Abstract
Introduction
We aimed to define prodromal Alzheimer's disease (AD) and AD dementia using normative neuropsychological data in a large population‐based cohort of adults with Down syndrome (DS).
Methods
Cross‐sectional study. DS participants were classified into asymptomatic, prodromal AD and AD dementia, based on neurologist's judgment blinded to neuropsychological data (Cambridge Cognitive Examination for Older Adults with Down's syndrome [CAMCOG‐DS] and modified Cued Recall Test [mCRT]). We compared the cutoffs derived from the normative data in young adults with DS to those from receiver‐operating characteristic curve (ROC) analysis.
Results
Diagnostic performance of the CAMCOG‐DS and modified Cued Recall Test (mCRT) in subjects with mild and moderate levels of intellectual disability (ID) was high, both for diagnosing prodromal AD and AD dementia (area under the curve [AUC] 0.73–0.83 and 0.90–1, respectively). The cutoffs derived from the normative data were similar to those derived from the ROC analyses.
Discussion
Diagnosing prodromal AD and AD dementia in DS with mild and moderate ID using population norms for neuropsychological tests is possible with high diagnostic accuracy.
Keywords: Down syndrome, Alzheimer's disease, dementia, cognitive testing, assessment, CAMCOG‐DS, Cued Recall Test, normative data
1. INTRODUCTION
Due to advances in medical care, life expectancy has increased significantly in people with Down syndrome (DS), now exceeding 60 years of age. 1 , 2 As a consequence, individuals with DS are now experiencing a high incidence of age‐associated health problems, 3 especially Alzheimer's disease (AD) dementia. Pathological studies show that by the age of 40 years, virtually all individuals with DS have AD neuropathology, 4 and longitudinal studies show that the cumulative incidence of dementia in adults with DS is in excess of 90% by age 65. 1 , 5 Symptomatic AD increases exponentially with age, with a mean age at dementia onset of between 53.7 and 55.8 years, 6 , 7 and approximately 50% of cases of dementia are being diagnosed in the sixth decade of life. 6 , 7 This association between DS and AD is explained mainly by the presence of an extra copy of the amyloid precursor protein (APP) gene, located on chromosome 21. 8
The diagnosis of prodromal AD and AD dementia in DS is a major challenge. Early symptoms of AD can be mistaken as part of the lifelong intellectual disability (ID), or they may be overlooked or misdiagnosed. In the general population, the diagnosis of mild cognitive impairment requires a change in cognition reported by the patient and/or caregiver, and is based on cognitive performance on neuropsychological tests relative to population norms, and dementia is diagnosed when cognitive decline affects the activities of daily living. In people with DS, the variable degree of ID problematizes these definitions. Furthermore, most test batteries commonly used in the general population are of limited use in DS, as many individuals score at floor and noncompletion rates are high. 9 , 10
Adapted tests have been developed to detect cognitive decline in DS, such as the Down Syndrome Mental Status Examination (DSMSE), the Test for Severe Impairment (TSI), the Cambridge Cognitive Examination for Older Adults with Down's Syndrome (CAMCOG‐DS), and the Arizona Cognitive Test Battery (ACTB) or the modified Cued Recall Test (mCRT). 3 , 9 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 However, unlike in the general population, in adults with DS we are lacking normative data due to the large sample sizes needed to account for the different levels of ID and its associated variability in cognitive abilities. Furthermore, although the diagnostic performance of several adapted tests has been assessed, most of the studies have used small sample sizes and did not take into account the level of ID. Therefore, the diagnosis of prodromal AD or AD dementia using neuropsychological tests in this population at the cross‐sectional level is difficult and cannot be made reliably on the basis of neuropsychological tests using population norms. 20
Taking advantage of the Down Alzheimer Barcelona Neuroimaging initiative (DABNI), a large population‐based cohort of adults with DS, the purpose of our study was to define population norms stratified by level of ID for the CAMCOG‐DS and the mCRT, a cognitive battery and an episodic memory test widely used in DS, and to assess their performance to diagnose prodromal AD and AD dementia in adults with DS.
2. METHODS
2.1. Participants
Single‐center cross‐sectional study. Adults with DS were recruited from February 1, 2013 to December 31, 2018 at the Alzheimer‐Down Unit from the Catalan Down Syndrome Foundation and Hospital de la Santa Creu i Sant Pau, in Barcelona, Spain. The Alzheimer‐Down Unit leads a population‐based health plan for adults with DS, which includes yearly neurological and neuropsychological assessments. All adults (≥18 years) with DS were eligible, irrespective of sex or level of ID. Patients showing any psychiatric or medical disorder that could affect cognition and/or functionality were excluded, as well as those with incomplete neuropsychological examinations (flow chart in Figure 1).
FIGURE 1.
