Abstract
Background
Some studies have shown associations of maternal age at delivery with asthma and food allergy in offspring. However, the relationship between maternal age at delivery and allergic rhinitis is largely unclear. This study aimed to investigate the association between maternal age at delivery and allergic rhinitis in a population sample of Asian children, and to explore potential effect modifiers.
Methods
A total of 1344 singleton-birth children (763 boys, 56.8%; mean age, 6.4 years) participating in the Longitudinal Investigation of Global Health in Taiwanese Schoolchildren (LIGHTS) cohort were evaluated by a modified International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire and interviewed by pediatricians. Allergic sensitization was determined by using Phadiatop Infant. Multiple logistic regression models with covariates adjustment were performed to investigate the association of maternal age at delivery with allergic rhinitis and allergic sensitization in offspring.
Results
Among 1344 study children, 793 (59%) had physician-diagnosed allergic rhinitis. Advanced maternal age at delivery (≥40 years) was significantly associated with increased odds of allergic rhinitis (adjusted odds ratio [AOR] = 4.58, 95% confidence interval [CI]: 1.90–11.03) and allergic sensitization (AOR = 2.86, 95% CI: 1.13–7.22) in offspring. A sex-stratified analysis revealed that the association of advanced maternal age with allergic rhinitis was statistically significant only in female offspring (AOR = 7.02, 95% CI: 1.89–26.14). Stratified analyses by birth order or environmental tobacco smoke exposure during pregnancy did not reveal any significant differences.
Conclusion
Advanced maternal age at delivery was associated with increased risk of allergic rhinitis in Asian children, probably more pronounced among girls.
Keywords: Allergic rhinitis, Allergic sensitization, Children, Maternal age at delivery
Abbreviations: ISAAC, International Study of Asthma and Allergies in Childhood; ETS, environmental tobacco smoke; LIGHTS, Longitudinal Investigation of Global Health in Taiwanese Schoolchildren; EMR, electronic medical records; IgE, immunoglobulin E; ARIA, allergic rhinitis and its impact on asthma; ANOVA, analysis of variance; SD, standard deviation; AOR, adjusted odds ratio; CI, confidence interval; NTD, New Taiwan Dollar
Introduction
The increasing prevalence of allergic diseases worldwide has been a concern in the past few decades.1, 2, 3 The International Study of Asthma and Allergies in Childhood (ISAAC) studies have reported a rising prevalence of allergic rhinoconjunctivitis in many countries around the world. Particularly, Taiwan has the highest prevalence of allergic rhinoconjunctivitis in children aged 6–7 years among 37 countries, and the prevalence has increased by 166% during a 7-year period.1 Rising prevalence of allergic rhinitis has led to substantial economic impact and dramatic reduction in quality of life in children with allergic rhinitis and their family.4,5 At present, the causes of allergic rhinitis still are not fully understood. Sex, birth order, environmental tobacco smoke (ETS) exposure, and family history of allergic diseases have been found to be associated with allergic rhinitis in children.6,7
There has been a trend toward increasing maternal age at delivery in many countries, including Taiwan.8, 9, 10 Growing attention has been paid in recent years toward ascertaining adverse outcomes in older mothers and their offspring.11,12 A study conducted by Hsieh et al in Taiwan revealed an increasing trend in the mean maternal age at delivery, as the proportion of women giving birth at age 35 or older increased from 11.4% in 1990 to 19.1% in 2003.12 They have also found that advanced maternal age at delivery was significantly associated with adverse pregnancy and perinatal complications. In a case-control study in the U.S., maternal age over 30 years at delivery was an independent predictor of food allergy in their children.13 Several studies have shown associations between maternal age at delivery and risk of asthma in offspring; however, this relationship was inconsistent across different countries,14, 15, 16, 17 suggesting that there may be ethnic differences in the relationship between maternal age at delivery and development of allergic diseases in offspring. To date, the relationship between maternal age at delivery and the development of childhood allergic rhinitis has not been fully explored.18, 19, 20, 21, 22, 23, 24
Although the exact cause of the rapid rise in prevalence of allergic rhinitis in children in Taiwan remains unclear, the trend toward increasing maternal age at delivery during the last decades coincides with the rising prevalence of allergic rhinitis, indicating a potential relationship between maternal age at delivery and allergic rhinitis in offspring. In a large prospective population-based cohort study, we aimed to investigate whether maternal age at delivery was associated with allergic rhinitis in Asian children in Taiwan and to assess potential effect modifiers, specifically, sex, birth order, and ETS exposure during pregnancy.
Methods
Study design and subjects
The study population included 1344 singleton-birth children participating in the Longitudinal Investigation of Global Health in Taiwanese Schoolchildren (LIGHTS) study, a prospective population-based cohort study designed to longitudinally investigate the effects of early-life environmental exposures and genetic predisposition on childhood health outcomes, particularly allergic diseases. A total of 1513 children born in 2010–2011 in the Chang Gung Memorial Hospital attended a follow-up visit of the LIGHTS cohort at the mean age of 6.5 years. After excluding multiple births (n = 162) and missing data on allergic rhinitis (n = 7), 1344 children were included in the current study. The flow chart for subject recruitment is depicted in Fig. 1. The perinatal health information was obtained from electronic medical records (EMR) in the Chang Gung Memorial Hospital. Demographic and epidemiologic data, general health information, and clinical data were collected using a modified ISAAC questionnaire, which was provided by parents of study children. An interview conducted by pediatricians was applied to all participants. Blood samples were drawn for subsequent measurement of serum allergen-specific immunoglobulin E (IgE). The study was approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 201600334A3). Written informed consent was provided by the parent or legal guardian of each participant.
