Matlab (Mathworks; Natick, MA) and SPSS version 26 (IBM; Armonk, NY).
3. Results
Descriptive statistics for the hippocampal stiffness measures (ipsilateral, contralateral, bilateral, and ratio) for both MTLE and control groups are reported in Table 2. The ipsilateral hippocampus was significantly stiffer than the contralateral hippocampus in MTLE patients (d = 0.96; p = 0.007), though not in the healthy control group (d = -0.12; p = 0.666). This result is illustrated in Fig. 1, which presents representative data from an MTLE patient exhibiting a stiffer hippocampus on the left (epileptogenic) side compared to the right (non-epileptogenic) side. Table 2 also includes effect sizes and p-values describing differences between groups. We did not observe any statistically significant differences between groups in unilateral or bilateral hippocampal stiffness. However, the ratio of hippocampal stiffness was significantly different between groups, such that stiffness of the hippocampus ipsilateral to epileptogenesis was stiffer than the contralateral side in MTLE (d = 0.97; p = 0.024). Additional MRE measures – damping ratio, ξ, storage modulus, G’, and loss modulus, G’’ – are included as Tables S1-3 in Supplemental Information.
Table 2.
Comparison of hippocampal stiffness and volume in MTLE (n = 12) vs. healthy (n = 13) groups. * indicates significance at the p < 0.025 level.
| MTLE | Healthy | Cohen’s d | p-value | |
|---|---|---|---|---|
| Age [yrs]: mean (range) | 45.8 (26-61) | 34.0 (20-60) | ||
| Sex (M/F) | 2/10 | 1/12 | ||
| Hippocampal Stiffness Measures | ||||
| Ipsilateral HC [kPa] | 3.13 ± 0.44 | 3.02 ± 0.53 | 0.23 | 0.570 |
| Contralateral HC [kPa] | 2.83 ± 0.30 | 3.07 ± 0.54 | -0.55 | 0.184 |
| Bilateral HC [kPa] | 2.95 ± 0.31 | 3.05 ± 0.48 | -0.24 | 0.555 |
| HC Ratio (ipsi./contra.) | 1.11 ± 0.11 | 0.99 ± 0.13 | 0.96 | 0.024* |
| Hippocampal Volume Measures | ||||
| Ipsilateral HC [cm3] | 3.66 ± 0.71 | 4.29 ± 0.27 | -1.20 | 0.007* |
| Contralateral HC [cm3] | 4.33 ± 0.50 | 4.41 ± 0.23 | -0.23 | 0.573 |
| Bilateral HC [cm3] | 7.99 ± 0.98 | 8.71 ± 0.46 | -0.95 | 0.027 |
| HC Ratio (ipsi./contra.) | 0.85 ± 0.15 | 0.97 ± 0.04 | -1.10 | 0.011* |
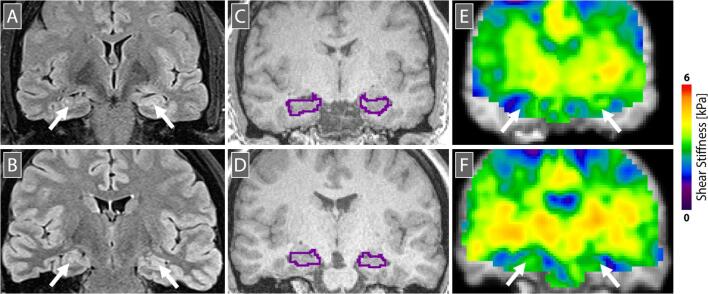
Fig. 1.
Representative data from (top) an MTLE patient (46 yo, female, left moderate MTLE) and (bottom) a healthy control participant (43 yo, female). (A,B) FLAIR image showing hyperintensity in left hippocampus in MTLE; (C,D) T1-weighted anatomical image used for segmentation of hippocampi, which are outlined; and (E,F) MRE stiffness map showing higher stiffness in left hippocampus in MTLE. Images are in radiological convention and are chosen to show approximately the same position on the anterior-posterior axis of the hippocampus.
Table 2 also summarizes measures of hippocampal volume – a known biomarker for MTLE – and analyses with hippocampal volume produced similar trends as observed in stiffness. The ipsilateral hippocampus was significantly smaller than the contralateral hippocampus in MTLE patients (d = -0.91; p = 0.009). Compared to healthy participants, MTLE patients exhibited smaller ipsilateral hippocampal volume and a smaller ratio of ipsilateral-to-hippocampal volume (d = -1.10; p = 0.011).
