Abstract
Background:
Patients undergoing cancer treatment often suffer from chemotherapy-induced neuropathic pain at their extremities, for which there is no FDA-approved drug. We hypothesized that local sympathetic blockade, which is used in the clinic to treat various pain conditions, can also be effective to treat chemotherapy-induced neuropathic pain.
Methods:
A local sympathectomy, i.e. cutting the ipsilateral gray rami entering the spinal nerves near the L3 and L4 dorsal root ganglia, was performed in mice receiving intraperitoneal injections every other day of the chemotherapeutic drug paclitaxel. Sympathectomy effects were then assessed in chemotherapy-induced pain-like behaviors (i.e. mechanical and cold allodynia), neuroimmune and electrophysiological responses.
Results:
Local microsympathectomy produced a fast recovery from mechanical allodynia (mean ± SD: sympathectomy vs. sham at day 5, 1.07 ± 0.34g vs. 0.51 ± 0.17g, n =5, p = 0.030 in male mice, and 1.08 ± 0.28g vs. 0.62 ± 0.16g, n=5, p = 0.036 in female mice) and prevented the development of cold allodynia in both male and female mice after paclitaxel. Mechanistically, microsympathectomy induced transcriptional increases in dorsal root ganglia of macrophage markers and anti-inflammatory cytokines, such as the transforming growth factor-β (TGFβ). Accordingly, depletion of monocytes/macrophages and blockade of TGFβ signaling reversed the relief of mechanical allodynia by microsympathectomy. In particular, exogenous TGFβ was sufficient to relieve mechanical allodynia after paclitaxel (TGFβ 100 ng/site vs. vehicle at 3 h, 1.21 ± 0.34g vs. 0.53 ± 0.14g, n = 5, p = 0.001 in male mice) and TGFβ signaling regulated neuronal activity in dorsal root ganglia.
Conclusion:
Local sympathetic nerves control the progression of immune responses in dorsal root ganglia and pain-like behaviors in mice after paclitaxel, raising the possibility that clinical strategies already in use for local sympathetic blockade may also offer an effective treatment for patients suffering from chemotherapy-induced neuropathic pain.
TOC Statement:
Local surgical sympathectomy relieved nociceptive and mechanical sensitization in a mouse model of paclitaxel-induced pain. Transforming growth factor-β was enhanced in mice after sympathectomy and was capable of reducing paclitaxel-induced mechanical sensitization.
Introduction
Chemotherapy-induced neuropathic pain is a disabling condition affecting up to 80% of patients during treatment with antineoplastic drugs, including the frontline chemotherapeutic agent paclitaxel.1,2 There are no FDA-approved drugs to treat this type of neuropathic pain and many drugs that are used for the treatment of other neuropathic pain states have shown poor or no analgesic effect on chemotherapy-induced neuropathic pain.1 Paclitaxel is a common drug for various solid cancers. However, it is often associated with neuropathic pain, which is generally localized in the distal extremities of the body and can persist for months or years after the end of the treatment.2
Although the exact mechanisms underlying paclitaxel-induced neuropathic pain remain incompletely known, there are preclinical lines of evidence indicating that immune cells in dorsal root ganglia (DRGs) play critical roles in the development and progression of chemotherapy-induced pain-like behaviors (i.e. mechanical and cold allodynia), which are reminiscent of its clinical symptoms.3 Interestingly, it has been reported that various chemotherapy agents induce the infiltration of monocytes into the DRGs and sciatic nerve, where they differentiate into inflammatory macrophages and contribute to pain-like behaviors in several animal models of chemotherapy-induced neuropathic pain,4–6 including in animals receiving paclitaxel. The association between monocyte/macrophage infiltration and pain-like behaviors in these models of chemotherapy-induced neuropathic pain has been reinforced pharmacologically. Depletion of macrophages using liposome-encapsulated clodronate alleviated paclitaxel-induced mechanical hypersensitivity, via the reduction of the paclitaxel-associated increase in macrophages and expression of the pro-inflammatory tumor necrosis factor alpha in the DRG.4 Similarly, minocycline, an antibiotic that inhibits monocyte/macrophage infiltration and pro-inflammatory cytokines alongside other actions, has been shown to prevent paclitaxel-induced mechanical allodynia.7 Unfortunately, a recent pilot study of minocycline in patients did not support its translation for the prevention of paclitaxel-induced neuropathy in clinic.8
Local sympathetic blockade is a well-accepted clinical procedure to treat various inflammatory pain conditions.9,10 Sympathetic nerves generally promote inflammation in the initial phase of immune responses, whereas they suppress inflammation in the later phase. However, these responses are variable depending on the disease, the involved immune cell receptor, and the local environment in particular tissues.11 Our recent study has shown that sympathetic nerves drive immune responses in organs such as the thymus, spleen and lymph nodes, and also regulate macrophage responses and cytokine expression levels in DRGs, maintaining inflammatory pain-like behaviors in rats.12 Other animal studies have reported sympathetic nerve sprouting around the DRG neurons after nerve injury, suggesting that sympathetic neurons also contribute to neuropathic pain-like behaviors by facilitating nociceptive transmission.13,14
Although various pain conditions seem to be exacerbated or maintained by sympathetic activity, whether and how localized sympathectomy influences the development and progression of chemotherapy-induced neuropathic pain remains to be investigated. We hypothesized that sympathetic nervous system activity also regulates the progression of immune response in DRGs and pain-like behaviors in mice treated with paclitaxel. Our preclinical study supports a novel therapeutic approach by which a clinically relevant local sympathectomy can provide relief of chemotherapy-induced neuropathic pain via anti-inflammatory responses and transforming growth factor-β (TGF-β1) signaling.
Materials and Methods
Animals and procedures
Male and female CD1 mice (8–10 weeks) from Charles River were used as indicated for behavioral and biochemical experiments. Mice were housed four per cage at 22 ± 0.5°C under a controlled 14/10 hours light/dark cycle with free access to food and water. To produce an animal model of chemotherapy-induced neuropathic pain, mice were treated with paclitaxel as previously described.15 Briefly, 6 mg/mL stock paclitaxel (Sigma-Aldrich, St. Louis, Mo) was diluted with Cremophor EL and 95% dehydrated ethanol (1:1 ratio, Sigma-Aldrich) and given at a dosage of 2 mg/kg diluted in saline intraperitoneally every other day for a total of 2 injections (days 0 and 2 with a final cumulative dose of 4 mg/kg). Control animals received an equivalent volume of the vehicle with proportional amounts of Cremophor EL and 95% dehydrated ethanol diluted in saline. Signs of peripheral neuropathy with a similar phenotype to that in patients have been validated by multiple investigators in this non–tumor-bearing animal model, including a time-dependent development of mechanical and cold allodynia. All experiments and procedures were performed in accordance with the guidelines recommended by the National Institutes of Health, the International Association for the study of Pain, the National Centre for the Replacement, Refinement, and Reduction of Animals in Research ARRIVE guidelines, and were approved by the Institutional Animal Care and Use Committee at University of Cincinnati. Animals were randomly assigned to experimental groups. Although no statistical power calculation was conducted, the sample size of each experimental group was based on our previous similar studies.12,16 All of the experimenters were blind to treatment condition.
Drugs and drug administration
We purchased the 6-hydroxydopamine (6-OHDA, cat# H4381) from MilliporeSigma (Burlington, MA), TGFβ (cat# TP723438) from Origene (Rockville, MD), the SB431542 (cat# S1067), a TGFβ inhibitor, from Selleckchem, the liposomal clodronate (cat# 283539) from Liposoma (The Netherlands, Amsterdam) and C-C chemokine receptor 2 (CCR2) antagonist INCB3344 (cat# A3494) from APExBIO (Houston, TX). The TGFβ and TGFβ inhibitor were administrated intrathecally to deliver reagent into cerebral spinal fluid and DRG tissues, as we have previously described.16,17 A valid spinal puncture was confirmed by a reflexive tail flick after the needle entry into subarachnoid space. The liposomal clodronate and INCB3344 were administrated intraperitoneally and intravenously, respectively.
Chemical sympathectomy
Mice were injected intraperitoneally with 6-OHDA (80 mg/kg) in 0.01% ascorbic acid in phosphate-buffered saline (PBS) at day −3. Control mice received injections of 0.01% ascorbic acid in PBS.
Local microsympathectomy
The proximal L3 and L4 spinal nerves and transverse processes on one side were exposed. The spinal nerves (ventral rami) were visualized and freed from surrounding tissue. The gray rami entering the L3 and L4 spinal nerves close to the DRGs were identified on the ventral side of the spinal nerve at the position very close to the intervertebral foramen. At this site (Suppl. Fig. 1A), where the gray ramus merges into the spinal nerve just across from the juncture where the dorsal ramus diverges from the ventral ramus, the gray rami and nearby connective tissue were gently dissected away from the nearby blood vessels and cut and disconnected from spinal nerve. Approximately 1 mm of gray ramus was further removed to make a gap and slow regeneration. Both L2 and L3 gray rami were cut in all microsympathectomy surgeries. Sham controls received similar exposure of the spinal nerves, but the gray rami were not cut. Two days after the microsympathectomy, the mice were treated with paclitaxel to mimic chemotherapy-induced neuropathic pain.
