Abstract
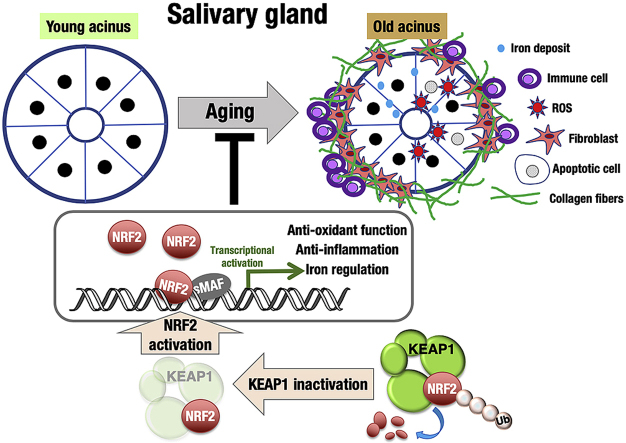
Saliva plays an essential role in the maintenance of oral health. The oral cavity environment changes during aging mainly due to alterations in the secretion and composition of saliva. In particular, unstimulated basal salivary flow decreases with age. The functional decline of the salivary glands impairs chewing and swallowing abilities and often becomes one of the predispositions for aging-related disorders, including aspiration pneumonia. The KEAP1-NRF2 system plays a central role in the regulation of the oxidative stress response. NRF2 is a transcription factor that coordinately regulates cytoprotective genes, and KEAP1 is a negative regulator of NRF2. Although NRF2 activation has been suggested to be advantageous for the prevention of aging-related diseases, its role in the course of physiological aging is not well understood. To investigate the impact of NRF2 activation on salivary gland aging, we compared the submandibular glands of Keap1-knockdown (KD) (Keap1FA/FA) mice in which NRF2 is activated with those of wild-type mice. Young mice did not show any apparent differences between the two genotypes, whereas in old mice, clear differences were observed. Aged wild-type submandibular glands exhibited iron and collagen depositions, immune cell infiltration and increased DNA damage and apoptosis accompanied by elevated oxidative stress, which were all markedly attenuated in Keap1-KD mice, suggesting that NRF2 activation has antiaging effects on salivary glands. We propose that appropriate activation of NRF2 is effective for the maintenance of healthy salivary gland conditions and for the prevention of hyposalivation in the elderly.
Keywords: Salivary glands, KEAP1, NRF2, Aging, Mouse
Graphical abstract
Highlights
-
•
NRF2 pathway activities are similar in young and old submandibular glands.
-
•
Keap1 knockdown increases NRF2 pathway activities in submandibular glands.
-
•
NRF2 activation attenuates oxidative stress increase in old submandibular glands.
-
•
NRF2 activation attenuates aging phenotypes in old submandibular glands.
1. Introduction
Saliva is an important fluid for oral cavity homeostasis and function. Human saliva not only consists of water but also other essential substances, including mucus, antibacterial compounds, electrolytes and various enzymes, thus playing multiple roles in the oral cavity and systemic health [1,2]. A decline in salivary function (hyposalivation) generally occurs in elderly people, often resulting in oral diseases, including dental caries and periodontal disease, which impair chewing and swallowing function, leading to impaired nutritional status. Hyposalivation causes difficulties not only in eating and tasting but also in speaking, seriously affecting quality of life [[3], [4], [5]]. Maintenance of salivary gland function is one of the important requirements for healthy aging.
Salivary gland aging in humans has been described from functional and structural aspects. A meta-analysis comparing saliva flow rates in young and old adults showed that whole saliva flow rates were reduced significantly in the old group [6]. Histological analysis showed an age-related decrease in the proportion of parenchymal tissue versus stromal tissue in salivary glands, which is likely to cause a decline in saliva production in the elderly [[6], [7], [8]]. Effective intervention to delay the progression of salivary gland aging has not been described thus far.
One of the important factors underlying aging is the accumulation of oxidative damage [[9], [10], [11]]. Their alleviation either by decreasing prooxidants or elevating antioxidants or both was able to extend the lifespan [12]. The KEAP1-NRF2 system plays a central role in the antioxidant response and defense against oxidative damage and is well conserved in all vertebrates and some invertebrates, such as flies and worms [13,14]. NRF2 is a potent transcription activator that coordinately regulates many cytoprotective genes, and KEAP1 is a negative regulator of NRF2 under unstressed conditions and is responsible for the inducible activation of NRF2 in response to oxidative and electrophilic stress [[15], [16], [17], [18]]. Although a number of studies have demonstrated that NRF2 activation is beneficial for our health [16,[19], [20], [21], [22], [23]], the impacts of NRF2 activation on physiological aging are not fully understood. The available literature only describes that NRF2 inactivation accelerates the progression of aging-associated phenotypes [[24], [25], [26], [27], [28], [29], [30], [31]]. Whether NRF2 activation counteracts the functional decline and morphological/histological alterations of tissues and organs during physiological aging remains to be elucidated.
To clarify the impact of NRF2 activation on the progression of aging phenotypes of the salivary gland, we exploited Keap1FA/FA mice, a Keap1 knockdown (KD) mouse model [[32], [33], [34]]. Due to the decreased expression of Keap1 from the Keap1FA allele, NRF2 is systemically activated even in unstressed conditions in Keap1 KD mice. In this study, we focused on submandibular glands that make major contributions to resting saliva production. Compared to stimulated saliva, resting saliva, which makes approximately two-thirds of the total saliva, is relevant to oral health and dental integrity. The resting saliva flow rate, reflecting the basal flow present throughout the majority of the day, correlates well with the severity of hyposalivation. Thus, we considered that antiaging interventions to the submandibular glands would be a key to the maintenance of oral health in elderly people. We found that NRF2 pathway activation in Keap1 KD mice effectively protected the submandibular glands from the accumulation of oxidative damage and smoldering inflammation during physiological aging.
2. Material and methods
2.1. Mice
Male wild-type and Keap1 knockdown (Keap1FA/FA) mice on a C57BL/6 genetic background were used in this study. Keap1FA is a floxed allele in which Keap1 expression is decreased [[32], [33], [34]]. Mice were genotyped by PCR using the following primers: Keap1FA forward 5’ - CAG CAG TTA AGG GCA CCA ATG C- 3′, and Keap1FA reverse 5′-CCT GCC TCA GCT TCC CAT CA-3’. All mice were bred and maintained under specific pathogen-free conditions according to the regulations of The Standards for Human Care and Use of Laboratory Animals of Tohoku University and The Guidelines for Proper Conduct of Animal Experiments by The Ministry of Education, Culture, Sports, Science, and Technology of Japan.