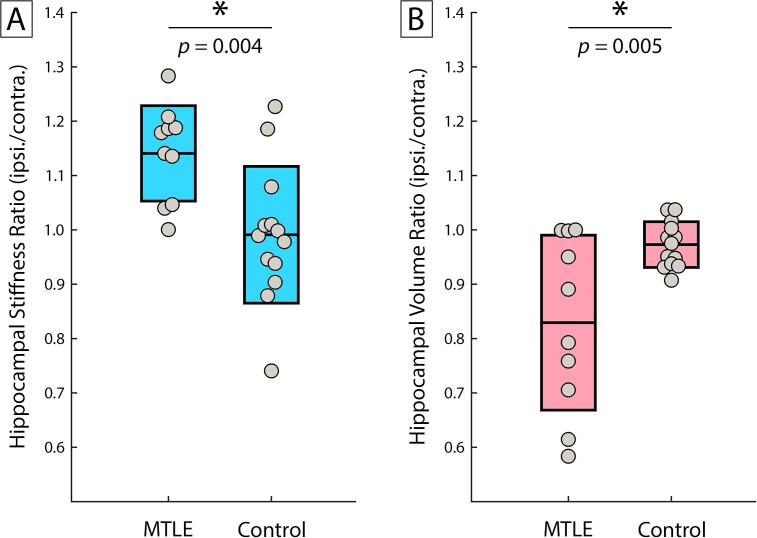
We performed a similar analysis that only included patients with left MTLE (n = 10). Fig. 2 compares left MTLE and healthy groups in both hippocampal stiffness ratio and hippocampal volume ratio. Both ratio measures were significantly different between groups. Specifically, the ipsilateral hippocampus was stiffer (d = 1.35; p = 0.004) and smaller (d = -1.31; p = 0.005) in left MTLE compared to healthy participants. Interestingly, the two right MTLE patients had the lowest stiffness ratios (0.90 and 0.98) in the patient group, and the only two ratios below 1.0.
Fig. 2.
Comparison of MTLE and healthy participants by (A) hippocampal stiffness ratio and (B) hippocampal volume ratio. MTLE significantly differed from controls in each measure exhibiting higher stiffness and lower volume in hippocampi ipsilateral to epileptogenesis. Only left MTLE were included (n = 10) for comparison with controls (n = 13). * indicates significance at the p < 0.025 level.
Table 3 presents descriptive statistics for hippocampal stiffness and volume ratios for the left MTLE and healthy comparison groups, as well as outcome of ANOVAs when including participant age as a covariate. Age was not a significant factor for either stiffness ratio or volume ratio, and, since these measures are internally referenced for each subject, we did not include participant age in our primary analyses. However, since our final MTLE and healthy groups had differing age distributions, we included the outcomes of tests with age as covariate for completeness. Both stiffness ratio (p = 0.038) and volume ratio (p = 0.015) remained significantly different between groups when accounting for age.
Table 3.
Comparison of hippocampal stiffness ratio and volume ratio in left MTLE (n = 10) vs. healthy (n = 13) groups. Statistical tests from ANOVA without and with participant age as a co-variate. * indicates significance at the p < 0.05 level.
| MTLE | Control | Cohen’s d | p-value | p (w/age) | |
|---|---|---|---|---|---|
| HC Stiffness Ratio | 1.14 ± 0.09 | 0.99 ± 0.13 | 1.35 | 0.004* | 0.038* |
| HC Volume Ratio | 0.83 ± 0.16 | 0.97 ± 0.04 | -1.31 | 0.005* | 0.015* |
Stepwise logistic regression was used to model the predictors to classify left MTLE patients vs. healthy participants. The overall model for classifying the group by both volume ratio and stiffness ratio was significant (χ2 = 15.37; p < 0.001; Nagelkerke R2 = 0.654). Volume ratio was included in the model as the first step (p = 0.049) followed by stiffness ratio (p = 0.038), indicating that the latter significantly improved model performance in classifying MTLE by offering additional diagnostic information. The two measures, stiffness ratio and volume ratio, were not significantly correlated (r = -0.30; p = 0.160), and thus it is unlikely partial volume effects strongly affected the MRE results.