Macrophage/monocyte depletion
Liposome-encapsulated clodronate was used to deplete phagocytic macrophages.18 Liposomal clodronate (15 ml/kg, ClodronateLiposomes.com) was intraperitoneally injected on day 5 and 7 after the first paclitaxel injection in mice with microsympathectomy. In response to peer review, INCB3344 was injected intravenously into the tail vein (100 μl of 0.18 mM solution = 18 nmol/injection) on day 5, 6 and 7 after the first paclitaxel injection in mice with microsympathectomy to partially deplete monocytes, as previously reported.19
Mechanical and cold allodynia
Mechanical allodynia was assessed as the hind paw withdrawal response to von Frey hair stimulation using the up-and-down method, as previously described.20 Briefly, the mice were first acclimatized (1 hour) in individual clear Plexiglas boxes on an elevated wire mesh platform to facilitate access to the plantar surface of the hind paws. Subsequently, a series of von Frey hairs (0.02, 0.07, 0.16, 0.4, 0.6, 1.0, and 1.4 g; Stoelting CO., Wood Dale, IL) were applied perpendicular to the plantar surface of hind paw. Testing began with the application of the 0.6 g hair. A positive response was defined as a clear paw withdrawal or shaking. Whenever a positive response occurred, the next lower hair was applied, and whenever a negative response occurred, the next higher hair was applied. The testing consisted of 6 stimuli, and the pattern of response was converted to a 50% von Frey threshold, using the method described previously.21 Cold allodynia was also assessed in response to peer review, and performed by the use of the cold plantar assay, as previously described.22 Briefly, animals were first placed individually into clear acrylic containers separated by black opaque dividers that were set on top of 3/16” borosilicate glass (Stemmerich Inc, St. Louis, MO) and allowed to acclimate for 20 minutes before testing. A dry ice pellet was applied to the hind paw through the glass. The time until hind paw withdrawal was recorded at 5-minute intervals per mouse, alternating paws, for a total of 3 trials, and the mean withdrawal latency was calculated. Withdrawal latencies were evaluated before and 1 to 14 days after the first injection of paclitaxel.
Real-time quantitative RT-PCR
Mice were terminally anesthetized with isoflurane and lumbar DRG (L3 and L4) were rapidly removed 7 days after paclitaxel treatment. In some experiments, spleen tissue was also isolated. Total RNA was extracted using Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA), which amount and quality were assessed by SimpliNano UV-Vis Spectrophotometer (General Electric, Boston, MA), and then converted into cDNA using a High-capacity RNA-to-cDNA kit (Thermo Fisher Scientific, Thermo Fisher Scientific, Waltham, MA). Specific primers for cytokines and markers of monocytes/macrophages, adrenergic and purinergic receptors, as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained from PrimerBank.23 Primer sequences are depicted in suppl. table 1. Real-time quantitative RT-PCR was performed on a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific) using PowerUp SYBR™ Green Master Mix (Thermo Fisher Scientific). All samples were analyzed at least in duplicate and normalized by GAPDH expression. The relative expression ratio per condition was calculated based on the method described by Pfaffl et al.24
Immunohistochemical analysis
Deeply anesthetized mice were perfused through the left ventricle with PBS solution, followed by 4% paraformaldehyde in PBS (PFA solution) and lumbar DRGs (L3 and L4) were collected 7 or 14 days after paclitaxel treatment. All tissues were post-fixed in PFA solution overnight and subsequently transferred into 30% sucrose in PBS for 24h. For ionized calcium binding adaptor molecule 1 (IBA1) immunohistochemical quantification, DRG tissues were sliced into 12 μm sections and placed on slides, which were then blocked for 1h at room temperature with 1% bovine serum albumin (BSA) with 0.2% Triton X-100 in PBS (BSA solution). Subsequently, sections were incubated with IBA1 primary antibody (goat, 1:1000, cat# NB100–1028, Novus) overnight at 4°C, followed by incubation with the secondary antibody anti-goat Alexa Fluor® 555 (1:1000, Thermo Fisher Scientific) for 1 h at room temperature. DAPI (4’,6-diamidino-2-phenylindole, Thermo Fisher Scientific) was used for counterstaining. For the quantification of sympathetic fibers, DRGs were sliced into 40 μm sections and sections were blocked for 1h at room temperature in the BSA solution, incubated with primary antibodies against tyrosine hydroxylase (Th, rabbit, 1:500, cat# P40101–0, Pel-Freez Biologicals, Rogers, AR) for 48 h at 4°C, and followed by incubation with the secondary antibodies anti-rabbit Alexa Fluor® 488 (1:1000, Thermo Fisher Scientific) for 1 h at room temperature. DAPI was used for counterstaining. For quantification, images from 4–5 sections of each DRG/group, selected at random, were captured under an Olympus BX63 fluorescent microscope using cellSens imaging acquisition software (Olympus, Center Valley, PA) by an investigator blinded to treatment conditions. Images of all DRG tissue were captured and intensity quantification was performed comparing samples from all experimental groups, prepared with the same staining solutions, then measured using identical display parameters.
Cytokine Array
The mouse cytokine array kits were purchased from R&D (cat# ARY006). Animals were terminally anesthetized with isoflurane and lumbar DRG (L3 and L4) were rapidly removed 7 days after paclitaxel treatment and homogenized in a lysis buffer containing a cocktail of protease inhibitors and phosphatase inhibitors. Concentration of protein was measured using Qubit TM (Invitrogen). Each reaction was performed according to manufacturer’s protocol.
Electrophysiology
Intracellular recording in current clamp mode was performed at 36 – 37°C using microelectrodes in sensory neurons near the dorsal surface of an acutely isolated whole DRG preparation, as previously described for rats.25 This preparation allows neurons to be recorded without enzymatic dissociation, with the surrounding satellite glia cells and neighboring neurons intact.26,27 The L4 DRG was isolated from mice 9 days after microsympathectomy and 7 days after paclitaxel treatment. The DRG was secured in the recording chamber and continuously perfused with artificial cerebro-spinal fluid (ACSF; in mM: NaCl 130, KCl 3.5, NaH2PO4 1.25, NaHCO3 24, Dextrose 10, MgCl2 1.2, CaCl2 1.2, 16 mM HEPES, pH = 7.3, bubbled with 95% O2/ 5% CO2). The TGF-βR1 inhibitor SB431542 (10 ng/ml) or vehicle was included throughout the recording period. Cells were classified as small/likely unmyelinated or large/likely myelinated, based on action potential duration <1.5 msec or >1.5 msec duration, respectively, as confirmed by conduction velocity measurements in a subset of cells. Excitability parameters were analyzed as described previously.28
Statistical analysis
Statistical analyses were performed with GraphPad Prism software (San Diego, CA) and all data were expressed as mean ± standard deviation. No outliers were excluded. Behavioral data were analyzed using two-way repeated measured analysis of variance (ANOVA) followed by Bonferroni post hoc test. Biochemical and electrophysiological data were analyzed by two-tailed, unpaired Student’s t-test or one-way ANOVA followed by Turkey post hoc test, or Mann-Whitney test for electrophysiological data that were not normally distributed according to the D’Agostino & Pearson omnibus normality test. The criterion for statistical significance was p < 0.05.
Results
Systemic chemical sympathectomy delays the development and resolution of paclitaxel-induced mechanical allodynia
We hypothesized that chemotherapy-induced neuropathic pain is exacerbated or maintained by sympathetic nervous system (SNS) activity. We first used a systemic approach to determine the role of the SNS by injecting the chemical 6-hydroxydopamine for a systemic sympathetic denervation or a vehicle control 3 days before paclitaxel treatment in mice (fig. 1A). Systemic sympathectomy had no effect on baseline paw mechanical withdrawal threshold. As previously reported15,16 and in vehicle control animals, treatment with paclitaxel produced a robust and transient (~2 weeks) mechanical allodynia (fig. 1A), a common readout of chemotherapy-induced neuropathic pain in animals.4,15. However, animals with systemic sympathectomy showed a delay in both the development and resolution of paclitaxel-induced mechanical allodynia (fig. 1B). This conflicting result is not due to the recovery of SNS activity, as indicated by the sustained loss of tyrosine hydroxylase (Th) up to 21 days (fig. 1C), a marker for sympathetic fibers. This result may due to contrasting systemic vs. local effects of a global sympathectomy.12
Figure 1. Systemic chemical sympathectomy delays the development and resolution of paclitaxel-induced mechanical allodynia.