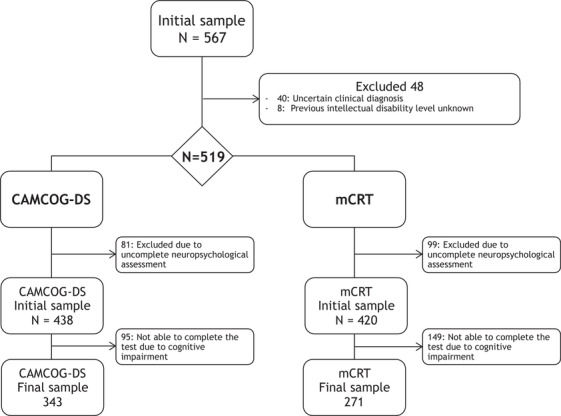
Study flow chart. mCRT, modified Cued Recall Test; CAMCOG‐DS, Cambridge Cognitive Examination for Older Adults with Down's Syndrome
The study was approved by the Sant Pau Ethics Committee following the standards for medical research in humans recommended by the Declaration of Helsinki and reported to the Minister of Justice according to the Spanish law for research in people with intellectual disabilities. All participants or their legally authorized representative gave written informed consent before enrollment.
2.2. Neurological assessment
The study procedures included a complete neurological examination with the participant and his/her main caregiver. The neurologist performed a physical exam, a structured medical history based on the DS‐Connect questionnaire, 21 a neurological exam, and a semi‐structured health questionnaire (Cambridge Examination for Mental Disorders of Older People with Down's Syndrome and others with intellectual disabilities [CAMDEX‐DS]) with the caregiver. The CAMDEX‐DS is a diagnostic tool based upon CAMDEX‐R and modified for the detection of dementia in people with ID. 20 It is also adapted and validated for the Spanish population with ID. 22
2.3. Neuropsychological assessment
The neuropsychological test battery for detecting dementia included the Cambridge Cognitive Examination for Older Adults with Down's Syndrome (CAMCOG‐DS) Spanish version 22 and the mCRT, 18 both directly administered to the patient. It also included the Dementia Questionnaire for People with Learning Disabilities (DLD), an informant‐based questionnaire to assess cognitive and functional decline due to dementia. 23
The CAMCOG‐DS has a maximum score of 109 points and comprises subscales for the following cognitive domains: orientation, language, memory, attention, praxis, abstract thinking, and perception.
The mCRT is an adapted test used to assess episodic memory in people with ID. Participants are asked to memorize 12 stimuli presented on three, 4‐item cards. The test consists of three trials of free and cued recall performed immediately after the learning phase to compute a free recall score and a total score (free recall + cued recall). For the present study we used the total score (maximum score of 36), as it was shown to be more sensitive to detect memory decline in DS. 19
2.4. Level of ID
The level of ID was categorized according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐V) as mild, moderate, severe, or profound, and was based on caregivers’ reports of the individuals’ best‐ever level of functioning. The Kauffman Brief Intelligence Test (K‐BIT) was also included to assess pre‐morbid intelligence level.
2.5. Diagnostic categories
In our center, participants with DS are initially clinically classified by neurologists and neuropsychologists in a consensus meeting after independent visits into the following diagnostic categories: (1) asymptomatic (aDS), when there is no clinical or neuropsychological suspicion of AD; (2) prodromal AD (pDS), when there is a suspicion of AD, but symptoms do not fulfill criteria for dementia; (3) AD dementia (dDS) in those subjects with DS with full blown dementia; and (4) uncertain, including those patients with medical, pharmacological, or psychiatric conditions significantly interfering in cognition and/or functional level. As mentioned previously, patients with an uncertain diagnosis were excluded from the analysis. It is important to note that to avoid circularity, in this study we used the initial neurologist's diagnosis blinded to neuropsychological assessment.
RESEARCH IN CONTEXT
Systematic review: Literature was reviewed through PubMed and meeting abstracts. Due to the variability of intellectual functioning in people with Down syndrome (DS), there are no accepted population‐based neuropsychological normative data and very few studies investigating the diagnostic performance of cognitive tests for diagnosing prodromal Alzheimer's disease (AD) and AD dementia in this population.
Interpretation: In a large population‐based cohort of adults with DS with mild and moderate levels of intellectual disability (ID), neuropsychological normative data for Cambridge Cognitive Examination for Older Adults with Down's Syndrome (CAMCOG‐DS) and modified Cued Recall Test (mCRT) were provided. We show that a diagnosis of prodromal AD and AD dementia can be done with high diagnostic accuracy.
Future directions: Our results support the use of the CAMCOG‐DS and mCRT as screening tools for the diagnosis of AD in people with DS with mild and moderate ID. Our cutoffs could be used for screening purposes in both clinical practice and research settings.
2.6. Statistical analysis
All statistical analyses were done in R version 3.4.3. First, to assess the applicability of the tests, we analyzed the completion rates by level of ID and clinical status. Second, we compared the neuropsychological performance between clinical groups stratifying by the level of ID using an analysis of variance (ANOVA) for normal variables or Kruskal‐Wallis for non‐normal variables. Third, we studied the relationship of the different neuropsychological tests with age. For this purpose, we fitted a local regression model stratified by level of ID. 7 Finally, to define cutoffs for the neuropsychological tests, we used two different approaches. The first approach consisted in defining the scores at percentile ranks of 1st, 5th, and 10th in the young (age ≤ 35) asymptomatic DS participants in mild and moderate ID separately. 24 This decision was based on the fact that all subjects with DS show the characteristic neuropathological signs of AD by the fourth decade of life. Subjects >35 years were expected to have AD neuropathology and thus could have undergone cognitive deterioration. The second approach consisted of using the receiver‐operating characteristic (ROC) curve analyses. The optimal cut‐point was determined using the Index of Union (IU) method, which is defined as the value whose sensitivity and specificity are the closest to the value of the area under the ROC curve and the absolute value of the difference between the sensitivity and specificity values is minimum. The criteria for optimality can change according to the aim of the study. However, as a general rule, minimizing the total misclassification rates is a good approach. With the IU method, since the difference between sensitivity and specificity values is minimum, this condition is met most of the time. 25
3. RESULTS
A total of 567 adults with DS were eligible. Figure 1 shows the study flow chart and the reasons for exclusion, mainly due to an uncertain diagnosis or incomplete neuropsychological assessment. The initial samples for the CAMCOG‐DS and the mCRT were composed of 438 and 420 subjects, respectively. Of note, age and level of ID differed between those subjects included and excluded from the study. Subjects who did not attend the neuropsychological visit were older and had more severe levels of ID (P < .05). Demographic and clinical characteristics of participants from the CAMCOG‐DS and mCRT initial sample are shown in the Appendix.