2.2. Submandibular gland preparation
Mice were sacrificed at 5 months (young) and 19 or 24 months (old) of age. For paraffin sections, salivary glands were fixed in 4% paraformaldehyde and embedded in paraffin. For frozen sections, salivary glands were fixed for 2 h at 4 °C in mixed fixative solution containing 1% formaldehyde/PBS, 0.2% glutaraldehyde/PBS, and 0.02% NP40/PBS supplemented with 2 mM MgCl2, washed with PBS supplemented with 2 mM MgCl2, soaked overnight at 4 °C in 20% sucrose/PBS supplemented with 2 mM MgCl2, embedded in OCT (Catalog No. 4583., Sakura Finetek Japan Inc, Tokyo, Japan) and kept at −80 °C.
2.3. Histological analysis
Paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E) for routine examination. For visualization of fibrotic tissue deposition, Picrosirius Red staining (Catalog No. 24901., Polysciences Inc, PA, US) was performed. For quantification of fibrosis, we defined fibrotic regions by setting a threshold for the Picrosirius Red staining intensity by using NIH ImageJ software (http://rsb.info.nih.gov/ij/). More precisely, the color image was converted to grayscale image, and the image segmentation method was used for measurement of the fibrotic areas that were above the threshold. The ratio of fibrotic areas against the whole area of the field was calculated. Four to nine representative fields were counted per sample. Three to four mice were analyzed per group. Prussian blue staining was performed to detect iron deposition. For quantification of iron deposition, the observation field (365 μm × 275 μm) was divided into 200 μm2 grids, and the grids with iron deposition were counted by using NIH ImageJ software. Seven to nineteen representative fields were counted per sample. Three to four mice were analyzed per group.
2.4. Immunofluorescence
8-OHdG and 4HNE immunofluorescence staining was accomplished using paraffin sections. As a first step, paraffin was removed using xylene, and afterwards rehydrated in graded alcohol. Antigen retrieval was performed using autoclave (121 °C for 1 min) in citric acid buffer and sodium citrate buffer solution. After cooling and washing steps using PBS, the slides were incubated for 10 min at room temperature in Protein Block Serum-Free Ready-to-use solution (Catalog No. X0909, DAKO, CA, US), for blocking non-specific staining. Primary antibody incubation was performed overnight at 4 °C in blocking solution. A suitable secondary antibody was reacted and counterstained with DAPI (Catalog No. 11034–56., 1:1000, Nacalai Tesque, Kyoto, Japan) in blocking solution for 1 h at room temperature. During the procedure, specimens were protected from light and mounted in Permafluor mountant (Catalog No. TA030FM, Thermo Fisher Scientific, MA, US).
γ-H2AX and CD45 immunofluorescence staining was accomplished using frozen sections. Frozen tissues embedded in OCT were sectioned (14 μm) and mounted on glass slides. The mounted tissues were air-dried overnight at room temperature and fixed in 4% paraformaldehyde for 10 min. Slides were washed with PBS and blocked in Protein Block Serum-Free Ready-to-use solution. From blocking to mounting steps, the same procedures with immunofluorescence using paraffin sections were performed.
Detailed information on the primary and secondary antibodies is provided in Supplementary Table S1.
2.5. TUNEL assay
The TUNEL assay for detecting apoptosis was also performed using paraffin sections with an In Situ Apoptosis Detection Kit (Catalog No. MK500., Takara, Ohtsu, Japan). The staining steps were performed according to a designated protocol provided by the manufacturer. The slides used for the TUNEL assay were counterstained with DAPI (Catalog No. 11034–56., 1:1000, Nacalai Tesque, Kyoto, Japan).
2.6. Image analysis
All histological samples were imaged by a Keyence BZ-9000 fluorescence microscope. Positive cells for γ-H2AX and TUNEL were counted directly in the area of investigation (365 μm × 275 μm) at a magnification of 400×. Five representative areas were counted per slide, using two slides per sample and four samples per group.
2.7. RNA extraction and RT-PCR
Salivary glands were harvested and snap frozen using liquid nitrogen and stored at −80 °C until further analysis. For RNA extraction, salivary glands were homogenized in Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions using Precellys 24 (Bertin Technology, Montigny-le-Bretonneux, France). RNA was reverse transcribed using random primers by Revertra Ace (Toyobo, Osaka, Japan). Quantitative PCR was performed using Thunderbird SYBR (Probe), qPCR mix (Toyobo, Osaka, Japan) and primers using the ABI 7300 system (Applied Biosystems, CA, US). The primers used in the quantitative PCR are described in Supplementary Table S2.
2.8. Statistical analysis
Quantitative data are presented as the means ± standard deviations (s.d.). Student's t-test and two-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test were utilized. For all tests, P values of <0.05 were considered statistically significant.
3. Results
3.1. The expression levels of NRF2 target genes are similar in young and old submandibular glands
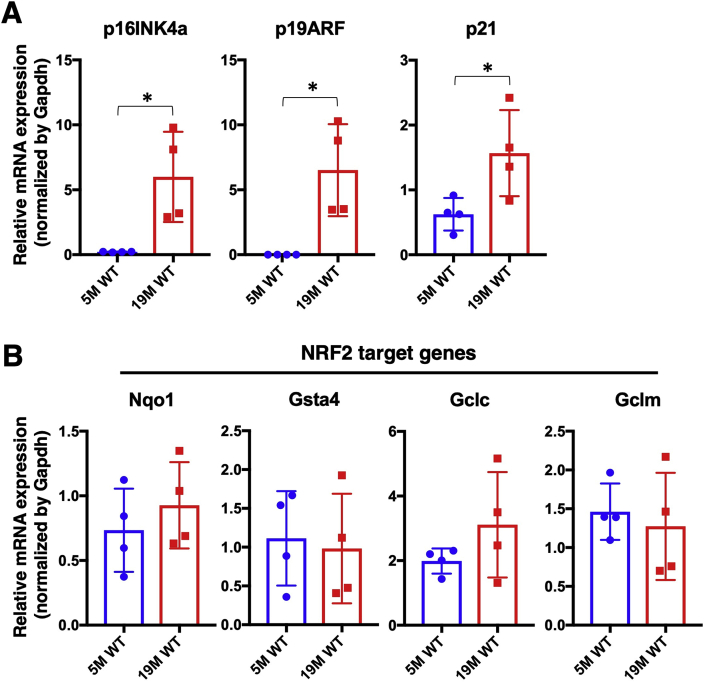
We first determined the appropriate timing for the analysis of submandibular glands during aging by checking the expression levels of the aging marker genes p16INK4a, p19ARF and p21. All three genes were significantly elevated in the submandibular glands of wild-type (WT) mice at 19 months compared with those at 5 months of age (Fig. 1A), suggesting that senescent cells accumulate in the submandibular glands by 19 months. Based on the increased levels of senescence markers, we decided to examine the aging phenotypes of the submandibular glands at 19 months and later.