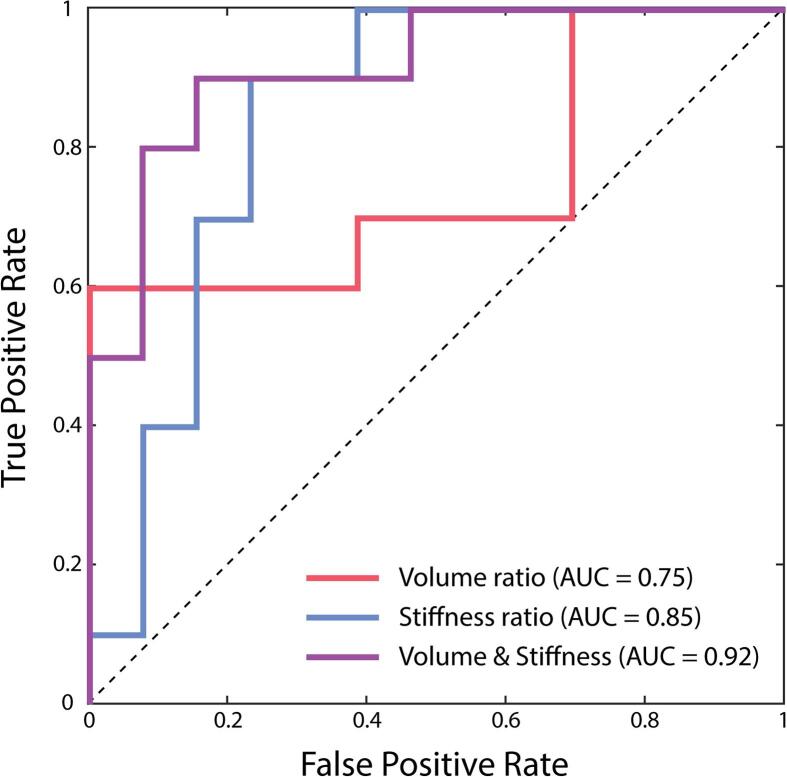
Fig. 3 presents ROC curves for stiffness ratio and volume ratio in classifying left MTLE patients vs. healthy participants, as well as for the combined (stiffness and volume ratios) prediction model. Volume ratio had an AUC of 0.75 (95% CI: 0.55-0.95) and stiffness ratio had an AUC of 0.85 (95% CI: 0.67-1.02), while the combined predictor had an AUC of 0.92 (95% CI: 0.79-1.05), and outperformed both individual measures. The overall model correctly classified 80% of MTLE patients (82.6% total accuracy).
Fig. 3.
Receiver operator characteristic (ROC) curves for each of the predictors in classifying left MTLE patients vs. healthy participants: hippocampal volume ratio, hippocampal stiffness ratio, and combined volume and stiffness predictor from logistic regression. Area under the curve (AUC) for each predictor included as a measure of predictive performance, with the combined model having the best performance.
Data for individual participants are included in Supplemental Information.
4. Discussion
In this work, we demonstrate that hippocampal stiffness measured with MRE is altered in patients with MTLE compared to healthy participants. Specifically, we found that the hippocampus ipsilateral to epileptogenesis is significantly stiffer than the contralateral hippocampus in MTLE patients, and the hippocampal stiffness ratio to be 14% higher in patients relative to healthy participants. This outcome supports our hypothesis that the epileptogenic hippocampus would be stiffer in MTLE due to sclerosis of tissue from seizure activity, and is consistent with previous findings of stiffer epileptogenic lesions observed with intraoperative ultrasound elastography (Chan et al., 2014, Mathon et al., 2019). Hippocampal sclerosis is marked by cell loss and reactive gliosis, and while it is not precisely known how these factors might affect MRE measures, reactive glial cells have been shown to be stiffer than normal cells (Lu et al., 2011), which would likely increase tissue stiffness. Our comparison of unilateral hippocampal stiffness measures, however, did not reveal significant differences between groups. Stiffness ratio, which is akin to a measure of asymmetry, is potentially more sensitive to group differences between patients and healthy participants because the contralateral hippocampus normalizes the data within an individual. We have previously shown that hippocampal stiffness can vary by more than 10% in healthy, young adults (Johnson et al., 2016), and inter-individual differences may obscure group differences in unilateral hippocampal stiffness measures.