As expected, participants with prodromal AD and AD dementia were older and had worse scores than asymptomatic subjects on the DLD (P < .001). There were no differences in the number of men and women between the groups. There was a higher proportion of subjects with severe/profound ID in the group with AD dementia.
3.1. Completion rates by level of ID and clinical diagnosis
Figure 2 shows the CAMCOG‐DS and mCRT completion rates by level of ID and clinical diagnosis. The most common reasons for not completing the test were not understanding the task and/or test instructions and/or severe attentional difficulties.
FIGURE 2.
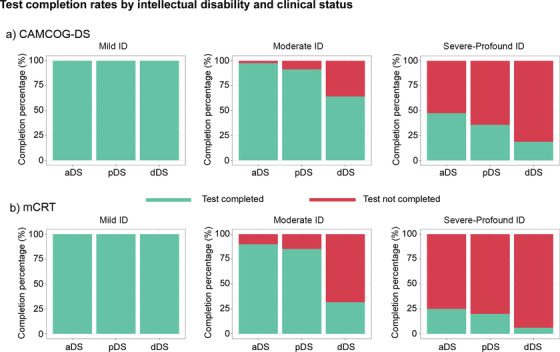
Completion rates for the CAMCOG‐DS and mCRT by level of intellectual disability and by diagnostic group. mCRT, modified Cued Recall Test; CAMCOG‐DS, Cambridge Cognitive Examination for Older Adults with Down's Syndrome; ID, intellectual disability; aDS, asymptomatic Down syndrome; pDS, prodromal Down syndrome; dDS, dementia Down syndrome
Overall, of the 438 subjects from the initial CAMCOG‐DS sample, 343 (78.3%) subjects were able to complete the test. Completion rates were lower for the mCRT, where 271 of 420 subjects (64.5%) could complete the task. All subjects with mild ID, regardless of clinical diagnosis, completed the tests, as did the majority of asymptomatic and prodromal AD subjects with moderate levels of ID. Completion rates were lower in those with pDS and dDS than in aDS. None of the subjects with profound ID and only a few subjects with severe ID were able to complete the tests. Therefore, subjects with severe and profound levels of ID were excluded from the subsequent analyses (normative data and ROC analysis).
3.2. Cognitive performance with aging and along the AD continuum by level of ID
Median scores and interquartile range for the cognitive and functional tests in those who completed the tests are presented in Table 1. Participants with mild ID obtained higher scores on the CAMCOG‐DS than subjects with moderate ID in the whole AD continuum (median scores in aDS: 89 vs 75, P < .0001; pDS: 79 vs 60.5, P = .0013; dDS: 76.5 vs 55, P = .021) (Figure 3). Total scores on the mCRT were significantly different between subjects with mild and moderate ID in aDS (median 36 vs 35, respectively, P = .0002), but not in pDS (28 vs 27, respectively, P = .92) and dDS (21 vs 15, respectively, P = .37) participants.
TABLE 1.
Demographic characteristics, median scores, and interquartile range (IQR) for the cognitive and functional tests of the participants who completed the CAMCOG‐DS and the mCRT
| CAMCOG‐DS subgroup | ||||
|---|---|---|---|---|
| aDS | pDS | dDS | TOTAL | |
| N | 277 | 31 | 35 | 343 |
| Sex (F/M) | 132/145 | 18/13 | 19/16 | 168/174 |
| Age (median years [IQR]) | 37 [15.0] | 51 [4.5] | 53 [8.5] | 41 [18.5] |
| ID (Mild/Moderate/Severe+Profound) | 82/159/36 | 5/21/5 | 4/25/6 | 91/205/47 |
| CAMCOG‐DS (median [IQR]) | 78 [25.0] | 58 [24.5] | 45 [26.5] | 73 [30.0] |
| DLD (median [IQR]) | 12 [14.0] | 21[19.5] | 43 [17.8] | 14 [17.0] |
| mCRT subgroup | ||||
| N | 220 | 31 | 20 | 271 |
| Sex (F/M) | 101/119 | 17/14 | 10/10 | 128/143 |
| Age (median years [IQR]) | 36 [15.0] | 51 [5.0] | 54 [7.0] | 39 [18.0] |
| ID (Mild/Moderate/Severe+Profound) | 75/125/20 | 5/23/3 | 5/13/2 | 85/161/25 |
| mCRT (median [IQR]) | 36.0 [2.0] | 27.0 [16.0] | 15.5 [11.3] | 35.0 [4.0] |
| DLD (median [IQR]) | 11 [12.0] | 17.5 [13.5] | 35 [24.0] | 13 [13.0] |
aDS, asymptomatic Down syndrome; CAMCOG‐DS, Cambridge Cognitive Examination for Older Adults with Down's Syndrome; dDS, dementia Down syndrome; DLD, Dementia Questionnaire for People with Learning Disabilities; F, female; ID, intellectual disability; IQR, interquartile range; M, male; mCRT, Modified Cued Recall Test; pDS, prodromal Down syndrome.