Fig. 1.
Gene expression in young and old WT submandibular glands.
Expression levels of aging marker genes (A) and NRF2 target genes (B) in the submandibular glands of WT mice at 5 months and 19 months of age. All samples were quantified against the same standard curve, and each expression level was normalized to Gapdh expression. The data represent the mean ± s.d. (n = 4). Unpaired two-tailed Student's t-test was applied. *P < 0.05. 5 M WT; 5 month-old wild type mice, 19 M WT; 19 month-old wild-type mice.
As an initial characterization of the NRF2 pathway during salivary gland aging, we examined the expression levels of four typical NRF2 target genes, namely, Nqo1, Gsta4, Gclc and Gclm, in the submandibular glands of WT mice at 5 and 19 months of age (Fig. 1B). No significant differences were observed between the two groups, suggesting that NRF2 activity is maintained in the submandibular glands during aging for at least 19 months after birth.
3.2. The NRF2 pathway is activated in the submandibular glands of Keap1 KD mice
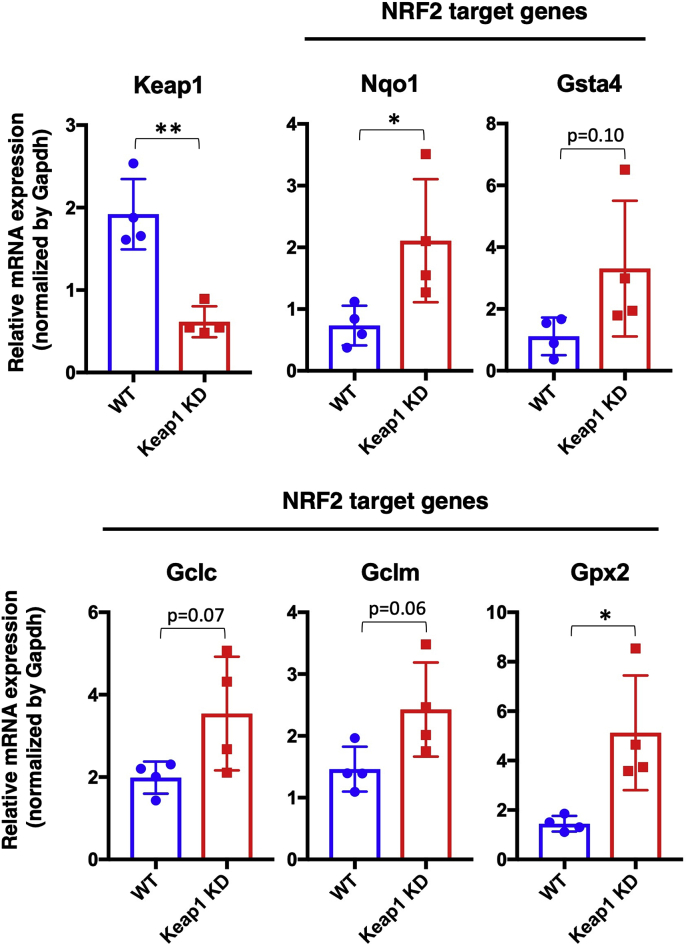
To clarify the impact of NRF2 activation on salivary gland aging, we utilized Keap1 KD mice [32]. We verified the decreased Keap1 expression in the submandibular glands of Keap1 KD mice (Fig. 2). As expected, NRF2 target genes were mostly upregulated in Keap1 KD mice, although the increase in Gsta4, Gclc and Gclm did not reach statistical significance. These results indicate that the NRF2 pathway is activated in Keap1 KD submandibular glands.
Fig. 2.
NRF2 target gene expression in Keap1 KD submandibular glands.
Expression levels of Keap1 and NRF2 target genes in the submandibular glands of WT and Keap1 KD mice at 5 months of age. The samples of WT mice are the same as those shown in Fig. 1B. All samples were quantified against the same standard curve, and each expression level was normalized to Gapdh expression. The data represent the mean ± s.d. (n = 4). Unpaired two-tailed Student's t-test was applied. *P < 0.05, **P < 0.01.
3.3. Keap1 knockdown attenuates the manifestation of aging phenotypes in submandibular glands
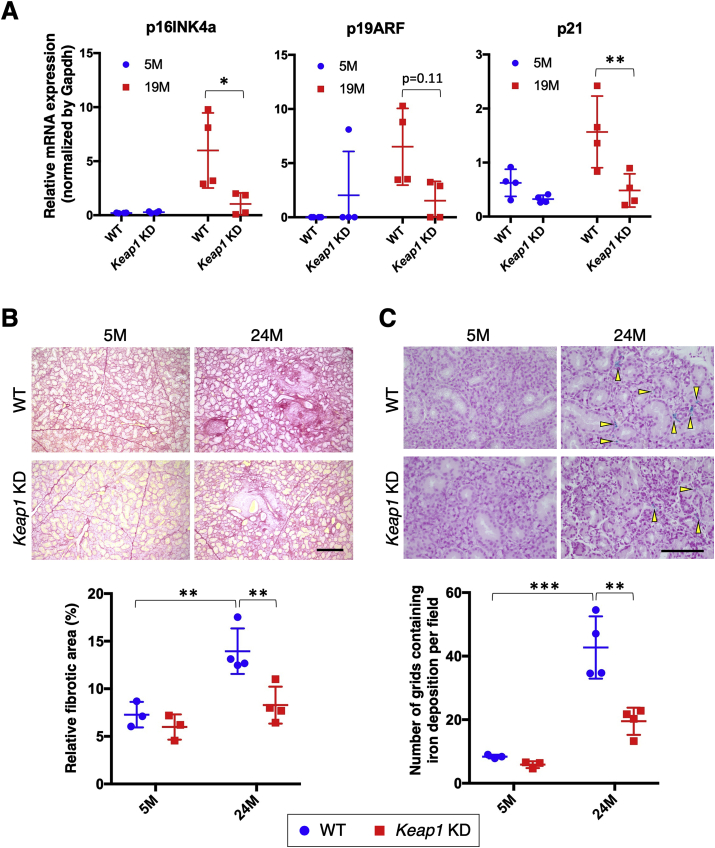
We started analyzing the impacts of NRF2 pathway activation on salivary gland aging from the expression levels of aging marker genes. The three aging marker genes, which were elevated in WT submandibular glands, were all robustly suppressed in Keap1 KD submandibular glands at 19 months of age (Fig. 3A). Although we could not detect cells showing senescence-associated beta-galactosidase activity, which is one of the markers of senescent cells, due to the high background activity in salivary glands (data not shown), this result suggested that the emergence and accumulation of senescent cells were suppressed by NRF2 activation. Consistently, the age-related progression of periductal and perilobular fibrosis and iron deposition in WT submandibular glands were ameliorated in Keap1 KD submandibular glands (Fig. 3B and C). At 5 months, no apparent differences were observed between the two genotypes, whereas age-related alterations by 24 months after birth were clearly less apparent in Keap1 KD mice than in WT mice. Thus, NRF2 pathway activation induced by Keap1 knockdown effectively suppressed the manifestation of aging phenotypes in submandibular glands.
