Abstract
An association between tyrosine-kinase inhibitor therapy for chronic myeloid leukemia and vascular adverse events including peripheral arterial disease, coronary artery disease, and pulmonary hypertension has been described. We present a patient who developed isolated pulmonary artery vasculitis resulting in left pulmonary artery stenosis, in addition to left coronary artery stenosis while on nilotinib. While monitoring for cardiovascular events is important, clinicians should also recognize possible drug-induced vasculitis during chronic nilotinib therapy.
Keywords: Chronic myeloid leukemia, Large vessel vasculitis, Pulmonary vasculitis, Pulmonary arteritis, Arteritis, Vasculitis, Nilotinib, Tyrosine kinase inhibitor, Vascular adverse events
1. Introduction
Tyrosine kinase inhibitors (TKIs) are considered mainstay treatment for patients diagnosed with chronic myeloid leukemia (CML). As CML requires lifelong treatment, selection of specific TKIs in the clinical setting requires careful consideration of toxicity profiles and patient comorbidities. Cardiovascular, pulmonary, and metabolic adverse events resulting from TKI therapy in CML have been documented and may complicate long-term therapy [1]. While not reported in initial clinical trials, cardiovascular toxicity from the second generation BCR-ABL1 TKI nilotinib has been an emerging problem in CML treatment [1], [2], [3], [4]. We report a case of a patient with CML on nilotinib who developed isolated pulmonary artery vasculitis with left pulmonary artery stenosis, and left coronary artery stenosis around the same time. To our knowledge, this is the first reported case of nilotinib-associated large-vessel vasculitis (LVV) and highlights a potentially new vascular complication of treatment with this TKI.
2. Case report
A previously heathy 47 year-old woman, without cardiovascular risk factors, was diagnosed with chronic phase CML in 2012. She was initiated on nilotinib 300 mg po bid, and has achieved Major Molecular Response (MMR) by Quantitative Real Time PCR (RQ-PCR). Baseline echocardiogram prior to initiation of CML treatment was normal with an estimated pulmonary artery pressure of 23–28 mmHg. Other comorbidities of note included seronegative inflammatory arthritis, gouty arthritis, and hyperparathyroidism treated with sulfasalazine, allopurinol, and thiazide diuretics. After six years of nilotinib, the patient developed new elevated erythrocyte sedimentation rate (ESR) (82 mg/hour; normal value <25 mm/h) and C-reactive protein (CRP) (2.5 mm/dL; normal value <0.3 mg/dL) that persisted for several months thereafter (Table 1). She reported fatigue, chest pressure, and dyspnea on exertion but no chronic cough or hemoptysis or other constitutional symptoms. Complete blood count (CBC) and comprehensive metabolic panel (CMP) were unremarkable. Urinalysis was normal. Her inflammatory arthritis was in remission. Given these unexplained elevated markers of inflammation, bone marrow biopsy was performed, and demonstrated normocellular marrow with trilenage hematopoesis.
Table 1.
Laboratory testing prior to, at the time of, and after the diagnosis and treatment of vasculitis.
