Abstract
Cancer cells have unlimited replicative potential, insensitivity to growth-inhibitory signals, evasion of apoptosis, cellular stress, and sustained angiogenesis, invasiveness and metastatic potential. Cancer cells adequately adapt cell metabolism and integrate several intracellular and redox signaling to promote cell survival in an inflammatory and hypoxic microenvironment in order to maintain/expand tumor phenotype. The administration of tyrosine kinase inhibitor (TKI) constitutes the recommended therapeutic strategy in different malignancies at advanced stages.
There are important interrelationships between cell stress, redox status, mitochondrial function, metabolism and cellular signaling pathways leading to cell survival/death. The induction of apoptosis and cell cycle arrest widely related to the antitumoral properties of TKIs result from tightly controlled events involving different cellular compartments and signaling pathways. The aim of the present review is to update the most relevant studies dealing with the impact of TKI treatment on cell function. The induction of endoplasmic reticulum (ER) stress and Ca2+ disturbances, leading to alteration of mitochondrial function, redox status and phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt)-mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) signaling pathways that involve cell metabolism reprogramming in cancer cells will be covered. Emphasis will be given to studies that identify key components of the integrated molecular pattern including receptor tyrosine kinase (RTK) downstream signaling, cell death and mitochondria-related events that appear to be involved in the resistance of cancer cells to TKI treatments.
Keywords: Autophagy, Cell death, Endoplasmic reticulum stress, mTOR, Redox status, PGC-1α
Abbreviations
- AIF
Apoptosis-inducing factor
- AML
Acute myeloid leukemia
- ALL
Acute lymphocytic leukemia
- AMPK
AMP-activated protein kinase
- ALK
Anaplastic lymphoma kinase
- APE1
Apurinic/apyrimidinic endonuclease 1
- BNIP3
BCL2 Interacting Protein 3
- BiP
Binding immunoglobulin protein
- NIX
BNIP3-like
- CAMKK2
Calcium/calmodulin-dependent protein kinase kinase 2
- CHOP
C/EBP homologous protein
- c-Met
Mesenchymal-epithelial transition factor receptor
- CML
Chronic myeloid leukemia
- CLL
Chronic lymphocytic leukemia
- JNK
c-Jun N-terminal kinase
- CSF-1R
CSF-1R Colony-stimulating factor receptor 1
- CRC
Colon and rectum carcinoma
- COX-2
Cyclooxygenase-2
- ER
Endoplasmic reticulum
- ENO2
Enolase 2
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-mesenchymal transition
- ERK
Extracellular signal-regulated kinases
- eIF2α
Eukaryotic Initiation Factor 2α
- FGFR
Fibroblast growth factor receptor
- Flt3
FMS-like tyrosine kinase 3
- Grp78
Glucose-regulated protein 78
- FoxM1
Forkhead Box M1
- GIST
Gastrointestinal stromal tumor
- GLUT1
Glucose transporter I
- GLUT3
Glucose transporter 3
- Grx1
Glutaredoxin 1
- HNSCC
Head and neck squamous cell carcinoma
- HCC
Hepatocellular carcinoma
- HGF
Hepatocyte growth factor
- HKII
Hexokinase II
- H2O2
Hydrogen peroxide
- HIF-1
Hypoxia-inducible factor-1
- IRE1α
Inositol-requiring enzyme-1α, IRE1
- JAK
Janus kinase
- mTOR
Mammalian target of rapamycin
- MMP
Matrix metalloproteinases
- MITF
Melanocyte lineage-specification transcription factor
- MAPK
Mitogen-activated protein kinase
- MEK
MAPK/ERK
- Mcl-1
Myeloid cell leukemia-1
- SIRT1
NAD-dependent deacetylase sirtuin-1
- NOX
NADPH oxidase
- NRTK
Non-receptor tyrosine kinase
- NSCLC
Non-small cell lung cancer
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2
Nuclear transcription factor erythroid 2-related factor 2
- LDH
Lactate dehydrogenase
- LKB1
Liver kinase B1
- PUMA
p53 upregulated modulator of apoptosis
- PPP
Pentose phosphate pathway
- Prdx2
Peroxiredoxin 2
- Prdx6
Peroxiredoxin 6
- PGC-1α
Peroxisome proliferator-activated receptor-coactivator 1α
- PI3K
Phosphatidylinositol 3-kinase
- PTEN
Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and tensin homolog
- PFK1
6-Phosphofructo-1-kinase
- PFKFB2
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2
- PDK-1
Phosphoinositide-dependent protein kinase-1
- PDGFR
Platelet derived growth factor receptor
- PARP-1
Poly (ADP-ribose) polymerase 1
- PKA
Protein kinase A
- Akt
Protein kinase B
- PERK
Protein kinase RNA-like endoplasmic reticulum kinase
- PPA2
Protein phosphatase A2
- PTPB1
Protein tyrosine phosphatase B1
- PDHA1
Pyruvate dehydrogenase E1 alpha 1
- PKM2
Pyruvate kinase isoenzyme type M2
- RHEB
Ras homolog enriched in brain
- ROS
Reactive oxygen species
- RTK
Receptor tyrosine kinase
- RCC
Renal cell carcinoma
- ROS1
ROS proto-oncogene 1
- SWH
Salvador-Warts-Hippo
- STAT3
Signal transducer and activator of transcription 3
- S6K1
S6 kinase 1
- SHP-1
Src homology region 2 domain-containing phosphatase-1
- SREBP1
Sterol regulatory element-binding transcription factor 1
- O2.−
Superoxide anion
- Trx1
Thioredoxin 1
- TrxR1
Thioredoxin reductase 1
- TGF-β
Transforming growth factor-β
- TCA
Tricarboxylic acid
- TK
Tyrosine kinase
- TKI
Tyrosine kinase inhibitor
- TSC
Tuberous sclerosis complex
- ULK1/2
Unc51-like kinases
- UPR
Unfolded protein response
- VEGFR
Vascular endothelial growth factor receptor
- XBP-1
X-Box binding protein 1
- XIAP
X-linked inhibitor of apoptosis protein
1. TK inhibition in cancer treatment
The activation of receptor tyrosine kinase (RTK) and non-receptor tyrosine kinase (NRTK) transmits downstream signaling events related to cell proliferation, growth, migration, angiogenesis or cell dedifferentiation [1]. The genetic alterations of RTKs involve different molecular mechanisms such as gain-of-function mutation, genomic amplifications, chromosomal translocations and autocrine activation are related to transforming-related abilities in cancer and resistance to treatments [2].
Chemotherapy and radiotherapy exert their antitumoral activity using common mechanisms in normal and tumor cells leading to noxious side effects in the surrounding tissues. The traditional anti-cancer chemotherapy targets dividing cells inducing DNA damage that leads to DNA damage response, leading to potential activation of DNA repair mechanisms, suppression of global general translation, cell cycle arrest and, ultimately, either cell survival or cell death [3,4]. Tyrosine kinase inhibitors (TKIs) research has gradually been replacing these conventional anti-tumoral therapies in order to achieve more specific targets with the promise to enhance positive results. TKIs target specific oncogenic signaling in tumor cells minimizing side effects in healthy cells [5]. The degree of efficacy and selectivity is variable among TKI treatments, and they can be differentially beneficial depending on the target disease in terms of preventing tumor cell dedifferentiation, proliferation and oncogenic signaling. Some of the advantages of TKIs over traditional chemotherapy are their high selectivity, few side effects and good oral administration, with an absorption grade that differs among TKI molecules [6]. Currently, more than 20 TKI drugs have been approved both as first- and second-line therapies in clinical oncology for the treatment of different cancer diseases (Table 1). Even TKIs employment have resulted in a breakthrough in the area of cancer treatment, the acquired resistance of tumoral cells is still a crucial barrier that avoids better results in the cure of cancer [7].
Table 1.
Tyrosine kinase inhibitors including targets, and their use as the recommended treatment in cancer. AML: Acute myeloid leukemia, ALL: Acute lymphocytic leukemia, CML: Chronic myeloid leukemia, CRC: Colon and rectum carcinoma, DTC: Differential thyroid carcinoma, GIST: Gastrointestinal stromal tumor, HCC: Hepatocellular carcinoma, MTC: Medullary thyroid carcinoma, c-Met: Mesenchymal-epithelial transition factor receptor, NSCLC: Non-small cell lung cancer, RCC: Renal cell carcinoma, STS: Soft tissue sarcoma.
| TKI | Target | Diseases |
|---|---|---|
| Alectinib | ALK | NSCLC |
| Afatinib | EGFR | NSCLC |
| Axitinib | VEGFR, PDGFR, c-Kit | RCC |
| Brigatinib | ALK | NSCLC |
| Bosutinib | Abl, Src | CML, MTC |
| Cabozantinib | VEGFR, c-Met, RET, Flt3, c-Kit, Axl, Tie-2 | HCC, MTC |
| Canertinib | EGFR | Breast cancer |
| Capmatinib | EGFR, c-Met | NSCLC |
| Ceritinib | ALK | NSCLC |
| Crizotinib | ALK, c-Met | NSCLC |
| Dasatinib | Abl, Src, PDGFR, c-Kit | CML |
| Dovitinib | VEGFR, FGFR, PDGFR, c-Kit, Flt3, CSF-1R | RCC |
| Erlotinib | EGFR | NSCLC |
| Gefitinib | EGFR | NSCLC |
| Herceptin | Her-2/neu | Breast Cancer |
| Imatinib | Abl, PDGFR, c-Kit | ALL, CML, CLL, GIST |
| Lapatinib | EGFR, ErbB2 | Breast cancer |
| Leflunomide | PDGFR | Prostate cancer |
| Lenvatinib | VEGFR, PDGFR, FGFR, RET, c-Kit | DTC, HCC |
| Neratinib | HER2 | Breast Cancer |
| Nilotinib | Bcr, Abl, PDGFR | CML |
| Osimertinib | EGFR | NSCLC |
| Pazopanib | VEGFR, PDGFR, FGFR, c-Kit | RCC, STS, NSCLC |
| Ponatinib | Bcr, Abl | CML, ALL |
| Regorafenib | VEGFR, PDGFR, FGFR, c-Kit, RET, Raf, Tie2 | HCC, CCR, GIST |
| Ruxolitinib | JAK1, JAK2 | Myelofibrosis |
| Semaxinib | VEGFR, c-Kit, Flt3 | AML |
| Sorafenib | Raf, VEGFR, PDGFR, c-Kit, Flt3 | RCC, HCC, Melanoma |
| Sunitinib | VEGFR, PDGFR, c-Kit, Flt3 | GIST, RCC |
| Sutent | Flt3 | AML |
| Vandetanib | VEGFR, EGFR | MTC |
| Vatalanib | VEGFR | CCR, Prostate cancer, RCC |
| Vemurafenib | B-Raf | Melanoma |
TKIs are low-weight molecules that act as receptor antagonists through interfering with ATP-γ-phosphate for the ATP binding site within the kinase catalytic domain preventing the autophosphorylation, dimerization and activation of the downstream cascade, thereby reducing tyrosine kinase (TK) phosphorylation [1]. TKIs can also occupy an adjacent site to their ATP binding domain, allowing both the inhibitor and ATP to bind to the kinase [8]. There are various TKIs that vary in their pharmacological effects, as well as in their side effects and target kinases, and the factors that define the effectiveness of inhibitors [9].
2. Alteration of ER stress and Ca2+ homeostasis by TKIs
The endoplasmic reticulum (ER) plays a crucial role in proteostasis, handling translocation during translation and folding of secretory proteins [10]. The accumulation of misfolded proteins in the lumen induces ER stress and triggers the unfolded protein response (UPR) to avoid cellular damage. Several situations including starvation, reduced glycosylation, drugs, alteration of redox status or mutant proteins might boost ER stress [11]. In mammals, inositol-requiring enzyme-1α (IRE1α), protein kinase RNA-like endoplasmic reticulum kinase (PERK) and ATF6 act as stress sensors governing restoration of homeostasis by attenuating translation, regulating ER lumen capacity and activating gene expression to reduce misfolding (Fig. 1).
Fig. 1.
Schematic representation of ER stress induction and downstream signaling events. Several factors might activate ER sensor chaperones inositol-requiring enzyme-1α (IRE1α), ATF6 and/or protein kinase RNA-like endoplasmic reticulum kinase (PERK), resulting in the inhibition of translation and induction of ER stress pathways. EIF2α: Eukaryotic initiation factor 2α, XBP-1: X-Box Binding Protein 1; CHOP: C/EBP homologous protein.