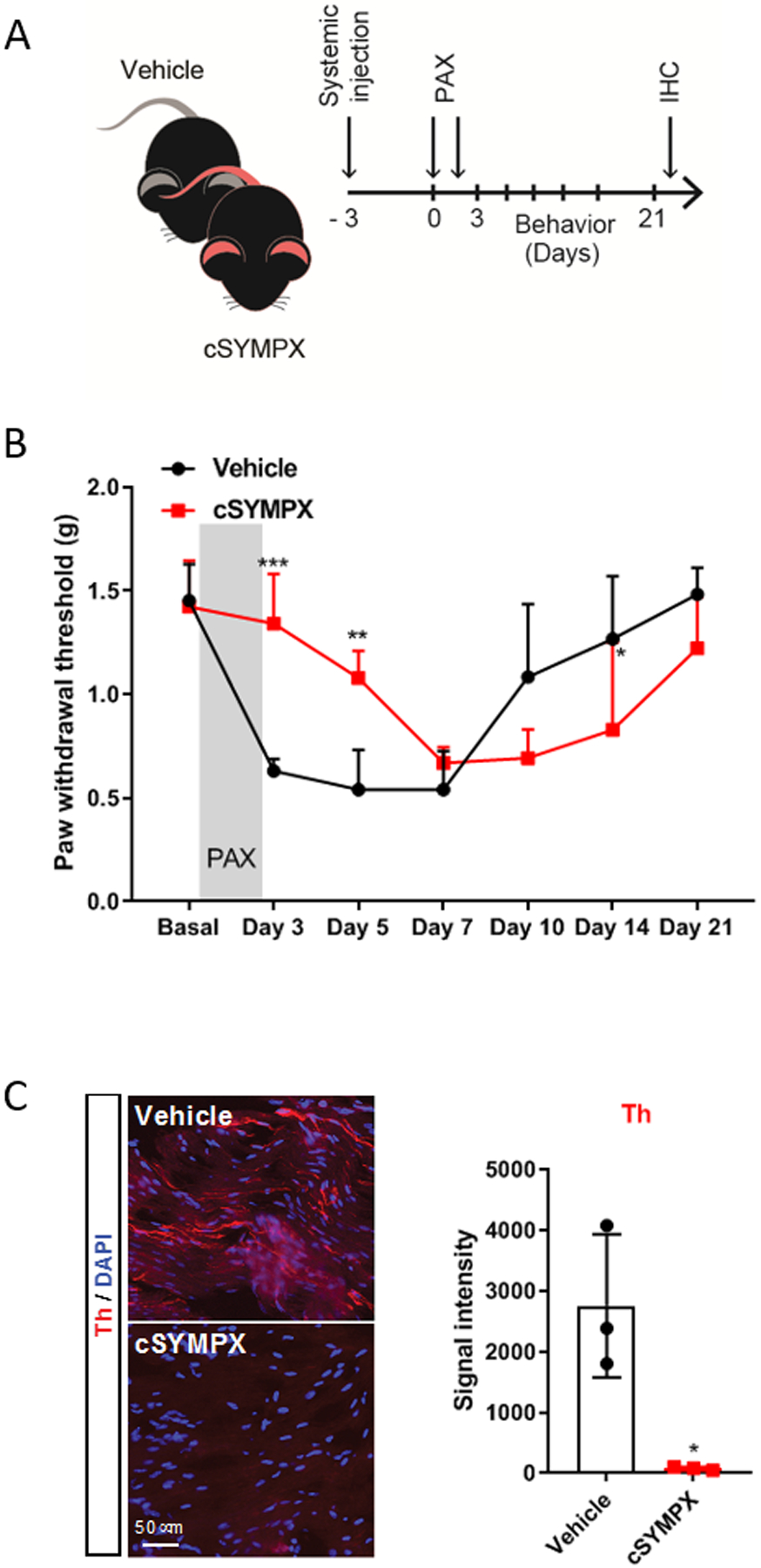
(A) Schematic illustration of the experiment showing the timeline of systemic chemical sympathectomy (cSYMPX), injections of paclitaxel (PAX), and behavioral and histochemical (IHC) assays. (B) Time course of paclitaxel-induced mechanical allodynia in mice (n=5 male mice/group, two-way ANOVA showed significant difference between cSYMPX and vehicle groups, Group × Time interaction: F6,48 = 12.02, p < 0.001; Bonferroni post hoc analysis revealed a significant difference between groups on day 3, 5 and 14. *p < 0.05, **p < 0.01, ***p < 0.001). (C) Representative images and quantification of tyrosine hydroxylase (Th) protein in DRG tissues of vehicle- and cSYMPX treated mice at day 21 of paclitaxel (n=3 male mice/group, t-test, *p = 0.017 compared to vehicle).
Local microsympathectomy reduces paclitaxel-induced mechanical and cold allodynia
Since chemotherapy-induced neuropathic pain generally develops in the distal extremities of the body, we investigated the effects of a local microsympathectomy, in which lumbar gray rami from sympathetic paravertebral ganglia were cut on one side (suppl. fig. 1), performed 2 days before paclitaxel treatment (fig. 2A) and resulting in the loss of peripheral sympathetic fibers innervated by those ganglia for at least 14 days (fig. 2B). Before implementing the paclitaxel-induced animal model, microsympathectomy and sham surgery did not affect baseline mechanical and cold allodynia (fig. 2C–F). Similar to vehicle control animals, sham control animals developed a marked decrease in the mechanical threshold lasting up-to two weeks. In contrast, microsympathectomy produced a fast and sustained recovery of mechanical allodynia in the ipsilateral hind paw of male and female mice (mean ± SD: microsympathectomy vs. sham at day 5, 1.07 ± 0.34g vs. 0.51 ± 0.17g, n =5, p = 0.030 in male mice, and 1.08 ± 0.28g vs. 0.62 ± 0.16g, n=5, p = 0.036 in female mice) after paclitaxel treatment (fig. 2C, D), whereas no effect was observed in the paw contralateral to the microsympathectomy of these same mice (suppl. fig. 2A, B). Paclitaxel is well-known to increase sensitivity to cold stimuli in patients.29 Using the cold plantar assay to evaluate the sensitivity to noxious cold stimuli, we demonstrated that microsympathectomy prevented the development of cold allodynia in the ipsilateral hind paw of male and female mice after paclitaxel treatment (fig. 2E, F). It is worth noting that we have observed similar effects in both males and females of prior microsympathectomy on mechanical behaviors in a nerve injury model and on both mechanical and cold behaviors in a back pain model that induces local inflammation of DRGs.12,13
Figure 2. Local surgical sympathectomy decreases paclitaxel-induced mechanical and cold allodynia.
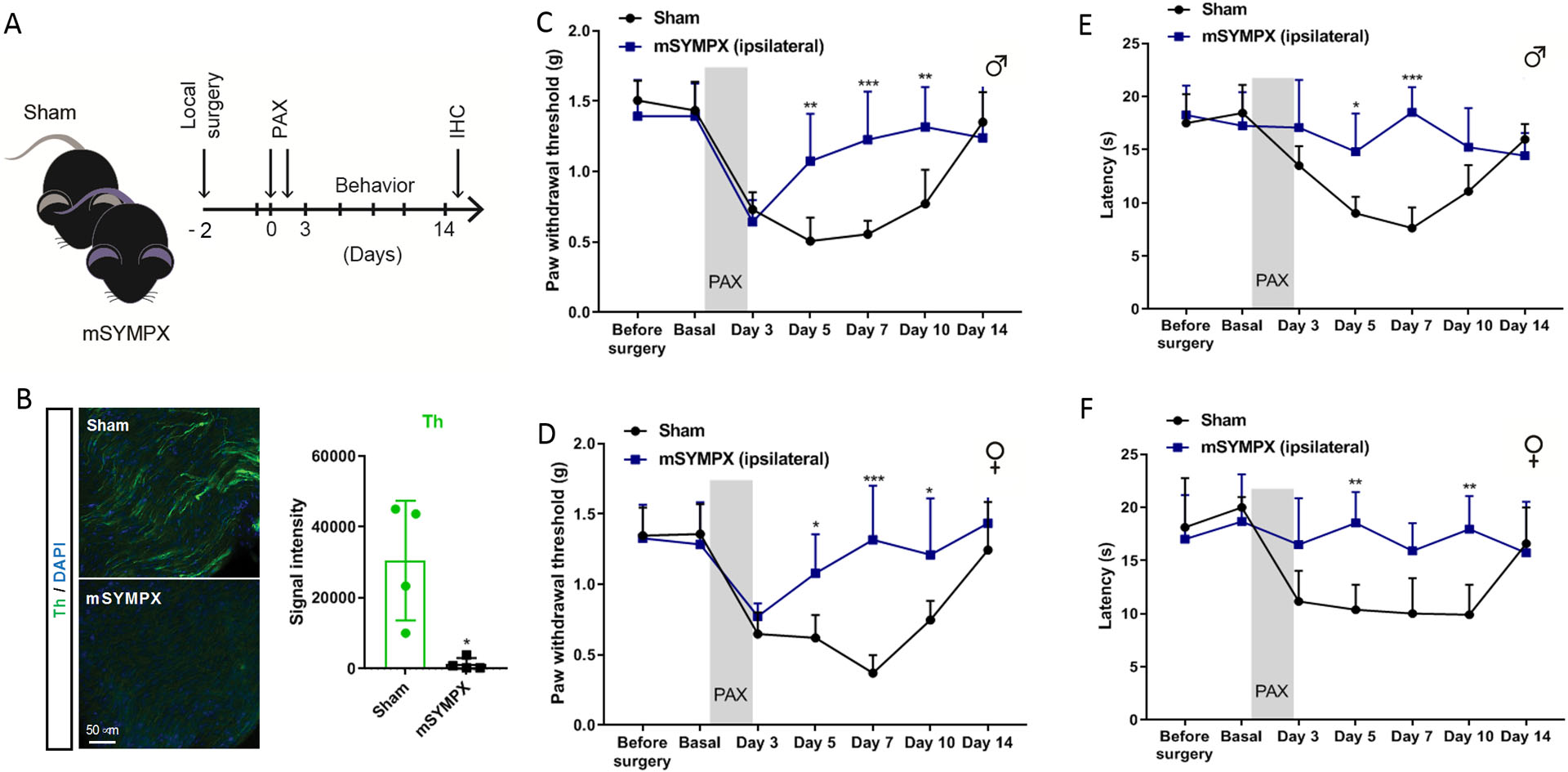
(A) Schematic illustration of the experiment showing the timeline of local surgical microsympathectomy (mSYMPX), injections of paclitaxel (PAX), and behavioral and histochemical (IHC) assays. (B) Representative images and quantification of tyrosine hydroxylase (Th) protein in DRG tissues of sham- and mSYMPX treated mice at day 14 of paclitaxel (n=4 male and female mice/group, t-test, *p = 0.013 compared to sham). (C, D) Time course of paclitaxel-induced mechanical allodynia tested in ipsilateral hind paw in (C) male mice (n=5 male mice/group, two-way ANOVA showed significant difference between mSYMPX and sham groups, Group × Time interaction: F6,48 = 5.19, p < 0.001; Bonferroni post hoc analysis revealed a significant difference between groups on day 3, 5 and 10. **p < 0.01, ***p < 0.001), and (D) female mice (n=5 female mice/group, for E the two-way ANOVA showed significant difference between mSYMPX and sham groups, Group × Time interaction: F6,48 = 5.6, p < 0.001; Bonferroni post hoc analysis revealed a significant difference between groups on day 5, 7 and 10. *p < 0.05, ***p < 0.001). (E, F) Time course of paclitaxel-induced cold allodynia tested in ipsilateral hind paws in (E) male mice (n=5 male mice/group, two-way ANOVA showed significant difference between mSYMPX and sham groups, Group × Time interaction: F6,48 = 6.92, p < 0.001; Bonferroni post hoc analysis revealed a significant difference between groups on day 5 and 7. *p < 0.05, ***p < 0.001), and (F) female mice (n=5 female mice/group, two-way ANOVA showed significant difference between mSYMPX and sham groups, Group × Time interaction: F6,48 = 4.91, p = 0.001; Bonferroni post hoc analysis revealed a significant difference between groups on day 5 and 10. **p < 0.01).