| March 2018 (Baseline) | June 2018 (First abnormality) | August 2018 (At diagnosis) | October 2018 (After treatment) | December 2019 (Last follow-up) | |
|---|---|---|---|---|---|
| C-reactive protein, (normal value <0.3 mg/dL) | <0.3 | 2.5 | 2.5 | <0.3 | 0.3 |
| Erythrocyte sedimentation rate (normal value <25 mm/h) | 42 | 82 | 99 | 27 | 26 |
| Hemoglobin (normal value 11.6–15.2 g/dL) | 11.6 | 12.7 | 11.5 | 13.4 | 12.3 |
| Hematocrit (normal value 34.9–45.2%) | 37.0 | 39.6 | 35.9 | 42.3 | 38.00 |
| Mean Corpuscular Value (normal value 79.3–98.6 fL) | 83.1 | 88.8 | 86.3 | 88.9 | 91.3 |
| White Blood Cell Count (normal value 4.16–9.95 × 103/uL) | 8.3 | 7.6 | 6.09 | 10.91 | 4.05 |
| Absolute Neutrophil Count (normal value 1.80–6.90 × 103/uL) | 6.18 | 5.49 | 4.51 | 6.57 | 2.10 |
| Platelet count (normal value 143–398 × 103/uL) | 234 | 252 | 248 | 228 | 215 |
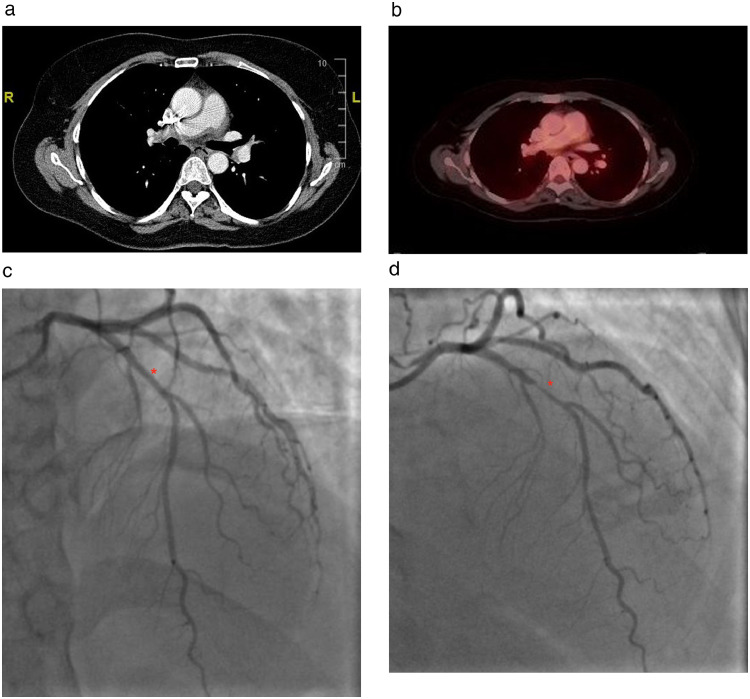
Chromosome banding analysis (CBA) of marrow cell metaphases, revealed Complete Cytogenetic Response (CCyR). Fluorescence in Situ Hybridization(FISH) analysis did not detect BCR-ABL rearrangement. Fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) was performed which showed soft tissue thickening of the main pulmonary artery and proximal bilateral pulmonary arteries (Fig. 1) with mild FDG uptake, and mild luminal narrowing of the left pulmonary artery concerning for vasculitis. Magnetic resonance angiography (MRA) of the chest confirmed wall thickening of the main pulmonary artery extending to the left and, to a lesser extent, right pulmonary artery and left pulmonary artery stenosis consistent with vasculitis. Further evaluation by rheumatology revealed no signs or symptoms of LVV or other autoimmune diseases that can cause pulmonary arteritis. Vascular examination was negative for carotid, subclavian, abdominal, or femoral bruits. Temporal and peripheral pulses were all present and symmetric. Laboratory testing included a borderline dilute Russel's viper venom test but normal antinuclear antibody, double stranded DNA, rheumatoid factor, anti-cyclic citrullinated peptides, anticardiolipin, beta-2-glycoprotein, cryoglobulins, total complement level, IgG subclasses, and ANCA antibodies. MRA of the abdomen and pelvis was performed to evaluate for LVV which was normal. Repeat echocardiography displayed new borderline elevated pulmonary artery systolic pressure (35 mmHg). She was diagnosed with isolated pulmonary vasculitis and started on prednisone 60 mg daily with gradual taper over several months. Markers of inflammation normalized with prednisone (ESR 15 mm/h and CRP <0.3 mg/dl). Around this time, given symptoms of dyspnea on exertion, she also underwent computerized tomography coronary angiogram which demonstrated a new high-grade stenosis (approximately 70–80%) of the proximal segment of the left anterior descending coronary artery (LAD) by diffuse low-density non-calcified plaque for which she underwent coronary angiogram and stenting of the mid-LAD (Fig. 1). Nilotininb was discontinued and dasitinib was started.