2.1. Impact of TKI on ER stress
The administration of TKI induces ER stress with different functional repercussions in cancer cells. Sorafenib inhibits RTKs such as vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR), NRTK such as c-kit and FMS-like tyrosine kinase (Flt3), as well as serine-threonine Raf kinase (Table 1), which increases markers of ER stress, including IRE1α, eukaryotic initiation factor 2α (eIF2α) and binding immunoglobulin protein (BiP)/glucose-regulated protein 78 (BiP/Grp78) in hepatic tissue from patients submitted to liver resection for hepatocellular carcinoma (HCC) [12]. In addition, the administration of Sorafenib (10 μM) has been related to the induction of ER stress, Ca2+ release, generation of reactive oxygen species (ROS), and apoptosis in human leukemia cells [13]. In this study, cell death is related to the activation of PERK and subsequent phosphorylation of eIF2α, as well as induction of IRE1α and X-Box Binding Protein 1 (XBP-1) mRNA processing, independent of the Raf/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) or MEK pathway [13]. We have recently showed that Sorafenib induces early (3–12 h) ER stress characterized by an increase of PERK and IRE1α signaling, but a decrease of ATF6 expression, temporally associated with the activation of c-Jun N-terminal kinase (JNK) and AMP-activated protein kinase (AMPK), and reduction of mammalian target of rapamycin (mTOR) and protein translation [14]. The downregulation of ER signaling using siRNA strategies has proved to reduce autophagy markers and increased apoptosis in Sorafenib-treated HepG2 cells [14]. The downregulation of ATF6 and autophagy mediated by Sorafenib [13,14] might be related to the disruption of the secretory pathway measured by Golgi fragmentation and phosphorylation of ATPase p97/VCP as the initiator of autophagy [15]. The induction of apoptosis by Sorafenib in renal cell carcinoma (RCC) has been related to the upregulation of PERK-related induction of ATF4, C/EBP homologous protein (CHOP) and p53 upregulated modulator of apoptosis (PUMA) [16].
Lenvatinib targets VEGFR, PDGFR, fibroblast growth factor receptor (FGFR), RET and c-Kit (Table 1), inducing an elevation of ER stress markers in nasopharyngeal cancer HK-1 cell line. Its combined treatment with iodine-131 potentiates ER stress and cell death, as well as reduces migration and invasiveness [17]. Differently, proapoptotic and antiproliferative properties induced by antiangiogenic drug Sunitinib with similar targets as Sorafenib (Table 1), are related to reduced expression of BiP/Grp78 and downregulation of PERK/eIF2α signaling in RCC [18]. The study supports the survival properties of ER stress in the model. In this sense, the knockdown of GRP78 inhibits cancer cell survival and induces apoptosis in cultured RCC [18]. Other study has shown that Sunitinib-related resistance is associated with IRE1α-dependent nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway [19].
The administration of Dasatinib, another TKI that targets mutated Abl, Src, PDGFR and c-Kit (Table 1), induces AMPK activation, epidermal growth factor receptor (EGFR) degradation and ER stress downregulation that lead to apoptosis in sensitive Ca9-22 and HSC3 cell lines [20]. Combined treatment of Dasatinib with metformin has a synergistic effect by boosting AMPK activation, ER stress and EGFR downregulation in order to overcome resistance to Dasatinib in head and neck squamous cell carcinoma cells (HNSCC) [20]. The inhibition of protein translation induced by ER stress and AMPK-mTOR inhibition might be an important anti-tumor mechanism in Dasatinib and metformin [20]. Correlative evidence suggests that the upregulation of EGFR limits the effectiveness of TKIs in advanced non-small cell lung cancer cells (NSCLC) [21]. In fact, EGFR-TKIs acquired resistance limits therapy in advanced NSCLC. To overcome this resistance, a combined treatment based on EGFR targeted Gefinitib or Erlotinib (Table 1) and phosphoinositide-dependent protein kinase-1 (PDK-1) inhibitor OSU-03012 could reduce protein kinase B (Akt) and increased pro-apoptotic ER signaling through upregulation of CHOP, without concomitant induction of cytoprotective chaperones like BiP/Grp78 nor Grp94 [22]. As sustained treatment with Erlotinib promotes pro-survival autophagy signaling contributing to tumor cell resistance, the inhibition of the autophagic flux during Erlotinib therapy modulates ER response and contributes to cell death [23]. Ca2+ signaling might play a crucial role in the adaptive resistance to EGFR inhibitors. In this sense, Afatinib (Table 1), shows alterations in the proteome and phosphoproteome associated with increased Ca2+-dependent adhesions and Ca2+ transporters in NSCLC PC9 cell line [24]. Ca+2 homeostasis could also be involved in resistant chronic myeloid leukemia (CML)-T1 cells to drugs that target Abl, PDGFR and c-Kit such as Imatinib (Table 1) [25].
3. Induction of mitochondrial dysfunction and ROS production by TKIs
ROS, mainly superoxide anion (O2.-) and hydrogen peroxide (H2O2), are generated during normal mitochondrial function playing a relevant role in both physiological functions and tumor development [26]. Exacerbated ROS levels are widely associated with mitochondrial dysfunction, which can lead to damage of different cellular constituents like proteins, DNA and lipids. Unrepaired damage to mitochondrial DNA leads to defective complexes I or III which can result in increased electron uncoupling and translocation of electrons to oxygen leading O2.- generation [27].
The administration of TKIs has been reported to induce cytotoxicity in different cancer cell lines [[28], [29], [30]]. Mitochondrial damage is usually considered as a drug's “off-target” effect contributing to adverse reactions, and appears to be commonly shared by many drugs and toxins [31,32]. Ponatinib (Bcr-Abl), Sorafenib and Regorafenib (VEGFR, PDGFR, FGFR, c-Kit, RET, Raf, Tie2), but not Crizotinib (mesenchymal-epithelial transition factor receptor or c-Met, and anaplastic lymphoma kinase or ALK) and Pazopanib (VEGFR, PDGFR, FGFR and c-Kit), were able to induce relevant mitochondrial dysfunction, uncoupling components of electron transport chain and promoting ROS generation [28,29,[33], [34], [35]].
The mitochondrial dysfunction is also associated with the occurrence of several complications or side effects related to diarrhea, fatigue, hand-foot skin reaction and hypertension that have been related to effectiveness of the treatment in the case of patients in advanced stage of HCC [36]. In close connection with TKI-induced ROS generation and Ca2+ disturbances by TKI administration, generation of cardiotoxicity is a major health problem despite antitumor effectiveness [37,38]. Sorafenib induces mitochondrial dysfunction related to the impairment of complexes I, III and V in the electronic respiratory chain and mitochondrial membrane depolarization [28,35,39] (Fig. 2). The generation of ROS by Sorafenib has been observed in different studies [28,40]. In addition, the presence of oxidative stress and ERK downregulation in peripheral blood mononuclear cells has been considered a good predictor of the evolution of patients with HCC treated with Sorafenib [41]. The induction of oxidative stress and depletion of antioxidant status by Sorafenib was related to Ca2+ disturbances that lead to its mitochondrial Ca2+ overload, which initiate lethal apoptotic events [42]. Regorafenib also directly uncouples oxidative phosphorylation and promotes Ca2+ accumulation and swelling of mitochondria [34]. Moreover, Regorafenib produces impairment of the respiratory chain that affects the maximal capacity of complex I [28] (Fig. 2). In this context, Dasatinib inhibits complexes IV and V of the electron transport chain in H9c2 rat cardiomyoblasts [29] (Fig. 2). Interestingly, the antitumoral properties of the antiangiogenic Sunitinib are related to the reduction of kidney toxicity, recovery of GSH levels and lipid peroxidation reduction induced by cisplatin in subcutaneous implantation of Ehrlich ascites carcinoma cells in mice [43].
Fig. 2.
Induction of mitochondrial dysfunction and reactive oxygen species (ROS) production by tyrosine kinase inhibitors (TKIs). TKIs act at the receptor tyrosine kinase (RTK), preventing Ras downstream events such as activation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) (MEK) and ERK phosphorylation that leads reduction of angiogenesis and tumor progression. TKIs might also induce mitochondrial dysfunction. Regorafenib impairs the activity of complex I of the respiratory chain. Dasatinib inhibits complexes IV and V. Sorafenib was demonstrated to behave as a mitochondrial uncoupler, and inhibits complexes I, III, V. The resulting ROS and products of the oxidative stress might be the cause and/or consequence of the mitochondrial dysfunction. Scheme extracted from elsewhere (190).
The antitumoral properties of Crizotinib, a TKI targeting ALK, ROS proto-oncogene 1 (ROS1) and c-Met [44], are related to cell death and ROS production in hepatocytes [45], cardiomyocytes [46], and alveolar rhabdomyosarcoma cells [47]. Gefitinib and Erlotinib, which target EGFR, have been also recently associated with the generation of oxidative stress [48]. The cytotoxicity of Erlotinib also involves NADPH oxidase (NOX) 4-induced oxidative stress in HNSCC cell lines [49]. The activity of Lapatinib, targeting a sub-class of EGFR 1 and/or 2 (ErbB1, ErbB2) [50], is also associated with ROS generation in inflammatory breast cancer models [51]. In concordance with the action of these TKIs, Imatinib that targets Abl, PDGFR and c-Kit [52], and is the recommended treatment for acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), CML and gastrointestinal stromal tumors (GIST), the induction of apoptosis is associated with ROS production and loss of the mitochondrial membrane potential [53].
4. Involvement of redox regulation in cancer
Oxidative stress, generated from ROS and antioxidant imbalance, is present in a large number of serious diseases including cancer [54]. A wide variety of stimuli derived from different signal molecules (growth factors, cytokines …) have been associated with H2O2 generation that can play a dual role, either in signaling or in oxidative stress, according to the local intracellular concentration attained [55]. During normal cell growth, levels of O2.- and H2O2 are kept under tight control by the intracellular redox regulatory mechanisms. In fact, several evidences support that the effect of intracellular ROS on oncogenesis is dependent on the ratio between intracellular O2.- and H2O2 in that a predominant increase in O2.- supports cell survival and promotes oncogenesis, and by contrast H2O2 prevents carcinogenesis by facilitating cell death signaling [56]. Alterations in redox regulatory mechanisms have been observed in different types of cancer together with the main prevalent genetic mutations related to telomerase, Wnt-β-catenin, PI3K/Akt/mTOR, p53 and MAPK signaling [57].
Despite the high production of ROS, cancer cells proliferate thanks to the induction of antioxidant defense systems through the activation of the nuclear transcription factor erythroid 2-related factor 2 (Nrf2) [58]. In these cells, the high rate of proliferation and apoptosis evasion may be achieved through the constitutive activation of redox-sensitive signal-transduction at the level of kinases such as MAPK family [59], phosphatidylinositol 3-kinase (PI3K)/Akt [60] and redox-sensitive transcription factors as NF-κB, Nrf2 and signal transducer and activator of transcription 3 (STAT3) [61], and inactivation of phosphatases as protein tyrosine phosphatase B1 (PTPB1) [62,63], protein phosphatase A2 (PPA2) [64] and phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and tensin homolog (PTEN) [65]. In this sense, and among other repercussions, the antitumoral activity of PPA2 [66] is reduced during oxidative and nitrosative stress when Tyr289 nitration within the B56ζ subunit of PP2A occurs, preventing the recruitment of its catalytic core, and consequently stabilizing the antiapoptotic properties of Bcl-2 [67].
A number of these signaling proteins that undergo redox changes are targets of redoxins, including thioredoxin 1 (Trx1) and glutaredoxin 1 (Grx1). In addition, it has been shown that peroxiredoxin 6 (Prdx6), Trx1 and thioredoxin reductase 1 (TrxR1) are often overexpressed in tumor cells and that high levels of Trx1 could be linked to drug resistance during cancer treatment [68,69]. As a matter of fact, it has been described that ROS production and NF-κB activation promote HCC progression [70] and that NF-κB DNA-binding activity is regulated by Trx1 [71,72].
STAT3 is an oncoprotein constitutively activated in several human cancers, what makes it a potential target for the development of cancer drugs [73]. STAT3 is activated by tyrosine phosphorylation which is influenced by its redox state. It has been described that STAT3 transcriptional activity can be modulated by S-glutathionylation [74] and it has also been found that its activity depends on its thiol redox state, which is influenced by H2O2 and peroxiredoxin 2 (Prdx2) levels and by the thioredoxin system activity [75]. The formation of Trx1-STAT3 disulfide exchange intermediates has been observed, suggesting that Trx1 may be a direct mediator of STAT3 disulfide reduction. Peroxiredoxin 6 (Prdx6) also promotes growth of lung tumors in mice through activation of Janus Kinase (JAK)2/STAT3 signaling [76].