Monocytes/macrophages are required for the relief of paclitaxel-induced mechanical allodynia by local microsympathectomy
We have reported that local microsympathectomy alleviated inflammatory pain in a back pain animal model by reducing inflammation and macrophage infiltration in DRGs.12 Infiltration and activation of macrophages has been reported to contribute to pain-like behaviors in animal models of chemotherapy-induced neuropathic pain and their systemic depletion shown to prevent the development of paclitaxel-induced mechanical allodynia.4 In agreement with previous studies in paclitaxel and vincristine models of chemotherapy-induced neuropathic pain,4,5 we did not observe transcriptional changes in macrophage markers (data not shown) and the canonical macrophage marker ionized calcium binding adaptor molecule 1 (IBA1) was unaffected in DRGs of mice with microsympathectomy compared to mice with sham surgery at day 7 of paclitaxel treatment (fig. 3A–C). However, transcripts for the macrophage activation marker CD68 (cluster of differentiation 68) and other macrophages markers such as EMR1 (EGF-like module-containing mucin-like hormone receptor-like 1, also known as F4/80) and ITGAM (integrin, alpha X), as well as the monocyte chemoattractant protein CCL2 (chemokine (C-C motif) ligand 2) and its receptor CCR2 (C-C chemokine receptor type 2) were significantly increased in mice with microsympathectomy after paclitaxel (fig. 3C), suggesting that the analgesic effect of microsympathectomy may emerge from the activation and infiltration of macrophages. To assess the involvement of infiltrated macrophages in the analgesic effect of microsympathectomy, we used three daily intravenous injections of the CCR2 antagonist INCB3344 (fig. 4A), which does not affect CCL2 regulation but it is known to reduce the number of circulating monocytes after nerve injury.19 This treatment significantly negated the analgesic effect of microsympathectomy (fig. 4B), revealing an active inhibition of the paclitaxel-induced mechanical allodynia by CCR2 signaling and potentially by infiltrating monocytes/macrophages. Of note, CCL2/CCR2 signaling is recognized to participate in microglia activation in the spinal cord and pain hypersensitivity.30 However, intrathecal injections of INCB3344 did not mitigate the analgesic effect of microsympathectomy (data not shown), suggesting that this effect is independent of local mechanisms. Concordantly, intraperitoneal injections of liposomal clodronate, which is known to mostly deplete circulating monocytes,18 significantly negated the analgesic effect of microsympathectomy (fig. 4C). Of note, liposomal clodronate minimally affected the expression of IBA1 in DRG (fig. 4D), but drastically diminished IBA1 and other monocyte/macrophage markers in the spleen (fig. 4D and suppl. fig. 3A–C), a major body reservoir of circulating monocytes. Together, our transcriptional and pharmacological results suggest a beneficial role in paclitaxel-induced neuropathic pain-like behaviors of circulating monocytes and potentially infiltrating macrophages after local microsympathectomy.
Figure 3. Transcriptional increases of macrophage markers in DRGs of paclitaxel-treated mice after local sympathectomy.
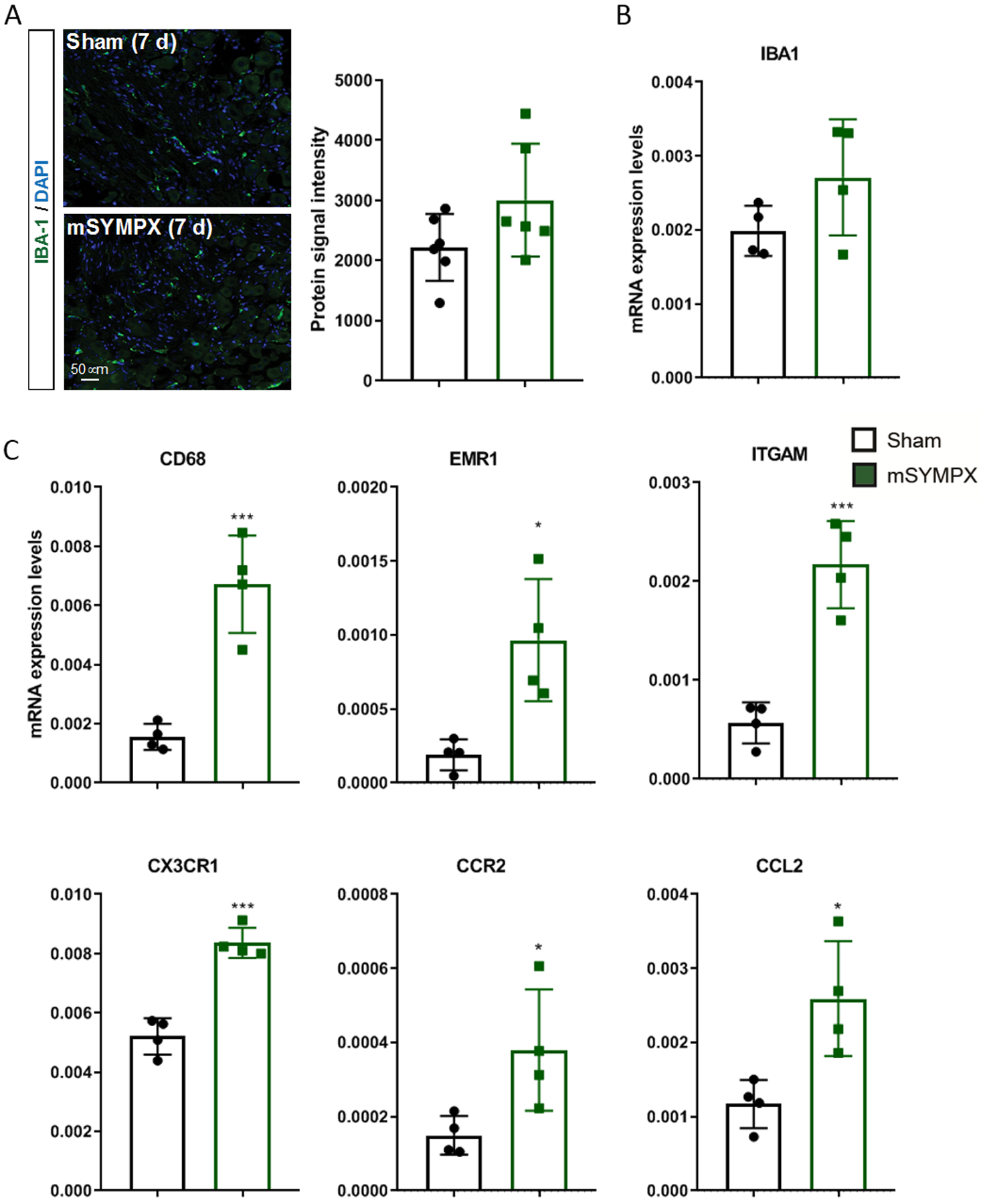
(A) Representative images and quantification of ionized calcium binding adaptor molecule 1 (IBA1) protein levels in DRG tissues of male mice with sham- and local microsympathectomy (mSYMPX) at day 7 after paclitaxel (n=6 male mice/group, t-test, p = 0.107 compared to sham). (B) The same tissues and conditions were also probed for transcriptional changes and similarly to the protein levels, the IBA1 mRNA was unchanged (n=4 male mice/group, t-test, p = 0.141 compared to sham). (C) In contrast, the macrophage markers cluster of differentiation 68 (CD68), EGF-like module-containing mucin-like hormone receptor-like 1 (EMR1), integrin alpha X (ITGAM) and C-X3-C motif chemokine receptor 1 (CX3CR1), as well as the monocyte chemotactic markers chemokine (C-C motif) ligand 2 (CCR2) and C-C chemokine receptor type 2 (CCL2) were significantly increased (n=4 male mice/group, t-test, *p<0.05, ***p < 0.001 compared to sham).
