Abstract
Rhombencephalitis (RE) refers to inflammatory diseases involving the brainstem and cerebellum. Although RE is a rare entity, it is associated with high morbidity and mortality. The management of such patients is often challenging in terms of identifying the etiology and defining prognosis. Infections, autoimmune and paraneoplastic conditions are commonly implicated. Patients with RE often present with a biphasic illness with an initial flu-like syndrome followed by brainstem dysfunction. CSF pleocytosis, abnormal brain MRI findings, isolation of organism or molecular (PCR/antigen) detection in CSF/blood cultures/stool samples and nasal/rectal swabs help in arriving at a definitive or probable diagnosis. Prompt aggressive treatment with antibacterial and antiviral drugs and/or immunoglobulins along with supportive therapy is crucial for avoiding a poor outcome.
We present a case report of a 28-year old female patient who developed RE and myelitis in the third trimester of pregnancy. We aim to highlight the highly suggestive radiological findings which corroborated with the clinical diagnosis of enterovirus infection. The patient's radiological follow-up and neurological sequalae are also described. To the best of our knowledge, ours is the first report which describes the MRI features of this clinical scenario in the third trimester of pregnancy, and also the subsequent clinico-radiological follow up.
Keywords: Brainstem, Rhombencephalitis, Myelitis, Anterior-horn cells, Enterovirus, Pregnancy
Introduction
The term ‘rhombencephalitis’ [RE] refers to inflammatory diseases of the brainstem and cerebellum. RE may often be challenging to diagnose and manage clinically. Infections, autoimmune and paraneoplastic conditions are common etiologies [1–7]. We present a case report of a young female patient who developed RE and myelitis in the third trimester of pregnancy. The pertinent clinical, laboratory and radiological features are highlighted along with a brief review of imaging literature.
Case report
In August 2019, a 28-year-old female patient who was previously healthy presented to a tertiary women's hospital at 35 weeks of gestation. She complained of fever, neck pain and sore throat for 2 days. She had no cough, shortness of breath, abdominal pain or diarrhoea. She did not report any other neurological symptoms like weakness, numbness, diplopia or photophobia. Up till then, her pregnancy had been uneventful. Clinical examination was unremarkable except for fever (38°C). She was evaluated for infection with blood cultures, dengue screen, influenza swab polymerase chain reaction (PCR), respiratory virus multiplex-PCR, urine analysis and urine cultures. All investigations were negative except for enterovirus RNA detected on nasal swab respiratory virus multiplex -PCR.
On day 2 of admission, the patient developed dysphagia to fluids. ENT evaluation was unremarkable. On day 4, she developed generalized tonic clonic seizures. She was given intravenous magnesium sulphate to treat presumptively for eclampsia and underwent an emergency Cesarean section. MRI brain performed at this time (MRI1) was reported as normal. The patient was started on intravenous acyclovir, ceftriaxone and vancomycin. Lumbar puncture (LP1) at this stage showed raised RBCs (10 cell/ul), raised WBCs (150 cells/ul), elevated protein (0.69g/L) and normal sugars (3.5mmol/L) [see Table 1]. No organisms were detected. As she remained confused, she was transferred to our tertiary neuroscience institute on day 6. She was admitted to the ICU and intubated in view of severe respiratory acidosis. A repeat LP (LP2) showed interval decrease in WBCs (48 cells/ul), persistently elevated protein (0.73 g/L) and normal sugar (3.8 mmol/L) [see Table 1]. CSF bacterial cultures, acid-fast bacillus smear and culture, fungal smear and culture, cryptococcal antigen, tuberculosis PCR, tetraplex (cytomegalovirus, herpes simplex virus, varicella zoster virus, toxoplasma) PCR and enterovirus PCR all returned negative. HIV screen, stool enterovirus PCR was also negative. A repeat MRI brain study at this point (MRI 2) showed ill-defined T1 hypointense and T2-FLAIR hyperintense lesions at the ponto-medullary junction. This signal abnormality was more prominent posteriorly, at the tegmentum of the pons (Fig 1). MRI cervical spine study showed longitudinally extensive T2 hyperintense signal in the cord, involving the central grey matter (predominantly the anterior horn cells) from C1 up to C7 level (Fig 2). No abnormal contrast enhancement was seen in the brain or cervical cord. The radiological diagnosis was RE and myelitis, possibly of infective or autoimmune etiology. The possibility of enterovirus infection was deemed more likely in view of typical posterior tegmental involvement of the pons and classic long segment central grey matter/anterior horn-cell involvement of the cervical cord. This corroborated with the clinical finding of positive enterovirus RNA on nasal swab.