Epithelial-mesenchymal transition (EMT) is an important de-differentiation process that occurs in normal development but also takes place in tumor cells and constitutes the first step towards metastasis during disease progression [77]. There are several proteins involved in EMT signaling pathways whose activity is ROS-dependent such as Smad, Snail, E-Cadherin, β-catenin and matrix metalloproteinases (MMP). It has also been described that transforming growth factor-β (TGFB) provokes an increase in the production of ROS, that results in the phosphorylation of Smad2, p38MAPK and ERK1/2 [78]. In addition, ROS may regulate EMT through a mechanism that involves NF-кB in close collaboration with hypoxia-inducible factor-1 (HIF-1) and cyclooxygenase-2 (COX-2) [79]. A critical molecular event of EMT is down-regulation of the cell adhesion molecule E-cadherin and its replacement by N-cadherin. It has been described by several authors that activation of Akt leads to a significant reduction in E-cadherin expression and nuclear localization of Snail, suggesting a role for the PI3K/Akt signaling pathway in the transient shift from E-cadherin to N-cadherin, and EMT progression in cancer [80]. The hyperactivation of PI3K/Akt signaling mediated by ROS and its relationship with tumor cell survival, apoptosis inhibition and resistance to chemotherapy has been described [81].
4.1. The role of redox in the resistance to TKIs
Given the role of redox status in tumorigenesis, we addressed how several studies have demonstrated a direct or indirect involvement of ROS in TKI-acquired resistance, usually through redox activation of the PI3K-Akt signaling pathways [1]. Akt is thought to be responsible for mediating the acquired resistance to Sorafenib via mTOR, but independent of PP2A, in HCC cells [82]. The overexpression of apurinic/apyrimidinic endonuclease 1 (APE1), a multifunctional enzyme that is involved in DNA repair and the redox regulation of transcription factors, significantly contributed to Gefitinib and Erlotinib resistance and Akt activation through a redox-dependent mechanism [83]. In addition, the combined treatment of Sorafenib and tetrandrine drastically increased apoptosis through ROS/Akt signaling in HCC cells [84].
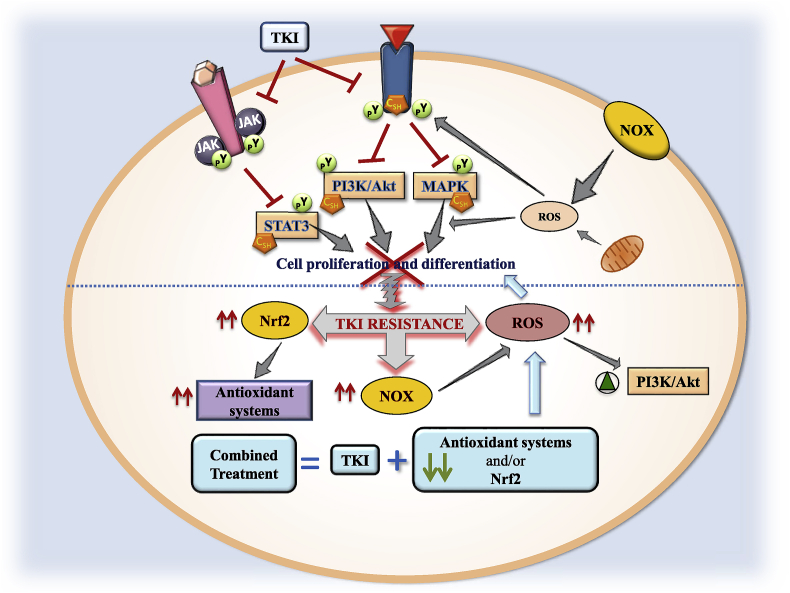
The high basal levels of ROS found in TKI-resistant cancer cells are mainly due to elevated NOX expression [85] although mitochondrial ROS production cannot be discarded (Fig. 3). CML and glioblastoma cancer cell resistance to Imatinib and Dasatinib might be associated to overexpression of components of the NOX2/Egr-1/Fyn signaling axis, which prompts the search for inhibitors of p47phox, the organizer subunit of NOX2, to treat TKI-resistant cancers [85]. In Erlotinib-resistant NSCLC cells, effective cell death induction is achieved by combined treatment with Erlotinib and ampelopsin, concomitant with ROS accumulation through upregulation of NOX2 expression [86]. Recently, it has been found that TKI-resistant lung cancer cells exhibit poly (ADP-ribose) polymerase 1 (PARP-1) dependence for survival through restriction of NOX-mediated cytotoxic ROS production [87].
Fig. 3.
Redox regulation by tyrosine kinase inhibitors (TKIs). Two receptor tyrosine kinase (RTK) are drawn inserted in the cell membrane with their respective bound ligands. The phosphorylated Tyr (pY) sites are also shown in RTK including redox sensitive intermediates (CSH, redox sensitive Cys in reduced state). The pathways inhibited by TKI are indicated with a red crossed line. Reactive oxygen species (ROS) generation by NADPH oxidase (NOX) and mitochondria impacts on redox sensitive sites in different targets showed with straight grey arrows. Transition from TKI-induced inhibition of cell proliferation and differentiation to resistance to TKI is indicated by a curly grey arrow and doted blue line. Events that accompany TKI resistance are displayed with vertical fine red arrows showing increased levels of nuclear transcription factor erythroid 2-related factor 2 (Nrf2)-dependent rise of antioxidant status, NOX and ROS, and activation of phosphatidylinositol 3-kinase (PI3K)/protein Kinase B (Akt) (green triangle inside a green circle). Grey arrows indicate an action. The lower part of the illustration reflects the contention that a combination of TKI treatment and down-regulation of antioxidant systems could counteract the development of TKI resistance in cancer cells (blue arrows). Janus kinase, JAK. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The combined treatment of Gefitinib- and Erlotinib-resistant k-Ras mutated NSCLC cells with Vorinostat has anti-proliferative and pro-apoptotic effects as well as increased ROS accumulation, Nrf2 downregulation and Keap1 upregulation [88]. Dysfunctional mutations in Keap1 gene provoke constitutive activation of Nrf2 leading to TKI resistance in NSCLC cells [89]. From these studies, Nrf2, a transcriptional factor playing a pivotal role in cellular antioxidant defense, emerges as a candidate target for anticancer therapy (Fig. 3).
4.2. The effectiveness of combined treatment with TKI and redoxin inhibitors
Reversible redox modification of key Cys residues constitutes a regulatory mechanism of protein function [90]. H2O2 acting as a second messenger modulates signaling cascades by reversible oxidation of redox sensitive cysteine residues in receptors and transduction proteins, including those using tyrosine phosphorylation signals like EGFR, MAPKs, PTEN, PI3K/Akt and STAT3 [91,92]. In particular, oxidation of Cys797 at the tyrosine kinase active site of EGFR enhances its activity [93] and coincidentally, mutation of C797S in EGFR has been described as a novel mechanism of acquired resistance to third-generation TKIs [94]. Peroxiredoxins may catalyze the oxidative modification of these cysteines while Trx and Grx are responsible for their reduction, as mentioned above. Hence, a role for redoxins in regulating cell signaling is to be expected.
Combination of TKI with down-regulation or inhibition of Grx/GSH or Trx/TrxR systems has been explored to treat TKI resistance. In a study carried out in Gefitinib-resistant NSCLC cells, Grx1 was upregulated and its inhibition enhanced the effects of Gefitinib on apoptosis an cell cycle arrest via the EGFR/Forkhead Box M1 (FoxM1) signaling pathway [95]. Trx1 has been shown to be overexpressed in a wide variety of cancers and its expression is associated with aggressive tumor growth, resistance to standard therapy and therefore decreased patient survival [96]. Recently, treatment with TKI has been combined with Trx1 inhibitors or downregulation of its levels to increase the effectiveness in TKI-resistant cancer cells [97]. In this study [97], the resistance of breast cancer cells to Trastuzumab, an antibody against Her2, appears to be related to the high levels of reduced Trx1 affecting the activity of the PTEN inhibitor. The combined treatment of Trastuzumab and a Trx1-inhibitor reduced cell viability, Akt phosphorylation and Bcl2 levels, as well as increased the levels of ROS, phosphorylated JNK, G1 arrest and apoptosis [97]. The downregulation of Trx1 could be a promising approach to improve the response to chemotherapy in HCC [98]. Our group has studied the role of Trx1 system in the sensitivity of three HCC cell lines (HepG2, SNU423 and SNU475) with different degrees of EMT to Sorafenib treatment. Basal Trx1/TrxR1 levels are markedly higher in poorly-differentiated SNU475 cells [99]. In these cells, Sorafenib treatment combined with Trx1 down-regulation using siRNA strategies dramatically induces inactivation of TGFβ and activation of tumor-suppressor Salvador-Warts-Hippo (SWH) signaling, as well as thiol oxidative changes in STAT3 [99]. In another study, the combination of Sorafenib with metformin decreases tumor invasiveness and cell motility associated with Trx1 downregulation [100]. Therefore, combination of Sorafenib with Trx1 inhibitors should be a good approach in the design of anticancer therapies. The panorama is not clear so far regarding the mechanisms of TKI resistance with the involvement of a varied panoply of signaling elements in different types of cancers. However, redox imbalance appears as a recurrent phenomenon in the majority, if not all cases studied so far. Consequently, combined therapy with TKI and other treatments aimed at either weakening the antioxidant defenses or stimulating ROS producing mechanisms can be envisaged as a promising therapeutic approach (Fig. 3).
5. Regulation of AMPK and mTOR during treatment with TKIs
The PI3K/Akt/mTOR pathway is one of the key pathways altered in cancer cells regulating relevant processes such as protein synthesis, cell growth, cell survival and, motility, as well as autophagy or angiogenesis. Several genes, such as PIK3K gene, are either overexpressed by amplification or activated by somatic point mutations in cancer [101]. The oncosuppressor PTEN is also frequently mutated [102] that leads to downstream Akt activation which, in turn, is often found to be somatically overexpressed and mutated [103]. The hyperactivation of PI3K/Akt/mTOR signaling is usually associated with resistance to different targeted-mediated therapies [104]. In this sense, mTOR can be regulated by different routes, being of special interest its inhibition by the AMPK, an energy sensor that regulates cellular metabolism while maintaining homeostasis, and which is involved in autophagy activation [105]. AMPK is activated in situations involving a rise of the AMP/ATP ratio, that might occur in stress induced by glucose deprivation, hypoxia, tissue ischemia, or during exercise. Thus, AMPK exerts stimulatory effects on glucose uptake, glycolysis, and fatty acid oxidation, while inhibits ATP-consuming cellular events, such as protein synthesis or fatty acid synthesis [106]. Liver kinase B1 (LKB1) is the upstream kinase activating AMPK by phosphorylating its Thr172 site, whereas calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) activates AMPK in response to calcium increase [107]. Although AMPK has been shown to promote Ser15p53 phosphorylation inducing cell cycle arrest, AMPKβ1 subunit is also upregulated by two downstream targets of p53 sestrin 1 and sestrin 2 [108]. The antitumoral properties of AMPK activation and mTOR inhibition have been related to antiproliferative and proapoptotic properties in leukemia [109]. Moreover, anti-apoptotic effects of AMPK have also been observed in other malignancies [110].
Autophagy is a highly conserved catabolic process that functions as a quality control system, facilitating the degradation of organelles and other damaged intracellular components in situations of metabolic stress. mTOR complex 1 works as a negative regulator of autophagy, exerting its inhibitory action by phosphorylating and inactivating Unc51-like kinases (ULK1/2) and Atg13 [111]. AMPK activation can subsequently trigger tuberous sclerosis complex (TSC)2 to repress mTOR complex 1 and, therefore, upregulates autophagy [112]. AMPK could also directly phosphorylate multiple sites in ULK1 that promotes autophagy [113] (Fig. 4).
Fig. 4.