Figure 4. Depletion of monocytes/macrophages by INCB3344 and liposomal clodronate unmasked the anti-allodynic effect of local sympathectomy in paclitaxel-treated mice.
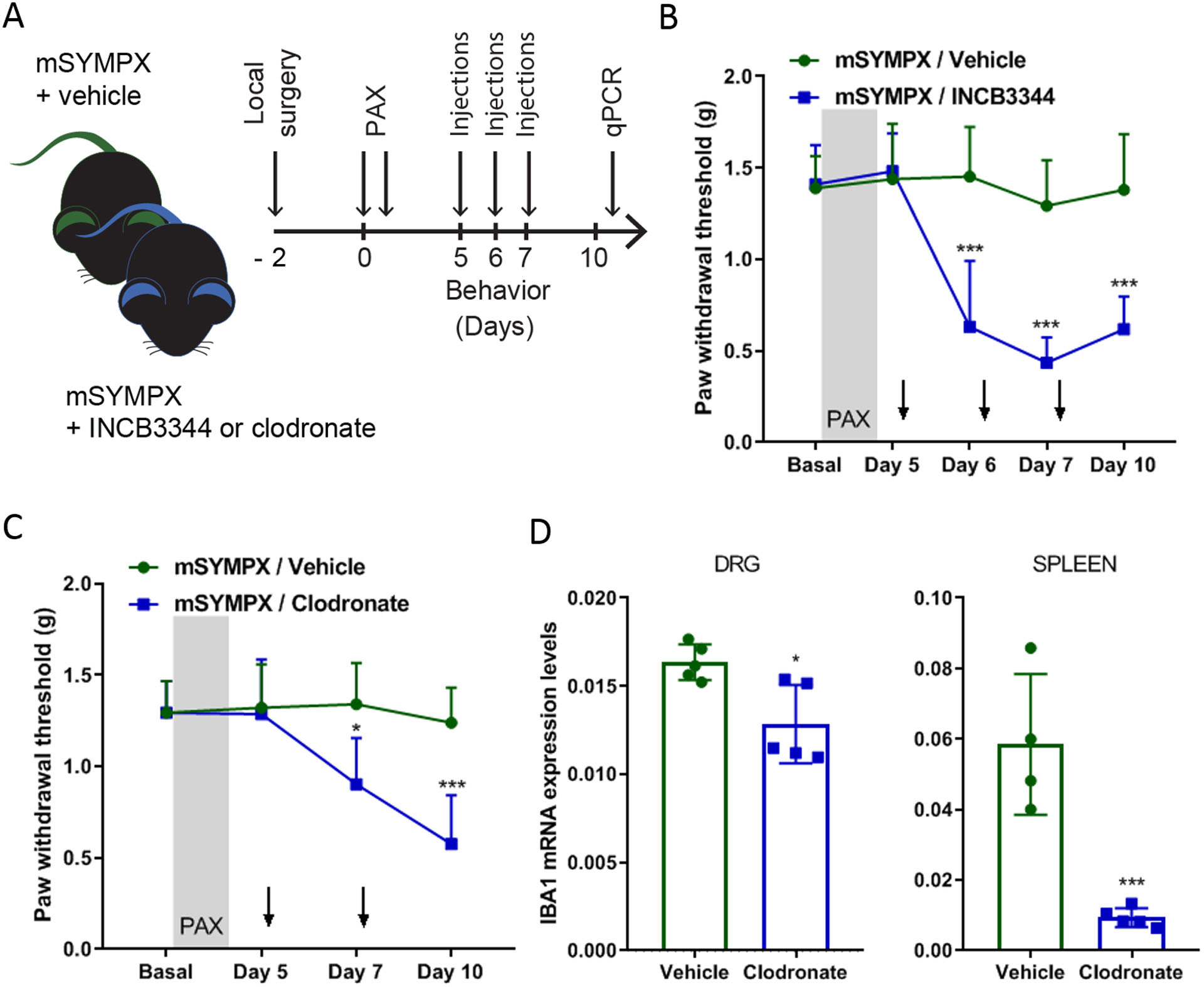
(A) Schematic illustration of the experiment showing the timeline of local microsympathectomy (mSYMPX), injections of paclitaxel (PAX), injections of INCB3344 (intravenously on day 5, 6, and 7) or liposomal clodronate (intraperitoneally on day 5 and 7), and behavioral and transcriptional (qPCR = real-time quantitative RT-PCR) assays. (B) Time course of paclitaxel-induced mechanical allodynia tested in ipsilateral hind paws in male mice treated with a vehicle control and INCB3344 (n=5 male mice/group, two-way ANOVA showed significant difference between mSYMPX –INCB3344 and -vehicle groups, Group × Time interaction: F4,32 = 10.14, p < 0.001; Bonferroni post hoc analysis revealed a significant difference between groups on day 6, 7 and 10. ***p < 0.001). (C) Time course of paclitaxel-induced mechanical allodynia tested in ipsilateral hind paws in male mice treated with a liposomal vehicle control and clodronate (n=5 male mice/group, two-way ANOVA showed significant difference between mSYMPX -clodronate and -vehicle groups, Group × Time interaction: F3,24 = 4.12, p = 0.017; Bonferroni post hoc analysis revealed a significant difference between groups on day 7 and 10. *p < 0.05, ***p < 0.001). (D) Liposomal clodronate treatment commonly used to deplete monocytes/macrophages showed a minimal effect on ionized calcium binding adaptor molecule 1 (IBA1) transcriptional expression in DRGs (Clodronate × Vehicle groups, p = 0.012), but a drastic transcriptional reduction of IBA1 in spleen tissues (Clodronate × Vehicle groups, p = 0.001) of mice 10 days after paclitaxel (n=4–5 male mice/group, t-test, *p<0.05, ***p < 0.001 compared to vehicle).
Local microsympathectomy promotes anti-inflammatory responses in DRGs and relief of paclitaxel-induced mechanical allodynia by TGF-β1 signaling
We have previously reported that local microsympathectomy limited the expression of pro-inflammatory cytokines favoring an anti-inflammatory response in locally inflamed DRGs.12 Transcriptional analyses of DRGs from mice with microsympathectomy compared to mice with sham surgery, examined 7 days after subsequent paclitaxel treatment, showed a significant increase of the anti-inflammatory macrophage marker arginase 1 (ARG1), whereas no effect was observed on the pro-inflammatory macrophage marker nitric oxide synthase 2 (NOS2) (fig. 5A). Concordantly, minimal or no effect was observed on pro-inflammatory cytokines (fig. 5B). However, transcriptional analyses of anti-inflammatory cytokines revealed that microsympathectomy significantly increased the expression of the interleukin (IL) IL-10, TGF-β and its receptor TGFβR1 (fig. 5C). Although protein analyses of 40 cytokines (suppl. fig. 4A) confirmed the minimal changes in pro-inflammatory cytokines, it did not validate the transcriptional increase of IL-10 (suppl. fig. 4B, C). In contrast, we showed that delivery of exogenous recombinant TGF-β dose-dependently after 7 days of paclitaxel (fig. 6A) significantly reduced mechanical allodynia (TGFβ 100 ng/site vs. vehicle at 3 h, 1.21 ± 0.34g vs. 0.53 ± 0.14g, n = 5, p = 0.001 in male mice) (fig. 6B), and this reduction was abolished by the co-injection of TGF-β with the potent and selective TGF-β receptor 1 (TGF-βR1) inhibitor SB431542 (fig. 6C). Consistently, SB431542 delivered 7 days after paclitaxel (fig. 6D) partially unmasked mechanical allodynia after microsympathectomy (fig. 6E). Microelectrode recordings using vehicle or SB431542, made in an isolated whole DRG preparation 7 d after microsympathectomy and paclitaxel, showed that SB431542 treatment resulted in a significant increase of the number of action potentials that could be evoked in small-sized (fig. 6F, G), but not large-sized DRG neurons (fig. 6H). Remarkably, SB431542 induced no other changes (e.g., resting potential and spontaneous activity) in the same preparations (suppl. table 2). Together these data suggest an essential involvement of TGF-β1 signaling in neuronal hyperexcitability and the analgesic effect of local microsympathectomy.
Figure 5. Transcriptional increases of anti-inflammatory cytokines in DRGs of paclitaxel-treated mice after local sympathectomy.