However, the stiffness ratio captures not only potential ipsilateral stiffening, but also potential contralateral softening. The contralateral hippocampus trended as softer in patients compared to healthy participants (though the difference was not significant; d = -0.55, p = 0.184). Contralateral damage to white and gray matter has been previously reported in MTLE (Ahmadi et al., 2009, Besson et al., 2014, Keller and Roberts, 2008, Kemmotsu et al., 2011, Seidenberg et al., 2005) and is likely caused by spreading of seizure activity from the epileptogenic source (Thom, 2014). While volume loss in the contralateral hippocampus is often not reported in MTLE (Coan et al., 2014, Keller and Roberts, 2008, Seidenberg et al., 2005), MRE may be sensitive to microstructural tissue alterations that occur prior to observable volume changes, such as reorganization of mossy fibers and fiber networks (Thom et al., 2009). Indeed, a previous diffusion MRI study found opposite trends in the diffusivity in ipsilateral and contralateral hippocampi in MTLE (Thivard et al., 2005). Further work is needed to identify the specific microstructural underpinnings of both ipsilateral and contralateral hippocampal stiffness in MTLE, likely using pathology samples following surgical resection and animal models of the disease.
We predominantly report and interpret results from a subgroup comprising patients with only left MTLE. This choice was in part practical; our sample was unbalanced with mostly left MTLE (n = 10) and few right MTLE (n = 2) cases, which impacted our ability to account for differences in outcome measures based on seizure lateralization. While the stiffness ratio in the entire group of left and right MTLE patients was still significantly greater than controls (d = 0.93; p = 0.024), the two patients with right MTLE were the only two subjects with ipsilateral-to-contralateral stiffness ratios less than 1.0. Given the small sample size of right MTLE patients, it is difficult to conclude that right MTLE exhibited different patterns of stiffness between hemispheres relative to left MTLE. However, previous reports describe greater extent of structural brain damage in left MTLE compared to right MTLE (Ahmadi et al., 2009, Besson et al., 2014, Kemmotsu et al., 2011), suggesting the presence of different pathological signatures that warrant separate analyses of the two disease groups. MRE data on a larger sample of right MTLE patients is needed confirm or reject this observation.
The hippocampal stiffness ratio is a potentially useful biomarker for detecting and characterizing MTLE and may provide information consistent with and complementary to other imaging measures. Here, we compare with hippocampal volume, since hippocampal volume loss is a hallmark of MTLE (Jack, 1994). We specifically examine the ipsilateral-to-contralateral ratio of hippocampal volume for convenient comparison with the stiffness ratio. This hippocampal volume ratio has been previously demonstrated to improve detection of MTLE (Coan et al., 2014). We find that both the stiffness ratio and volume ratio behave similarly in differentiating patients from healthy participants – ipsilateral hippocampi are both stiffer and smaller than their contralateral counterparts. Combining both measures improves classification, and the addition of stiffness ratio provides a significant improvement over volume ratio alone. This result suggests that inclusion of MRE may improve diagnostic performance when added to a neuroimaging protocol for MTLE patients.
While MRE measures in this paper appear to be useful in examining MTLE in addition to volume, there have also been many studies using other quantitative MRI contrasts to examine changes in the hippocampus due to MTLE pathology, including from diffusion MRI (Thivard et al., 2005), quantitative T1 and T2 relaxometry (Bernhardt et al., 2018, Coan et al., 2014, Mueller et al., 2007), functional MRI (Golby et al., 2002, Haneef et al., 2014), and others. It is not yet clear how the information gained from stiffness measured with MRE will agree with or differ from these other measures due to sensitivity to underlying hippocampal tissue microstructure. One previous study of hippocampal stiffness in Alzheimer’s disease found that combining this measure with those from diffusion MRI improved diagnostic performance (Gerischer et al., 2018), while we have previously found that MRE measures revealed a hippocampal structure–function relationship not observed with diffusion MRI in young adults (Schwarb et al., 2016). These findings suggest MRE is at least complementary to diffusion measures, similar to our finding comparing MRE with volume in this study, likely owing to the unique sensitivity of MRE to how tissue components interact when forced (Sack et al., 2013). A complete analysis with other imaging biomarkers is warranted and will be useful in understanding the impact of MRE in MTLE care.
The primary limitation of this study is the small sample size, and, as such, the results presented here must be considered as preliminary. A larger study with more patients, and a more even distribution of age, sex, and lateralization, is necessary to confirm these findings. An additional limitation was the greater-than-average loss of scans from low signal-to-noise ratio likely due to incorrect positioning of the head on the pillow actuator. While MRE is generally reliable with minimal failures, real-time quality control measures are currently lacking and will be explored in future studies. Additionally, our sample was cross-sectional and included only patients with moderate or severe MTLE confirmed through additional biomarkers; thus, we are not able to infer how hippocampal stiffness would manifest in mild MTLE or change with disease progression. The sensitivity of stiffness to hippocampal pathology in MTLE suggests potential for early detection, which indicates a longitudinal study of individuals with likely MTLE may reveal MRE signatures that support decision-making for early intervention. Lastly, we lack confirmatory pathology from surgically-resected hippocampal tissue in the patient sample. Future studies of MRE in this setting should focus on MTLE at hippocampi sites routinely resected surgically, which would link stiffness outcomes more completely with histopathological results.