Regulation of AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) during the treatment with tyrosine kinase inhibitors (TKIs). Regulation of cell proliferation, differentiation, angiogenesis and tumorigenesis signaling pathways by TKIs are closely related to the regulation of AMPK and mTOR. The binding of a mitogen to a receptor tyrosine kinase (RTK) stimulates the Ras/Raf/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK) (MEK) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling that target mTOR pathways. Among other downstream events, mTOR phosphorylates Unc51-like kinase (ULK) and inhibits autophagy. Akt negatively regulates AMPK, a protein involved in mTOR inhibition and ULK phosphorylation that promotes autophagy. Different TKIs are able to activate (green line) or inhibit (red line) specific pathways involved in downregulation of survival pathways. The activation of AMPK and inhibition of mTOR by TKIs induce autophagy and/or apoptosis that leads to the blockage of tumorigenesis and cell proliferation processes. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Controversial reports exist on the antitumoral properties of autophagy during TKI treatments. It has been suggested that normalization of autophagy may be relevant to avoid cellular resistance to cancer drugs [114]. However, the baseline apoptotic priming is a key determinant of the reliable cell killing during TKI treatment [115]. It appears that response to targeted therapies can be predicted with the dynamic BH3 profiling technique to measure early changes in net pro-apoptotic signaling at the mitochondrion (‘priming’) [116]. Autophagy has been considered to be the mechanism regulating the proximity of tumor cells to apoptotic cliff during drug administration. In this sense, the inhibition of autophagy by drugs, that downregulates the expression of pro-apoptotic transcription regulators such as Foxo3a, reduces the threshold to the initiation of apoptosis [117].
Sorafenib and Regorafenib have been extensively demonstrated to induce autophagy [118]. We have recently observed that the early Sorafenib-induced ER stress and transitory upregulation of JNK and AMPK-dependent signaling are associated with the inhibition of mTOR and activation of autophagy [14]. The activation of AMPK by Sorafenib is related to inhibition of mitochondrial metabolism, and increase of AMP/ADP and ADP/ATP ratios [119]. The inhibition of PERK, IRE1α and ATF6 branches of ER stress reduces autophagy, which played a survival role in Sorafenib-treated HepG2 cells [14].
Imatinib induces AMPK activation and suppression of S6 kinase 1 (S6K1) and mTOR, that appears to be related to sensitization of CML cells [120]. However, it is known that activation of AMPK has antileukemic effects, since even being an autophagy activator, it can lead to cell death when ATP concentrations are sufficiently low [121]. Lapatinib inhibits Akt/mTOR signaling and activates AMPK, being both aspects related to anticancer effects in breast cancer cells [122]. SU6656, a Src kinase inhibitor, activates AMPK pathway by enhancing its phosphorylation by LKB1 independently of cellular energy state [119]. The inhibition of mTOR by AMPK activation induced by different TKI, such as Erlotinib, Imatinib, Lapatinib, Sorafenib, and other preclinical drugs such as SU6656 (Src) or AEE788 (VEGFR and ErbB2) is relevant for the antitumoral properties of the drugs. The antitumoral impact of mTOR inhibition in autophagy process is related to the cell type and drug target. All in all, targeting autophagy has also become an important drug discovery approach to increase cancer chemosensitivity (Fig. 4).
6. Regulation of PGC-1α during TKI
The peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) regulates the activity of transcription factors and nuclear receptors that targets genes involved in mitochondrial biogenesis, oxidative metabolism, thermogenesis, anabolic pathways, glucose and lipid metabolisms, muscle fiber regeneration and antioxidant status [[123], [124], [125], [126]]. The expression of PGC-1α is upregulated by p53 and the oncogenic melanocyte lineage-specification transcription factor (MITF), and downregulated by c-Myc and HIF-1 [127]. The activity of PGC-1α can be regulated by phosphorylation through AMPK, protein kinase A (PKA), Akt, and p38 MAPK, which modulates its transcription regulatory properties [128]. AMPK enhances mitochondrial biogenesis inducing PGC-1α transcription, and its phosphorylation Thr177,Ser538PGC-1α which promotes its co-transcriptional activity [129]. p38 MAPK activates PGC-1α by phosphorylation at Thr262, Ser265 and Thr298, increasing its half-life in cytoplasm [130]. By contrast, Akt inhibits PGC-1α activity through inducing its phosphorylation at Ser570PGC-1α [131]. In conditions of low cellular energy status, NAD-dependent deacetylase sirtuin-1 (SIRT1) deacetylates and activates PGC-1α. However, when the energy cellular status is high, histone acetylase GCN5 induces PGC-1α acetylation, that inhibits its activity [126]. In the context of HCC, the overexpression of SIRT1 was correlated with microvascular invasion, metastasis and predicted poor outcomes [132].
Opposite reports have been published assessing the expression of PGC-1α expression in tumors [[133], [134], [135], [136]]. Although mitochondrial dysfunction is generally associated with the activation of glycolysis, many cancer cells possess intact mitochondrial respiration being some cancers subtypes dependent on mitochondrial respiration [127]. Mitochondrial biogenesis and metabolic reprogramming influence the phenotype of cancer cells and resistance to targeted therapy [137]. Interestingly, Hirpara et al. [138] showed that resistance to Gefitinib is associated with the upregulation of PGC1α and TFAM expression, and increased formation of supercomplexes I and II, oxygen consumption rate and mitochondrial mass in NSCLC cell line. Vemurafenib, an inhibitor of mutated serine/threonine kinase B-Raf, increases the MITF/PGC-1α signaling axis resulting in metabolic reprogramming towards oxidative phosphorylation and conferring cellular intrinsic resistance in melanoma cells [139]. Both studies identify OPB-51602 [138] and Gamitrinib [139] as targets of mitochondrial components to circumvent cellular resistance to TKI. The transient overexpression of SIRT1, as well as NAD repletion, decreases Sorafenib-induced apoptosis suggesting that SIRT1 could be an underlying mechanism of resistance to Sorafenib treatment in HCC [39].
7. Role of cell metabolism in cancer
The process of tumorigenesis and cancer progression involves alteration of cellular metabolism. During the last decades, numerous works have reported how metabolic reprogramming supports the anabolic requirements for cancer cell proliferation in terms of energetic demand for cell proliferation, increased macromolecules biosynthesis, new biomass building and tumor progression. Both genetic and microenvironmental factors are involved in the deregulation of tumor cells metabolism, which in turn are able to modulate the microenvironment inside the tumor driving cancer evolution, invasiveness or metastatic potential [[140], [141], [142]].
One of the hallmarks of cancer is the deregulation of cellular energetics towards aerobic glycolysis to generate ATP and metabolic intermediates even in presence of normal oxygen concentration, known as “Warburg effect” [143]. This effect is supported by tightly regulated molecular pathways including MAPK, PI3K, HIF-1, p53, c-Myc or AMPK pathways. Despite this shift from oxidative phosphorylation to glycolys for ATP production, mitochondria are required for tumor development and their regulation is a promising field for combined anticancer therapies. The activation of RTK/NRTK regulates PI3K-Akt-TSC1/2-Ras homolog enriched in brain (RHEB)-mTOR pathway in order to maintain a cellular balance between anabolism and catabolism. mTOR complex 1 regulates protein and nucleotide synthesis, lipogenesis and glycolysis. The stimulation of protein synthesis involves an increase of ribosome biogenesis and mRNA translation [144]. mTOR increases nucleotide synthesis by increasing ATF4-dependent expression of mitochondrial bifunctional enzyme methylenetetrahydrofolate dehydrogenase/cyclohydrolase, the key enzyme in mitochondrial tetrahydrofolate cycle, increasing the production of purine nucleotides [145]. mTOR augments lipogenesis in Sterol regulatory element-binding transcription factor 1 (SREBP1)-dependent pathway either through S6K1 phosphorylation [146] or by controlling the nuclear entry of lipin 1, a phosphatidic acid phosphatase, involved on SREBP1 gene regulation [147]. mTOR signaling also stimulates the expression of genes involved in nearly every step of glycolysis and pentose phosphate pathway (PPP) [146]. Several key enzymes of glucose metabolism, such as enolase 2 (ENO2), hexokinase II (HKII), lactate dehydrogenase (LDH) A, 6-phosphofructo-1-kinase (PFK1), and glucose transporter I (GLUT1) are described to be regulated by c-Myc [148], however, they are not upregulated in TSC-deficient cells [146].
7.1. Impact of TKIs in cancer metabolic pathways
TKIs can deregulate glycolysis in malignant tissue through its impact on EGFR, VEGFR, c-Met, Bcr-Abl, HER2 or ALK signaling in many different cancer types [149]. The inhibition of PDGFR, c-Kit and Bcr-Abl by Imatinib leads pyruvate entering into the tricarboxylic acid (TCA) cycle providing these cells with an alternative mechanism of glucose metabolism to compensate for the inhibition of glycolysis [150], and reduced GLUT1 in leukemic cell [151]. Thus, Imatinib induces a shift in the glucose metabolism between anaerobic glycolysis to aerobic mitochondrial TCA cycle depending on dosage (Fig. 5). In this sense, combined adjuvant therapies based on targeting mitochondrial metabolism are relevant to prevent treatment-acquired resistance [138,139].
Fig. 5.
Metabolic impact of tyrosine kinase inhibitor (TKI) administration. The inhibition of different receptor tyrosine kinase (RTK) alters all cancer hallmarks. Dovitinib, Imatinib, Gefitinib, Erlotinib and Capmatinib reduce glycolytic pathway, while Sorafenib promoted mitochondrial dysfunction. Lipid synthesis and protein synthesis were profoundly altered by TKIs. ETC; Electron transport chain.
Different studies using Erlotinib and Gefitinib, which specifically target EGFR, have shown that these drugs reduce extracellular acidification rate, lactate production and glucose consumption in lung adenocarcinoma cell lines [152]. These effects might be the consequence of c-Myc downregulation during EGFR inhibition [153]. Moreover, metabolomic analysis of TKI-treated cells shows a reduced presence of intermediate metabolites related to glucose metabolism [153].
The overexpression of EGFR and HER2 is associated with aggressive phenotypes and tumor progression in breast cancer. Lapatinib exerts a dual inhibition of EGFR and HER2, although its effects on HER2 appears to be more critical [154]. Among other interactions, Lapatinib blocks HER2 signaling inhibiting the phosphorylation of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 (PFKFB2) in different cancer cell lines, decreasing the amount of intermediate metabolites in PPP and blocking the glucose-dependent EGFR/HER2 signaling [155]. Consequently, current studies are trying to determine how HER2 expression could be considered as a predictive biomarker of clinical prognosis.
The inhibition of c-Met reduces intracellular NADPH content which plays an essential role during lipid and nucleic acid biosynthesis, as well as by enzymatic systems regulating oxidative system [149]. In addition, c-Met inhibition modulates glucose metabolism preventing the expression of different mitochondrial proteins [156]. The increased tumor growth activity of Cabozantinib, that targets VEGFR, c-Met, Tie2, Axl, RET, c-Kit and Flt3 (Table 1), compared to Regorafenib is related to decreased glycolysis in a colon and rectum carcinoma (CRC) xenograft mouse model [157]. Cabozantinib significantly reduces glucose uptake and PI3K/mTOR-dependent signaling pathway in CRC098 and CRC162 cells [157]. Crizotinib, which targets c-Met and ALK, reduces the expression of phosphorylated pyruvate kinase isoenzyme type M2 (PKM2), lactate release, phosphorylated LDH and glucose uptake in lymphoma cells [158].
The administration of Sorafenib induces a glycolytic cell reprogramming that may represent a cellular resistance strategy potentially targetable by combination therapies. Sorafenib induces the expression of glucose transport 3 (GLUT3), ENO2, and PFK1 that are related to increased uptake of glucose and lactate production as a response to mitochondrial dysfunction [159] (Fig. 5). In addition, Sorafenib downregulates the expression of the mitochondrial enzyme pyruvate dehydrogenase E1 alpha 1 (PDHA1) [159].
The resistance to Axitinib treatment, which targets VEGFR, PDGFR and c-Kit, has been related to upregulation of cell surface exposure of GLUT1 and extracellular acidification rate, both reflecting an increased glycolytic rate [160]. Dovitinib, targeting VEGFR, FGFR, PDGFR, c-Kit, Flt3 and colony-stimulating factor receptor 1 (CSF-1R), is able to hinder glucose metabolism with combined downregulation of Akt-mTOR pathway [161]. Imatinib shifts glycolysis to mitochondrial glucose metabolism inducing a reduction of glucose uptake and an increment of cell energetic state [162]. The inhibition of EGFR- and/or c-Met-related pathways by Capmatinib, Gefitinib and Erlotinib reverts aerobic glycolysis and reactivates mitochondrial oxidative phosphorylation in NSCLC through the concerted downregulation of HKII and phosphorylated PKM2, and upregulation of mitochondrial-dependent oxidative phosphorylation [163].