Table 1-.
Results of CSF studies.
| Test Name | UoM | Ref. range | LP1(day 4 of illness) | LP2(day 6 of illness) | LP3(day 17 of illness) |
|---|---|---|---|---|---|
| RBC, Fluid (RBCF1) | cells/uL | <=0 | 10 | 1 | 3 |
| Nucleated Cell (NC) | cells/uL | 0-5 | 150 | 48 | 9 |
| Protein, CSF (TPC) | g/L | 0.10–0.40 | 0.69 | 0.73 | 0.67 |
| Glucose, CSF (GLUC) | mmol/L | 2.4–4.3 | 3.5 | 3.8 | 3.0 |
| Basophils (FBAS) | % | * | 0 | * | |
| Eosinophils (FEOS) | % | * | 0 | * | |
| Lymphocytes (FLYM) | % | * | 76 | * | |
| Monocytes (FMON) | % | * | 24 | * | |
| Neutrophils (FNEU) | % | * | 0 | * | |
| Organism | 0 | 0 | 0 |
Unable to perform manual differential count due to degeneration of cellular morphology
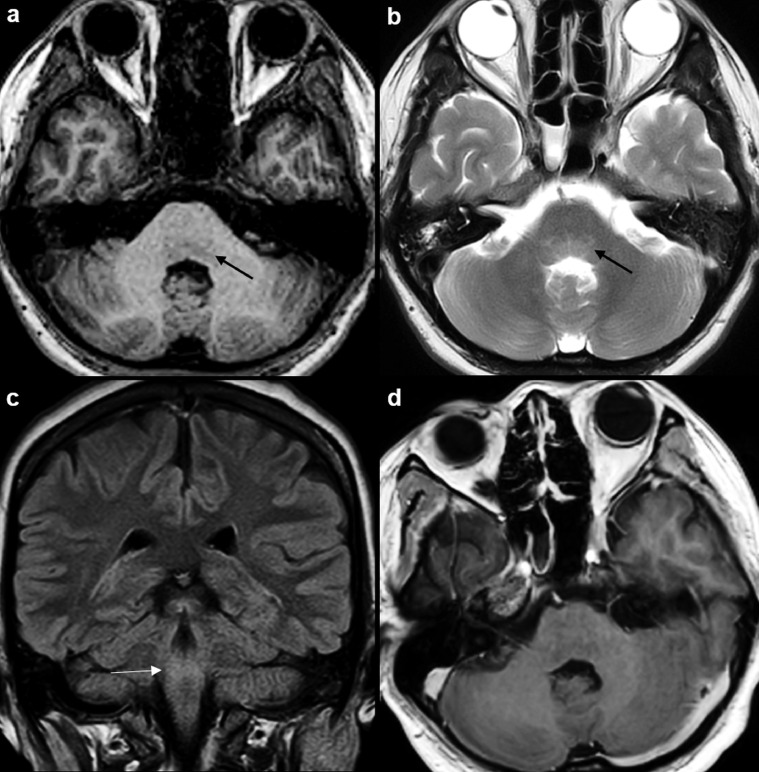
Fig. 1.
MRI brain study at time of admission in ICU of our institute (MRI 2). Axial T1W MR image (a) at the level of the pons shows ill-defined hypointense signal in the pontine tegmentum (arrow). There is mild T2 hyperintense signal in this region (arrow) on the corresponding axial T2W MR image (b). Coronal FLAIR image (c) shows corresponding ill-defined hyperintense signal in the tegmentum (arrow). Contrast-enhanced axial T1W MR image (d) shows no abnormal enhancement in the involved posterior brainstem.