5. Conclusion
Hippocampal stiffness measured with MRE in MTLE patients revealed the hippocampus ipsilateral to epileptogenesis is stiffer than its contralateral counterpart, likely due to altered tissue microstructure due to pathology, including reactive gliosis. Stiffness ratio from MRE provides a new imaging biomarker that appears to be sensitive to MTLE and may offer a unique contrast when added to a comprehensive neuroimaging battery due to its sensitivity to how tissue components act and interact when forced, thus potentially allowing for more accurate diagnosis and earlier detection of the disease. Here we show that hippocampal stiffness ratio is complementary to hippocampal volume ratio, an established imaging biomarker for the disease, and future studies comparing it to other quantitative imaging biomarkers will reveal the additional diagnostic information to be gained from this technique.
CRediT authorship contribution statement
Graham R. Huesmann: Conceptualization, Data curation, Funding acquisition, Project administration, Writing - original draft. Hillary Schwarb: Conceptualization, Data curation, Formal analysis, Writing - review & editing. Daniel R. Smith: Formal analysis, Writing - review & editing. Ryan T. Pohlig: Formal analysis, Writing - review & editing. Aaron T. Anderson: Data curation, Writing - review & editing. Matthew D.J. McGarry: Resources, Writing - review & editing. Keith D. Paulsen: Resources, Writing - review & editing. Tracey Mencio Wszalek: Data curation, Project administration, Writing - review & editing. Bradley P. Sutton: Data curation, Writing - review & editing. Curtis L. Johnson: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Writing - original draft.
Acknowledgments
Acknowledgements
This research was partially supported by the Carle Neuroscience Institute, the Delaware CTR ACCEL Program (NIH/NIGMS U54-GM104941), NIH/NIA (R01-AG058853), and NIH/NIBIB (R01-EB027577).
Disclosure
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. GRH has received research support from Eisai and has participated in advisory boards for Eisai. None of the support from Eisai was used in the conduct of the research for this paper. None of the other authors have any conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102313.
Contributor Information
Graham R. Huesmann, Email: graham.huesmann@carle.com.
Hillary Schwarb, Email: schwarb2@illinois.edu.
Curtis L. Johnson, Email: clj@udel.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmadi M.E., Hagler D.J., McDonald C.R., Tecoma E.S., Iragui V.J., Dale A.M., Halgren E. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. Am. J. Neuroradiol. 2009;30:1740–1747. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arani A., Murphy M.C., Glaser K.J., Manduca A., Lake D.S., Kruse S.A., Jack C.R., Ehman R.L., Huston J. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage. 2015;111:59–64. doi: 10.1016/j.neuroimage.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A.T., Vickrey B.G., Testa F.M., Levy S.R., Shinnar S., DiMario F., Smith S. How long does it take for epilepsy to become intractable? A prospective investigation. Ann. Neurol. 2006;60:73–79. doi: 10.1002/ana.20852. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Fadaie F., Vos de Wael R., Hong S.J., Liu M., Guiot M.C., Rudko D.A., Bernasconi A., Bernasconi N. Preferential susceptibility of limbic cortices to microstructural damage in temporal lobe epilepsy: A quantitative T1 mapping study. Neuroimage. 2018;182:294–303. doi: 10.1016/j.neuroimage.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Besson P., Dinkelacker V., Valabregue R., Thivard L., Leclerc X., Baulac M., Sammler D., Colliot O., Lehéricy S., Samson S., Dupont S. Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage. 2014;100:135–144. doi: 10.1016/j.neuroimage.2014.04.071. [DOI] [PubMed] [Google Scholar]
- Beyenburg S., Mitchell A.J., Schmidt D., Elger C.E., Reuber M. Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav. 2005;7:161–171. doi: 10.1016/j.yebeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Chan H.W., Pressler R., Uff C., Gunny R., St Piers K., Cross H., Bamber J., Dorward N., Harkness W., Chakraborty A. A novel technique of detecting MRI-negative lesion in focal symptomatic epilepsy: Intraoperative ShearWave Elastography. Epilepsia. 2014;55:30–33. doi: 10.1111/epi.12562. [DOI] [PubMed] [Google Scholar]