The contribution of reprogramming amino acids metabolism in malignant tissues is relevant for its requirement for protein synthesis. The impact of Sorafenib in mTOR and protein synthesis has also been already described [14]. Erlotinib, Gefitinb and Capmatinib have also been reported as negative regulators of amino acid levels [149]. Altered levels of lipids are widely related to carcinogenesis and cancer metastasis [164]. In fact, the levels of total cholesterol, triglycerides, HDL and LDL in plasma from breast cancer patients are significantly higher than those of control group [165]. Treatment of Bcr-Abl-positive cells with Imatinib decreases phosphocholine concentrations [162]. Erlotinib reduces the expression of fatty acid genes in MDA-MB-231 and Hs578T cells, while Capmatinib sensitizes MDA-MB-468 cells to knockdown of arachidonic and linoleic acid metabolism rate limiting enzymes [166]. The different response to TKI in sensitive or resistant cells is evidence that metabolic reprogramming is relevant to revert malignancy, and combined TKI-based strategies can be a very promising clinical tool for more effective targeted therapies to treat previously characterized subgroups of patients.
8. Induction of cell death by TKIs in cancer cells
The resistance to apoptosis is one of the main characteristics of tumor cells, characterized by their unlimited replicative potential, insensitivity to cellular stress, sustained angiogenesis, invasiveness and metastatic potential [167]. Apoptosis is a form of genetically programmed death process that can be mediated by ligand-dependent receptor activation (Type I) or mitochondrial outer membrane permeabilization (Type II), which could lead to an uncontrolled cell death process under limiting energy conditions termed necrosis, which involves cell swelling and sudden membrane rupture.
Sorafenib can induce apoptosis of cancer cells through regulation of multiple proapoptotic and antiapoptotic factors, including Bad, Bax, Bim, myeloid cell leukemia-1 (Mcl-1), and X-linked inhibitor of apoptosis protein (XIAP) [168]. The expression of these factors may be regulated at either transcriptional, translational, or posttranslational levels, and may involve mechanisms independent on ERK activity. The inhibition of Mcl-1 expression, which accounts for Sorafenib-induced apoptosis in multiple cancer types, has been found independent on Raf/MEK/ERK inhibition [169]. Recently, the proapoptotic factor Bim has been identified as an important factor in Sorafenib-induced apoptosis [14,170]. In fact, the progressive increase of ER stress and PERK-dependent CHOP expression, and reduction of Thr308Akt/Akt and Ser473Akt/Akt ratios are associated with the reduction of autophagic flux and an additional upregulation of BimEL expression and caspase-3 activity [14]. Small interfering-RNA (si-RNA) assays show that Bim, but not Bak and Bax, is involved in the induction of caspase-3 in Sorafenib-treated HepG2 cells [14]. The increase in Bad and decrease in Mcl-1 expression have also been shown to account for Sorafenib-induced apoptosis [171]. Bim exerts its proapoptotic activity through binding to several antiapoptotic Bcl-2 family members [172]. The mechanisms accounting for downregulation of Mcl-1 expression by Sorafenib may be cell type specific, and involve both ERK-dependent and ERK-independent mechanisms [173,174]. Sorafenib can also inhibit phosphorylation of eIF4B, which may lead to decreased translation of antiapoptotic factors such and Mcl-1 and cFLIP, and could also inhibit the nuclear translocation of the apoptosis-inducing factor (AIF) [175,176]. Beclin-1 contains a BH3 domain which binds to multiple antiapoptotic proteins belonging to the Bcl-2 family, such as Bcl-2, Bcl-xL, or Mcl-1 [177]. This interaction represses Beclin-1 activity and significantly reduces initiation of autophagy. When the BH3 domain is phosphorylated, there is a decrease in the binding affinity between these two proteins, resulting in Beclin-1 release and its subsequent activation [178]. Another significant signaling pathway related to reduced apoptotic cell death involves HIF-1α. HIF-1α levels are higher in Sorafenib-resistant HCC tissue samples than observed in Sorafenib-sensitive ones since, through HIF-1α resistant cells upregulate genes implicated in cell proliferation, angiogenesis, migration and autophagy [179]. Sorafenib reduces the expression of HIF-1α targets BCL2 Interacting Protein 3 (BNIP3) and BNIP3-like (NIX) that delay mitophagy, as revealed by their mitochondria and lysosomes colocalization and the autophagosome markers p62 and LC3-II [180].
The induction of apoptosis by Lenvatinib has also been related to reduction of tumor growth in a glioma-derived tumor bearing mice [181]. Lenvatinib significantly reduces Akt and ERK1/2 phosphorylation, cyclin D1 expression and cell proliferation, as well as increases the percentage of apoptotic cells in cultured anaplastic thyroid cancer cells [181]. The induction of ER stress has also been associated with the induction of apoptosis, reduced cell migration and invasiveness in Lenvatinib-treated nasopharyngeal carcinoma cells [17].
The reduction of phospho-ERK and phospho-JNK by Regorafenib have been associated with decreased cell proliferation and induction of apoptosis in cultured liver cancer cells [182]. The induction of apoptosis by Sorafenib [183] and Regorafenib [184] is related to the activation of Src homology region 2 domain-containing phosphatase-1 (SHP-1) and inhibition of STAT3 phosphorylation. Analysis of Bcl-2 family members reveals dose- and time-dependent depletion of antiapoptotic Mcl-1 in Regorafenib-treated HCT116 cells. A delayed and/or attenuated Mcl-1 degradation is observed in resistant Regorafenib-treated CRC [185]. Cabozantinib reduces cell proliferation and promotes apoptosis in vitro and in vivo in breast, lung, and glioma tumor cells [186]. Cabozantinib blocks hepatocyte growth factor (HGF)-stimulated c-Met pathway, and inhibits cell migration and invasiveness in cultured liver cancer cells, as well as reduces tumor growth and angiogenesis, and promotes apoptosis in xenograft-mouse model [187]. The reduced phosphorylation of c-Met RET and AXL is related to downregulation of PI3K/mTOR-dependent signaling pathway and increased ATG3, LC3 and Beclin-1 expression upon Cabozantinib treatment in CRC patient-derived tumor xenograft models [157].
9. Concluding remarks
Downregulation of RTK and NRTK by TKIs administration drastically alters cancer hallmarks involving cell survival/death, cellular stress, and metabolism. The alteration of TK-related signaling by TKIs involves the activation of ER stress and UPR that affect the expression of key proteins involved in mitochondrial function, PI3K/TSC/mTOR and AMPK that impact cell metabolism and death (Fig. 6). The balance between O2.- and H2O2 is tightly controlled, and proteins regulating redox status that change the activation/deactivation state of proteins involved in cellular signaling are altered during TKI treatment. The shift between pro- and antitumoral role of autophagy and mitochondria-related events can be involved in the resistance of cancer cells to treatments. In addition, the proximity of tumor cells to the apoptotic cliff promoted by TKI treatment can also limit the induction of cell death in cancer cells. In conclusion, the specific genetic pattern of cancer cells and the prevailing molecular signaling status upon drug pressure that drive resistance to cancer-related hallmarks, support the use of combined TKI treatments.
Fig. 6.
Graphical Abstract. Tyrosine kinase inhibitor (TKI) induced endoplasmic reticulum (ER) stress promoting unfolded protein response (UPR), Ca2+ release, translation blockage, autophagy and apoptosis. Furthermore, other mechanisms of TKIs involve mitochondrial dysfunction, generation of reactive oxygen species (ROS), AMP-activated protein kinase (AMPK) activation and mammalian target of rapamycin (mTOR) inhibition. These cellular pathways are interconnected and result in the induction of autophagy and apoptosis.
Acknowledgments
This study was funded by Institute of Health Carlos III (ISCiii) (PI16/00090, PI19/00838 and PI19/01266), Spanish Ministry of Economy and Competitiveness (BFU2016-80006-P), Andalusian Ministry of Economy, Innovation, Science and Employment (BIO-216 and CTS-6264), Andalusian Ministry of Equality, Health and Social Policies (PI-0198-2016) and Valencian Ministry of Education, Culture and Sports (PROMETEO/2019/027). P de la C-O was supported by FPU predoctoral fellowship (FPU17/00026) from Spanish Ministry of Education, Culture and Sports. E N-V was supported by the the predoctoral i-PFIS IIS-enterprise contract in science and technologies in health (IFI18/00014) from ISCiii. We thank the Biomedical Research Network Center for Cardiovascular Diseases (CIBERcv), and the Biomedical Research Network Center for Liver and Digestive Diseases (CIBERehd) founded by the ISCiii and co-financed by European Regional Development Fund (ERDF) "A way to achieve Europe" for their financial support.
References
- 1.Jiao Q., Bi L., Ren Y., Song S., Wang Q., Wang Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Canc. 2018;17:36. doi: 10.1186/s12943-018-0801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemmon M.A., Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods D., Turchi J.J. Chemotherapy induced DNA damage response: convergence of drugs and pathways. Canc. Biol. Ther. 2013;14:379–389. doi: 10.4161/cbt.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lomax M.E., Folkes L.K., O'Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin. Oncol. 2013;25:578–585. doi: 10.1016/j.clon.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Bhullar K.S., Lagaron N.O., McGowan E.M., Parmar I., Jha A., Hubbard B.P., Rupasinghe H.P.V. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol. Canc. 2018;17:48. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenihan D.J., Kowey P.R. Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist. 2013;18:900–908. doi: 10.1634/theoncologist.2012-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Togashi Y., Hayashi H., Okamoto K., Fumita S., Terashima M., de Velasco M.A., Sakai K. Chronic nicotine exposure mediates resistance to EGFR-TKI in EGFR-mutated lung cancer via an EGFR signal. Lung Canc. 2015;88:16–23. doi: 10.1016/j.lungcan.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Hojjat-Farsangi M. Small-molecule inhibitors of the receptor tyrosine kinases: promising tools for targeted cancer therapies. Int. J. Mol. Sci. 2014;15:13768–13801. doi: 10.3390/ijms150813768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmann J.T., Haap M., Kopp H.G., Lipp H.P. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr. Drug Metabol. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport T.A., Jungnickel B., Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu. Rev. Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- 11.Hampton R.Y. ER stress response: getting the UPR hand on misfolded proteins. Curr. Biol. 2000;10:R518–R521. doi: 10.1016/s0960-9822(00)00583-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu M., Zhou R., Wu X., Xu X., Su M., Yang B. Clinicopathologic charcterization of sorafenib-induced endoplasmic reticulum stress in human liver cancer cells. J. Physiol. Pharmacol. 2018;69 doi: 10.26402/jpp.2018.4.08. [DOI] [PubMed] [Google Scholar]
- 13.Rahmani M., Davis E.M., Crabtree T.R., Habibi J.R., Nguyen T.K., Dent P., Grant S. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol. Cell Biol. 2007;27:5499–5513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Hernandez M.A., Gonzalez R., de la Rosa A.J., Gallego P., Ordonez R., Navarro-Villaran E., Contreras L. Molecular characterization of autophagic and apoptotic signaling induced by sorafenib in liver cancer cells. J. Cell. Physiol. 2018;234:692–708. doi: 10.1002/jcp.26855. [DOI] [PubMed] [Google Scholar]
- 15.Yi P., Higa A., Taouji S., Bexiga M.G., Marza E., Arma D., Castain C. Sorafenib-mediated targeting of the AAA(+) ATPase p97/VCP leads to disruption of the secretory pathway, endoplasmic reticulum stress, and hepatocellular cancer cell death. Mol. Canc. Therapeut. 2012;11:2610–2620. doi: 10.1158/1535-7163.MCT-12-0516. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q., Wu G., Che X., Li Q., Zhang Z., Tang Q. Sorafenib induces renal cell carcinoma apoptosis via upregulating activating transcription factor 4. Pharmazie. 2018;73:156–160. doi: 10.1691/ph.2018.7855. [DOI] [PubMed] [Google Scholar]