Fig. 1.
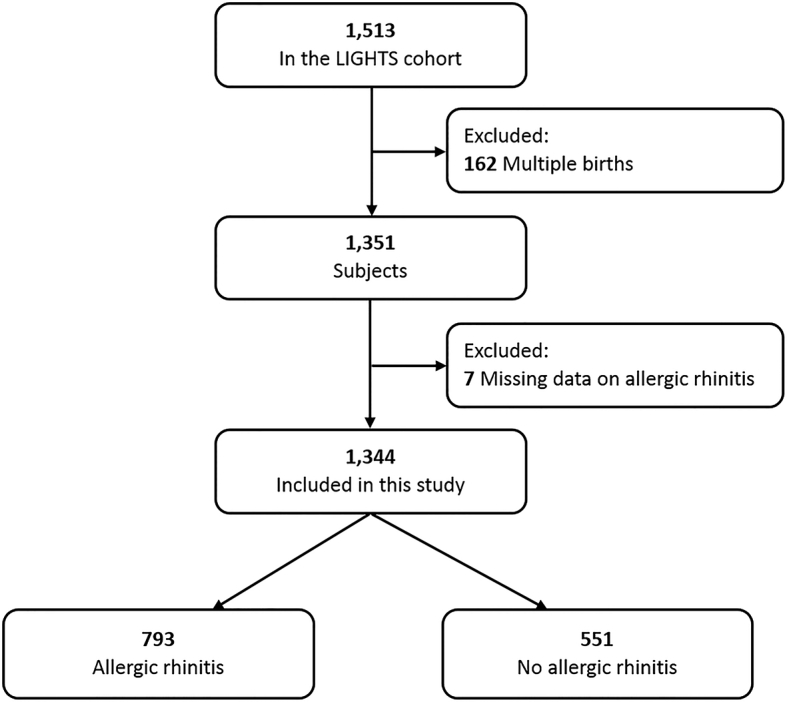
Schematic presentation of the recruitment process of the study participants. LIGHTS: Longitudinal Investigation of Global Health in Taiwanese Schoolchildren
Maternal age at delivery
Information of maternal birth date was obtained from the EMR in the Chang Gung Memorial Hospital. The maternal age at delivery was calculated based on the date of delivery and categorized into the following five groups: <25, 25–29, 30–34, 35–39, and ≥40 years, accordingly. The group of 30–34 years was treated as the reference group in subsequent analyses.
Outcome assessment
The main outcome of this study was allergic rhinitis. Information on symptoms of allergic rhinitis was collected using the modified ISAAC questionnaire. Parents of study children answered the following rhinitis related questions: “Has your child ever had a problem with sneezing, or a runny or blocked nose when he/she did not have a cold?” (defined as ever rhinitis); and “In the past 12 months, has your child had a problem with sneezing, or a runny or blocked nose when he/she did not have a cold?” (defined as current rhinitis). The diagnosis of allergic rhinitis was made through a comprehensive history and physical examination during an interview by pediatricians using the allergic rhinitis and its impact on asthma (ARIA) guideline25 (defined as physician-diagnosed allergic rhinitis). Allergic sensitization was defined based on Phadiatop Infant test result (≥0.35 PAU/L) (Phadia, Uppsala, Sweden), a reliable alternative to skin prick test for measuring allergen-specific IgE against the following allergens: house dust mite, cat, dog, birch, timothy, ragweed, wall pellitory, egg white, cow's milk, peanut, and shrimp.26
Statistical analysis
For demographic characteristics, categorical variables were compared and tested using chi-squared test; continuous variables were tested using analysis of variance (ANOVA). Multiple logistic regression analyses were applied to examine the associations between maternal age at delivery and outcomes of interest, including physician-diagnosed allergic rhinitis, ever rhinitis, current rhinitis, and allergic sensitization, with adjustment of relevant covariates. Maternal age at delivery was analyzed either as a categorical variable with five groups (<25, 25–29, 30–34 [reference group], 35–39, and ≥40 years) or as a continuous variable. The adjusted covariates were listed as follows: child's age, sex, prematurity (based on gestational age [GA]: term [ ≥37 weeks], late preterm [32 – <37 weeks], very preterm [28 – <32 weeks], and extremely preterm [<28 weeks]),27 birth order, cesarean delivery, pregnancy-related complications (hypertension, preeclampsia, and gestational diabetes mellitus), parental allergic diseases, parental ethnicity, parental smoking status, parental education, and household income. To evaluate potential effect modification, we performed subgroup analysis, stratified by child's sex, birth order, and ETS exposure during pregnancy, separately. P-values less than 0.05 were considered statistically significant. We used the statistical program SPSS 22.0 (IBM Corp., Armonk, NY, USA) for data analysis.
Results
Subject characteristics by maternal age at delivery
A total of 1344 children were included in this study. The mean age was 6.4 years (standard deviation [SD]: 0.4) and 56.8% of participants were male. The prevalence of physician-diagnosed allergic rhinitis, ever rhinitis, current rhinitis, and allergic sensitization was 59.0%, 68.1%, 65.7%, and 65.1%, respectively. With increasing maternal age at delivery, participants tended to have higher proportions of cesarean delivery, higher parental education level and higher household income, and lower proportions of being a first-born child and ETS exposure during pregnancy (Table S1).