- Chen C., Shih Y.H., Yen D.J., Lirng J.F., Guo Y.C., Yu H.Y., Yiu C.H. Olfactory auras in patients with temporal lobe epilepsy. Epilepsia. 2003;44:257–260. doi: 10.1046/j.1528-1157.2003.25902.x. [DOI] [PubMed] [Google Scholar]
- Coan A.C., Cendes F. Epilepsy as progressive disorders: What is the evidence that can guide our clinical decisions and how can neuroimaging help? Epilepsy Behav. 2013;26:313–321. doi: 10.1016/j.yebeh.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Coan A.C., Kubota B., Bergo F.P.G., Campos B.M., Cendes F. 3T MRI quantification of hippocampal volume and signal in mesial temporal lobe epilepsy improves detection of hippocampal sclerosis. Am. J. Neuroradiol. 2014;35:77–83. doi: 10.3174/ajnr.A3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.S. Imaging in the surgical treatment of epilepsy. Nat. Rev. Neurol. 2010;6:537–550. doi: 10.1038/nrneurol.2010.131. [DOI] [PubMed] [Google Scholar]
- Engel J. Surgical treatment for epilepsy: too little, too late? J. Am. Med. Assoc. 2008;300:2548–2550. doi: 10.1001/jama.2008.756. [DOI] [PubMed] [Google Scholar]
- Engel J. Surgery for seizures. N. Engl. J. Med. 2002;334:647–653. doi: 10.1056/nejm199603073341008. [DOI] [PubMed] [Google Scholar]
- Engel J. Mesial temporal lobe epilepsy: what have we learned? Neuroscience. 2001;7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- Engel J., McDermott M.P., Wiebe S., Langfitt J.T., Stern J.M., Dewar S., Sperling M.R., Gardiner I., Erba G., Fried I., Jacobs M., Vinters H.V., Mintzer S., Kieburtz K. Early surgical therapy for drug-resistant temporal lobe epilepsy. J. Am. Med. Assoc. 2012;307:922–930. doi: 10.1097/01.UC.0000428885.17795.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Wiebe S., French J., Sperling M. Practice parameter: Temporal lobe and localized neocortical resections for epilepsy Report of the Quality Standards Subcommittee of the American Academy of Neurology, in Association with the. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van Der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Gerischer L.M., Fehlner A., Köbe T., Prehn K., Antonenko D., Grittner U., Braun J., Sack I., Flöel A. Combining viscoelasticity, diffusivity and volume of the hippocampus for the diagnosis of Alzheimer’s disease based on magnetic resonance imaging. NeuroImage Clin. 2018;18:485–493. doi: 10.1016/j.nicl.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G.H. Simple analytic spiral K-space algorithm. Magn. Reson. Med. 1999;42:412–415. doi: 10.1002/(SICI)1522-2594(199908)42:2<412::AID-MRM25>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Golby A.J., Poldrack R.A., Illes J., Chen D., Desmond J.E., Gabrieli J.D.E. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia. 2002;43:855–863. doi: 10.1046/j.1528-1157.2002.20501.x. [DOI] [PubMed] [Google Scholar]
- Haneef Z., Lenartowicz A., Yeh H.J., Levin H.S., Engel J., Stern J.M. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 2014;55:137–145. doi: 10.1111/epi.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox L.V., Johnson C.L., Barnhill E., McGarry M.D.J., Huston J., Van Beek E.J.R., Starr J.M., Roberts N. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys. Med. Biol. 2016;61:R401–R437. doi: 10.1088/0031-9155/61/24/R401. [DOI] [PubMed] [Google Scholar]
- Hiscox, L. V, Johnson, C.L., McGarry, M.D.J., Marshall, H., Ritchie, C.W., Beek, E.J.R. Van, Roberts, N., Starr, J.M., 2020. Mechanical property alterations across the cerebral cortex due to Alzheimer’s disease. Brain Commun. 2, fcz049. https://doi.org/10.1093/braincomms/fcz049. [DOI] [PMC free article] [PubMed]