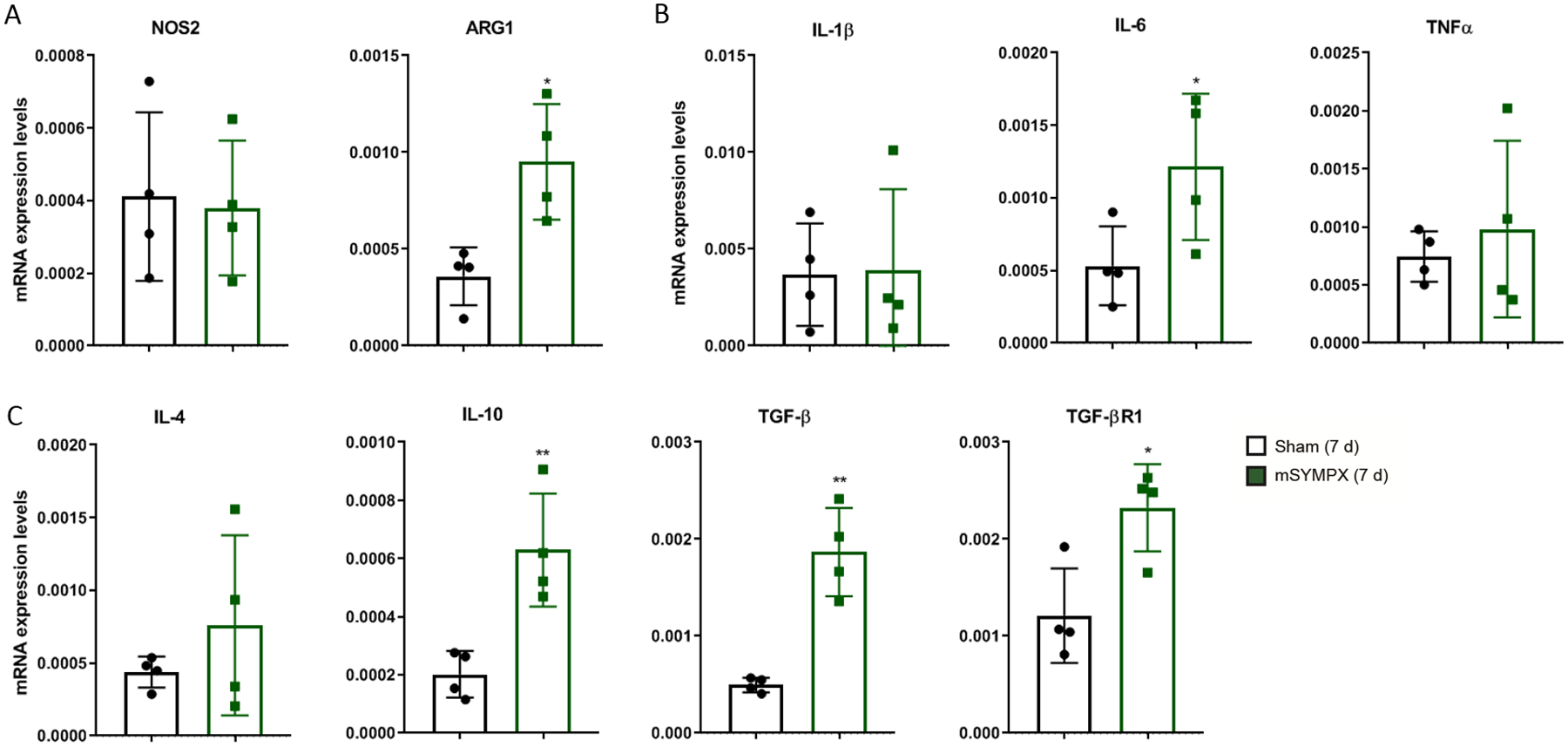
Transcriptional expression levels in DRGs 7 days after paclitaxel treatment in male mice with sham surgery or local microsympathectomy (mSYMPX). Transcriptional analyses of: (A) the macrophage pro-inflammatory marker nitric oxide synthase 2 (NOS2) and anti-inflammatory marker arginase 1 (ARG1); (B) pro-inflammatory cytokines interleukins (IL) IL-1β, IL-6 and tumor necrosis factor alpha (TNFα); as well as (C) anti-inflammatory cytokines IL-4, IL-10, and TGF-β, and its receptor TGF-βR1. For A-C, n=4 male mice/group, t-test, *p < 0.05, **p < 0.01 compared to sham.
Figure 6. Local sympathectomy accelerates the resolution of paclitaxel-induced mechanical allodynia through anti-inflammatory TGF-β signaling.
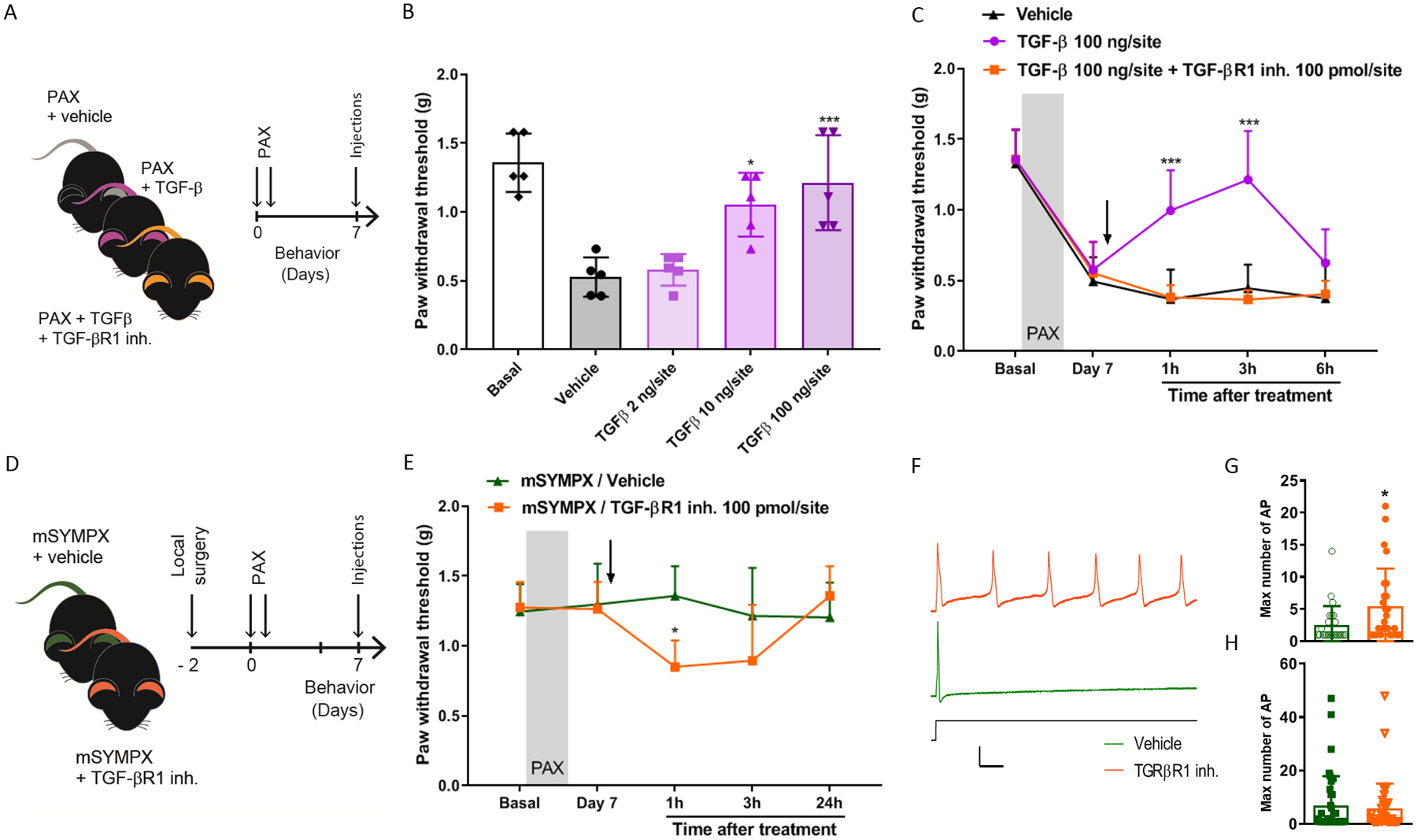
(A) Schematic illustration of the experiment showing the timeline of the injections of paclitaxel (PAX), intrathecal injections of recombinant TGF-β protein or TGF-β + TGF-β receptor 1 inhibitor (TGF-βR1 inh.), and the behavioral assay that was carried out at 7 day after paclitaxel. (B) Reversal of paclitaxel-induced mechanical allodynia tested in ipsilateral hind paws in male mice after 3 h of treatment with different doses of recombinant TGF-β (n=5 mice/group, one-way ANOVA showed significant difference between TGF-β and vehicle groups, Group: F4,20 = 14.02, p < 0.001; Turkey post hoc analysis revealed a significant difference at concentrations of 10 ng and 100 ng. *p < 0.05, *p < 0.001). (C) Time course of paclitaxel-induced mechanical allodynia showed that the anti-allodynic effect of TGF-β was abolished by the intrathecal co-injection of the TGF-βR1 inhibitor (n=5 male mice/group, two-way ANOVA showed significant difference between TGF-β and vehicle groups, Group × Time interaction: F8,48 = 5.63, p < 0.001; Bonferroni post hoc analysis revealed a significant difference between groups at 1 and 3 hours. *p < 0.001). (D) Schematic illustration of the experiment showing the timeline of local microsympathectomy (mSYMPX), injections of paclitaxel (PAX), injections of TGF-βR1 inh., and the behavioral assay that was carried out at 7 day after paclitaxel. (E) TGF-βR1 inhibitor also reverses the anti-allodynic effect of mSYMPX on paclitaxel-induced mechanical allodynia in mice (n=5 male mice/group, two-way ANOVA showed significant difference between mSYMPX -SB431542 and -vehicle groups, Group × Time interaction: F4,32 = 3.25, p = 0.024; Bonferroni post hoc analysis revealed a significant difference between groups at 1 hour. *p < 0.05). (F) Sample traces of action potential firing in response to suprathreshold currents in small cells isolated after chemotherapy and mSYMPX, with TGFβ1R inhibitor (orange) or vehicle (green) present during the recording session. Shown is response to stimulus of 2.4 nA which evoked maximum number of action potentials in the TGFβ1R inhibitor cell; and response to stimulus of 3.4 nA in the vehicle treated cell, where it was not possible to evoke more than one action potential. Scale bars 25 msec, 50 mV. (G, H) Quantification of the TGF-βR1 inhibitor effect on the maximum number of action potentials evoked by suprathreshold currents in small-size DRG neurons (G) and large-size DRG neurons (H) isolated from mSYMPX mice treated with paclitaxel (n=28–29 small neurons/group, or 41–43 large neurons per group; Mann-Whitney test, *p < 0.05 compared to vehicle). Additional electrophysiological details can be found in suppl. table 2.