Association between maternal age at delivery and allergic rhinitis
Table 1 shows the characteristics of the study participants grouped by allergic rhinitis status. Children with allergic rhinitis had higher proportions of being born to mothers aged 40 years and above, male, being a first-born child, and parental allergic diseases, compared to those without allergic rhinitis.
Table 1.
Characteristics of study participants by allergic rhinitis.
| Characteristic | All (n = 1344) | Allergic rhinitis |
Pa | ||||
|---|---|---|---|---|---|---|---|
| Yes (n = 793) | No (n = 551) | ||||||
| Maternal age at delivery, n (%) | 0.027 | ||||||
| <25 years | 48 | (3.6) | 26 | (3.3) | 22 | (4.0) | |
| 25–29 years | 337 | (25.1) | 204 | (25.7) | 133 | (24.1) | |
| 30–34 years | 654 | (48.7) | 392 | (49.4) | 262 | (47.5) | |
| 35–39 years | 262 | (19.5) | 138 | (17.4) | 124 | (22.5) | |
| ≥40 years | 43 | (3.2) | 33 | (4.2) | 10 | (1.8) | |
| Age, year (mean ± SD) | 6.4 ± 0.4 | 6.5 ± 0.4 | 6.4 ± 0.4 | 0.127 | |||
| Male, n (%) | 763 | (56.8) | 468 | (59.0) | 295 | (53.5) | 0.046 |
| Prematurityb, n (%) | 0.334 | ||||||
| Term | 1139 | (84.7) | 671 | (84.6) | 468 | (84.9) | |
| Late preterm | 156 | (11.6) | 96 | (12.1) | 60 | (10.9) | |
| Very preterm | 11 | (0.8) | 8 | (1.0) | 3 | (0.5) | |
| Extremely preterm | 38 | (2.8) | 18 | (2.3) | 20 | (3.6) | |
| Birth order, n (%) | <0.001 | ||||||
| First | 752 | (56.0) | 487 | (61.4) | 265 | (48.1) | |
| Second | 481 | (35.8) | 259 | (32.7) | 222 | (40.3) | |
| Third or later | 111 | (8.3) | 47 | (5.9) | 64 | (11.6) | |
| Cesarean delivery, n (%) | 479 | (35.6) | 278 | (35.1) | 201 | (36.5) | 0.592 |
| Pregnancy-related complications, n (%) | 83 | (6.2) | 48 | (6.1) | 35 | (6.4) | 0.823 |
| Maternal allergic diseases, n (%) | 613 | (45.9) | 424 | (53.9) | 189 | (34.4) | <0.001 |
| Paternal allergic diseases, n (%) | 683 | (51.0) | 446 | (56.6) | 237 | (43.1) | <0.001 |
| Maternal ethnicity, n (%) | 0.504 | ||||||
| Chinese | 1318 | (98.1) | 776 | (97.9) | 542 | (98.4) | |
| Non-Chinese | 26 | (1.9) | 17 | (2.1) | 9 | (1.6) | |
| Paternal ethnicity, n (%) | 0.714 | ||||||
| Chinese | 1340 | (99.7) | 791 | (99.7) | 549 | (99.6) | |
| Non-Chinese | 4 | (0.3) | 2 | (0.3) | 2 | (0.4) | |
| Maternal smoking, n (%) | 44 | (3.3) | 27 | (3.4) | 17 | (3.1) | 0.751 |
| Paternal smoking, n (%) | 401 | (29.8) | 232 | (29.3) | 169 | (30.7) | 0.577 |
| ETS exposure during pregnancy, n (%) | 547 | (41.7) | 314 | (40.7) | 233 | (43.1) | 0.397 |
| Maternal university education, n (%) | 1075 | (80.3) | 639 | (80.9) | 436 | (79.4) | 0.506 |
| Paternal university education, n (%) | 1013 | (76.8) | 607 | (78.3) | 406 | (74.6) | 0.118 |
| Household income per year, n (%) | 0.395 | ||||||
| <300,000 NTD | 43 | (3.2) | 20 | (2.5) | 23 | (4.2) | |
| 300,000~600,000 NTD | 221 | (16.6) | 125 | (15.9) | 96 | (17.6) | |
| 600,000~900,000 NTD | 291 | (21.8) | 178 | (22.6) | 113 | (20.7) | |
| 900,000~1,200,000 NTD | 371 | (27.8) | 223 | (28.3) | 148 | (27.1) | |
| >1,200,000 NTD | 408 | (30.6) | 242 | (30.7) | 166 | (30.4) | |
n: number, SD: standard deviation, NTD: New Taiwan Dollar.
P value is obtained from ANOVA test (continuous variables) or chi-squared test (categorical variables). P < 0.05 is bold.
Classified based on gestational age: term (≥37 weeks), late preterm (32 – <37 weeks), very preterm (28 – <32 weeks), and extremely preterm (<28 weeks).