- Hiscox L.V., Johnson C.L., McGarry M.D.J., Perrins M., Littlejohn A., van Beek E.J.R., Roberts N., Starr J.M. High-resolution magnetic resonance elastography reveals differences in subcortical gray matter viscoelasticity between young and healthy older adults. Neurobiol. Aging. 2018;65:158–167. doi: 10.1016/j.neurobiolaging.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox L.V., Johnson C.L., McGarry M.D.J., Schwarb H., van Beek E.J.R., Roberts N., Starr J.M. Hippocampal viscoelasticity and episodic memory performance in healthy older adults examined with magnetic resonance elastography. Brain Imaging Behav. 2020;14:175–185. doi: 10.1007/s11682-018-9988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.D., Fattahi N., Van Gompel J., Arani A., Meyer F., Lanzino G., Link M.J., Ehman R., Huston J. Higher-resolution magnetic resonance elastography in meningiomas to determine intratumoral consistency. Neurosurgery. 2015;77:653–658. doi: 10.1227/NEU.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J., Murphy M.C., Boeve B.F., Fattahi N., Arani A., Glaser K.J., Manduca A., Jones D.T., Ehman R.L. Magnetic resonance elastography of frontotemporal dementia. J. Magn. Reson. Imaging. 2016;43:474–478. doi: 10.1002/jmri.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr. MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35(Suppl 6):S21–S29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P.R., Brady M., Smith S.M. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson C.L., Holtrop J.L., McGarry M.D.J., Weaver J.B., Paulsen K.D., Georgiadis J.G., Sutton B.P. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn. Reson. Med. 2014;71:477–485. doi: 10.1002/mrm.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., Schwarb H., McGarry M.D.J., Anderson A.T., Huesmann G.R., Sutton B.P., Cohen N.J. Viscoelasticity of subcortical gray matter structures. Hum. Brain Mapp. 2016;37:4221–4233. doi: 10.1002/hbm.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.S., Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N., Girard H.M., Bernhardt B.C., Bonilha L., Lin J.J., Tecoma E.S., Iragui V.J., Hagler D.J., Halgren E., McDonald C.R. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52:2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp M.J., Woermann F.G. Imaging structure and function in refractory focal epilepsy. Lancet Neurol. 2005;4:42–53. doi: 10.1016/S1474-4422(04)00965-2. [DOI] [PubMed] [Google Scholar]
- Labate A., Aguglia U., Tripepi G., Mumoli L., Ferlazzo E., Baggetta R., Quattrone A., Gambardella A. Long-term outcome of mild mesial temporal lobe epilepsy: a prospective longitudinal cohort study. Neurology. 2016;86:1904–1910. doi: 10.1212/WNL.0000000000002674. [DOI] [PubMed] [Google Scholar]
- Leys C., Ley C., Klein O., Bernard P., Licata L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013;49:764–766. doi: 10.1016/j.jesp.2013.03.013. [DOI] [Google Scholar]
- Lu Y., Iandiev I., Hollborn M., Körber N., Ulbricht E., Hirrlinger P.G., Pannicke T., Wei E., Bringmann A., Wolburg H., Wilhelmsson U., Pekny M., Wiedemann P., Reichenbach A., Käs J.A. Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J. 2011;25:624–631. doi: 10.1096/fj.10-163790. [DOI] [PubMed] [Google Scholar]
- Manduca A., Oliphant T.E., Dresner M.A., Mahowald J.L., Kruse S.A., Amromin E., Felmlee J.P., Greenleaf J.F., Ehman R.L. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med. Image Anal. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- Mathon B., Amelot A., Carpentier A., Clemenceau S. Intraoperative real-time guidance using ShearWave Elastography for epilepsy surgery. Seizure. 2019;71:24–27. doi: 10.1016/j.seizure.2019.06.001. [DOI] [PubMed] [Google Scholar]
- McGarry M., Johnson C.L., Sutton B.P., Van Houten E.E., Georgiadis J.G., Weaver J.B., Paulsen K.D. Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE Trans. Med. Imaging. 2013;32:1901–1909. doi: 10.1109/TMI.2013.2268978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M.D.J., Van Houten E.E.W. Use of a Rayleigh damping model in elastography. Med. Biol. Eng. Comput. 2008;46:759–766. doi: 10.1007/s11517-008-0356-5. [DOI] [PubMed] [Google Scholar]
- McGarry M.D.J., Van Houten E.E.W., Johnson C.L., Georgiadis J.G., Sutton B.P., Weaver J.B., Paulsen K.D. Multiresolution MR elastography using nonlinear inversion. Med. Phys. 2012;39:6388–6396. doi: 10.1118/1.4754649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M.D.J., Van Houten E.E.W., Perrĩez P.R., Pattison A.J., Weaver J.B., Paulsen K.D. An octahedral shear strain-based measure of SNR for 3D MR elastography. Phys. Med. Biol. 2011;56 doi: 10.1088/0031-9155/56/13/N02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward J.M., Guo J., Berndt D., Braun J., Sack I., Infante-Duarte C. Tissue structure and inflammatory processes shape viscoelastic properties of the mouse brain. NMR Biomed. 2015;28:831–839. doi: 10.1002/nbm.3319. [DOI] [PubMed] [Google Scholar]