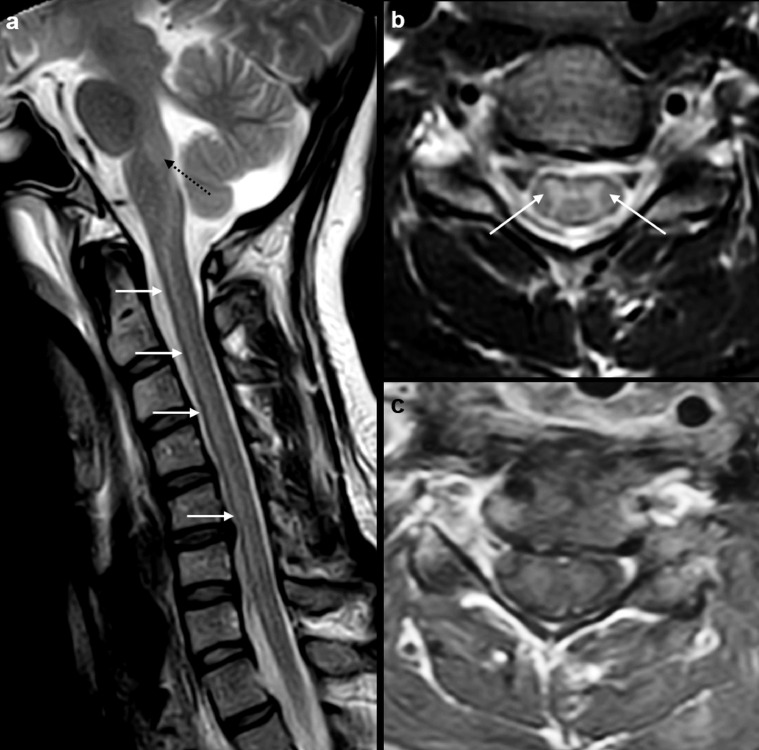
Fig. 2.
MRI cervical spine study at time of admission in ICU of our institute. Sagittal T2W MR image (a) shows T2 hyperintense signal in the posterior pons and medulla (dotted arrow). There is extensive T2 hyperintense signal involving the anterior aspect of the entire cervical cord (arrows). Axial T2W MR image (b) at C5 level shows hyperintense signal in the central grey matter, especially anterior horn cells (arrow). Contrast-enhanced axial T1W MR image at C5 (c) shows no abnormal enhancement in the central grey matter or in the surrounding white matter of cord.
Retrospective history taking at this point revealed that her 2-year-old daughter (who resided with her) was diagnosed with ‘hand-foot-mouth-disease’ (HFMD) 4 days prior to her onset of symptoms. There was no history of consumption of unpasteurized milk, meat or soft cheeses. Postnatally, the newborn remained healthy. After 4 days in the ICU, the patient improved in mentation and ventilation and was transferred to a general ward. At this point, she developed transient diplopia and ataxia, but these gradually improved over the next few days.
A repeat LP (LP 3) on day 17 of illness showed significant improvement in lymphocytosis [see Table 1]. CSF bacterial cultures, acid-fast bacillus cultures, fungal smears, and cultures, cryptococcal antigen, TB PCR, tetraplex PCR, and meningitis/encephalitis PCR all returned negative again. Autoimmune workup returned negative for paraneoplastic panel, autoimmune encephalitis panel (serum and CSF), anti-aquaporin-4 (neuromyelitis optica), and myelin oligodendrocyte glycoprotein (MOG) antibody. CSF cytology showed no malignant cells. MRI of the brain (MRI 3) at this time showed only minimal residual T2/FLAIR hyperintensity in the pons and medulla (Fig 3). MRI of the cervical spine showed stable longitudinally extensive T2 hyperintense signal in anterior horn cell region of the cervical spinal cord (Fig 3). Again, no abnormal enhancement was seen in the brain or cervical cord. Although MRI findings at this time were still abnormal, clinically the patient did not have any weakness. She had improved in terms of ataxia, diplopia and dysphagia and hence was discharged.