A significant positive association was found between advanced maternal age at delivery (≥40 years) and physician-diagnosed allergic rhinitis (adjusted odds ratio [AOR] = 4.58, 95% confidence interval [CI]: 1.90–11.03), after adjusting for child's age, sex, prematurity, birth order, cesarean delivery, pregnancy-related complications, parental allergic diseases, parental ethnicity, parental smoking status, parental education, and household income (Table 2). Maternal age ≥40 years was also associated with a significantly increased odds of physician-diagnosed allergic rhinitis (AOR = 5.24, 95% CI: 2.14–12.81) compared to maternal age 35–39 years. In the model with maternal age at delivery treated as a continuous variable, every five year increase in maternal age was associated with a 18% increase (AOR = 1.18, 95% CI: 1.01–1.39; P_trend = 0.045) in odds of physician-diagnosed allergic rhinitis.
Table 2.
Associations of maternal age at delivery with allergic rhinitis and rhinitis symptoms in offspring.
| Outcome | Maternal age at delivery, years | AOR (95% CI)a | P |
|---|---|---|---|
| Allergic rhinitis | <25 | 0.56 (0.28–1.10) | 0.093 |
| 25–29 | 0.91 (0.68–1.23) | 0.559 | |
| 30–34 | Reference | – | |
| 35–39 | 0.88 (0.64–1.20) | 0.406 | |
| ≥40 | 4.58 (1.90–11.03) | 0.001 | |
| per 5-year continuous increase | 1.18 (1.01–1.39) | 0.045 | |
| Ever rhinitis | <25 | 0.58 (0.29–1.18) | 0.133 |
| 25–29 | 0.92 (0.67–1.27) | 0.627 | |
| 30–34 | Reference | – | |
| 35–39 | 0.86 (0.62–1.19) | 0.358 | |
| ≥40 | 3.62 (1.43–9.13) | 0.006 | |
| per 5-year continuous increase | 1.12 (0.94–1.32) | 0.205 | |
| Current rhinitis | <25 | 0.62 (0.30–1.25) | 0.180 |
| 25–29 | 0.87 (0.64–1.19) | 0.381 | |
| 30–34 | Reference | – | |
| 35–39 | 0.87 (0.63–1.21) | 0.405 | |
| ≥40 | 2.26 (1.01–5.08) | 0.049 | |
| per 5-year continuous increase | 1.10 (0.93–1.30) | 0.250 | |
| Allergic sensitization | <25 | 1.03 (0.50–2.12) | 0.946 |
| 25–29 | 0.82 (0.61–1.12) | 0.209 | |
| 30–34 | Reference | – | |
| 35–39 | 1.09 (0.79–1.52) | 0.594 | |
| ≥40 | 2.86 (1.13–7.22) | 0.026 | |
| per 5-year continuous increase | 1.16 (0.98–1.37) | 0.081 |
AOR: adjusted odds ratio, CI: confidence interval.
Covariates included in the adjusted models are child's age, sex, prematurity, birth order, cesarean delivery, pregnancy-related complications, parental allergic diseases, parental ethnicity, parental smoking status, parental education, and household income. P < 0.05 is bold.
Consistent associations were observed between advanced maternal age at delivery and symptoms of allergic rhinitis (AOR = 3.62, 95% CI: 1.43–9.13 for ever rhinitis and AOR = 2.26, 95% CI: 1.01–5.08 for current rhinitis) (Table 2). The relationships between covariates and physician-diagnosed allergic rhinitis were shown in Table 3. Age, male, first-born child, and parental allergic diseases were significantly associated with physician-diagnosed allergic rhinitis (Table 3).
Table 3.
Subject's characteristics and perinatal factors in relation to allergic rhinitis.
| Characteristic | AOR (95% CI)a | P | |
|---|---|---|---|
| Child's age | 1.39 | (1.04–1.86) | 0.027 |
| Sex | |||
| Female | Reference | – | |
| Male | 1.27 | (1.00–1.61) | 0.046 |
| Prematurityb | |||
| Term | Reference | – | |
| Late preterm | 1.15 | (0.79–1.68) | 0.475 |
| Very preterm | 2.66 | (0.66–10.74) | 0.170 |
| Extremely preterm | 0.62 | (0.31–1.26) | 0.185 |
| Birth order | |||
| First | Reference | – | |
| Second | 0.59 | (0.46–0.76) | <0.001 |
| Third or later | 0.41 | (0.27–0.65) | <0.001 |
| Cesarean delivery | 0.92 | (0.71–1.18) | 0.504 |
| Pregnancy-related complications | 1.03 | (0.62–1.73) | 0.904 |
| Maternal allergic diseases | 2.24 | (1.76–2.85) | <0.001 |
| Paternal allergic diseases | 1.63 | (1.29–2.06) | <0.001 |
| Maternal ethnicity | |||
| Chinese | Reference | – | |
| Non-Chinese | 2.08 | (0.78–5.58) | 0.145 |
| Paternal ethnicity | |||
| Chinese | Reference | – | |
| Non-Chinese | 0.35 | (0.04–3.01) | 0.336 |
| Maternal smoking | 1.24 | (0.58–2.65) | 0.572 |
| Paternal smoking | 1.02 | (0.77–1.34) | 0.914 |
| Maternal university education | 0.73 | (0.50–1.04) | 0.084 |
| Paternal university education | 1.13 | (0.81–1.57) | 0.490 |
| Household income per year | |||
| <300,000 NTD | 0.56 | (0.27–1.18) | 0.129 |
| 300,000~600,000 NTD | 0.78 | (0.53–1.15) | 0.208 |
| 600,000~900,000 NTD | Reference | – | |
| 900,000~1,200,000 NTD | 0.86 | (0.61–1.20) | 0.364 |
| >1,200,000 NTD | 0.84 | (0.60–1.20) | 0.340 |
The model included maternal age at delivery, child's age, sex, prematurity, birth order, cesarean delivery, pregnancy-related complications, parental allergic diseases, parental ethnicity, parental smoking status, parental education, and household income. P < 0.05 is bold.