Discussion
The prevalence of neuropathic pain is very high after chemotherapy. Currently, there is no FDA-approved analgesic for chemotherapy-induced neuropathic pain, often leaving clinicians to decrease the dose or duration of an otherwise life-saving therapy. Using the well-characterized paclitaxel-induced animal model of chemotherapy-induced neuropathic pain, we have demonstrated the analgesic effect of a local sympathectomy. This is particularly relevant because local sympathetic blocks (e.g., via local anesthetic injections) are standard procedures in the clinic to treat some pain conditions, notably complex regional pain syndrome, but also phantom limb pain, herpes zoster and postherpetic neuralgia, ischemic pain, postmasectomy pain, and cancer pain.9,10
The mechanisms by which sympathectomy alleviates neuropathic pain are incompletely known. Because peripheral nerve injury induces sprouting of sympathetic fibers into DRGs, many preclinical studies focus in abnormal interactions between these fibers and sensory neurons.14,31–33 Indeed, it has been found that sympathectomy can reduce the hyperexcitability of sensory neurons and pain-like behavior in a lumbar radiculopathy animal model.34 However, the analgesic effects of sympathectomy in various neuropathic pain animal models are contradictory.35,36 These contradictions may due to the use of global surgical and chemical methods of sympathectomy, timing of the intervention, and the contrasting effects of the sympathetic denervation of various immune tissues (e.g., thymus, spleen, and lymph nodes).37,38 We have observed that the use of a systemic chemical sympathectomy results in the delay of both the development and the resolution of paclitaxel-induced mechanical allodynia. Previous studies have demonstrated that distinct T cells and anti-inflammatory cytokines are important for the attenuation and resolution of paclitaxel-induced mechanical allodynia,15,39 and sympathetic denervation of immune tissues by systemic approaches can potentially interfere with these immune responses. For instance, sympathetic denervation of the spleen or depletion of the sympathetic neurotransmitter norepinephrine by treatment with reserpine prevents the vagally-stimulated anti-inflammatory responses,40 reinforcing our rationale for localized sympathetic interventions.
Our preclinical studies have shown that a local microsympathectomy, consisting of cutting the grey rami near the lumbar DRGs, is highly effective in reducing neuronal excitability and pain-like behaviors in animal models of neuropathic pain and low back pain.12,13 Furthermore, this surgery has been shown to mitigate the increase of macrophage and pro-inflammatory cytokines and decrease of anti-inflammatory cytokines associated with a low back pain model.12 Although previous studies have found no changes in transcriptional levels of macrophage markers,4,5 we found that several macrophage markers are increased by microsympathectomy after paclitaxel. Most importantly, similarly to the low-back pain study, we observed a change in the balance between pro-inflammatory cytokines and decrease of anti-inflammatory cytokines in our paclitaxel-induced animal model, and provided several lines of evidence to demonstrate that this analgesic effect is mediated by monocytes/macrophages and anti-inflammatory TGF-β signaling. First, local microsympathectomy increased the expression of macrophage marker CD68, as well as the monocyte chemotactic markers CCL2 and CCR2 in DRGs, suggesting an increase of infiltrating macrophages. Second, monocyte/macrophage depletion significantly diminished the microsympathectomy analgesic effect, suggesting a beneficial role of these cells in this animal model, as previously reported.41,42 We observed a similar effect with the systemic delivery of the CCR2 agonist INCB3344, which is known to partially deplete circulating monocytes.19 However, intrathecal delivery to spinal and DRG tissues of INCB3344 presented no analgesic effects suggesting that local CCR2 is probably not required for the recruitment of monocytes and attenuation of paclitaxel-induced mechanical allodynia by microsympathectomy. This result may be due to the previously reported observation that the regulation of the monocyte recruiting cytokine CCL2 is unaltered by INCB334.19 Third, TGF-β1 levels in DRGs dramatically increased after microsympathectomy. Fourth, administration of exogenous TGF-β1 potently inhibited mechanical allodynia after paclitaxel, and a TGF-βR1 inhibitor partially blocked both TGF-β1 and microsympathectomy analgesic effects.
The question arises whether monocytes/macrophages are responsible for the increase in TGF-β1 in DRG after local microsympathectomy. Depletion of monocytes/macrophages has been shown to delay the resolution of inflammatory pain-like behaviors via the impairment of the production of the anti-inflammatory cytokine IL-10 in DRGs.41 However, we were unable to shown a similar result or impairment of any anti-inflammatory cytokines, including TGF-β1, after our depletion of monocytes/macrophages (data not shown). Future studies will attempt to investigate whether there is a link between monocytes/macrophages and TGF-β1, or whether they are distinct, and TGF-β1 may be indeed produced by other DRG cells.43 This may be achieved using cell sorting or other single-cell analysis.39,44 Another question that arises is: how does TGF-β1 alleviate paclitaxel-induced mechanical allodynia? Although TGF-β1 may protect against nerve degeneration resulting from the toxicity of paclitaxel, we showed that blocking TGF-β1 signaling rapidly unmasked DRG neuronal hyperexcitability. This rapid action is inconsistent with the canonical nerve protective and transcriptional roles of TGF-β1 signaling. We propose that at least part of the beneficial effect of TGF-β1 is mediated via direct effects on the TGF-βR1 that is broadly expressed in DRG neurons.44 We have previously shown that local microsympathectomy reduced the hypersensitivity of large-sized DRG neurons induced by local DRG inflammation12. However, we have demonstrated that the analgesia associated with the microsympathectomy in this paclitaxel-induced animal model is mediated, at least in part, by the TGF-β1 signaling and reduction of ectopic action potentials in small-sized DRG neurons. Consistently, TGF-β1 was previously reported to result in a rapid block of ectopic action potentials in small sized DRG neurons, and relief of pain-like behaviors in an animal model of nerve injury.45 IL-10 may also block these ectopic action potentials after paclitaxel.15 Although we failed to confirm the transcriptional increase of IL-10 at the protein level, we should not rule out that IL-10 and other anti-inflammatory mediators may be implicated in the prevention and decrease of paclitaxel-induced mechanical and cold allodynia by local microsympathectomy.
Our and other previous studies have shown that systemic depletion of monocytes/macrophages prevents and alleviates paclitaxel-induced mechanical allodynia, but this strategy is unfortunately often accompanied with compromised immune response and higher risks of infection and immune diseases. Alternatively, the detrimental adrenergic communication between sympathetic fibers and macrophages could be avoided by targeting noradrenergic signaling using beta-blockers (e.g., carvedilol), which have been proposed as adjuvants for cancer chemotherapy to protect against cardiotoxicity.46 However, selective serotonin and noradrenaline reuptake inhibitors (e.g., venlafaxine and duloxetine), which increase the extracellular levels of noradrenaline and potentiate noradrenergic signaling, seem also to have beneficial effects in reducing paresthesia and pain in cancer patients treated with paclitaxel.47,48 Although many studies focus on catecholamines, ignoring other sympathetic co-transmitters, a number of additional sympathetic co-transmitters such as adenosine triphosphate (ATP) have been shown to play a pivotal role in pain and can potentially elicit the activation of neurons, macrophages and pro-inflammatory responses in DRGs.13 Catecholamines and ATP may have synergistic effects, and we have previously found that a cocktail of norepinephrine and ATP inhibitors was effective in blocking the effects of stimulation of the dorsal ramus (that also contains sympathetic fibers) in DRG neurons.13 This is consistent with a high expression of purinergic receptors in DRG tissues, and suggests that targeting both adrenergic and purinergic signaling may represent a better therapeutic option for chemotherapy-induced neuropathic pain.
In summary, our results unravel a previously unrecognized link whereby sympathetic fibers obstruct the emergence of anti-inflammatory responses and support the development of paclitaxel-induced mechanical and cold allodynia. Local sympathectomy promotes anti-inflammatory responses and faster resolution of mechanical allodynia, as well as preventing cold allodynia, after paclitaxel treatment. Together these results suggest that sympathetic blocks, already used in the clinic for various pain conditions,9,10 may represent a novel therapeutic strategy to effectively relief chemotherapy-induced neuropathic pain.