Fig. 1.
Computed tomography chest obtained during positron emission tomography demonstrating soft tissue thickening of the proximal (panel a, coronal view) with mild FDG uptake (panel b). Coronary angiogram in 2016 showed normal left anterior descending artery (panel c, asterisk) while repeat coronary angiogram 2 years later, showed a high-grade stenosis of the proximal segment of the left anterior descending coronary artery (panel d, asterisk).
MRA of the chest, abdomen, and pelvis after discontinuation of steroids showed improvement in pulmonary arteritis and no evidence of active vasculitis or progression to new areas. Repeat echocardiography showed resolution of elevated pulmonary artery pressure which was now 25 mmHg. At the time of last follow-up, the patient's vasculitis has been in clinical and biochemical remission off prednisone. She has continued dasitinib without new vascular events and also follows with cardiology and rheumatology regularly.
3. Discussion
Novel BCR/ABL1 TKIs, such as nilotinib, offer major antileukemic effects, but add complexity to the treatment paradigm of CML as their targets are not exclusive to hematopoietic cells and may induce vascular damage [2]. There is growing literature about TKI-related VAEs including coronary thrombosis, peripheral arterial disease, and pulmonary hypertension [1], [2], [3], [4]. Recently, a report of carotid artery stenosis in a patient treated with nilotinib was published adding to the list of VAEs [5]. Multiple mechanisms may contribute. Nilotinib may induce vascular vasospasm and stenosis, cause pro-atherogenic effects on vascular endothelial cells, and impair vascular repair via anti-angiogenic mechanisms in addition to affecting cells like mast cells which are important in vascular repair [2,6]. Furthermore, nilotinib also has metabolic effects including hyperglycemia and dyslipidemia that mediate atherosclerosis development, although the exact cellular interactions behind these metabolic changes remain unknown [2,6]. Clinical risk factors associated with TKI VAEs include duration of therapy, higher doses, and preexisting cardiovascular risk factors [2].
This is the first case report of isolated pulmonary vasculitis in association with nilotinib for CML.
Reports of other VAEs like vasculitis with TKIs are scant. Cases of nilotinib-associated arteritis and arterial stenosis were reported in a study using the US Food and Drug Adverse Event Reporting System database, but specifics on the type of arteritis or the arteries affected were not provided [3]. While pulmonary artery involvement is seen in certain forms of vasculitis, isolated pulmonary vasculitis is exceedingly rare [7]. The elevated markers of inflammation, PET/CT findings, and vessel wall thickening on MRA support a diagnosis of isolated pulmonary artery vasculitis. While nilotinib has been associated with atherosclerosis which can also cause FDG uptake on PET, the pulmonary artery involvement would be atypical for atherosclerosis. CT findings didn't show calcifications or other abnormalities seen with atherosclerosis and the only area of uptake on initial PET/CT was the pulmonary arteries. There are several features in this case which point toward a drug-induced vasculitis. She had no manifestations consistent with autoimmune conditions that cause pulmonary artery involvement. In particular, there were no features of Takayasu arteritis which would be the main consideration in someone her age and imaging of the entire aorta and branches did not show other areas of vasculitis. The concurrent coronary artery thrombosis, which is a well-recognized complication of nilotinib, also argues for a drug adverse event and suggests that these VAEs may share a common mechanism. Finally, manifestations resolved with prednisone monotherapy and withdrawal of the medication without recurrence, as opposed to the more typical relapsing course of large-vessel vasculitis.