Classified based on gestational age: term (≥37 weeks), late preterm (32 – <37 weeks), very preterm (28 – <32 weeks), and extremely preterm (<28 weeks).
We further performed stratified analyses to evaluate whether the detrimental effects of advanced maternal age at delivery on allergic rhinitis differed by child's sex, birth order, or ETS exposure during pregnancy. Results from sex-stratified analysis showed that the positive association of advanced maternal age at delivery with risk of allergic rhinitis was statistically significant in female offspring (AOR = 7.02, 95% CI: 1.89–26.14), but only marginally significant in male counterpart (AOR = 2.90, 95% CI: 0.85–9.86) (Table 4). Stratified analyses by birth order or ETS exposure during pregnancy did not reveal significant differences.
Table 4.
Associations of maternal age at delivery with allergic rhinitis in offspring, stratified by sex, birth order, and environmental tobacco smoke (ETS) exposure during pregnancy.
| Maternal age at delivery, years | n (%)a | AOR | (95% CI)b | P | n (%)a | AOR | (95% CI)b | P |
|---|---|---|---|---|---|---|---|---|
|
Female (n = 581) |
Male (n = 763) |
|||||||
| <25 | 13 (56.5) | 0.93 | (0.31–2.83) | 0.903 | 13 (52.0) | 0.44 | (0.17–1.14) | 0.091 |
| 25–29 | 75 (54.7) | 0.77 | (0.48–1.22) | 0.268 | 129 (64.5) | 0.99 | (0.66–1.48) | 0.950 |
| 30–34 | 162 (55.5) | Reference | – | 230 (63.5) | Reference | – | ||
| 35–39 | 57 (53.3) | 1.15 | (0.70–1.89) | 0.582 | 81 (52.3) | 0.71 | (0.46–1.09) | 0.117 |
| ≥40 |
18 (81.8) |
7.02 |
(1.89–26.14) |
0.004 |
15 (71.4) |
2.90 |
(0.85–9.86) |
0.089 |
|
First-born child (n = 752) |
Second or later child (n = 592) |
|||||||
| <25 | 24 (57.1) | 0.54 | (0.29–1.47) | 0.132 | 2 (33.3) | 0.31 | (0.04–2.20) | 0.241 |
| 25–29 | 158 (66.4) | 0.98 | (0.71–1.53) | 0.928 | 46 (46.5) | 0.75 | (0.45–1.26) | 0.284 |
| 30–34 | 236 (64.8) | Reference | – | 156 (53.8) | Reference | – | ||
| 35–39 | 56 (59.6) | 0.91 | (0.62–1.72) | 0.723 | 82 (48.8) | 0.82 | (0.54–1.26) | 0.369 |
| ≥40 |
13 (92.9) |
8.62 |
(1.07–69.46) |
0.043 |
20 (69.0) |
4.12 |
(1.44–11.79) |
0.008 |
|
ETS exposure during pregnancy (n = 547) |
No ETS exposure during pregnancy (n = 765) |
|||||||
| <25 | 15 (51.7) | 0.51 | (0.20–1.32) | 0.164 | 9 (56.3) | 0.50 | (0.15–1.62) | 0.247 |
| 25–29 | 102 (62.2) | 1.06 | (0.66–1.70) | 0.810 | 96 (59.3) | 0.84 | (0.55–1.26) | 0.393 |
| 30–34 | 139 (57.9) | Reference | – | 247 (60.8) | Reference | – | ||
| 35–39 | 46 (47.9) | 0.76 | (0.44–1.32) | 0.324 | 85 (54.1) | 0.88 | (0.58–1.32) | 0.529 |
| ≥40 | 12 (66.7) | 4.32 | (1.18–15.75) | 0.027 | 20 (83.3) | 6.57 | (1.77–24.33) | 0.005 |
AOR: adjusted odds ratio, CI: confidence interval. ETS: environmental tobacco smoke.
n (%) refers to the numbers (%) of subjects with physician-diagnosed allergic rhinitis.
Covariates included in the adjusted models are child's age, sex, prematurity, birth order, cesarean delivery, pregnancy-related complications, parental allergic diseases, parental ethnicity, parental smoking status, parental education, and household income. P < 0.05 is bold.
Association between maternal age at delivery and allergic sensitization
In addition to allergic rhinitis and related symptoms, we also investigated the relationship between maternal age at delivery and allergic sensitization in 1294 children receiving measurement of serum allergen-specific IgE. Table 2 indicates that advanced maternal age at delivery was significantly associated with allergic sensitization in offspring (AOR = 2.86, 95% CI: 1.13–7.22). There was an estimated 16% increase (AOR = 1.16, 95% CI: 0.98–1.37; P_trend = 0.081) in odds of allergic sensitization per 5-year increase in maternal age at delivery, which was marginally significant (Table 2).