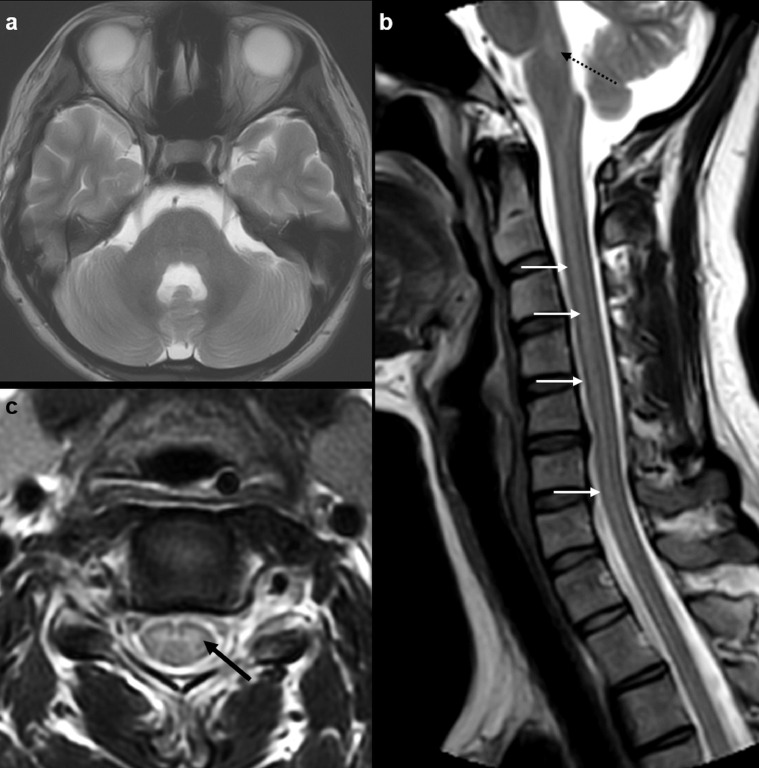
Fig. 3.
MRI brain and cervical spine study on day 17 of illness (MRI 3). Axial T2W MR image (a) at the level of the pons shows complete resolution of previously seen T2 hyperintense signal in the tegmentum. Sagittal T2W MR image (a) shows minimal residual T2 hyperintense signal in the posterior medulla (dotted arrow). There is stable extensive T2 hyperintense signal involving the anterior aspect of the entire cervical cord (arrows). Axial T2W MR image at C5 level (b) again shows stable hyperintense signal in the central grey matter, especially anterior horn cells (arrow).
In September 2019, 10 days after being discharged, the patient presented with intention tremors in both upper limbs. Power in both upper limbs was normal. A repeat MRI (MRI 4) of the brain and cervical spine at this time showed a tiny, well-defined T2-FLAIR hyperintense lesion in the left side of the medulla with no enhancement (Fig 4). Stable long-segment T2 hyperintensity in the anterior horn cell region of the cervical cord was seen again, now showing contrast enhancement (Fig 4). These imaging findings in the medulla and the cord were deemed to be sequelae/evolving changes of previous inflammation. Despite these imaging findings, she remained clinically well with no focal weakness. At a subsequent clinical review 3 months post illness, the tremors had resolved and she was completely asymptomatic.
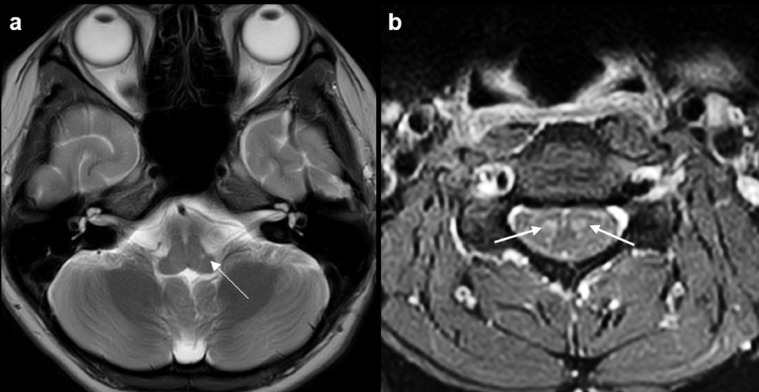
Fig. 4.
MRI brain and cervical spine study on day 10 after discharge (MRI 4). Axial T2W MR image (a) at the level of the lower medulla shows a tiny T2 bright residual lesion at the left lateral aspect (arrow). Contrast-enhanced axial T1W MR image (b) of the cervical cord at C5 level shows prominent enhancement in bilateral anterior horn cells (arrows).
Discussion
The term ‘RE’ refers to inflammatory diseases of the hindbrain (brainstem and cerebellum). RE and brainstem encephalitis are often used as interchangeable terms. Although RE is an uncommon entity, it may be potentially life-threatening if not diagnosed and treated early. RE has a wide variety of etiologies, the most frequent being infections, autoimmune and paraneoplastic conditions (see Table 2) [1–4]. Listeria monocytogenes is the most frequently implicated infectious agent, followed by enterovirus and herpes simplex virus [1–5]. Of the autoimmune conditions, Bechet's disease is the most common [1–4,6], whereas, majority of paraneoplastic syndromes causing RE are associated with small cell lung cancer [1–4,7]. RE typically presents in a biphasic time course with an initial respiratory or gastro-intestinal prodrome followed by brainstem dysfunction. Brainstem dysfunction is manifest by lower cranial nerve palsies, ophthalmoplegia, ataxia, limb weakness and cardiorespiratory failure [1–10]. The diagnosis of RE is often difficult as clinical findings can be confounding and laboratory results may be normal or nonspecific. CSF pleocytosis with elevated protein, abnormal brain MRI findings, detection of organism in CSF/blood cultures/PCR or nasal/rectal swab/stool samples and/or positive antibody tests may help in arriving at a definitive or probable diagnosis [1–10].