Expanding literature has also demonstrated an association between pulmonary hypertension and TKIs including nilotinib [8]. In a recent pharmacovigilance analysis, Cornet et al. identified the potential role of proto-oncogene tyrosine-protein kinase Src (c-Src) and tyrosine-protein kinase Tec in pulmonary arterial hypertension induced by protein kinase inhibitors (PKIs) [9]. The authors suggested that c-Src inhibition by PKIs may enhance vasoconstriction and vascular remodeling but this would not explain the finding of vasculitis [9]. Pulmonary artery stenosis from large-vessel vasculitis can cause pulmonary hypertension [10]. In the present case, we suspect the pulmonary hypertension was in part related to the vasculitis. The occurrence of pulmonary hypertension in other patients treated with nilotinib, and to a much larger extent other TKIs, does not include vascular imaging. It is unclear if some of the previously reported cases of pulmonary artery hypertension may be secondary to pulmonary vasculitis as in our case.
In conclusion, the development of isolated pulmonary vasculitis, as well as coronary artery stenosis, in our patient was likely related to long-term nilotinib therapy. This case illustrates the importance of remaining vigilant for nilotinib-induced vascular adverse effects including, based on this report, vasculitis. Recognition of potential drug-associated adverse effects like vasculitis is important because treatment includes the cessation of the medication, if feasible, and avoids exposure to unnecessary immunosuppressive therapy.
Authors’ contributions
All authors have made a substantial contribution to the drafting/revision of the article and final approval.
Declaration of Competing Interests
The authors declare that they have no competing interests.
Funding sources
None.
Acknowledgments
None.
References
- 1.Medeiros B.C., Possick J., Fradley M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: strategies for monitoring, detecting, and managing. Blood Rev. 2018;32(4):289–299. doi: 10.1016/j.blre.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Valent P., Hadzijusufovic E., Hoermann G. Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leuk Res. 2017;59:47–54. doi: 10.1016/j.leukres.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes J., Mauro M., Steegmann J.L. Cardiovascular and pulmonary adverse events in patients treated with BCR-ABL inhibitors: data from the FDA Adverse Event Reporting System. Am. J. Hematol. 2015;90(4):E66–E72. doi: 10.1002/ajh.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh K.J., Lee J.Y., Shin D.Y. Analysis of adverse events associated with dasatinib and nilotinib treatments in chronic-phase chronic myeloid leukemia patients outside clinical trials. Int. J. Hematol. 2017;106(2):229–239. doi: 10.1007/s12185-017-2225-1. [DOI] [PubMed] [Google Scholar]
- 5.Hersant J., Gardembas M., Leftheriotis G., Vandeputte P., Abraham P., Henni S. Tyrosine kinase inhibitor-induced carotid stenosis: a case report. Leuk Res. Rep. 2019;12 doi: 10.1016/j.lrr.2019.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haguet H., Douxfils J., Chatelain C., Graux C., Mullier F., Dogné J.M. BCR-ABL tyrosine kinase inhibitors: which mechanism(s) may explain the risk of thrombosis? TH Open. 2018;2(1):e68–e88. doi: 10.1055/s-0038-1624566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riancho-Zarrabeitia L., Zurbano F., Gómez-Román J. Isolated pulmonary vasculitis: case report and literature review. Semin. Arthritis Rheum. 2015;44(5):514–517. doi: 10.1016/j.semarthrit.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Minami M., Arita T., Iwasaki H. Comparative analysis of pulmonary hypertension in patients treated with imatinib, nilotinib and dasatinib. Br. J. Haematol. 2017;177(4):578–587. doi: 10.1111/bjh.14608. [DOI] [PubMed] [Google Scholar]
- 9.Cornet L., Khouri C., Roustit M. Pulmonary arterial hypertension associated with protein kinase inhibitors: a pharmacovigilance-pharmacodynamic study. Eur. Respir. J. 2019;53(5) doi: 10.1183/13993003.02472-2018. [DOI] [PubMed] [Google Scholar]
- 10.Hagan G., Gopalan D., Church C. Isolated large vessel pulmonary vasculitis as a cause of chronic obstruction of the pulmonary arteries. Pulm. Circ. 2011;1(3):425–429. doi: 10.4103/2045-8932.87312. [DOI] [PMC free article] [PubMed] [Google Scholar]