Supplementary Material
What we already know about this topic
Chemotherapy-induced neuropathic pain is a common and difficult to treat problem.
Inflammation may support chemotherapy-induced pain by interacting with sensory neurons.
What this article tells us that is new
Local surgical sympathectomy relieved nociceptive and mechanical sensitization in a mouse model of paclitaxel-induced pain.
Transforming growth factor-β was enhanced in mice after sympathectomy and was capable of reducing paclitaxel-induced mechanical sensitization.
Acknowledgments and funding statement:
This work was supported by NIH-NINDS grants NS106264 and NS113243 to T.B., and NS045594 and NS055860 to J.-M.Z. We also acknowledge the support of institutional grants from the University of Cincinnati (UC) and UC Gardner Neuroscience Institute to T.B.
Footnotes
Conflicts of interest: The authors declare no competing interests.
References
- 1.Sisignbrennerano M, Baron R, Scholich K, Geisslinger G: Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain 2014; 10:pp 694–707 [DOI] [PubMed] [Google Scholar]
- 2.Flatters SJL, Dougherty PM, Colvin LA: Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review 2017; 119:pp 737–49 [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Kavelaars A, Dougherty PM, Heijnen CJ: Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source 2018; 124:pp 2289–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Li Y, Carvalho-Barbosa M De, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM: Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J Pain 2016; 17:775–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montague K, Simeoli R, Valente J, Malcangio M: A novel interaction between CX 3 CR 1 and CCR 2 signalling in monocytes constitutes an underlying mechanism for persistent vincristine-induced pain. J Neuroinflammation 2018 151 2018; 15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Old EA, Nadkarni S, Grist J, Gentry C, Bevan S, Kim KW, Mogg AJ, Perretti M, Malcangio M: Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest 2014; 124:2023–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CC, Lu N, Cui Y, Yang T, Zhao ZQ, Xin WJ, Liu XG: Prevention of Paclitaxel-induced allodynia by Minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain 2010; 6:1744–8069-6–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pachman DR, Dockter T, Zekan PJ, Fruth B, Ruddy KJ, Ta LE, Lafky JM, Dentchev T, Le-Lindqwister NA, Sikov WM, Staff N, Beutler AS, Loprinzi CL: A pilot study of minocycline for the prevention of paclitaxel-associated neuropathy: ACCRU study RU221408I. Support Care Cancer 2017; 25:3407–16 [DOI] [PubMed] [Google Scholar]
- 9.Sekhadia MP, Nader A, Benzon HT: Peripheral sympathetic blocks, Essentials of Pain Medicine. Elsevier, 2011, pp 621–8 doi: 10.1016/B978-1-4377-2242-0.00088-2 [DOI] [Google Scholar]
- 10.Harden RN, Oaklander AL, Burton AW, Perez RSGM, Richardson K, Swan M, Barthel J, Costa B, Graciosa JR, Bruehl S: Complex regional pain syndrome: Practical diagnostic and treatment guidelines, 4th edition. Pain Med (United States) 2013; 14:180–229 [DOI] [PubMed] [Google Scholar]
- 11.Chavan SS, Pavlov VA, Tracey KJ: Mechanisms and Therapeutic Relevance of Neuro-immune Communication 2017; 46:pp 927–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie W, Chen S, Strong JA, Li A-L, Lewkowich IP, Zhang J-M: Localized Sympathectomy Reduces Mechanical Hypersensitivity by Restoring Normal Immune Homeostasis in Rat Models of Inflammatory Pain. J Neurosci 2016; 36:8712–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie W, Strong JA, Zhang JM: Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain 2010; 151:447–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramer MS, Bisby MA: Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain 1997; 70:237–44 [DOI] [PubMed] [Google Scholar]
- 15.Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A: CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 2016; 36:11074–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonello R, Lee SH, Berta T: Monoclonal Antibody Targeting the Matrix Metalloproteinase 9 Prevents and Reverses Paclitaxel-Induced Peripheral Neuropathy in Mice. J Pain 2019; 20:515–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SH, Cho PS, Tonello R, Lee HK, Jang JH, Park GY, Hwang SW, Park CK, Jung SJ, Berta T: Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J Allergy Clin Immunol 2018; 142:1349–1352.e16 [DOI] [PubMed] [Google Scholar]
- 18.Peng J, Gu N, Zhou L, B Eyo U, Murugan M, Gan W-B, Wu L-J: Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 2016; 7:12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steenwinckel J Van, Auvynet C, Sapienza A, Reaux-Le Goazigo A, Combadière C, Melik Parsadaniantz S: Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve. Brain Behav Immun 2015; 45:198–210 [DOI] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL: Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53:55–63 [DOI] [PubMed] [Google Scholar]
- 21.Dixon WJ: Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20:441–62 [DOI] [PubMed] [Google Scholar]
- 22.Brenner DS, Golden JP, Gereau IV RW: A novel behavioral assay for measuring cold sensation in mice. PLoS One 2012; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Spandidos A, Wang H, Seed B: PrimerBank: A PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res 2012; 40:D1144–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:45e–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie W, Strong JA, Kim D, Shahrestani S, Zhang JM: Bursting activity in myelinated sensory neurons plays a key role in pain behavior induced by localized inflammation of the rat sensory ganglion. Neuroscience 2012; 206:212–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JM, Song XJ, LaMotte RH: Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 1999; 82:3359–66 [DOI] [PubMed] [Google Scholar]
- 27.Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH: Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol 1999; 82:3347–58 [DOI] [PubMed] [Google Scholar]
- 28.Xie W, Strong JA, Zhang JM: Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience 2015; 291:317–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starobova H, Vetter I: Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci 2017; 10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LDF, Thompson SWN, Marchand F, McMahon SB: CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain 2009; 13:263–72 [DOI] [PubMed] [Google Scholar]
- 31.Lee BH, Yoon YW, Chung K, Chung JM: Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp Brain Res 1998; 120:432–8 [DOI] [PubMed] [Google Scholar]
- 32.Chung K, Kim HJ, Na HS, Park MJ, Chung JM: Abnormalities of sympathetic innervation in the area of an injured peripheral nerve in a rat model of neuropathic pain. Neurosci Lett 1993; 162:85–8 [DOI] [PubMed] [Google Scholar]
- 33.McLachlan EM, Hu P: Inflammation in dorsal root ganglia after peripheral nerve injury: effects of the sympathetic innervation. Aut Neurosci 2014; 182:108–17 [DOI] [PubMed] [Google Scholar]
- 34.Iwase T, Takebayashi T, Tanimoto K, Terashima Y, Miyakawa T, Kobayashi T, Tohse N, Yamashita T: Sympathectomy attenuates excitability of dorsal root ganglion neurons and pain behaviour in a lumbar radiculopathy model. Bone Joint Res 2012; 1:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pertin M, Allchorne AJ, Beggah AT, Woolf CJ, Decosterd I: Delayed sympathetic dependence in the spared nerve injury (SNI) model of neuropathic pain. Mol Pain 2007; 3:1744–8069-3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringkamp M, Eschenfelder S, Grethel EJ, Häbler HJ, Meyer RA, Jänig W, Raja SN: Lumbar sympathectomy failed to reverse mechanical allodynia- and hyperalgesia-like behavior in rats with L5 spinal nerve injury. Pain 1999; 79:143–53 [DOI] [PubMed] [Google Scholar]
- 37.Kenney MJ, Ganta CK: Autonomic nervous system and immune system interactions. Compr Physiol 2014; 4:1177–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D: Sympathetic modulation of immunity: Relevance to disease 2008; 252:pp 27–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu XJ, Zhang YL, Liu T, Xu ZZ, Park CK, Berta T, Jiang DH, Ji RR: Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res 2014; 24:1374–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellinger DL, Lorton D: Sympathetic nerve hyperactivity in the spleen: Causal for nonpathogenic-driven chronic immune-mediated inflammatory diseases (IMIDS)? 2018; 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willemen HLDM, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A: Monocytes/macrophages control resolution of transient inflammatory pain. J Pain 2014; 15:496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR: GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 2018; 128:3568–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Gu X, Yi S: The Regulatory Effects of Transforming Growth Factor-β on Nerve Regeneration. Cell Transplant 2016; 26:381–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P: Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2014; 18:145–53 [DOI] [PubMed] [Google Scholar]
- 45.Chen G, Park C-K, Xie R-G, Ji R-R: Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest 2015; 125:3226–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avila MS, Ayub-Ferreira SM, Barros Wanderley Junior MR de, Cruz F das D, Gonçalves Brandão SM, Carvalho Rigaud VO, Higushi-dos-Santos M, Hajjar LA, Filho RK, Hoff PM, Sahade M, Ferrari MSM, Paula Costa RL de, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA: Carvedilol for Prevention of Chemotherapy Related Cardiotoxicity. J Am Coll Cardiol 2018; 71:2281–90 [DOI] [PubMed] [Google Scholar]
- 47.Durand JP, Goldwasser F: Dramatic recovery of paclitaxel-disabling neurosensory toxicity following treatment with venlafaxine. Anticancer Drugs 2002; 13:777–80 [DOI] [PubMed] [Google Scholar]
- 48.Smith EML, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL: Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA - J Am Med Assoc 2013; 309:1359–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