Fig. 3.
Aging phenotypes in the submandibular glands of WT and Keap1 KD mice.
A. Expression levels of aging marker genes. The samples of WT mice are the same as those shown in Fig. 1A. All samples were quantified against the same standard curve, and each expression level was normalized to Gapdh expression. The data represent the mean ± s.d. (n = 4). B. Representative images of Picrosirius Red staining showing collagen deposition (red color) in submandibular glands (top) and ratios of fibrotic areas (bottom). The experiments were performed on three to four samples in each group. A scale bar corresponds to 250 μm. C. Representative images of Prussian blue staining showing iron deposition (yellow arrowheads) in submandibular glands (top) and the number of grids containing iron deposition per field. The experiments were performed on three or four samples in each group. A scale bar corresponds to 100 μm. 5 M; 5 month-old mice, 19 M; 19 month-old mice; 24 M; 24 month-old mice. Two-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test was applied. *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. Keap1 knockdown suppresses smoldering inflammation in old submandibular glands
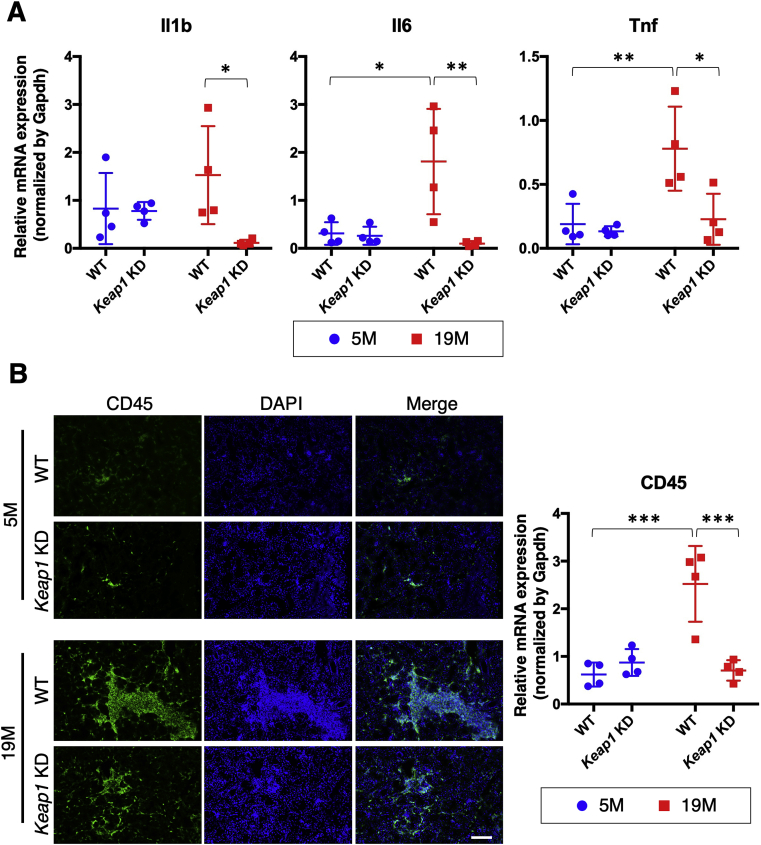
One of the common features of aging status is low-grade chronic smoldering inflammation. Based on previous reports describing that NRF2 has potent anti-inflammatory activity [22,35], we hypothesized that NRF2 activation exerts its antiaging function by suppressing the smoldering inflammation of salivary glands. As we expected, age-related upregulation of the proinflammatory cytokine genes Il1b, Il6 and Tnf in WT submandibular glands was robustly suppressed by Keap1 knockdown (Fig. 4A). To examine the infiltration of inflammatory cells into the submandibular glands, we conducted immunofluorescence analysis using an antibody against CD45, a common marker of myeloid and lymphoid cells (Fig. 4B). At 5 months, very few CD45-positive cells were observed in the submandibular glands of both genotypes. At 19 months, massive accumulation of CD45-positive cells was observed in WT mice, which was markedly suppressed in Keap1 KD mice. These observations suggest that the accumulation of inflammatory cells might be one of the reasons for the elevated expression of proinflammatory cytokine genes in old WT submandibular glands. NRF2 pathway activation indeed suppressed smoldering inflammation in old submandibular glands.
Fig. 4.
Age-dependent chronic inflammation in the submandibular glands of WT and Keap1 KD mice.
A. Expression levels of proinflammatory cytokine genes. All samples were quantified against the same standard curve, and each expression level was normalized to Gapdh expression. The data represent the mean ± s.d. (n = 4). B. Immunofluorescence with CD45 antibody (left) and CD45 mRNA quantification (right). The experiments were performed on four samples in each group. A scale bar corresponds to 100 μm. 5 M; 5 month-old mice, 19 M; 19 month-old mice. Two-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test was applied. *P < 0.05, **P < 0.01, ***P < 0.001.