Discussion
This study of 1344 Asian children in a prospective population-based cohort is one of the first to demonstrate that advanced maternal age at delivery, specifically age equal to or over 40 years, is significantly associated with allergic rhinitis in offspring. The results remain significant after adjusting for a range of factors, including child's age, sex, prematurity, birth order, cesarean delivery, pregnancy-related complications, parental allergic diseases, parental ethnicity, parental smoking status, parental education and household income. This study further adds new evidence that the detrimental effect of advanced maternal age at delivery on allergic rhinitis is more pronounced among female offspring. This study also provides supportive evidence that advanced maternal age at delivery is positively associated with allergic sensitization (measured objectively by allergen-specific IgE).
Our findings are in line with some previous studies. In a cross-sectional study of children aged 4–6 years in China, Wang et al showed that maternal age over 30 years was significantly associated with increased risk of allergic rhinitis in offspring.18 In a retrospective cohort study of young adult men in Sweden, Bråbäck et al found that young maternal age (<29 years) was significantly related to decreased risk of allergic rhinitis.19 Similarly, Strachan et al also observed a protective effect of maternal youth (<30 years) on hay fever in offspring.20,21 In contrast, a population-based cohort study of children aged 4 years in Norway reported a decrease in the risk of allergic rhinitis with increasing maternal age.22 No association between maternal age at delivery and allergic rhinitis in children and adolescents was reported by other studies.23,24 These inconsistent findings across different studies may reflect ethnic differences in the relationship between maternal age and development of allergic rhinitis in offspring.
Our study provides an objective evidence that advanced maternal age at delivery was associated with allergic sensitization, which has been recognized as an important risk factor to the development of allergic rhinitis.28 None of previous studies evaluating the relationship between maternal age at delivery and allergic rhinitis conducted objective measures of IgE-mediated allergy (skin testing and/or serum allergen-specific IgE). In a prospective cohort study of 184 atopic mothers and their neonates, maternal age was found to be positively associated with higher neonatal total serum IgE levels, a well-established predictive factor of later allergic sensitization.29 To the best of our knowledge, there is lack of direct evidence supporting the relationship between maternal age at delivery and allergic sensitization until the current study. The association of advanced maternal age at delivery with both allergic rhinitis and allergic sensitization found in the current study implies that the impact of maternal age on the development of allergic rhinitis in offspring may be mediated through IgE-associated mechanisms.
It is interesting to note the sex difference in the relationship between advanced maternal age at delivery and allergic rhinitis, suggesting that girls may be more vulnerable to the detrimental effect of advanced maternal age at delivery on allergic rhinitis, compared to their counterpart. In a multinational cohort study in Europe, Gomez et al have documented that advance maternal age at delivery may affect offspring health in a sex-specific manner.14 In particular, advance maternal age at delivery is related to better lung function and lower risk of asthma in adult offspring, but only among females.14 The underlying biological mechanisms related to the observed sex-specific effects are largely unclear and worth further investigation.
Our findings were in line with previous studies that reported having older siblings as a protective factor against allergic rhinitis.30 It can be argued that the potential confounding effect of older siblings, which are more common among births to older mothers, would mask or dilute the increased risk of allergic rhinitis in children born to mothers with advanced age at delivery. It is therefore reassuring to note that the associations between advance maternal age at delivery and allergic rhinitis are remarkably consistent among children with and without older sibling(s).
It remains possible that maternal age at delivery influencing allergic rhinitis in offspring may be due to other factors, such as prematurity, birth order, mode of delivery, pregnancy complications, ethnicity, or socio-economic status. However, our study demonstrates that the link between maternal age at delivery and allergic rhinitis in offspring remains statistically significant after adjustment of the above factors, suggesting that maternal age at delivery may independently, at least partly, affect the development of allergic rhinitis in offspring. Previous studies have suggested that maternal age at delivery may influence the development and health outcomes of their offspring through affecting cytokine levels in maternal amniotic fluid31 and epigenetic signature in offspring,32 which we speculate might also influence the subsequent development of allergic rhinitis. Further studies are required to elucidate the biological mechanisms behind the link between maternal age at delivery and allergic rhinitis. Our study demonstrated that the detrimental effect of maternal aging on allergic rhinitis was mainly confined to the upper extreme of reproductive age, rather than an obvious linear dose-response relationship. Previous studies reported a similar phenomenon of more adverse pregnancy outcomes in extremes of reproductive age,12,33 although the exact mechanisms remain unknown.
The low proportion of births to mothers under 25 years of age reflected the recent demographic transition toward increasing maternal age at delivery in Taiwan. It is interesting to note the relatively lower risk of allergic rhinitis in offspring born to mothers aged less than 25 years, although the association was only marginally significant due to the small sample size in this age group. Taken together with the detrimental effect of advanced maternal age at delivery on allergic rhinitis, the recent rising prevalence of allergic rhinitis in Taiwan may be partly contributed by both detrimental effect from more births to women of advanced age and loss of protective effect from fewer births to women younger than age 25.
The present study has several strengths. First, the study was conducted in a large population-based cohort of Asian children with measurement of serum allergen-specific IgE as an objective marker of allergic sensitization. Second, the information of maternal age at delivery and perinatal data were obtained directly from EMR in the hospital instead of self-reported data, avoiding potential recall bias. Third, the diagnosis of allergic rhinitis was reliable since the information was evaluated and confirmed by pediatricians during an interview. This study has some potential limitations. First, the study cohort only included an Asian pediatric population. Whether the results can be generalized to other ethnic populations remains to be confirmed. Second, although several pertinent factors have been controlled and adjusted in the analyses, it remains possible that the observed increased risk might be partially explained by unmeasured confounding factors. Third, we did not have information on cytokine levels or epigenetic signature, which might be varied by different maternal age at delivery. Fourth, the small sample sizes for maternal subjects in the two extreme age groups have resulted in the wide 95% confidence intervals. Further studies with larger sample sizes are needed before firm conclusions can be drawn.