Table 2–
Most common etiologies of RE (summarized from references 3, 4, 6, 7).
| Infection |
Bacterial:
|
Viral:
|
| Autoimmune |
|
|
| Paraneoplastic |
|
|
Our patient had presented with a prodrome of upper respiratory symptoms. Although she did complain of neck pain, the lack of objective stiffness, lack of neurological deficits and her non-toxic clinical status made the initial clinical diagnosis challenging. Initially her dysphagia was deemed to be due to globus sensation, however on retrospective evaluation, it indicated the onset of brainstem dysfunction, which was subsequently followed by respiratory failure requiring intubation. The patient also developed diplopia and ataxia. In summary, the clinical features although confounding at the beginning, eventually fitted into the picture of RE.
Further, determining the cause of RE in our case proved to be challenging as well. Listeria was initially high on the suspicion list, considering that is the most common infectious cause and also common in pregnant patients. However, risk factors and exposures did not match those of listeriosis i.e. there was no history of consumption of raw cheeses or unpasteurized dairy products [1–5]. No organism was isolated in CSF or blood smears or cultures, or PCR tests. Also, autoimmune work-up was negative. In view of the positive contact history (daughter diagnosed with HFMD), positive nasal swab for enterovirus RNA, and lymphocytic predominant CSF studies, enterovirus was considered to be the most likely etiological agent. The characteristic MRI findings of T2-FLAIR hyperintense lesions at the ponto-medullary junction especially in the pontine tegmentum [2,8–18] and long-segment T2 hyperintensity involving the cervical anterior-horn cells [8–19] corroborated with the clinical suspicion of enterovirus infection. Absence of rim-enhancing lesions and absence of supratentorial lesions further helped to rule out listeria RE [2,4,5].
Enterovirus infections typically affect the paediatric population causing epidemic outbreaks of HFMD or herpangina, which are mild self-limiting diseases. A small number of these infections progress to neurological conditions like aseptic meningitis, RE and polio-like myelitis/acute flaccid paralysis. EV-D68 and EV-A71 are the most common subtypes associated with neurological complications [8], [9], [10], [11], [12], [12], [14], [15], [16], [17]–18]. Enterovirus encephalitis can occur in adults as well [18,20]; also, enterovirus meningitis has been reported in pregnancy [21]. Enterovirus RNA yield from CSF and blood is known to be poor [15,17,18]. Hence it is recommended that in hospitalized patients (often with positive contact history/or in epidemic outbreaks) with clinical features of brainstem dysfunction/acute flaccid paralysis, CSF pleocytosis, negative PCR of CSF/blood sample, but a positive PCR of nasal/throat or rectal swab should be considered as diagnostic of enterovirus infection [18,22]. Keeping in view these guidelines, in conjunction with the classic imaging findings, our case was conclusively diagnosed as enterovirus associated RE and myelitis.