3.5. Keap1 knockdown prevents DNA damage and cell death in old submandibular glands
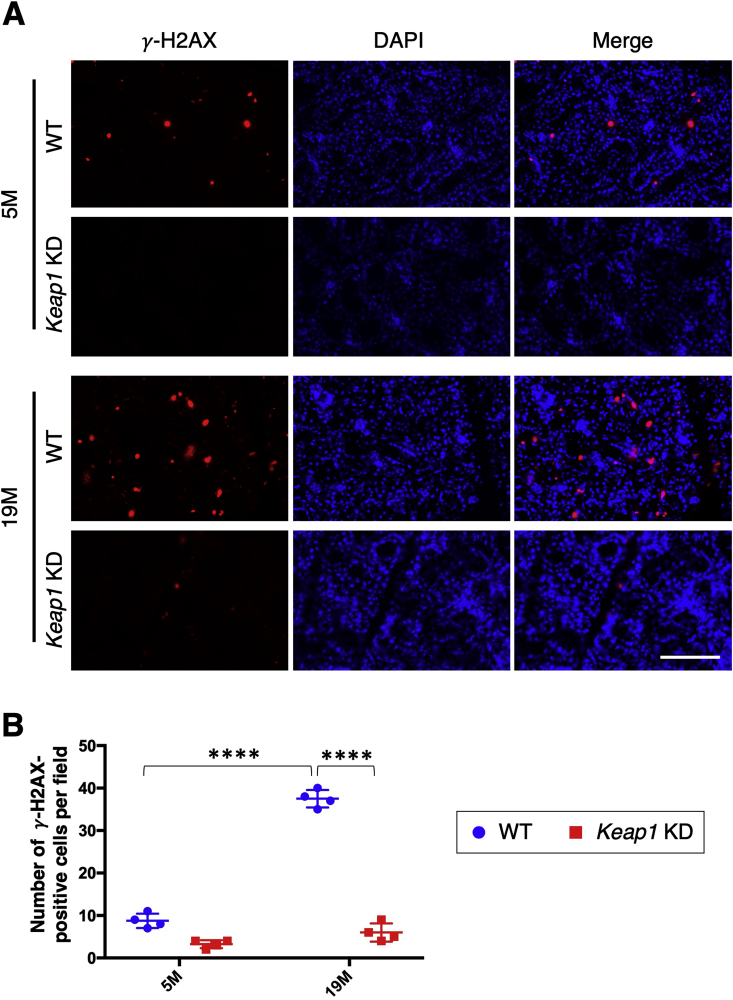
The accumulation of DNA damage is another feature of aging status [[36], [37], [38], [39], [40], [41], [42]]. We conducted immunofluorescence analysis of γ-H2AX, a well-established DNA damage marker, to examine DNA damage accumulation in the submandibular glands during aging (Fig. 5). A small number of γ-H2AX-positive cells were observed in WT mice at 5 months, and their number was dramatically increased at 19 months. Keap1 KD submandibular glands exhibited very few γ-H2AX-positive cells at both young and old stages. These results suggested that NRF2 activation inhibited the age-related accumulation of DNA damage in salivary glands.
Fig. 5.
Detection of γ-H2AX in the submandibular glands of WT and Keap1 KD mice.
A. Immunofluorescence with γ-H2AX antibody. The experiments were performed on four samples in each group. A scale bar corresponds to 100 μm. B. Quantification of γ-H2AX-positive cells. The results are presented as the means ± s.d. Two-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test was applied. ****P < 0.0001. 5 M; 5 month-old mice, 19 M; 19 month-old mice.
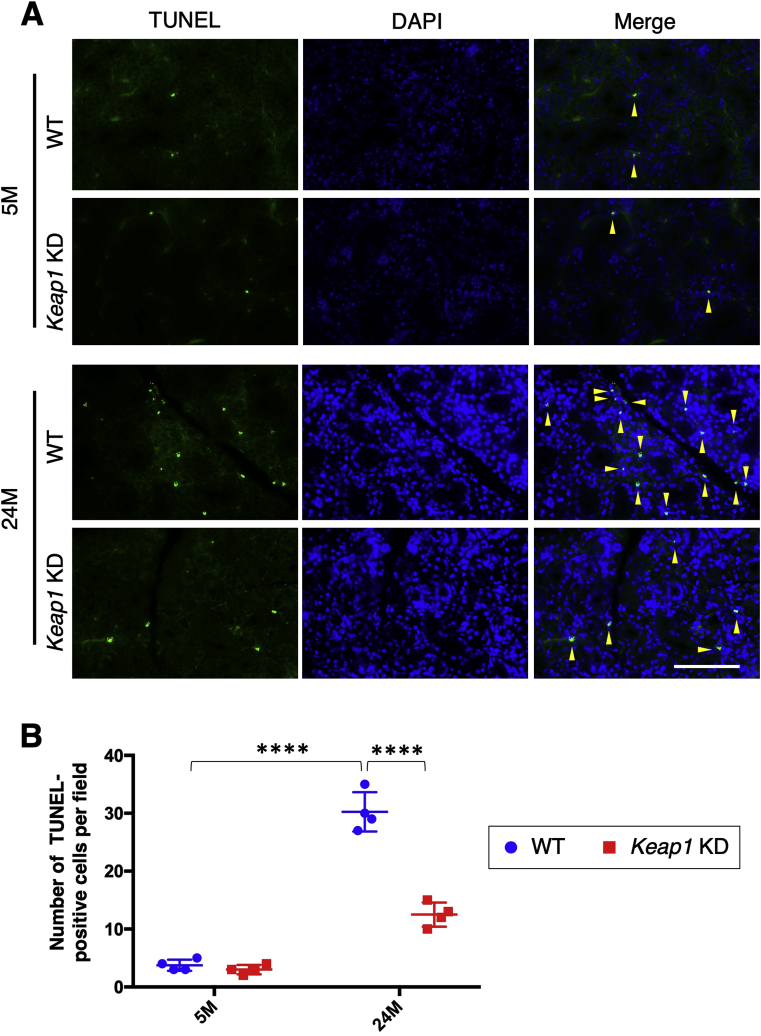
Because DNA damage, especially double strand breaks indicated by γ-H2AX, often results in apoptotic cell death, we conducted a TUNEL assay to detect apoptosis. The increase in TUNEL-positive cells in WT submandibular glands during aging was suppressed in Keap1 KD submandibular glands (Fig. 6). NRF2 activation successfully reduced the age-related accumulation of DNA damage and possibly consequent apoptosis in submandibular glands.
Fig. 6.
Detection of apoptotic cells in the submandibular glands of WT and Keap1 KD mice.
A. TUNEL assay for the detection of apoptotic cells. The experiments were performed on four samples in each group. Yellow arrowheads indicate TUNEL-positive cells. A scale bar corresponds to 100 μm. B. Quantification of TUNEL-positive cells. The results are presented as the means ± s.d. Two-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test was applied. ****P < 0.0001. 5 M; 5 month-old mice, 24 M; 24 month-old mice.