- 17.Wang G., Zhuang J., Ni J., Ye Y., He S., Xia W. Combined effects of Lenvatinib and iodine-131 on cell apoptosis in nasopharyngeal carcinoma through inducing endoplasmic reticulum stress. Exp. Ther. Med. 2018;16:3325–3332. doi: 10.3892/etm.2018.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han K.S., Li N., Raven P.A., Fazli L., Frees S., Ettinger S., Park K.C. Inhibition of endoplasmic reticulum chaperone protein glucose-regulated protein 78 potentiates anti-angiogenic therapy in renal cell carcinoma through inactivation of the PERK/eIF2alpha pathway. Oncotarget. 2015;6:34818–34830. doi: 10.18632/oncotarget.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makhov P., Naito S., Haifler M., Kutikov A., Boumber Y., Uzzo R.G., Kolenko V.M. The convergent roles of NF-kappaB and ER stress in sunitinib-mediated expression of pro-tumorigenic cytokines and refractory phenotype in renal cell carcinoma. Cell Death Dis. 2018;9:374. doi: 10.1038/s41419-018-0388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y.C., Wu M.H., Wei T.T., Lin Y.C., Huang W.C., Huang L.Y., Lin Y.T. Metformin sensitizes anticancer effect of dasatinib in head and neck squamous cell carcinoma cells through AMPK-dependent ER stress. Oncotarget. 2014;5:298–308. doi: 10.18632/oncotarget.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maione P., Gridelli C., Troiani T., Ciardiello F. Combining targeted therapies and drugs with multiple targets in the treatment of NSCLC. Oncologist. 2006;11:274–284. doi: 10.1634/theoncologist.11-3-274. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y.C., Kulp S.K., Wang D., Yang C.C., Sargeant A.M., Hung J.H., Kashida Y. Targeting endoplasmic reticulum stress and Akt with OSU-03012 and gefitinib or erlotinib to overcome resistance to epidermal growth factor receptor inhibitors. Canc. Res. 2008;68:2820–2830. doi: 10.1158/0008-5472.CAN-07-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z., Du T., Dong X., Li Z., Wu G., Zhang R. Autophagy inhibition facilitates erlotinib cytotoxicity in lung cancer cells through modulation of endoplasmic reticulum stress. Int. J. Oncol. 2016;48:2558–2566. doi: 10.3892/ijo.2016.3468. [DOI] [PubMed] [Google Scholar]
- 24.Mulder C., Prust N., van Doorn S., Reinecke M., Kuster B., van Bergen En, Henegouwen P., Lemeer S. Adaptive resistance to EGFR-targeted therapy by calcium signaling in NSCLC cells. Mol. Canc. Res. 2018;16:1773–1784. doi: 10.1158/1541-7786.MCR-18-0212. [DOI] [PubMed] [Google Scholar]
- 25.Toman O., Kabickova T., Vit O., Fiser R., Polakova K.M., Zach J., Linhartova J. Proteomic analysis of imatinib-resistant CML-T1 cells reveals calcium homeostasis as a potential therapeutic target. Oncol. Rep. 2016;36:1258–1268. doi: 10.3892/or.2016.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg F., Chandel N.S. Reactive oxygen species-dependent signaling regulates cancer. Cell. Mol. Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Houten B., Woshner V., Santos J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2006;5:145–152. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Paech F., Mingard C., Grunig D., Abegg V.F., Bouitbir J., Krahenbuhl S. Mechanisms of mitochondrial toxicity of the kinase inhibitors ponatinib, regorafenib and sorafenib in human hepatic HepG2 cells. Toxicology. 2018;395:34–44. doi: 10.1016/j.tox.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Will Y., Dykens J.A., Nadanaciva S., Hirakawa B., Jamieson J., Marroquin L.D., Hynes J. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol. Sci. 2008;106:153–161. doi: 10.1093/toxsci/kfn157. [DOI] [PubMed] [Google Scholar]
- 30.Force T., Krause D.S., Van Etten R.A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Canc. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 31.Jones D.P., Lemasters J.J., Han D., Boelsterli U.A., Kaplowitz N. Mechanisms of pathogenesis in drug hepatotoxicity putting the stress on mitochondria. Mol. Interv. 2010;10:98–111. doi: 10.1124/mi.10.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah R.R., Morganroth J., Shah D.R. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 33.Mingard C., Paech F., Bouitbir J., Krahenbuhl S. Mechanisms of toxicity associated with six tyrosine kinase inhibitors in human hepatocyte cell lines. J. Appl. Toxicol. 2018;38:418–431. doi: 10.1002/jat.3551. [DOI] [PubMed] [Google Scholar]
- 34.Weng Z., Luo Y., Yang X., Greenhaw J.J., Li H., Xie L., Mattes W.B. Regorafenib impairs mitochondrial functions, activates AMP-activated protein kinase, induces autophagy, and causes rat hepatocyte necrosis. Toxicology. 2015;327:10–21. doi: 10.1016/j.tox.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 35.French K.J., Coatney R.W., Renninger J.P., Hu C.X., Gales T.L., Zhao S., Storck L.M. Differences in effects on myocardium and mitochondria by angiogenic inhibitors suggest separate mechanisms of cardiotoxicity. Toxicol. Pathol. 2010;38:691–702. doi: 10.1177/0192623310373775. [DOI] [PubMed] [Google Scholar]
- 36.Rimola J., Diaz-Gonzalez A., Darnell A., Varela M., Pons F., Hernandez-Guerra M., Delgado M. Complete response under sorafenib in patients with hepatocellular carcinoma: relationship with dermatologic adverse events. Hepatology. 2018;67(2):612–622. doi: 10.1002/hep.29515. [DOI] [PubMed] [Google Scholar]
- 37.Ma W., Liu M., Liang F., Zhao L., Gao C., Jiang X., Zhang X. Cardiotoxicity of sorafenib is mediated through elevation of ROS level and CaMKII activity and dysregulation of calcium homoeostasis. Basic Clin. Pharmacol. Toxicol. 2020;126(2):166–188. doi: 10.1111/bcpt.13318. [DOI] [PubMed] [Google Scholar]
- 38.Bouitbir J., Alshaikhali A., Panajatovic M.V., Abegg V.F., Paech F., Krahenbuhl S. Mitochondrial oxidative stress plays a critical role in the cardiotoxicity of sunitinib: running title: sunitinib and oxidative stress in hearts. Toxicology. 2019;426:152281. doi: 10.1016/j.tox.2019.152281. [DOI] [PubMed] [Google Scholar]
- 39.Garten A., Grohmann T., Kluckova K., Lavery G.G., Kiess W., Penke M. Sorafenib-induced apoptosis in hepatocellular carcinoma is reversed by SIRT1. Int. J. Mol. Sci. 2019;20:4048. doi: 10.3390/ijms20164048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coriat R., Nicco C., Chereau C., Mir O., Alexandre J., Ropert S., Weill B. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol. Canc. Therapeut. 2012;11:2284–2293. doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- 41.Caraglia M., Giuberti G., Marra M., Addeo R., Montella L., Murolo M., Sperlongano P. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis. 2011;2:e150. doi: 10.1038/cddis.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiou J.F., Tai C.J., Wang Y.H., Liu T.Z., Jen Y.M., Shiau C.Y. Sorafenib induces preferential apoptotic killing of a drug- and radio-resistant Hep G2 cells through a mitochondria-dependent oxidative stress mechanism. Canc. Biol. Ther. 2009;8:1904–1913. doi: 10.4161/cbt.8.20.9436. [DOI] [PubMed] [Google Scholar]
- 43.Suddek G.M. Sunitinib improves chemotherapeutic efficacy and ameliorates cisplatin-induced nephrotoxicity in experimental animals. Canc. Chemother. Pharmacol. 2011;67:1035–1044. doi: 10.1007/s00280-010-1402-1. [DOI] [PubMed] [Google Scholar]
- 44.Christensen J.G., Zou H.Y., Arango M.E., Li Q., Lee J.H., McDonnell S.R., Yamazaki S. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Canc. Therapeut. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 45.Yan H., Du J., Chen X., Yang B., He Q., Yang X., Luo P. ROS-dependent DNA damage contributes to crizotinib-induced hepatotoxicity via the apoptotic pathway. Toxicol. Appl. Pharmacol. 2019:114768. doi: 10.1016/j.taap.2019.114768. [DOI] [PubMed] [Google Scholar]
- 46.Doherty K.R., Wappel R.L., Talbert D.R., Trusk P.B., Moran D.M., Kramer J.W., Brown A.M. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol. Appl. Pharmacol. 2013;272:245–255. doi: 10.1016/j.taap.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Solomon B.J., Mok T., Kim D.W., Wu Y.L., Nakagawa K., Mekhail T., Felip E. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 48.Teppo H.R., Soini Y., Karihtala P. Reactive oxygen species-mediated mechanisms of action of targeted cancer therapy. Oxid .Med. Cell Longev. 2017;2017:1485283. doi: 10.1155/2017/1485283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orcutt K.P., Parsons A.D., Sibenaller Z.A., Scarbrough P.M., Zhu Y., Sobhakumari A., Wilke W.W. Erlotinib-mediated inhibition of EGFR signaling induces metabolic oxidative stress through NOX4. Canc. Res. 2011;71:3932–3940. doi: 10.1158/0008-5472.CAN-10-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee A., Dhadda A.S., Shehata M., Chan S. Lapatinib: a tyrosine kinase inhibitor with a clinical role in breast cancer. Expet Opin. Pharmacother. 2007;8:2189–2204. doi: 10.1517/14656566.8.13.2189. [DOI] [PubMed] [Google Scholar]
- 51.Aird K.M., Allensworth J.L., Batinic-Haberle I., Lyerly H.K., Dewhirst M.W., Devi G.R. ErbB1/2 tyrosine kinase inhibitor mediates oxidative stress-induced apoptosis in inflammatory breast cancer cells. Breast Canc. Res. Treat. 2012;132:109–119. doi: 10.1007/s10549-011-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchdunger E., Cioffi C.L., Law N., Stover D., Ohno-Jones S., Druker B.J., Lydon N.B. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J. Pharmacol. Exp. Therapeut. 2000;295:139–145. [PubMed] [Google Scholar]
- 53.Chang S.P., Shen S.C., Lee W.R., Yang L.L., Chen Y.C. Imatinib mesylate induction of ROS-dependent apoptosis in melanoma B16F0 cells. J. Dermatol. Sci. 2011;62:183–191. doi: 10.1016/j.jdermsci.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Durackova Z. Some current insights into oxidative stress. Physiol. Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 55.Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. Signal. 2000;2000 doi: 10.1126/stke.2000.53.pe1. pe1--pe1. [DOI] [PubMed] [Google Scholar]
- 56.Pervaiz S., Clement M.V. Superoxide anion: oncogenic reactive oxygen species? Int. J. Biochem. Cell Biol. 2007;39:1297–1304. doi: 10.1016/j.biocel.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Schulze K., Imbeaud S., Letouze E., Alexandrov L.B., Calderaro J., Rebouissou S., Couchy G. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du J., Sun C., Hu Z., Yang Y., Zhu Y., Zheng D., Gu L. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PloS One. 2010;5 doi: 10.1371/journal.pone.0015940. e15940-e15940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruhul Amin A.R.M., Senga T., Oo M.L., Thant A.A., Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: a role for the dual signalling pathways, Akt and Erk. Gene Cell. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 61.Hornsveld M., Dansen T.B. The hallmarks of cancer from a redox perspective. Antioxidants Redox Signal. 2016;25:300–325. doi: 10.1089/ars.2015.6580. [DOI] [PubMed] [Google Scholar]
- 62.Lou Y.-W., Chen Y.-Y., Hsu S.-F., Chen R.-K., Lee C.-L., Khoo K.-H., Tonks N.K. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 63.Barrett W.C., DeGnore J.P., König S., Fales H.M., Keng Y.F., Zhang Z.Y., Yim M.B. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 64.Murata H., Ihara Y., Nakamura H., Yodoi J., Sumikawa K., Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J. Biol. Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 65.Kwon J., Lee S.R., Yang K.S., Ahn Y., Kim Y.J., Stadtman E.R., Rhee S.G. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Switzer C.H., Glynn S.A., Cheng R.Y., Ridnour L.A., Green J.E., Ambs S., Wink D.A. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol. Canc. Res. 2012;10:1203–1215. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Low I.C., Loh T., Huang Y., Virshup D.M., Pervaiz S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood. 2014;124:2223–2234. doi: 10.1182/blood-2014-03-563296. [DOI] [PubMed] [Google Scholar]