FIGURE 3.
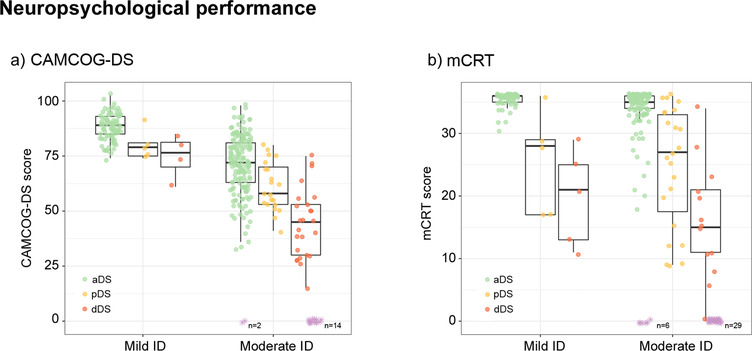
Neuropsychological performance of Down syndrome by clinical groups according to level of intellectual disability. ID, intellectual disability; aDS, asymptomatic Down syndrome; pDS, prodromal Down syndrome; dDS, dementia Down syndrome; mCRT, modified Cued Recall Test. CAMCOG‐DS, Cambridge Cognitive Examination for Older Adults with Down's Syndrome. A subset of participants with moderate ID had difficulties understanding the instructions of the CAMCOG‐DS and/or the mCRT due to cognitive difficulties, and thus received a score of “0” on these tasks (represented in purple at the bottom of the figure)
We found no differences between male and female participants in the cognitive scores. There was an age effect on CAMCOG‐DS and mCRT scores, both in the whole cohort and in aDS individuals (Figure 4). There was a progressive decline on both the CAMCOG‐DS and mCRT after age 40, and especially for the subgroup of participants with moderate ID.
FIGURE 4.
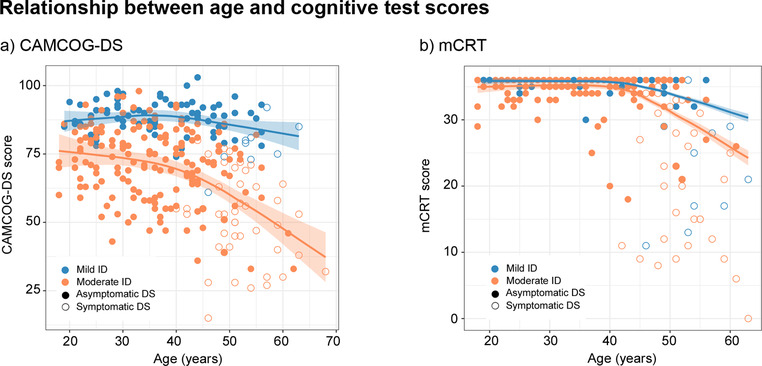
Relationship between age and cognitive scores in subjects with mild and moderate ID for the whole cohort. Footnote: mCRT, modified Cued Recall Test; CAMCOG‐DS, Cambridge Cognitive Examination for Older Adults with Down's Syndrome; ID, intellectual disability; DS, Down syndrome
3.3. Normative data for the CAMCOG‐DS and mCRT in asymptomatic DS individuals
To exclude preclinical AD, we derived normative data in the younger subjects (≤35 years: 107 subjects for the CAMCOG‐DS and 89 subjects for the mCRT). Normative data were generated in aDS individuals in mild and moderate ID separately. Scores corresponding to the 1st, 5th, and 10th percentiles were used to define pathological performances (see Appendix for further details).
For the CAMCOG‐DS, cut‐points corresponding to the 1st, 5th, and 10th percentiles for subjects with mild ID were, respectively, 77, 78, and 80. In subjects with moderate ID, cut‐points corresponding to these percentiles were as follows: 48, 53, and 59, respectively.
For the mCRT, cut‐points for the participants with mild ID were 34 for the 1st percentile, and 35 for the 5th and 10th percentiles. For those subjects with moderate ID, these percentiles corresponded to a score of 30, 32, and 33, respectively.
3.4. Diagnostic performance
To assess the diagnostic performance to detect prodromal AD and AD dementia we performed ROC curve analyses. All subjects with DS with mild ID and a very high proportion of subjects with moderate ID were able to complete the tests. However, a subset of participants with moderate ID and symptomatic AD had difficulties understanding the instructions of the CAMCOG‐DS and/or the mCRT due to cognitive difficulties, and thus received a score of “0” (represented in purple in Figure 3). These subjects were excluded from these analyses due to concerns about construct validity.