- Mueller S.G., Laxer K.D., Schuff N., Weiner M.W. Voxel-based T2 relaxation rate measurements in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2007;48:220–228. doi: 10.1111/j.1528-1167.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder T., Pfeffer A., Schreyer S., Guo J., Braun J., Sack I., Steiner B., Klein C. MR elastography detection of early viscoelastic response of the murine hippocampus to amyloid β accumulation and neuronal cell loss due to Alzheimer’s disease. J. Magn. Reson. Imaging. 2018;47:105–114. doi: 10.1002/jmri.25741. [DOI] [PubMed] [Google Scholar]
- Murphy M.C., Huston J., Glaser K.J., Manduca A., Meyer F.B., Lanzino G., Morris J.M., Felmlee J.P., Ehman R.L. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J. Neurosurg. 2013;118:643–648. doi: 10.3171/2012.9.JNS12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.C., Jones D.T., Jack C.R., Glaser K.J., Senjem M.L., Manduca A., Felmlee J.P., Carter R.E., Ehman R.L., Huston J. Regional brain stiffness changes across the Alzheimer’s disease spectrum. NeuroImage Clin. 2016;10:283–290. doi: 10.1016/j.nicl.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthupillai R., Lomas D.J., Rossman P.J., Greenleaf J.F., Manduca A., Ehman R.L. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- Pauli C., Schwarzbold M.L., Diaz A.P., de Oliveira Thais M.E.R., Kondageski C., Linhares M.N., Guarnieri R., de Lemos Zingano B., Ben J., Nunes J.C., Markowitsch H.J., Wolf P., Wiebe S., Lin K., Walz R. Predictors of meaningful improvement in quality of life after temporal lobe epilepsy surgery: a prospective study. Epilepsia. 2017;58:755–763. doi: 10.1111/epi.13721. [DOI] [PubMed] [Google Scholar]
- Sack I., Jöhrens K., Wuerfel J., Braun J. Structure-sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter. 2013;9:5672–5680. doi: 10.1039/c3sm50552a. [DOI] [Google Scholar]
- Schregel, K., Wuerfel nee Tysiak, E., Garteiser, P., Gemeinhardt, I., Prozorovski, T., Aktas, O., Merz, H., Petersen, D., Wuerfel, J., Sinkus, R., 2012. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc. Natl. Acad. Sci. 109, 6650–6655. https://doi.org/10.1073/pnas.1200151109. [DOI] [PMC free article] [PubMed]
- Schwarb H., Johnson C.L., McGarry M.D.J., Cohen N.J. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage. 2016;132:534–541. doi: 10.1016/j.neuroimage.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M., Kelly K.G., Parrish J., Geary E., Dow C., Rutecki P., Hermann B. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46:420–430. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- Semah F., Picot M.C., Adam C., Broglin D., Arzimanoglou A., Bazin B., Cavalcanti D., Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/WNL.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Sutton B.P., Noll D.C., Fessler J.A. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans. Med. Imaging. 2003;22:178–188. doi: 10.1109/TMI.2002.808360. [DOI] [PubMed] [Google Scholar]
- Thivard L., Lehéricy S., Krainik A., Adam C., Dormont D., Chiras J., Baulac M., Dupont S. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005;28:682–690. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Thom M. Review: Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol. Appl. Neurobiol. 2014;40:520–543. doi: 10.1111/nan.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M., Martinian L., Catarino C., Yogarajah M., Koepp M.J., Caboclo L., Sisodiya S.M. Bilateral reorganization of the dentate gyrus in hippocampal sclerosis: a postmortem study. Neurology. 2009;73:1033–1040. doi: 10.1212/WNL.0b013e3181b99a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S., Blume W.T., Girvin J.P., Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N. Engl. J. Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wiebe S., Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat. Rev. Neurol. 2012;8:669–677. doi: 10.1038/nrneurol.2012.181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.