3.6. Keap1 knockdown reduces oxidative stress in old submandibular glands
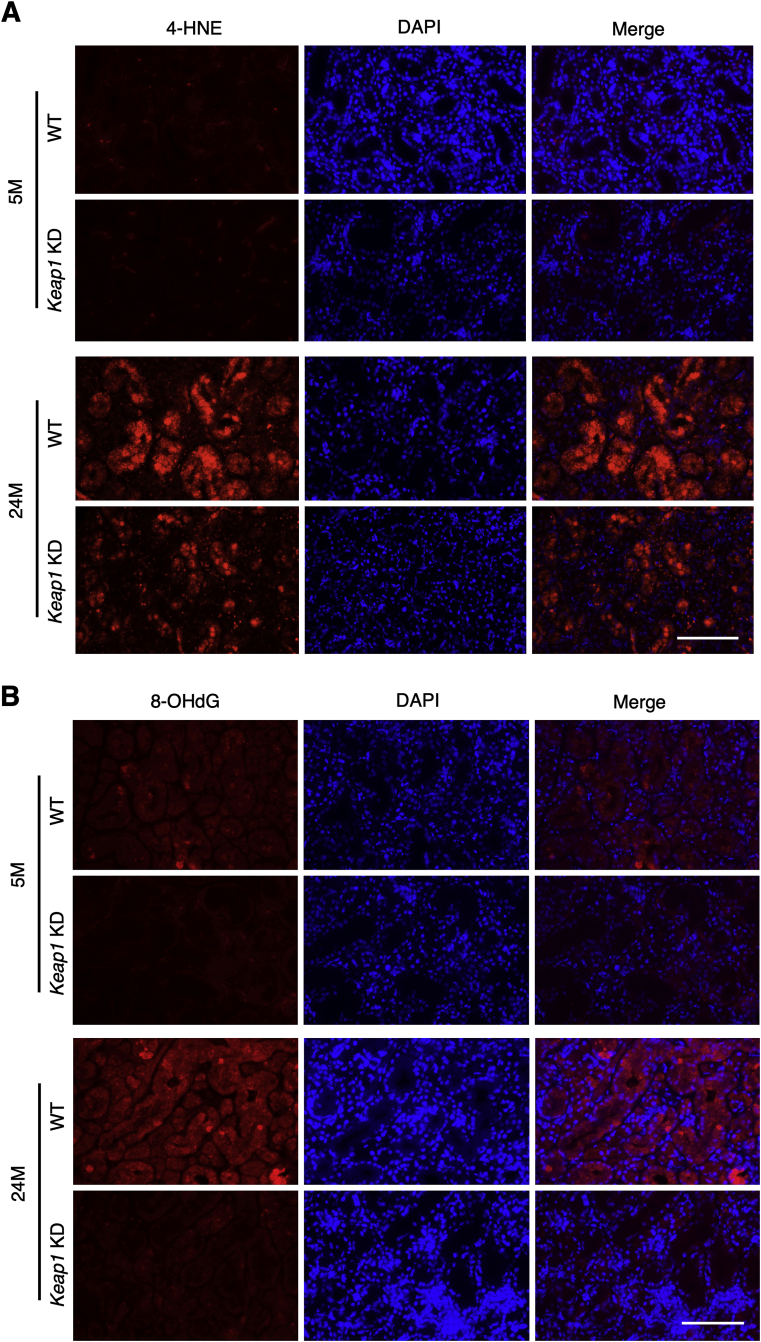
Since NRF2 is known to exert a potent antioxidant function, NRF2 was expected to reduce DNA damage and apoptosis via regulation of oxidative stress. We examined the oxidative stress markers 4-hydroxynonenal (4-HNE) and 8-OHdG by immunofluorescence. At 5 months, 4-HNE staining was similarly weak in WT and Keap1 KD submandibular glands (Fig. 7A). At 24 months, WT submandibular glands showed markedly intense staining, whereas the staining intensity was much weaker in Keap1 KD submandibular glands. For the staining of 8-OHdG, WT submandibular glands exhibited a dramatic increase in intensity during aging, whereas the staining intensity in Keap1 KD submandibular glands was low and did not show any obvious age-related changes (Fig. 7B). Thus, NRF2 pathway activation by Keap1 knockdown attenuated the age-related increase in oxidative stress in submandibular glands.
Fig. 7.
Evaluation of oxidative stress in the submandibular glands of WT and Keap1 KD mice.
Immunofluorescence with 4HNE (A) and 8OHdG (B) antibodies. The experiments were performed on four samples in each group. Scale bars correspond to 100 μm. 4-HNE; 4-hydroxynonenal, 5 M; 5 month-old mice, 24 M; 24 month-old mice.
4. Discussion
This study unequivocally demonstrated that KEAP1 inhibition and resultant NRF2 pathway activation attenuated the progression of submandibular gland aging. Multiple parameters evaluating the aging of submandibular glands were examined, including fibrosis, iron deposition, cell senescence, inflammation and oxidative stress, and age-related changes in these factors were all attenuated by Keap1 knockdown and consequent NRF2 pathway activation. This is the first report revealing the antiaging effects of the NRF2 pathway in physiological aging. We propose that NRF2-activating intervention maintains oral health by invigorating salivary glands.
Several studies described that NRF2 activity is decreased with age [43,44]. In contrast, we did not observe any significant differences in the expression levels of NRF2 target genes in young and aged submandibular glands. This result suggests that progression of age-related phenotypes detected in this study is not primarily caused by the decline of protective function by NRF2 pathway. Increased production rather than decreased quenching capacity of reactive oxygen species (ROS) is likely to drive the aging phenotypes of submandibular glands.
Higher NRF2 activity was reported to correlate with longer lifespan in various organisms, including C. elegans [45], Drosophila, [13] naked-mole rat [46], and snell dwarf mice [15]. However, hyperactivation of NRF2 was rather unfavorable and shown to accelerate the aging of Drosophila [47]. In line with this report, we previously observed that Keap1 disruption in mouse hematopoietic stem cells accelerated their exhaustion [48]. These results suggest that the extent of NRF2 activation needs to be appropriately adjusted to obtain maximum benefits, such as better maintenance of tissue integrity and organismal fitness. Because whole-body deletion of Keap1 leads to lethality at weaning, tissue-specific Keap1 disruption is a way to examine an impact of NRF2 activation on the age-related changes in the tissue homeostasis. Compared with the tissue-specific Keap1 knockout mice, Keap1 KD mice, which we used in this study, exhibit mild activation of NRF2 in the tissue of interest. This may be a reason why we observed beneficial effects of NRF2 pathway activation on the submandibular gland during aging.
Chronic smoldering inflammation is one of the important factors associated with aging phenotypes and aging-related diseases [[49], [50], [51]]. We indeed observed that infiltration of CD45-positive cells and the expression of proinflammatory cytokine genes were increased in aged salivary glands. As expected from the well-documented potent anti-inflammatory action of NRF2 [22,35], the inflammation parameters were significantly reduced in the aged submandibular glands by systemic KEAP1 reduction and consequent NRF2 activation. However, we have not determined in which cell lineages NRF2 substantially contributes to the anti-inflammatory and antiaging effects in the submandibular glands of Keap1 KD mice. NRF2 in acinar and ductal cells of submandibular glands may have delayed the development of aging phenotypes. Alternatively, vascular endothelial cells may have maintained the healthy condition of the parenchymal cells, considering a previous report that describes systemic antiaging effects of endothelial cell-specific inhibition of inflammatory signaling mediated by NFkB [52].