We extensively reviewed literature describing imaging features of enterovirus related RE and myelitis [2,4,8–19]. Almost all of these reports describe paediatric cases. To the best of our knowledge, ours is the first case report describing the imaging features of this clinical scenario in a pregnant patient in the third trimester. MRI scans are known to be abnormal in almost 70%-75% of RE cases caused by enterovirus, compared to 100% of cases caused by Listeria and 67% of cases caused by herpes simplex virus [2,4]. Enterovirus RE typically involves the posterior aspects of the pons and the medulla, most notably the pontine tegmentum. The lesions appear iso-hypointense on T1 and hyperintense on T2/FLAIR sequences. The other favoured sites include the dentate nuclei of the cerebellum and substantia nigra of the midbrain. Supratentorial involvement is uncommon; very few reports mention involvement of the thalami and putamina. Restricted diffusion and contrast enhancement are uncommon in brain lesions. The characteristic imaging feature of enterovirus-related myelitis is longitudinally extensive spinal cord lesions restricted to the grey matter or with predominant anterior-horn cell involvement. The T2-bright appearance of the involved anterior horn cells is classically described as the ‘owl's eye’ sign [19]. The cervical cord is commonly involved, cord swelling may be seen. Restricted diffusion is uncommon in cord lesions; however, enhancement of grey matter and ventral spinal roots has been described [11,13,16,18]. Our case demonstrated classic tegmental involvement in the brainstem and anterior horn cell involvement in the cervical cord. Other T2-bright brainstem lesions (eg, multiple sclerosis, osmotic myelinolysis, brainstem glioma) and other T2-bright anterior horn-cell lesions (eg, neuromyelitis optica, cord infarction) were very unlikely in our clinical scenario. The brainstem lesions had largely resolved over the subsequent follow-up MRI studies, save for a well-defined, tiny T2-bright lesion in the left side of the medulla (Fig 4). This was ascribed to residual cavitatory change which is a well-known sequel of enterovirus RE [9,10]. The last follow-up cervical spine MRI study showed persistent cervical cord T2 hyperintensity, now with appearance of contrast-enhancement in the anterior horn cells. Signal abnormalities due to enterovirus myelitis are known to persist for over a few years [16].
Our patient was empirically treated for meningitis with intravenous antibiotics and acyclovir at the onset of seizures. Unlike listeria RE or HSV RE where ampicillin and acyclovir are used as specific treatments, no specific antimicrobial drug is available for the treatment of enterovirus RE. Intravenous immunoglobulins, steroids and plasmapheresis have been used with varying response. Supportive therapy, especially cardiopulmonary support forms the mainstay of treatment [1,3–5,8,11,16–18,22].
Neurologic sequalae of EV-D68 and EV-A71 infections in children are well described. Children with mild neurological involvement such as aseptic meningitis often recover completely. Children with RE along with cardiopulmonary failure have the highest rate of long-term sequelae such as residual limb weakness, delayed neurodevelopment, impaired cognition etc [9,11,15–18]. Neurological sequalae of enterovirus-RE in adults are not well described in literature. Our patient developed intention tremors (known sequel of rhombencephalon involvement) [16] a few weeks after discharge. However, they were transient and had resolved by her last clinical review. In conclusion, our patient had no long-term residual neurological deficits.
Conclusion
Although enterovirus RE is typically perceived as a paediatric disease, it may occur in adults as well, including in pregnant patients. A high index of clinical suspicion should be maintained in patients presenting with a positive contact history, classic biphasic clinical picture of RE and CSF pleocytosis. Isolation of enterovirus PCR in respiratory or stool and/or blood /CSF samples helps in reaching the conclusive diagnosis. From a neuroradiologist's perspective, awareness of these clinical features and knowledge of typical MRI findings namely posterior pontomedullary lesions and involvement of the anterior horn cells would help in avoiding diagnostic pitfalls. Also, awareness of clinico-radiological sequalae as described in our report would help in furthering our understanding of this entity.
Acknowledgments
Consent to publish
Informed written consent obtained and documented. No ethics committee approval is required for case report publication as per our institutional policy.
Author Contributions
Sumit Kumar Sonu: study concept, data collection, drafting manuscript, literature review. Yi Wai Lai: data collection, drafting manuscript, literature review. Kamal Verma: critical revision of manuscript, literature review. Yih Yian Sitoh: critical revision of manuscript. Bela Purohit: study concept, drafting and revising manuscript, literature review, image formatting, guarantor of integrity of study.
Footnotes
Grants information: No grants were applied or received. No conflict of interests.