- 68.Du Y., Zhang H., Lu J., Holmgren A. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J. Biol. Chem. 2012;287:38210–38219. doi: 10.1074/jbc.M112.392225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher A.B. The phospholipase A2 activity of peroxiredoxin 6. JLR (J. Lipid Res.) 2018;59:1132–1147. doi: 10.1194/jlr.R082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F., Yang J.-L., Yu K-k, Xu M., Xu Y-z, Chen L., Lu Y-m. Activation of the NF-kB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinoma. Mol. Canc. 2015;14 doi: 10.1186/s12943-014-0274-0. 10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matthews J.R., Wakasugi N., Virelizier J.-L., Yodoi J., Hay R.T. Thioredoxin regulates the DNA binding activity of NF-$χ$B by reduction of a disulphid bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirota K., Murata M., Sachi Y., Nakamura H., Takeuchi J., Mori K., Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. J. Biol. Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 73.Darnell J.E. Validating Stat3 in cancer therapy. Nat. Med. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 74.Butturini E., Darra E., Chiavegato G., Cellini B., Cozzolino F., Monti M., Pucci P. S-Glutathionylation at Cys328 and Cys542 impairs STAT3 phosphorylation. ACS Chem. Biol. 2014;9:1885–1893. doi: 10.1021/cb500407d. [DOI] [PubMed] [Google Scholar]
- 75.Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T., Scharf A.N.D. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015;11:64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- 76.Yun H.-M., Park K.-R., Park M.H., Kim D.H., Jo M.R., Kim J.Y., Kim E.-C. PRDX6 promotes tumor development via the JAK2/STAT3 pathway in a urethane-induced lung tumor model. Free Radic. Biol. Med. 2015;80:136–144. doi: 10.1016/j.freeradbiomed.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 77.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rhyu D.Y., Yang Y., Ha H., Lee G.T., Song J.S., Uh S.T., Lee H.B. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 79.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 80.Larue L., Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 81.Helfinger V., Schröder K. Redox control in cancer development and progression. Mol. Aspect. Med. 2018;63:88–98. doi: 10.1016/j.mam.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Dong J., Zhai B., Sun W., Hu F., Cheng H., Xu J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PloS One. 2017;12:1–16. doi: 10.1371/journal.pone.0185088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu G.S., Li M., Xu C.X., Wang D. APE1 stimulates EGFR-TKI resistance by activating Akt signaling through a redox-dependent mechanism in lung adenocarcinoma. Cell Death Dis. 2018;9:1111. doi: 10.1038/s41419-018-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wan J., Liu T., Mei L., Li J., Gong K., Yu C., Li W. Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling. Br. J. Canc. 2013;109:342–350. doi: 10.1038/bjc.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Irwin M.E., Johnson B.P., Manshouri R., Amin H.M., Chandra J. A NOX2/Egr-1/Fyn pathway delineates new targets for TKI-resistant malignancies. Oncotarget. 2015;6:23631–23646. doi: 10.18632/oncotarget.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong S.W., Park N.S., Noh M.H., Shim J.A., Ahn B.N., Kim Y.S., Kim D. Combination treatment with erlotinib and ampelopsin overcomes erlotinib resistance in NSCLC cells via the Nox2-ROS-Bim pathway. Lung Canc. 2017;106:115–124. doi: 10.1016/j.lungcan.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 87.Marcar L., Bardhan K., Gheorghiu L., Dinkelborg P., Pfäffle H., Liu Q., Wang M. Acquired resistance of EGFR-mutated lung cancer to tyrosine kinase inhibitor treatment promotes PARP inhibitor sensitivity. Cell Rep. 2019;27:3422–3432. doi: 10.1016/j.celrep.2019.05.058. e3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leone A., Roca M.S., Ciardiello C., Terranova-Barberio M., Vitagliano C., Ciliberto G., Mancini R. Vorinostat synergizes with EGFR inhibitors in NSCLC cells by increasing ROS via up-regulation of the major mitochondrial porin VDAC1 and modulation of the c-Myc-NRF2-KEAP1 pathway. Free Radic. Biol. Med. 2015;89:287–299. doi: 10.1016/j.freeradbiomed.2015.07.155. [DOI] [PubMed] [Google Scholar]
- 89.Yamadori T., Ishii Y., Homma S., Morishima Y., Kurishima K., Itoh K., Yamamoto M. Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancers. Oncogene. 2012;31:4768–4777. doi: 10.1038/onc.2011.628. [DOI] [PubMed] [Google Scholar]
- 90.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Schieber M., Chandel Navdeep S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Östman A., Frijhoff J., Sandin Å., Böhmer F.-D. Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 2011;150:345–356. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- 93.Paulsen C.E., Truong T.H., Garcia F.J., Homann A., Gupta V., Leonard S.E., Carroll K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niederst M.J., Hu H., Mulvey H.E., Lockerman E.L., Garcia A.R., Piotrowska Z., Sequist L.V. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin. Canc. Res. : Off. J. Am. Assoc. Canc. Res. 2015;21:3924–3933. doi: 10.1158/1078-0432.CCR-15-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L., Liu J., Liu J., Chen X., Chang M., Li J., Zhou J. GLRX inhibition enhances the effects of geftinib in EGFR-TKI-resistant NSCLC cells through FoxM1 signaling pathway. J. Canc. Res. Clin. Oncol. 2019;145:861–872. doi: 10.1007/s00432-019-02845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Powis G., Kirkpatrick D.L. Thioredoxin signaling as a target for cancer therapy. Curr. Opin. Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 97.Sadeghirizi A., Yazdanparast R., Aghazadeh S. Combating trastuzumab resistance by targeting thioredoxin-1/PTEN interaction. Tumor Biol. 2016;37:6737–6747. doi: 10.1007/s13277-015-4424-9. [DOI] [PubMed] [Google Scholar]
- 98.Li W., Shi J., Zhang C., Li M., Gan L., Xu H., Yang X. Co-delivery of thioredoxin 1 shRNA and doxorubicin by folate-targeted gemini surfactant-based cationic liposomes to sensitize hepatocellular carcinoma cells. J. Mater. Chem. B. 2014;2:4901–4910. doi: 10.1039/c4tb00502c. [DOI] [PubMed] [Google Scholar]
- 99.López-Grueso M.J., González R., Muntané J., Bárcena J.A., Padilla C.A. Thioredoxin downregulation enhances sorafenib effects in hepatocarcinoma cells. Antioxidants. 2019;8 doi: 10.3390/antiox8100501. 501-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo Z., Cao M., You A., Gao J., Zhou H., Li H., Cui Y. Metformin inhibits the prometastatic effect of sorafenib in hepatocellular carcinoma by upregulating the expression of TIP30. Canc. Sci. 2016;107:507–513. doi: 10.1111/cas.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murugan A.K., Munirajan A.K., Tsuchida N. Genetic deregulation of the PIK3CA oncogene in oral cancer. Canc. Lett. 2013;338:193–203. doi: 10.1016/j.canlet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 102.Leslie N.R. PTEN: an intercellular peacekeeper? Sci. Signal. 2012;5:pe50. doi: 10.1126/scisignal.2003685. [DOI] [PubMed] [Google Scholar]
- 103.Mundi P.S., Sachdev J., McCourt C., Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. Br. J. Clin. Pharmacol. 2016;82:943–956. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller T.W., Balko J.M., Arteaga C.L. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J. Clin. Oncol. 2011;29:4452–4461. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo Z., Saha A.K., Xiang X., Ruderman N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z., Wang N., Liu P., Xie X. AMPK and cancer. Exper. Suppl. 2016;107:203–226. doi: 10.1007/978-3-319-43589-3_9. [DOI] [PubMed] [Google Scholar]
- 108.Budanov A.V., Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Visnjic D., Dembitz V., Lalic H. The role of AMPK/mTOR modulators in the therapy of acute myeloid leukemia. Curr. Med. Chem. 2019;26:2208–2229. doi: 10.2174/0929867325666180117105522. [DOI] [PubMed] [Google Scholar]
- 110.Baumann P., Mandl-Weber S., Emmerich B., Straka C., Schmidmaier R. Inhibition of adenosine monophosphate-activated protein kinase induces apoptosis in multiple myeloma cells. Anti Canc. Drugs. 2007;18:405–410. doi: 10.1097/CAD.0b013e32801416b6. [DOI] [PubMed] [Google Scholar]
- 111.Chan E.Y. mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci. Signal. 2009;2:pe51. doi: 10.1126/scisignal.284pe51. [DOI] [PubMed] [Google Scholar]
- 112.Tripathi D.N., Chowdhury R., Trudel L.J., Tee A.R., Slack R.S., Walker C.L., Wogan G.N. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2950–E2957. doi: 10.1073/pnas.1307736110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao M., Klionsky D.J. AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metabol. 2011;13:119–120. doi: 10.1016/j.cmet.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu J., Fan L., Wang H., Sun G. Autophagy, a double-edged sword in anti-angiogenesis therapy. Med. Oncol. 2016;33:10. doi: 10.1007/s12032-015-0721-9. [DOI] [PubMed] [Google Scholar]
- 115.Letai A. Cell death and cancer therapy: don't forget to kill the cancer cell! Clin. Canc. Res. 2015;21:5015–5020. doi: 10.1158/1078-0432.CCR-15-1204. [DOI] [PubMed] [Google Scholar]
- 116.Montero J., Sarosiek K.A., DeAngelo J.D., Maertens O., Ryan J., Ercan D., Piao H. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160:977–989. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fitzwalter B.E., Towers C.G., Sullivan K.D., Andrysik Z., Hoh M., Ludwig M., O'Prey J. Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. Dev. Cell. 2018;44:555–565 e553. doi: 10.1016/j.devcel.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prieto-Dominguez N., Ordonez R., Fernandez A., Garcia-Palomo A., Muntane J., Gonzalez-Gallego J., Mauriz J.L. Modulation of autophagy by sorafenib: effects on treatment response. Front. Pharmacol. 2016;7:151. doi: 10.3389/fphar.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ross F.A., Hawley S.A., Auciello F.R., Gowans G.J., Atrih A., Lamont D.J., Hardie D.G. Mechanisms of paradoxical activation of AMPK by the kinase inhibitors SU6656 and sorafenib. Cell Chem. Biol. 2017;24:813–824 e814. doi: 10.1016/j.chembiol.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hirao T., Yamaguchi M., Kikuya M., Chibana H., Ito K., Aoki S. Altered intracellular signaling by imatinib increases the anti-cancer effects of tyrosine kinase inhibitors in chronic myelogenous leukemia cells. Canc. Sci. 2018;109:121–131. doi: 10.1111/cas.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vakana E., Altman J.K., Glaser H., Donato N.J., Platanias L.C. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood. 2011;118:6399–6402. doi: 10.1182/blood-2011-01-332783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu X., Wu L., Qiao H., Han T., Chen S., Liu X., Jiang R. Autophagy stimulates apoptosis in HER2-overexpressing breast cancers treated by lapatinib. J. Cell. Biochem. 2013;114:2643–2653. doi: 10.1002/jcb.24611. [DOI] [PubMed] [Google Scholar]
- 123.Bhalla K., Hwang B.J., Dewi R.E., Ou L., Twaddel W., Fang H.B., Vafai S.B. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Canc. Res. 2011;71:6888–6898. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bost F., Kaminski L. The metabolic modulator PGC-1alpha in cancer. Am. J. Canc. Res. 2019;9:198–211. [PMC free article] [PubMed] [Google Scholar]
- 125.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 126.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 127.Vyas S., Zaganjor E., Haigis M.C. Mitochondria and cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fernandez-Marcos P.J., Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011;93:884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jager S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Puigserver P., Rhee J., Lin J., Wu Z., Yoon J.C., Zhang C.Y., Krauss S. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol. Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 131.Li X., Monks B., Ge Q., Birnbaum M.J. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 132.Li Y., Xu S., Li J., Zheng L., Feng M., Wang X., Han K. SIRT1 facilitates hepatocellular carcinoma metastasis by promoting PGC-1alpha-mediated mitochondrial biogenesis. Oncotarget. 2016;7:29255–29274. doi: 10.18632/oncotarget.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feilchenfeldt J., Brundler M.A., Soravia C., Totsch M., Meier C.A. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARgamma-coactivator 1 (PGC-1) Canc. Lett. 2004;203:25–33. doi: 10.1016/j.canlet.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 134.Cormio A., Guerra F., Cormio G., Pesce V., Fracasso F., Loizzi V., Cantatore P. The PGC-1alpha-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem. Biophys. Res. Commun. 2009;390:1182–1185. doi: 10.1016/j.bbrc.2009.10.114. [DOI] [PubMed] [Google Scholar]
- 135.Liu R., Zhang H., Zhang Y., Li S., Wang X., Wang X., Wang C. Peroxisome proliferator-activated receptor gamma coactivator-1 alpha acts as a tumor suppressor in hepatocellular carcinoma. Tumour Biol. 2017;39 doi: 10.1177/1010428317695031. 1010428317695031. [DOI] [PubMed] [Google Scholar]