It should be noted that the age-related increase in iron deposition was attenuated in Keap1 KD submandibular glands. The iron homeostatic system is altered during aging, and disturbance in iron regulation is closely related to aging phenotypes in various organs, such as the brain [[53], [54], [55]], liver [56], kidney [57], and muscle [58,59]. Iron is considered a prooxidant and catalyst for the formation of ROS in biological systems [60,61], and iron-associated oxidative damage often underlies aging-related diseases [58]. NRF2 directly activates genes encoding ferritin heavy chain and light chain (Fth and Ftl) [62], which are subunits of ferritin, an iron-binding protein complex responsible for iron storage and, at the same time, for protection of cellular components from ROS generated by the Fenton reaction triggered by unbound iron ion. NRF2 also directly activates Slc40a1 [63], whose product ferroportin is an iron transporter that plays a role in cellular iron release. With these activities, we suppose that NRF2 activation decreases iron-derived ROS generation in addition to increasing antioxidant capacity for quenching ROS, resulting in the suppression of age-related increases in ROS in old submandibular glands [64,65].
In this study, we could not detect significant alterations in salivary gland function due to aging in either WT or Keap1 KD mice (unpublished observation). This may be because precise quantification of basal saliva secretion that is mainly performed by submandibular glands was rather difficult due to the small volume. Other salivary glands, such as the parotid and sublingual glands, may compensate for the reduced saliva secretion from the submandibular glands, as histological alterations of parotid and sublingual glands were not as obvious as those of submandibular glands at 19–24 months of age (unpublished observation). Functional alterations could be detected in mice older than those we examined in this study.
Considering the multiple roles of saliva, such as helping with digestion, swallowing and taste perception and its antibacterial/antifungal activities, maintaining the structural and functional integrity of the salivary glands is essential for not only oral health but also systemic health. Phytochemicals, such as sulforaphane from broccoli sprouts and phenethyl isothiocyanate from watercress [66], are expected to be effective for attenuating the aging phenotypes of salivary glands by inducing moderate levels of NRF2 activation. Intriguingly, anethole trithione, which has been shown to increase salivary flow and clinically used for the treatment of hyposalivazation [67,68], has an NRF2 inducing activity [69]. The stimulatory effect of anethole trithione on the salivary gland may depend on NRF2.
5. Conclusions
KEAP1 inhibition and resultant NRF2 pathway activation attenuated the progression of submandibular gland aging in mice. NRF2-activating intervention is expected to maintain oral health by invigorating salivary glands. For this purpose, phytochemicals, such as sulforaphane contained in broccoli sprout, would be a preferable reagent inducing moderate levels of NRF2 activation.
Author contributions
S.M.W. conducted the experiments, analyzed the data and wrote the paper. D.M. designed the study, provided critical biomaterials and analyzed the data. H.M. designed the study, supervised the research, analyzed the data and wrote the paper.
Declaration of competing interest
The authors declare no competing financial or non-financial interests.
Acknowledgments
We thank Prof. Masi Yamamoto for providing us Keap1 knockdown mice, Ms. Nao Ota for the support of mouse breeding and the Biomedical Research Cores of the Tohoku University Graduate School of Medicine and Institute of Development, Aging and Cancer for their technical supports. This work was supported by JSPS [grant numbers 19K07361 (DM), 18H02621 (HM) and 18H04794 (HM)], the Naito Foundation (HM), a research grant from the Princess Takamatsu Cancer Research Fund [grant number 15-24728 (HM)], the Uehara Memorial Foundation (HM), AMED [grant number JP19gm5010002 (HM)] and Japan-Sweden Research Cooperative Program between JSPS and STINT [grant number JPJSBP120195402 (HM)]. The funders had no role in the study design, data collection and analysis, decision to publish or manuscript preparation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101603.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sejdini M. The effect of Ca and Mg concentrations and quantity and their correlation with caries intensity in school-age children. Int. J. Dent. 2018;2018:2759040. doi: 10.1155/2018/2759040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porcheri, Mitsiadis Physiology, pathology and regeneration of salivary glands. Cells. 2019;8:976. doi: 10.3390/cells8090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner M.D., Ship J.A. Dry mouth and its effects on the oral health of elderly people. J. Am. Dent. Assoc. 2007;138:S15–S20. doi: 10.14219/jada.archive.2007.0358. [DOI] [PubMed] [Google Scholar]
- 4.Satoh-Kuriwada S. Hyposalivation strongly influences hypogeusia in the elderly. J. Health Sci. 2009;55:689–698. [Google Scholar]
- 5.Islas-Granillo H. Relationship of hyposalivation and xerostomia in Mexican elderly with socioeconomic, sociodemographic and dental factors. Sci. Rep. 2017;7:40686. doi: 10.1038/srep40686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Affoo R.H., Foley N., Garrick R., Siqueira W.L., Martin R.E. Meta-analysis of salivary flow rates in young and older adults. J. Am. Geriatr. Soc. 2015;63:2142–2151. doi: 10.1111/jgs.13652. [DOI] [PubMed] [Google Scholar]
- 7.Scott J. Quantitative age changes in the histological structure of human submandibular salivary glands. Arch. Oral Biol. 1977;22:221–227. doi: 10.1016/0003-9969(77)90158-3. [DOI] [PubMed] [Google Scholar]
- 8.Scott J., Flower E.A., Burns J. A quantitative study of histological changes in the human parotid gland occurring with adult age. J. Oral Pathol. Med. 1987;16:505–510. doi: 10.1111/j.1600-0714.1987.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 9.Edrey Y.H., Salmon A.B. Revisiting an age-old question regarding oxidative stress. Free Radic. Biol. Med. 2014;71:368–378. doi: 10.1016/j.freeradbiomed.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.J. Idh2 deficiency accelerates renal dysfunction in aged mice. Biochem. Biophys. Res. Commun. 2017;493:34–39. doi: 10.1016/j.bbrc.2017.09.082. [DOI] [PubMed] [Google Scholar]
- 11.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Salmon A.B., Richardson A., Pérez V.I., Manuscript A. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 2010;48:1–32. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sykiotis G.P., Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sykiotis G.P., Habeos I.G., Samuelson A.V., Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiser S.F., Miller R.A. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol. Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uruno A., Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide - Biol. Chem. 2011;25:153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S.M.U., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Bellezza I. Oxidative stress in age-related macular degeneration: NRF2 as therapeutic target. Front. Pharmacol. 2018;9:1–7. doi: 10.3389/fphar.2018.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Blake D.J. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am. J. Respir. Cell Mol. Biol. 2010;42:524–536. doi: 10.1165/rcmb.2009-0054OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshino T. Protective role of Nrf2 in age-related hearing loss and gentamicin ototoxicity. Biochem. Biophys. Res. Commun. 