Conclusion
This large prospective population-based cohort study of Asian children demonstrates an association of advanced maternal age at delivery with increased risk of allergic rhinitis in Asian children, probably more pronounced among girls. It might be worth paying particular attention and administering promising strategies to prevent allergic rhinitis in young children born to mothers of advanced age. Further works are needed to confirm this interpretation.
Funding
This work was supported by grants from the Ministry of Science and Technology of Taiwan (PI: Yao, MOST 106-2314-B-182-051-MY3; PI: Tsai, MOST 107-2314-B-400-031-MY3) and Chang Gung Medical Foundation, Taiwan (PI: Yao, CMRPG3F1711~3F1713, CMRPG3E1201~3E1205, CORPG3F0081~3F0083, CORPG3F0361, and CMRPG3J0121).
Author contributions
H-Y. Lu performed data analysis, interpreted the results, and drafted the manuscript. C-W. Chiu and H-Y. Huang assisted in data analysis and interpretation. P-H. Kao, Z-T. Tsai, C-C. Gau, W-F. Lee, C-Y. Wu, Y-T. Lan, C-C. Hung, F-Y. Chang, Y-W. Huang, H-Y. Huang, J. Chang-Chien contributed to participant recruitment, cohort maintenance, and data collection. H-J. Tsai provided thoughtful input in study design and interpretation of the results and participated in drafting and critically revising the manuscript. T-C. Yao conceptualized, designed, and supervised the study, raised funding for the study, interpreted results, and drafted the manuscript. All authors contributed to the interpretation and discussion of the results, and read and approved the final manuscript.
Ethics statement
The study was approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 201600334A3). Written informed consent was provided by the parent or legal guardian of each participant. The procedures followed in this study were in accordance with the ethical standards of The Institutional Review Boards of Chang Gung Memorial Hospital and with the Helsinki Declaration of 1975, as revised in 1983.
Consent for publication
All authors consented to the publication of this work.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, T-C. Yao, upon reasonable request.
Submission declaration
We confirm that this manuscript has not been submitted or is not simultaneously being submitted elsewhere, and that no portion of the data has been or will be published in proceedings or transactions of meetings or symposium volumes.
Acknowledgements
Members of the LIGHTS study group are: Tsung-Chieh Yao (Principle Investigator), Hui-Ju Tsai (Co-Principle Investigator), Chao-Yi Wu, Hung-Yi Lu, Chun-Chun Gau, Wan-Fang Lee, Li-Lun Lin, Shu-Jung Huang, Yu-Tung Lan, Chung-Chieh Hung, I-Chun Lu, Fang-Yu Chang, Chun-Hui Chu, Chi-Yen Hung, Kun-Lin Lu, Yu-Wen Huang, Yin-Shan Huang, Ching-Hua Lin, Hsin Fang, Zhao-Ting Tsai, Po-Hsiang Kao, Cha-Shien Yen, Yen-Ju Shen, Chi-Wei Chiu, Tzu-Hsiang Weng, Chia-Hua Ho, Chi-Wen Huang, Yu-Tang Juan, Ju Chang-Chien, Hsin-Yi Huang, Yu-Lung Tseng, Wan-Chin Lin, and Shih-Ling Wang. The authors thank the study participants and their parents for their active participation in the study.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100127.
Contributor Information
Hui-Ju Tsai, Email: tsaihj@nhri.org.tw.
Tsung-Chieh Yao, Email: yao@adm.cgmh.org.tw.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Asher M.I., Montefort S., Björkstén B. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Brozek G., Lawson J., Szumilas D., Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: four repeated surveys from 1993-2014. Respir Med. 2015;109:982–990. doi: 10.1016/j.rmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Yao T.C., Ou L.S., Yeh K.W., Lee W.I., Chen L.C., Huang J.L. Associations of age, gender, and BMI with prevalence of allergic diseases in children: PATCH study. J Asthma. 2011;48:503–510. doi: 10.3109/02770903.2011.576743. [DOI] [PubMed] [Google Scholar]
- 4.van Oene C.M., van Reij E.J., Sprangers M.A., Fokkens W.J. Quality-assessment of disease-specific quality of life questionnaires for rhinitis and rhinosinusitis: a systematic review. Allergy. 2007;62:1359–1371. doi: 10.1111/j.1398-9995.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer E.O., Bukstein D.A. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106:S12–S16. doi: 10.1016/j.anai.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Yao T.C., Chang S.W., Chang W.C. Exposure to tobacco smoke and childhood rhinitis: a population-based study. Sci Rep. 2017;7:42836. doi: 10.1038/srep42836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong S.N., Chew F.T. Epidemiology of allergic rhinitis and associated risk factors in Asia. World Allergy Org J. 2018;11 doi: 10.1186/s40413-018-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyama K., Pinto R., Ray J.G. Association of maternal age with severe maternal morbidity and mortality in Canada. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saloojee H., Coovadia H. Maternal age matters: for a lifetime, or longer. Lancet Glob Health. 2015;3:e342–e343. doi: 10.1016/S2214-109X(15)00034-0. [DOI] [PubMed] [Google Scholar]
- 10.Martin J.A., Hamilton B.E., Osterman M.J.K., Driscoll A.K., Drake P. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1–55. [PubMed] [Google Scholar]