- 136.Tennakoon J.B., Shi Y., Han J.J., Tsouko E., White M.A., Burns A.R., Zhang A. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene. 2014;33:5251–5261. doi: 10.1038/onc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lyons A., Coleman M., Riis S., Favre C., O'Flanagan C.H., Zhdanov A.V., Papkovsky D.B. Insulin-like growth factor 1 signaling is essential for mitochondrial biogenesis and mitophagy in cancer cells. J. Biol. Chem. 2017;292:16983–16998. doi: 10.1074/jbc.M117.792838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirpara J., Eu J.Q., Tan J.K.M., Wong A.L., Clement M.-V., Kong L.R., Ohi N. Metabolic reprogramming of oncogene-addicted cancer cells to OXPHOS as a mechanism of drug resistance. Redox Biol. 2019;25:101076. doi: 10.1016/j.redox.2018.101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haq R., Shoag J., Andreu-Perez P., Yokoyama S., Edelman H., Rowe G.C., Frederick D.T. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Canc. Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gentric G., Mieulet V., Mechta-Grigoriou F. Heterogeneity in cancer metabolism: new concepts in an old field. Antioxidants Redox Signal. 2017;26:462–485. doi: 10.1089/ars.2016.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Justus C.R., Sanderlin E.J., Yang L.V. Molecular connections between cancer cell metabolism and the tumor microenvironment. Int. J. Mol. Sci. 2015;16:11055–11086. doi: 10.3390/ijms160511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metabol. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Warburg O. On the origin of cancer cells. Science. 1956;123:309. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 144.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ben-Sahra I., Hoxhaj G., Ricoult S.J.H., Asara J.M., Manning B.D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–733. doi: 10.1126/science.aad0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., Triantafellow E. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peterson T.R., Sengupta S.S., Harris T.E., Carmack A.E., Kang S.A., Balderas E., Guertin D.A. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Osthus R.C., Shim H., Kim S., Li Q., Reddy R., Mukherjee M., Xu Y. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 149.Poliaková M., Aebersold D.M., Zimmer Y., Medová M. The relevance of tyrosine kinase inhibitors for global metabolic pathways in cancer. Mol. Canc. 2018;17 doi: 10.1186/s12943-018-0798-9. 27-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alvarez-Calderon F., Gregory M.A., Pham-Danis C., DeRyckere D., Stevens B.M., Zaberezhnyy V., Hill A.A. Tyrosine kinase inhibition in leukemia induces an altered metabolic state sensitive to mitochondrial perturbations. Clin. Canc. Res. : Off. J. Am. Assoc. Canc. Res. 2015;21:1360–1372. doi: 10.1158/1078-0432.CCR-14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barnes K., McIntosh E., Whetton A.D., Daley G.Q., Bentley J., Baldwin S.A. Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene. 2005;24:3257–3267. doi: 10.1038/sj.onc.1208461. [DOI] [PubMed] [Google Scholar]
- 152.Lim S.-O., Li C.-W., Xia W., Lee H.-H., Chang S.-S., Shen J., Hsu J.L. EGFR signaling enhances aerobic glycolysis in triple-negative breast cancer cells to promote tumor growth and immune escape. Canc. Res. 2016;76:1284–1296. doi: 10.1158/0008-5472.CAN-15-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Makinoshima H., Takita M., Matsumoto S., Yagishita A., Owada S., Esumi H., Tsuchihara K. Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J. Biol. Chem. 2014;289:20813–20823. doi: 10.1074/jbc.M114.575464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oakman C., Pestrin M., Zafarana E., Cantisani E., Di Leo A. Role of lapatinib in the first-line treatment of patients with metastatic breast cancer. Canc. Manag. Res. 2010;2:13–25. [PMC free article] [PubMed] [Google Scholar]
- 155.Ruprecht B., Zaal E.A., Zecha J., Wu W., Berkers C.R., Kuster B., Lemeer S. Lapatinib resistance in breast cancer cells is accompanied by phosphorylation-mediated reprogramming of glycolysis. Canc. Res. 2017;77:1842. doi: 10.1158/0008-5472.CAN-16-2976. [DOI] [PubMed] [Google Scholar]
- 156.Guo T., Zhu Y., Gan C.S., Lee S.S., Zhu J., Wang H., Li X. Quantitative proteomics discloses MET expression in mitochondria as a direct target of MET kinase inhibitor in cancer cells. Mol. Cell. Proteomics : MCP. 2010;9:2629–2641. doi: 10.1074/mcp.M110.001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Scott A.J., Arcaroli J.J., Bagby S.M., Yahn R., Huber K.M., Serkova N.J., Nguyen A. Cabozantinib exhibits potent antitumor activity in colorectal cancer patient-derived tumor xenograft models via autophagy and signaling mechanisms. Mol. Canc. Therapeut. 2018;17:2112–2122. doi: 10.1158/1535-7163.MCT-17-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McDonnell S.R.P., Hwang S.R., Rolland D., Murga-Zamalloa C., Basrur V., Conlon K.P., Fermin D. Integrated phosphoproteomic and metabolomic profiling reveals NPM-ALK-mediated phosphorylation of PKM2 and metabolic reprogramming in anaplastic large cell lymphoma. Blood. 2013;122:958–968. doi: 10.1182/blood-2013-01-482026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tesori V., Piscaglia A.C., Samengo D., Barba M., Bernardini C., Scatena R., Pontoglio A. The multikinase inhibitor Sorafenib enhances glycolysis and synergizes with glycolysis blockade for cancer cell killing. Sci. Rep. 2015;5 doi: 10.1038/srep09149. 9149-9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hudson C.D., Hagemann T., Mather S.J., Avril N. Resistance to the tyrosine kinase inhibitor axitinib is associated with increased glucose metabolism in pancreatic adenocarcinoma. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.125. e1160-e1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fumarola C., Cretella D., La Monica S., Bonelli M.A., Alfieri R., Caffarra C., Quaini F. Enhancement of the anti-tumor activity of FGFR1 inhibition in squamous cell lung cancer by targeting downstream signaling involved in glucose metabolism. Oncotarget. 2017;8:91841–91859. doi: 10.18632/oncotarget.19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gottschalk S., Anderson N., Hainz C., Eckhardt S.G., Serkova N.J. Imatinib (STI571)-Mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin. Canc. Res. 2004;10:6661. doi: 10.1158/1078-0432.CCR-04-0039. [DOI] [PubMed] [Google Scholar]
- 163.De Rosa V., Iommelli F., Monti M., Fonti R., Votta G., Stoppelli M.P., Del Vecchio S. Reversal of warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non–small cell lung cancer. Clin. Canc. Res. 2015;21:5110. doi: 10.1158/1078-0432.CCR-15-0375. [DOI] [PubMed] [Google Scholar]
- 164.Long J., Zhang C.-J., Zhu N., Du K., Yin Y.-F., Tan X., Liao D.-F. Lipid metabolism and carcinogenesis, cancer development. Am. J. Canc. Res. 2018;8:778–791. [PMC free article] [PubMed] [Google Scholar]
- 165.Zhu Y., Aupperlee M.D., Zhao Y., Tan Y.S., Kirk E.L., Sun X., Troester M.A. Pubertal and adult windows of susceptibility to a high animal fat diet in Trp53-null mammary tumorigenesis. Oncotarget. 2016;7:83409–83423. doi: 10.18632/oncotarget.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lanning N.J., Castle J.P., Singh S.J., Leon A.N., Tovar E.A., Sanghera A., MacKeigan J.P. Metabolic profiling of triple-negative breast cancer cells reveals metabolic vulnerabilities. Canc. Metabol. 2017;5 doi: 10.1186/s40170-017-0168-x. 6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 168.Zhang W., Konopleva M., Ruvolo V.R., McQueen T., Evans R.L., Bornmann W.G., McCubrey J. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 169.Yu C., Bruzek L.M., Meng X.W., Gores G.J., Carter C.A., Kaufmann S.H., Adjei A.A. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 170.Rahmani M., Nguyen T.K., Dent P., Grant S. The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant bcr/abl+ human leukemia cells in association with signal transducer and activator of transcription 5 inhibition and myeloid cell leukemia-1 down-regulation. Mol. Pharmacol. 2007;72:788–795. doi: 10.1124/mol.106.033308. [DOI] [PubMed] [Google Scholar]
- 171.Ou D.L., Shen Y.C., Liang J.D., Liou J.Y., Yu S.L., Fan H.H., Wang D.S. Induction of Bim expression contributes to the antitumor synergy between sorafenib and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitor CI-1040 in hepatocellular carcinoma. Clin. Canc. Res. 2009;15:5820–5828. doi: 10.1158/1078-0432.CCR-08-3294. [DOI] [PubMed] [Google Scholar]
- 172.Chen L., Willis S.N., Wei A., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 173.Kim S.H., Ricci M.S., El-Deiry W.S. Mcl-1: a gateway to TRAIL sensitization. Canc. Res. 2008;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 174.Ding Q., Huo L., Yang J.Y., Xia W., Wei Y., Liao Y., Chang C.J. Down-regulation of myeloid cell leukemia-1 through inhibiting Erk/Pin 1 pathway by sorafenib facilitates chemosensitization in breast cancer. Canc. Res. 2008;68:6109–6117. doi: 10.1158/0008-5472.CAN-08-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rosato R.R., Almenara J.A., Coe S., Grant S. The multikinase inhibitor sorafenib potentiates TRAIL lethality in human leukemia cells in association with Mcl-1 and cFLIPL down-regulation. Canc. Res. 2007;67:9490–9500. doi: 10.1158/0008-5472.CAN-07-0598. [DOI] [PubMed] [Google Scholar]
- 176.Panka D.J., Wang W., Atkins M.B., Mier J.W. The Raf inhibitor BAY 43-9006 (Sorafenib) induces caspase-independent apoptosis in melanoma cells. Canc. Res. 2006;66:1611–1619. doi: 10.1158/0008-5472.CAN-05-0808. [DOI] [PubMed] [Google Scholar]
- 177.Germain M., Nguyen A.P., Le Grand J.N., Arbour N., Vanderluit J.L., Park D.S., Opferman J.T. MCL-1 is a stress sensor that regulates autophagy in a developmentally regulated manner. EMBO J. 2011;30:395–407. doi: 10.1038/emboj.2010.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tai W.T., Shiau C.W., Chen H.L., Liu C.Y., Lin C.S., Cheng A.L., Chen P.J. Mcl-1-dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC-59 in hepatocellular carcinoma cells. Cell Death Dis. 2013;4:e485. doi: 10.1038/cddis.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu L.P., Ho R.L., Chen G.G., Lai P.B. Sorafenib inhibits hypoxia-inducible factor-1alpha synthesis: implications for antiangiogenic activity in hepatocellular carcinoma. Clin. Canc. Res. 2012;18:5662–5671. doi: 10.1158/1078-0432.CCR-12-0552. [DOI] [PubMed] [Google Scholar]
- 180.Prieto-Dominguez N., Mendez-Blanco C., Carbajo-Pescador S., Fondevila F., Garcia-Palomo A., Gonzalez-Gallego J., Mauriz J.L. Melatonin enhances sorafenib actions in human hepatocarcinoma cells by inhibiting mTORC1/p70S6K/HIF-1alpha and hypoxia-mediated mitophagy. Oncotarget. 2017;8:91402–91414. doi: 10.18632/oncotarget.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li J.Z.C., Zhang Z.M., Lv L.J., Qiao H.B., Chen X.J. A multi-targeted tyrosine kinase inhibitor lenvatinib for the treatment of mice with advanced glioblastoma. Mol. Med. Rep. 2017;16(5):7105–7111. doi: 10.3892/mmr.2017.7456. [DOI] [PubMed] [Google Scholar]
- 182.Carr B.I., Cavallini A., Lippolis C., D'Alessandro R., Messa C., Refolo M.G., Tafaro A. Fluoro-Sorafenib (Regorafenib) effects on hepatoma cells: growth inhibition, quiescence, and recovery. J. Cell. Physiol. 2013;228:292–297. doi: 10.1002/jcp.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen K.F., Su J.C., Liu C.Y., Huang J.W., Chen K.C., Chen W.L., Tai W.T. A novel obatoclax derivative, SC-2001, induces apoptosis in hepatocellular carcinoma cells through SHP-1-dependent STAT3 inactivation. Canc. Lett. 2012;321:27–35. doi: 10.1016/j.canlet.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 184.Tai W.T., Chu P.Y., Shiau C.W., Chen Y.L., Li Y.S., Hung M.H., Chen L.J. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin. Canc. Res. 2014;20:5768–5776. doi: 10.1158/1078-0432.CCR-14-0725. [DOI] [PubMed] [Google Scholar]
- 185.Tong J., Tan S., Zou F., Yu J., Zhang L. FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene. 2017;36:787–796. doi: 10.1038/onc.2016.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yakes F.M., Chen J., Tan J., Yamaguchi K., Shi Y., Yu P., Qian F. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Canc. Therapeut. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 187.Xiang Q., Chen W., Ren M., Wang J., Zhang H., Deng D.Y., Zhang L. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin. Canc. Res. 2014;20:2959–2970. doi: 10.1158/1078-0432.CCR-13-2620. [DOI] [PubMed] [Google Scholar]