2011;415:94–98. doi: 10.1016/j.bbrc.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi E.H. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:1–14. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honkura Y. NRF2 is a key target for prevention of noise-induced hearing loss by reducing oxidative damage of cochlea. Sci. Rep. 2016;6:1–2. doi: 10.1038/srep19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoh K. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 25.Gounder S.S. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PloS One. 2012;7:e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecker L. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narasimhan M. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radic. Biol. Med. 2014;71:402–414. doi: 10.1016/j.freeradbiomed.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarantini S. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2018;73:853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulop G.A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. GeroScience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn B. Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol. 2018;17:47–58. doi: 10.1016/j.redox.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valcarcel-Ares M.N. Interaction of obesity and Nrf2 deficiency accelerates cerebromicrovascular aging and impairs neurovascular coupling responses. Faseb. J. 2017;31(No. 1_Supplement) Abstract Number: 681.5. [Google Scholar]
- 32.Okawa H. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagishita Y., Uruno A., Chartoumpekis D.V., Kensler T.W., Yamamoto M. Nrf2 represses the onset of type 1 diabetes in non-obese diabetic mice. J. Endocrinol. 2019;240:403–416. doi: 10.1530/JOE-18-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki T. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol. Cell Biol. 2017;37:1–18. doi: 10.1128/MCB.00063-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J.H., Hales C.N., Ozanne S.E. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res. 2007;35:7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumacher B., Garinis G.A., Hoeijmakers J.H.J. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Hoeijmakers J.H.J. DNA damage, aging, and cancer. N. Engl. J. Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 39.Soares J.P. Aging and DNA damage in humans: a meta-analysis study. Aging (Albany. NY) 2014;6:432–439. doi: 10.18632/aging.100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maynard S., Fang E.F., Scheibye-Knudsen M., Croteau D.L., Bohr V.A. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Med. 2015;5:a025130. doi: 10.1101/cshperspect.a025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ou H.L., Schumacher B. DNA damage responses and p53 in the aging process. Blood. 2018;131:488–495. doi: 10.1182/blood-2017-07-746396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.da Silva P.F.L., Schumacher B. DNA damage responses in ageing. Open Biol. 2019;9:190168. doi: 10.1098/rsob.190168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suh J.H. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corenblum M.J. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging Cell. 2016;15:725–736. doi: 10.1111/acel.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S.K., Tedesco P.M., Johnson T.E. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 2009;8:258–269. doi: 10.1111/j.1474-9726.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis K.N. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc. Natl. Acad. Sci. U.S.A. 2015;112:3722–3727. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsakiri E.N. Hyperactivation of Nrf2 increases stress tolerance at the cost of aging acceleration due to metabolic deregulation. Aging Cell. 2019;18:1–15. doi: 10.1111/acel.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murakami S. NRF2 activation impairs quiescence and bone marrow reconstitution capacity of hematopoietic stem cells. Mol. Cell Biol. 2017;37:1–16. doi: 10.1128/MCB.00086-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franceschi C., Campisi J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 51.Lasry A., Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Hasegawa Y. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125:1122–1133. doi: 10.1161/CIRCULATIONAHA.111.054346. [DOI] [PubMed] [Google Scholar]
- 53.Ramos P. Iron levels in the human brain: a post-mortem study of anatomical region differences and age-related changes. J. Trace Elem. Med. Biol. 2014;28:13–17. doi: 10.1016/j.jtemb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Ashraf A., Clark M., So P.W. The aging of iron man. Front. Aging Neurosci. 2018;10:1–23. doi: 10.3389/fnagi.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams R., Buchheit C.L., Berman N.E.J., Levine S.M. Pathogenic implications of iron accumulation in multiple sclerosis. J. Neurochem. 2012;120:7–25. doi: 10.1111/j.1471-4159.2011.07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arruda L.F., Arruda S.F., Campos N.A., de Valencia F.F., Siqueira E.M. de A. Dietary iron concentration may influence aging process by altering oxidative stress in tissues of adult rats. PloS One. 2013;8:e61058. doi: 10.1371/journal.pone.0061058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bloomer S.A., Brown K.E., Kregel K.C. Renal iron accumulation and oxidative injury with aging: effects of treatment with an iron chelator. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:680–684. doi: 10.1093/gerona/glz055. [DOI] [PubMed] [Google Scholar]
- 58.Xu J., Knutson M.D., Carter C.S., Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PloS One. 2008;3:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Picca A. Advanced age is associated with iron dyshomeostasis and mitochondrial DNA damage in human skeletal muscle. Cells. 2019;8:1525. doi: 10.3390/cells8121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 61.Bresgen N., Eckl P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules. 2015;5:808–847. doi: 10.3390/biom5020808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pietsch E.C., Chan J.Y., Torti F.M., Torti S.V. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J. Biol. Chem. 2003;278:2361–2369. doi: 10.1074/jbc.M210664200. [DOI] [PubMed] [Google Scholar]
- 63.Marro S. Heme controls ferroportin 1 (FPN1) transcription involving Bach 1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95:1261–1268. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerins M.J., Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxidants Redox Signal. 2018;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palliyaguru D.L., Yuan J.M., Kensler T.W., Fahey J.W. Isothiocyanates: translating the power of plants to people. Mol. Nutr. Food Res. 2018;62:1–23. doi: 10.1002/mnfr.201700965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamada T. Treatment of xerostomia with the bile secretion-stimulating drug anethole trithione: a clinical trial. Am. J. Med. Sci. 1999;318:146–151. doi: 10.1097/00000441-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Nagano T., Takeyama M. Enhancement of salivary secretion and neuropeptide (substance P, α-calcitonin gene-related peptide) levels in saliva by chronic anethole trithione treatment. J. Pharm. Pharmacol. 2001;53:1697–1702. doi: 10.1211/0022357011778098. [DOI] [PubMed] [Google Scholar]
- 69.Holland R. Hydrogen peroxide is a second messenger in phase 2 enzyme induction by cancer chemopreventive dithiolethiones. Chem. Res. Toxicol. 2009;22:1427–1434. doi: 10.1021/tx900110n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.