- 11.Kanmaz A.G., Inan A.H., Beyan E., Ogur S., Budak A. Effect of advanced maternal age on pregnancy outcomes: a single-centre data from a tertiary healthcare hospital. J Obstet Gynaecol. 2019;39:1104–1111. doi: 10.1080/01443615.2019.1606172. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh T.T., Liou J.D., Hsu J.J., Lo L.M., Chen S.F., Hung T.H. Advanced maternal age and adverse perinatal outcomes in an Asian population. Eur J Obstet Gynecol Reprod Biol. 2010;148:21–26. doi: 10.1016/j.ejogrb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Dioun A.F., Harris S.K., Hibberd P.L. Is maternal age at delivery related to childhood food allergy? Pediatr Allergy Immunol. 2003;14:307–311. doi: 10.1034/j.1399-3038.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 14.Gomez Real F., Burgess J.A., Villani S. Maternal age at delivery, lung function and asthma in offspring: a population-based survey. Eur Respir J. 2018;51 doi: 10.1183/13993003.01611-2016. [DOI] [PubMed] [Google Scholar]
- 15.Abid Z., Oh S.S., Hu D. Maternal age and asthma in Latino populations. Clin Exp Allergy. 2016;46:1398–1406. doi: 10.1111/cea.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laerum B.N., Svanes C., Wentzel-Larsen T. Young maternal age at delivery is associated with asthma in adult offspring. Respir Med. 2007;101:1431–1438. doi: 10.1016/j.rmed.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Metsala J., Kilkkinen A., Kaila M. Perinatal factors and the risk of asthma in childhood-a population-based register study in Finland. Am J Epidemiol. 2008;168:170–178. doi: 10.1093/aje/kwn105. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Liu W., Hu Y., Zou Z., Shen L., Huang C. Home environment, lifestyles behaviors, and rhinitis in childhood. Int J Hyg Environ Health. 2016;219:220–231. doi: 10.1016/j.ijheh.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Bråbäck L., Hedberg A. Perinatal risk factors for atopic disease in conscripts. Clin Exp Allergy. 1998;28:936–942. doi: 10.1046/j.1365-2222.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 20.Strachan D.P., Taylor E.M., Carpenter R.G. Family structure, neonatal infection, and hay fever in adolescence. Arch Dis Child. 1996;74:422–426. doi: 10.1136/adc.74.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strachan D.P. Epidemiology of hay fever: towards a community diagnosis. Clin Exp Allergy. 1995;25:296–303. doi: 10.1111/j.1365-2222.1995.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 22.Nafstad P., Magnus P., Jaakkola J.J. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. J Allergy Clin Immunol. 2000;106:867–873. doi: 10.1067/mai.2000.110558. [DOI] [PubMed] [Google Scholar]
- 23.Miyake Y., Yura A., Iki M. Cross-sectional study of allergic disorders in relation to familial factors in Japanese adolescents. Acta Paediatr. 2004;93:380–385. doi: 10.1080/08035250410022819. [DOI] [PubMed] [Google Scholar]
- 24.Gerlich J., Benecke N., Peters-Weist A.S. Pregnancy and perinatal conditions and atopic disease prevalence in childhood and adulthood. Allergy. 2018;73:1064–1074. doi: 10.1111/all.13372. [DOI] [PubMed] [Google Scholar]
- 25.Brozek J.L., Bousquet J., Agache I. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Ballardini N., Nilsson C., Nilsson M., Lilja G. ImmunoCAP Phadiatop Infant-a new blood test for detecting IgE sensitisation in children at 2 years of age. Allergy. 2006;61:337–343. doi: 10.1111/j.1398-9995.2005.00936.x. [DOI] [PubMed] [Google Scholar]
- 27.Quinn J.A., Munoz F.M., Gonik B. Preterm birth: case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34:6047–6056. doi: 10.1016/j.vaccine.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu C.Y., Huang Y.L., Tsai M.H. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PloS One. 2014;9 doi: 10.1371/journal.pone.0102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Gool C.J., Thijs C., Dagnelie P.C. Determinants of neonatal IgE level: parity, maternal age, birth season and perinatal essential fatty acid status in infants of atopic mothers. Allergy. 2004;59:961–968. doi: 10.1111/j.1398-9995.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 30.McKeever SAL T.M., Smith C., Collins J., Heatlie H., Frischer M., Hubbard R. vol. 56. 2001. pp. 758–762. (Siblings, Multiple Births, and the Incidence of Allergic Disease: A Birth Cohort Study Using the West Midlands General Practice Research Database). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissenbacher T., Laubender R.P., Witkin S.S. Influence of maternal age, gestational age and fetal gender on expression of immune mediators in amniotic fluid. BMC Res Notes. 2012;5:375. doi: 10.1186/1756-0500-5-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markunas C.A., Wilcox A.J., Xu Z. Maternal age at delivery is associated with an epigenetic signature in both newborns and adults. PloS One. 2016;11 doi: 10.1371/journal.pone.0156361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Londero A.P., Rossetti E., Pittini C., Cagnacci A., Driul L. Maternal age and the risk of adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. 2019;19:261. doi: 10.1186/s12884-019-2400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, T-C. Yao, upon reasonable request.