Figure 5 shows the ROC analyses. The AUC for CAMCOG‐DS for the aDS versus pDS comparison in mild ID was 0.81 (95% CI 0.55–1), and the optimal cutoff point was 82, with a sensitivity of 80% and a specificity of 80.5%. Comparing aDS versus dDS, we obtained an AUC of 0.91 (95% CI 0.78–1) and an optimal cutoff point of 80, with a sensitivity of 75% and a specificity of 87.8%. In the subgroup of participants with moderate ID, the AUC in the aDS versus pDS comparison was 0.73 (95% CI 0.63–0.83), with a cutoff point of 64, a sensitivity of 66.7%, and a specificity of 72.3%. When comparing aDS versus dDS, we found an AUC of 0.90 (95% CI 0.83–0.97) and a cutoff point of 56, with a sensitivity of 84% and a specificity of 84.3%.
FIGURE 5.
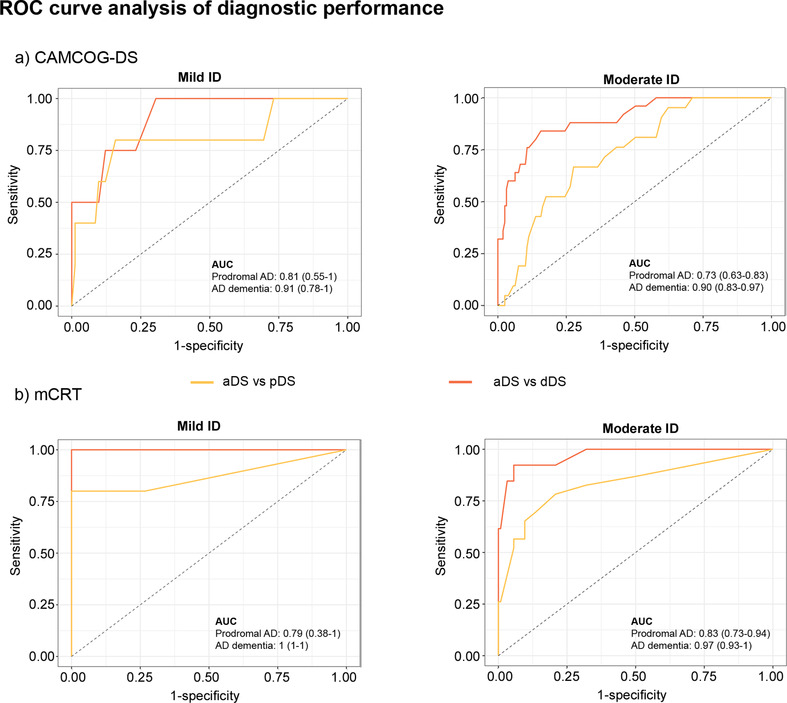
ROC curve analysis of diagnostic performance of CAMCOG‐DS total score (a) and the mCRT total immediate recall score (b) for Down syndrome clinical groups (prodromal DS and dementia DS). pDS, prodromal Down syndrome; dDS, dementia Down syndrome; mCRT, modified Cued Recall Test; CAMCOG‐DS, Cambridge Cognitive Examination for Older Adults with Down's Syndrome; ID, intellectual disability
In the ROC analysis for the mCRT scores (Figure 5), the AUC for the comparisons between aDS and pDS with mild ID was 0.79 (95% CI 0.38–1), with a cutoff point of 35, a sensitivity of 66.7%, and a specificity of 73.3%. Comparing aDS versus dDS, we obtained an AUC of 1 (95% CI 1–1), with a cutoff point of 29, a sensitivity of 100%, and a specificity of 100%. In the subgroup of participants with moderate ID, we obtained an AUC for the comparison between aDS and pDS of 0.83 (95% CI 0.73–0.94) and a cutoff point of 33, with a sensitivity of 78.3% and a specificity of 79.2%. When comparing aDS versus dDS, we found an AUC of 0.97 (95% CI 0.93–1) and a cutoff point of 28, with a sensitivity of 92.3% and a specificity of 94.4%.
Normative values corresponding to the 1st, 5th, and 10th percentiles obtained in young aDS with DS were comparable to those cut‐points obtained by ROC analysis for the detection of symptomatic AD for both CAMCOG‐DS and mCRT (Appendix, Table S2).
4. DISCUSSION
This is the first study to show the applicability of the mCRT and the CAMCOG‐DS in a large population‐based cohort stratified by level of ID, and also the first to show the diagnostic performance to detect prodromal AD and AD dementia. We showed that it is possible to diagnose symptomatic AD using population norms in people with DS through the administration of neuropsychological tests in adults with mild and moderate ID. The cutoff points derived from the normative data were in agreement with the thresholds in the ROC analyses.
Completion rates for the CAMCOG‐DS and mCRT greatly varied by the severity of ID and along the AD continuum, with the mCRT showing lower completion rates. This was an expected result, as these tests were designed to assess cognition in mild and moderate ID. Virtually all aDS with mild and moderate ID completed the tests compared to less than 50% of subjects with severe ID and none of the subjects with profound ID. Similarly, symptomatic AD also affected completion rates. Although most pDS with mild or moderate levels of ID could complete the tests, completion rates decreased in dDS with moderate ID. These results show that assessing cognition in mild to moderate ID is feasible along the AD continuum, but that other instruments with lower floor effects should be used in severe ID.