References
- 1.Wasenko J.J., Park B.J., Jubelt B. Magnetic Resonance Imaging of mesenrhombencephalitis. J Clin Imaging. 2002;26(4):237–242. doi: 10.1016/s0899-7071(02)00427-8. [DOI] [PubMed] [Google Scholar]
- 2.Campos L.G., Trindade R.A.R., Faistauer A. Rhombencephalitis: Pictorial essay. Radiol Bras. 2016;49(5):329–336. doi: 10.1590/0100-3984.2015.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan I.L., Mowry E.M., Steele S.U. Brainstem Encephalitis: etiologies, treatment, and predictors of outcome. J Neurology. 2013;260(9):2312–2319. doi: 10.1007/s00415-013-6986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jubelt B., Mihai C., Li T.M. Rhombencephalitis / brainstem encephalitis. Curr Neurol Neurosci Rep. 2011;11(6):543–552. doi: 10.1007/s11910-011-0228-5. [DOI] [PubMed] [Google Scholar]
- 5.Mansbridge C.T., Grecua I., Chong J.S.W.L.V. Two cases of listeria rhombencephalitis. IDcases. 2018;11:22–25. doi: 10.1016/j.idcr.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang P., Bucelli R. Reversible rhombencephalitis in neuro-Behcet's disease. The Neurohospitalist. 2017;7(3):148–149. doi: 10.1177/1941874416670070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett M., Prosser J., Sutton I. Paraneoplastic brainstem encephalitis in a woman with anti-Ma 2 antibody. J Neurol, Neurosurg & Psychiatry. 2001;70(2):222–225. doi: 10.1136/jnnp.70.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelgawad M.S., El-Nekidy A.E., Abouyoussef R.A.M. MRI Findings of Enteroviral Encephalomyelitis. The Egyptian Journal of Radiology and Nuclear Medicine. 2016;47(3):1031–1036. [Google Scholar]
- 9.Shen W.-C., Chiu H.-H., Chow K.-C. MR imaging findings of enteroviral encephalomyelitis: An outbreak in Taiwan. Am J Neuroradiol. 1999;20(10):1889–1895. [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng H., Huang W., Wen F. MRI signal intensity differentiation of brainstem encephalitis induced by Enterovirus 71: a classification approach for acute and convalescence stages. BioMed Eng Online. 2016;15:25. doi: 10.1186/s12938-016-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K.Y., Lee Y.-J., Kim T.H. Clinico-radiological spectrum in enterovirus 71 infection involving the central nervous system in children. J Clin Neuroscience. 2014;21(3):416–420. doi: 10.1016/j.jocn.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Chen F., Liu T. MRI findings of neurological complications in hand-foot-mouth disease by Enterovirus 71 infection. Int J Neuroscience. 2012;122(7):338–344. doi: 10.3109/00207454.2012.657379. [DOI] [PubMed] [Google Scholar]
- 13.Maloney J.A., Mirsky D.M., Messacar K. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the Enterovirus D 68 outbreak. AJNR Am J Neuroradiol. 2015;36(2):245–250. doi: 10.3174/ajnr.A4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C.Y., Chang Y.C., Huang C.C. MR imaging findings of epidemic enterovirus 71 encephalitis in infants and young children. Chinese J Radiology. 2000;25(2):45–52. [Google Scholar]
- 15.Chang L.-Y., Lin H.-Y., Gau S.S.-F. Enterovirus A71 neurologic complications and long term sequelae. J Biomed Sci. 2019;26(1):57. doi: 10.1186/s12929-019-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C.-C., Liu C.-C., Chang Y.-C. Neurologic complications in children with enterovirus 71 infection. The New Eng J Medicine. 1999;341(13):936–942. doi: 10.1056/NEJM199909233411302. [DOI] [PubMed] [Google Scholar]
- 17.Hellferich J., Knoester M., Leer-Buter C.C.V. Acute flaccid myelitis and enterovirus D68: lessons from the past and present. European J Paediatrics. 2019;178(9):1305–1315. doi: 10.1007/s00431-019-03435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoester M., Helfferich J., Poelman R. Twenty-nine cases of enterovirus-D68-associated acute flaccid myelitis in Europe 2016. The Paediatric Infectious Diseases. 2019;38(1):16–21. doi: 10.1097/INF.0000000000002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golub D., Williams F., Wong T. A longitudinally extensive spinal cord lesion restricted to gray matter in an adolescent male. Front Neurol. 2019;10:270. doi: 10.3389/fneur.2019.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowlkes Ashley L. Enterovirus-associated encephalitis in the California Encephalitis Project, 1998-2005. The J Infectious Diseases. 2008;198(11):1685–1691. doi: 10.1086/592988. [DOI] [PubMed] [Google Scholar]
- 21.Khediri Z., Vauloup-Fellous C., Benachi A. Adverse effects of maternal enterovirus infection on the pregnancy outcome: a prospective and retrospective pilot study. Virol J. 2018;15:70. doi: 10.1186/s12985-018-0978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Velez C.M., Anderson M.S., Robinson C.C. Outbreak of neurologic Enterovirus type 71 disease: a diagnostic challenge. Clin Infect Dis. 2007;45(8):950–957. doi: 10.1086/521895. [DOI] [PubMed] [Google Scholar]