The level of ID had a greater impact on CAMCOG‐DS scores than on mCRT scores. Subjects with mild ID obtained significantly higher scores on the CAMCOG‐DS than subjects within the moderate range. The mCRT, however, was less sensitive. As in our previous report on the natural history of AD in DS, cognitive decline was detectable after age 40, especially in moderate ID, 7 in agreement with previous studies. 26 , 27 , 28 The median age at diagnosis in our aforementioned study was 50.2 years for pDS and 53.7 years for dDS. 7 Therefore, cognitive decline is detectable cross‐sectionally 10 years before symptomatic AD and occurs in parallel to hippocampal atrophy, 7 after amyloid and tau biomarkers become abnormal. These results reinforce that most of the decline associated with aging in DS is AD related. It is important to consider that all subjects with DS show the characteristic neuropathological signs of AD by the fourth decade of life. 4 , 29 , 30 For this reason, in order to derive the population norms, we chose the younger individuals (≤35 years).
There was a clear decrease in both the CAMCOG‐DS and mCRT values along the AD continuum. ROC analyses showed good diagnostic performance for the CAMCOG‐DS and the mCRT. The cutoff score for the CAMCOG‐DS derived from the ROC analyses achieved high sensitivity and specificity to diagnose AD dementia in both mild and moderate ID. Prodromal AD could also be diagnosed with good accuracy in mild ID, but not in moderate levels of ID. The mCRT showed higher diagnostic performance than the CAMCOG‐DS. To the best of our knowledge, only one group has reported on CAMCOG‐DS cutoff scores for the diagnoses of AD dementia in people with DS. 22 In the validation study of the CAMDEX‐DS in Spain, Esteba et al. found a cutoff score of 68 in mild ID and 52 in moderate ID, 22 lower than in our study (we obtained cutoff scores of 80 and 56, respectively). The inclusion of subjects with less cognitive decline in our study, which is based on a population health plan with active screening for AD, might explain the discrepancies. Cued recall tasks 31 are commonly used in the general population to differentiate between memory decline related to aging and AD‐related memory deficits. 32 , 33 aDS individuals often performed at ceiling on the total score of the test. It is notable that this test was less sensitive to the level of ID than the CAMCOG‐DS, and the cutoffs were very similar in the mild and moderate levels of ID. This lower variability associated to the level of ID facilitates diagnosis. Cognitive functions have been shown to decline sequentially in people with DS, with different cognitive domains being affected at different stages of the disease. 15 , 34 , 35 A longitudinal study conducted by Krinsky‐McHale et al. 36 found that participants with DS with early stage AD dementia showed severely diminished episodic memory. Again, our cutoff scores of 29 and 28 (for the mild and moderate level of ID, respectively), derived from the ROC analyses, are higher than that reported by Devenny et al., 18 who proposed a provisional cutoff score of 23 to identify dementia due to AD in DS populations. 18 Similar to the CAMCOG‐DS results, these differences could be explained by the inclusion in our sample of subjects with earlier stages of AD, and thus with less impairment in memory functions.
We provide several thresholds for the normative data for the CAMCOG‐DS and the mCRT obtained in younger asymptomatic subjects with DS (aged ≤ 35 years) with mild and moderate levels of ID (1st, 5th, and 10th percentiles). These cutoffs were in agreement with those established for the diagnosis of prodromal AD and AD dementia using ROC analyses (with the exception of the mCRT cutoffs derived in the ROC analyses for AD dementia). CAMCOG‐DS scores below 80 in subjects with mild ID and below 59 in those with moderate ID were indicative of a pathological performance on this test (corresponding to the 10th percentile). In the case of the mCRT, total immediate scores below 35 and 33 for those subjects with mild and moderate ID, respectively, could be considered at risk for symptomatic AD (both prodromal AD and AD dementia). Therefore, the 10th percentile cutoffs could be used for screening purposes in clinical practice. However, to diagnose AD dementia, more stringent thresholds should be used, especially for the CRT.
Taking into account the sensitivity and specificity obtained in both the normative data and from the ROC curve analysis, the cutoff points that we would recommend for the diagnosis of AD dementia in people with DS would be the following: In mild ID, CAMCOG‐DS scores of 80 and mCRT scores of 29; in moderate ID, CAMCOG‐DS scores of 56 and mCRT scores of 28.
The main strengths of this study are the large sample size and the fact that it comes from a large population‐based cohort of adults with DS representative of the DS population in Catalonia. This enabled us to establish robust population‐based norms in young aDS stratified by the level of ID. Another strength is that we could compare the thresholds derived from this approach to those of the ROC analyses, showing that the thresholds were comparable and yielded good diagnostic performance. This study also has some limitations. First, it is based on cross‐sectional data. Second, it is a single‐center study, and thus needs to be replicated in other cohorts and populations to confirm the generalizability of our results. Third, despite the large overall sample size, the number of subjects with prodromal AD and AD dementia with mild ID was reduced. For this reason we did not perform an internal cross‐validation in the ROC approach. Fourth, to avoid circularity, we used the neurologist's initial diagnosis, blinded to neuropsychological assessment, and this could have led to a misidentification of prodromal AD cases as asymptomatic or to the inclusion in the asymptomatic group of subjects with medical, pharmacological, or psychiatric conditions that could have effects on cognition not detected by the neurologist. Of note this might have underestimated the diagnostic performance of the tests. Further studies with longitudinal follow‐up and/or biomarkers would allow for more reliable diagnosis. 37
In summary, our study shows that, similar to the general population, a diagnosis of prodromal AD and AD dementia can be done reliably in adults with DS based on the observation of low levels of cognitive test performance relative to population norms when stratifying by the level of ID.
CONFLICTS OF INTEREST
Dr. Fortea has received compensation for consultancies to Novartis and AC Immune. The remaining authors have no conflicts of interest to declare that are relevant for this article.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors would like to thank all the participants with Down's syndrome, their families, and their carers for their support of and dedication to this research. We also acknowledge the Fundació Catalana Síndrome de Down for global support; Reyes Alcoverro, Marta Salinas, and Tania Martínez for administrative support; Concepción Escolá and Diana Garzón for nursing handling. We also thank the PET imaging technologist, radiographers, technicians, and radiochemists at the Wolfson Brain Imaging Centre, as well as the clinicians for their help in acquiring the data reported in this article.
This study was supported by the Fondo de Investigaciones Sanitario (FIS), Instituto de Salud Carlos III (PI14/01126 and PI17/01019 to Juan Fortea, PI13/01532 and PI16/01825 to Rafael Blesa, PI18/00335 to Maria Carmona‐Iragui, INT19/00016 and PI18/00435 to Daniel Alcolea, and PI14/1561 and PI17/01896 to Alberto Lleó) and the CIBERNED program (Program 1, Alzheimer Disease to Alberto Lleó and SIGNAL study, www.signalstudy.es), partly jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una manera de hacer Europa. This work was also supported by the National Institutes of Health (NIA grants 1R01AG056850–01A1; R21AG056974 and R01AG061566 to Juan Fortea), Departament de Salut de la Generalitat de Catalunya, Pla Estratègic de Recerca i Innovació en Salut (SLT002/16/00408 to Alberto Lleó), Fundació La Marató de TV3 (20141210 to Juan Fortea and 044412 to Rafael Blesa); Fundació Catalana Síndrome de Down and Fundació Víctor Grífols i Lucas partially supported this work. This work was also supported by Generalitat de Catalunya (SLT006/17/00119 to Juan Fortea, SLT006/17/95 to Eduard Vilaplana and SLT006/17/00125 to Daniel Alcolea) and a grant from the Fundació Bancaria La Caixa to Rafael Blesa.
The article describes independent research, and the views expressed are those of the authors and not necessarily those of the funders. The sponsors of the study did not take part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; writing and review of the report; or the decision to submit the article for publication.
Benejam B, Videla L, Vilaplana E, et al. Diagnosis of prodromal and Alzheimer's disease dementia in adults with Down syndrome using neuropsychological tests. Alzheimer's Dement. 2020;12:e12047 10.1002/dad2.12047
Bessy Benejam and Laura Videla have equally contributed to this study.
REFERENCES
- 1. McCarron M, McCallion P, Reilly E, Mulryan N. A prospective 14‐year longitudinal follow‐up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2014;58:61‐70. [DOI] [PubMed] [Google Scholar]
- 2. Bittles AH, Bower C, Hussain R, Glasson EJ. The four ages of Down syndrome. Eur J Public Health. 2007;17:221‐225. [DOI] [PubMed] [Google Scholar]
- 3. Krinsky‐McHale SJ, Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev Disabil Res Rev. 2013;18:31‐42. [DOI] [PubMed] [Google Scholar]
- 4. Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278‐282. [DOI] [PubMed] [Google Scholar]
- 5. Zis P, Strydom A. Clinical aspects and biomarkers of Alzheimer's disease in Down syndrome. Free Radic Biol Med. 2018;114:3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sinai A, Mokrysz C, Bernal J, et al. Predictors of age of diagnosis and survival of Alzheimer's disease in Down syndrome. J Alzheimer's Dis. 2017;61:717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fortea J, Vilaplana E, Carmona‐Iragui M, et al. Clinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: a cross‐sectional study. Lancet. 2020; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiseman FK, Al‐Janabi T, Hardy J, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16:564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. 2019;15:135‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caoimh RO, Clune Y, Molloy DW. Screening for Alzheimer's disease in Downs Syndrome. J Alzheimers Dis Parkinsonism. 2019. 10.4172/2161-0460.S7-001 [DOI] [Google Scholar]
- 11. Tyrrell J, Cosgrave M, Mccarron M, et al. Dementia in people with Down's syndrome. Int J Geriatr Psychiatry. 2001;16:1168‐1174. [DOI] [PubMed] [Google Scholar]
- 12. Ball S L, Holland AJ, Huppert FA, Treppert P, Watson P, Hon J. Personality and behaviour changes mark the early stages of Alzheimer's disease in adults with Down's syndrome: findings from a prospective population‐based study. Int J Geriatr Psychiatry. 2006;21:661‐673. [DOI] [PubMed] [Google Scholar]
- 13. Ballard C, Mobley W, Hardy J, Williams G, Corbett A. Dementia in Down's syndrome. Lancet Neurol. 2016;15:622‐636. [DOI] [PubMed] [Google Scholar]
- 14. Hithersay R, Hamburg S, Knight B, Strydom A. Cognitive decline and dementia in Down syndrome. Curr Opin Psychiatry. 2017;30:102‐107. [DOI] [PubMed] [Google Scholar]
- 15. Lautarescu BA, Holland AJ, Zaman SH. The early presentation of dementia in people with Down syndrome: a systematic review of longitudinal studies. Neuropsychol Rev. 2017;27:31‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinai A, Hassiotis A, Rantell K, Strydom A. Assessing specific cognitive deficits associated with dementia in older adults with down syndrome: use and validity of the Arizona Cognitive Test Battery (ACTB). PLoS One. 2016;11:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edgin JO, Anand P, Rosser T, et al. The Arizona cognitive test battery for Down syndrome: test‐retest reliability and practice effects. Am J Intellect Dev Disabil. 2017;122:215‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Devenny DA, Zimmerli EJ, Kittler P, Krinsky‐McHale SJ. Cued recall in early‐stage dementia in adults with Down's syndrome. J Intellect Disabil Res. 2002;46:472‐483. [DOI] [PubMed] [Google Scholar]
- 19. Benejam B, Fortea J, Molina‐López R, Videla S. Patterns of performance on the modified Cued Recall Test in Spanish adults with Down syndrome with and without dementia. Am J Intellect Dev Disabil. 2015;120:481‐489. [DOI] [PubMed] [Google Scholar]
- 20. Ball SL, Holland AJ, Huppert FA, Treppner P, Watson P, Hon J. The modified CAMDEX informant interview is a valid and reliable tool for use in the diagnosis of dementia in adults with Down's syndrome. J Intellect Disabil Res. 2004;48:611‐620. [DOI] [PubMed] [Google Scholar]
- 21. Peprah EK, Parisi MA, Kaeser L, Bardhan S, Oster‐Granite M, Maddox YT. DS‐connect: a promising tool to improve lives and engage Down syndrome communities worldwide. Glob Heart. 2015;10:337‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Esteba‐Castillo S, Dalmau‐Bueno A, Ribas‐Vidal N, Vilà‐Alsina M, Novell‐Alsina R, García‐Alba J. Adaptation and validation of CAMDEX‐DS (Cambridge Examination for Mental Disorders of Older People with Down's Syndrome and others with intellectual disabilities) in Spanish population with intellectual disabilities. Rev Neurol. 2013;57:337‐346. [PubMed] [Google Scholar]
- 23. Evenhuis HM. Evaluation of a screening instrument for dementia in ageing mentally retarded persons. J Intellect Disabil Res. 1992;36:337‐347. [DOI] [PubMed] [Google Scholar]
- 24. Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th ed New York, NY: Oxford University Press; 2004. [Google Scholar]
- 25. Unal I. Defining an optimal cut‐point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghezzo A, Salvioli S, Solimando MC, et al. Age‐related changes of adaptive and neuropsychological features in persons with Down syndrome. PLoS One. 2014;9:e113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliver C, Crayton L, Holland A, Hall S, Bradbury J. A four year prospective study of age‐related cognitive change in adults with Down's syndrome. Psychol Med. 1998;28:1365‐1377. [DOI] [PubMed] [Google Scholar]
- 28. Devenny DA, Silverman WP, Hill AL, Jenkins E, Sersen EA, Wisniewski KE. Normal ageing in adults with Down's syndrome: a longitudinal study. J Intellect Disabil Res. 1996;40(Pt 3):208‐221. [PubMed] [Google Scholar]
- 29. Mann DM. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988;43:99‐136. [DOI] [PubMed] [Google Scholar]
- 30. Hof PR, Bouras C, Perl DP, Sparks DL, Mehta N, Morrison JH. Age‐related distribution of neuropathologic changes in the cerebral cortex of patients with Down's syndrome. Quantitative regional analysis and comparison with Alzheimer's disease. Arch Neurol. 1995;52:379‐391. [DOI] [PubMed] [Google Scholar]
- 31. Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6:433‐440. [DOI] [PubMed] [Google Scholar]
- 32. Grober E, Buschke H. Genuine memory deficits in dementia. J Dev Neuropsychol. 1987; 3: 13‐36. [Google Scholar]
- 33. Petersen RC, Smith GE, Ivnik RJ, Kokmen E, Tangalos EG. Memory function in very early Alzheimer's disease. Neurology. 1994;44:867‐872. [DOI] [PubMed] [Google Scholar]
- 34. Devenny DA, Krinsky‐McHale SJ, Sersen G, Silverman WP. Sequence of cognitive decline in dementia in adults with Down's syndrome. J Intellect Disabil Res. 2000;44(pt 6):654‐665. [DOI] [PubMed] [Google Scholar]
- 35. Cosgrave MP, Tyrrell J, McCarron M, Gill M, Lawlor BA. A five year follow‐up study of dementia in persons with Down's syndrome: early symptoms and patterns of deterioration. Ir J Psychol Med. 2000;17:5‐11. [Google Scholar]
- 36. Krinsky‐McHale SJ, Devenny DA, Silverman WP. Changes in explicit memory associated with early dementia in adults with Down's syndrome. J Intellect Disabil Res. 2002;46:198‐208. [DOI] [PubMed] [Google Scholar]
- 37. Fortea J, Carmona‐Iragui M, Benejam B, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer's disease in adults with Down syndrome: a cross‐sectional study. Lancet Neurol. 2018;17:860‐869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


